Impact of Transglutaminase-Mediated Crosslinking on the Conformational Changes in a Dual-Protein System and IgE Reactivity of Soy Protein
Abstract
:1. Introduction
2. Results and Discussion
2.1. SDS-PAGE
2.2. TGase Crosslinking on the Ultraviolet Spectrum of Dual-Protein Samples
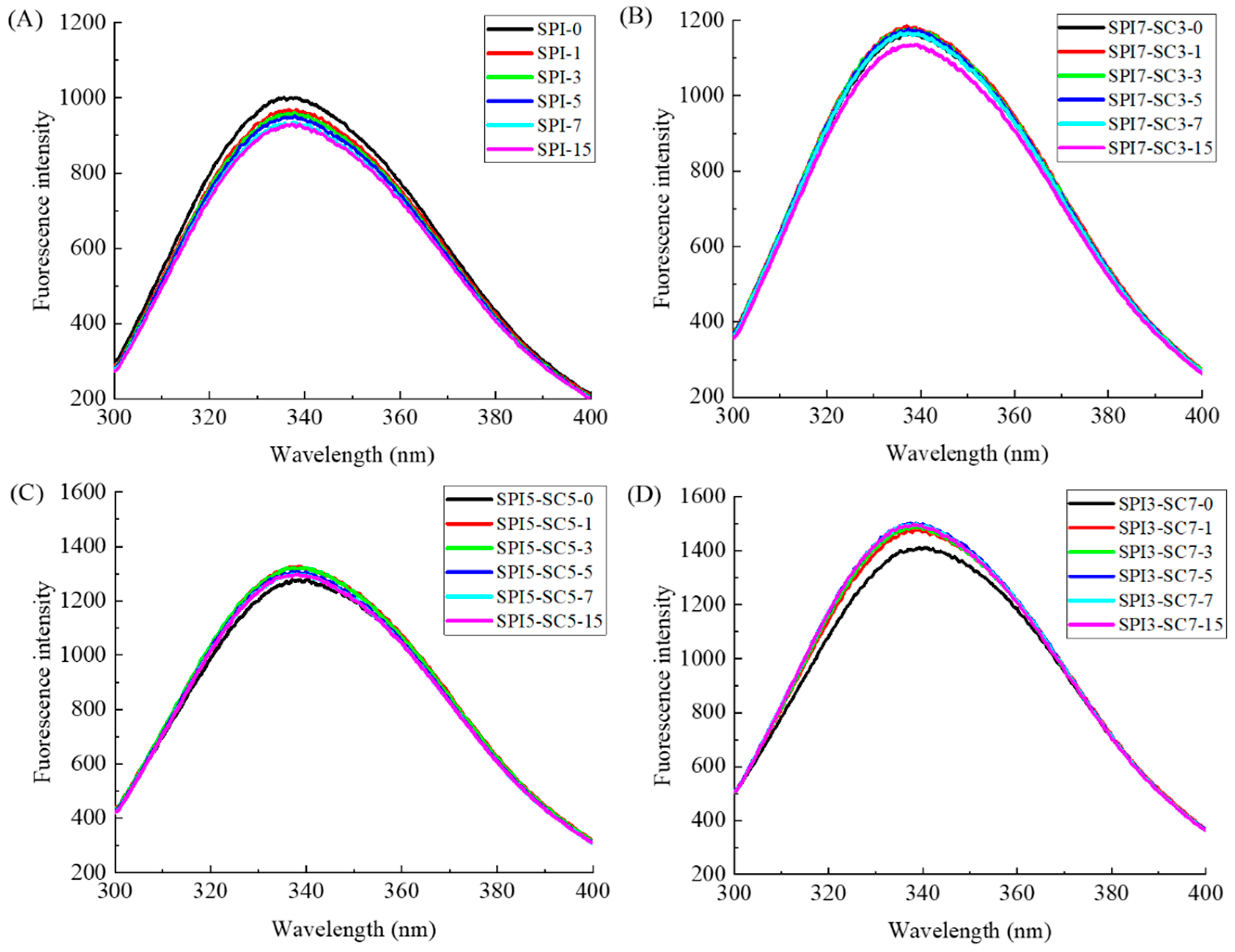
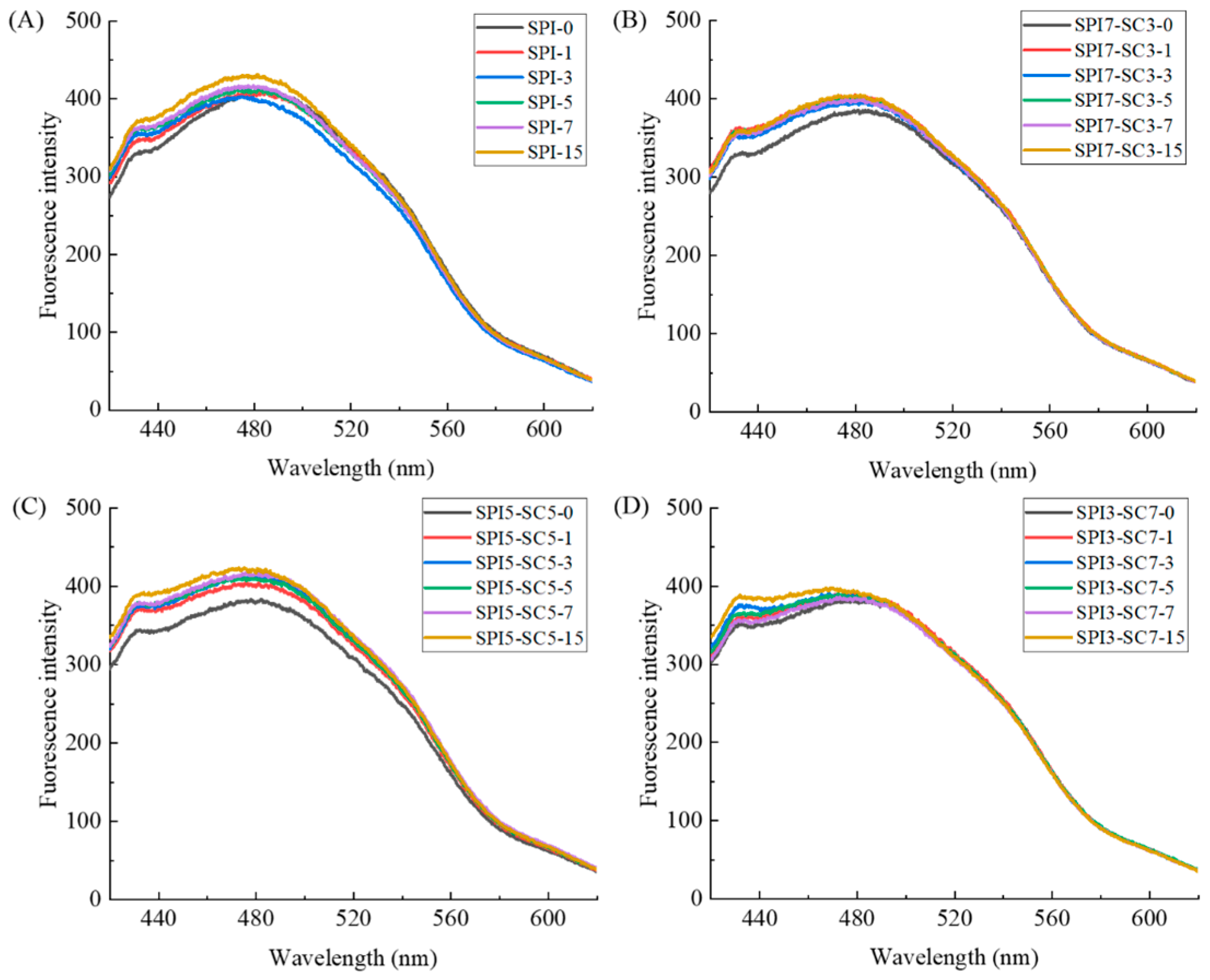
2.3. Endogenous Fluorescence Spectroscopic Analysis
2.4. Surface Hydrophobicity Analysis
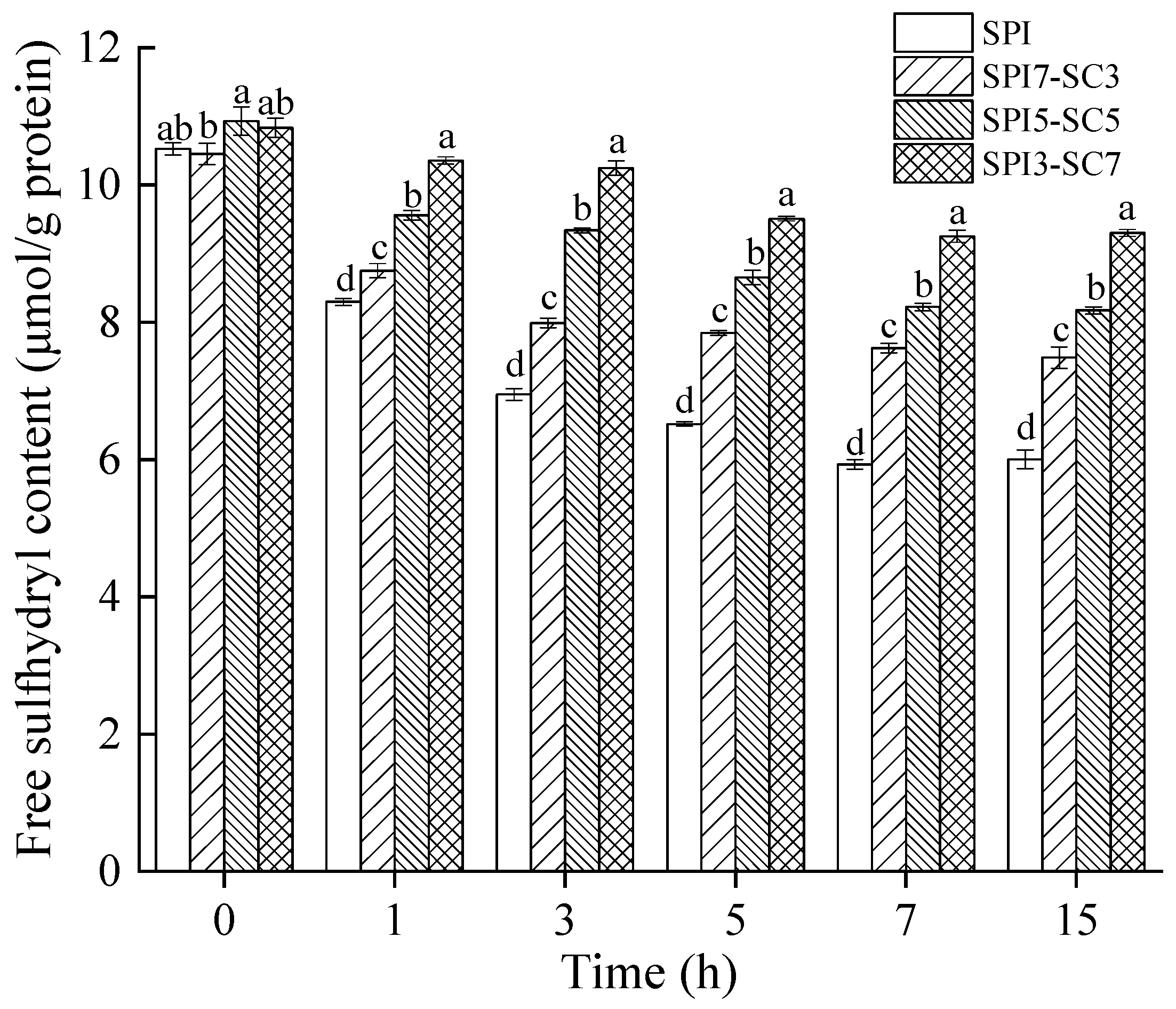
2.5. Quantification of Free Sulfhydryl (-SH)
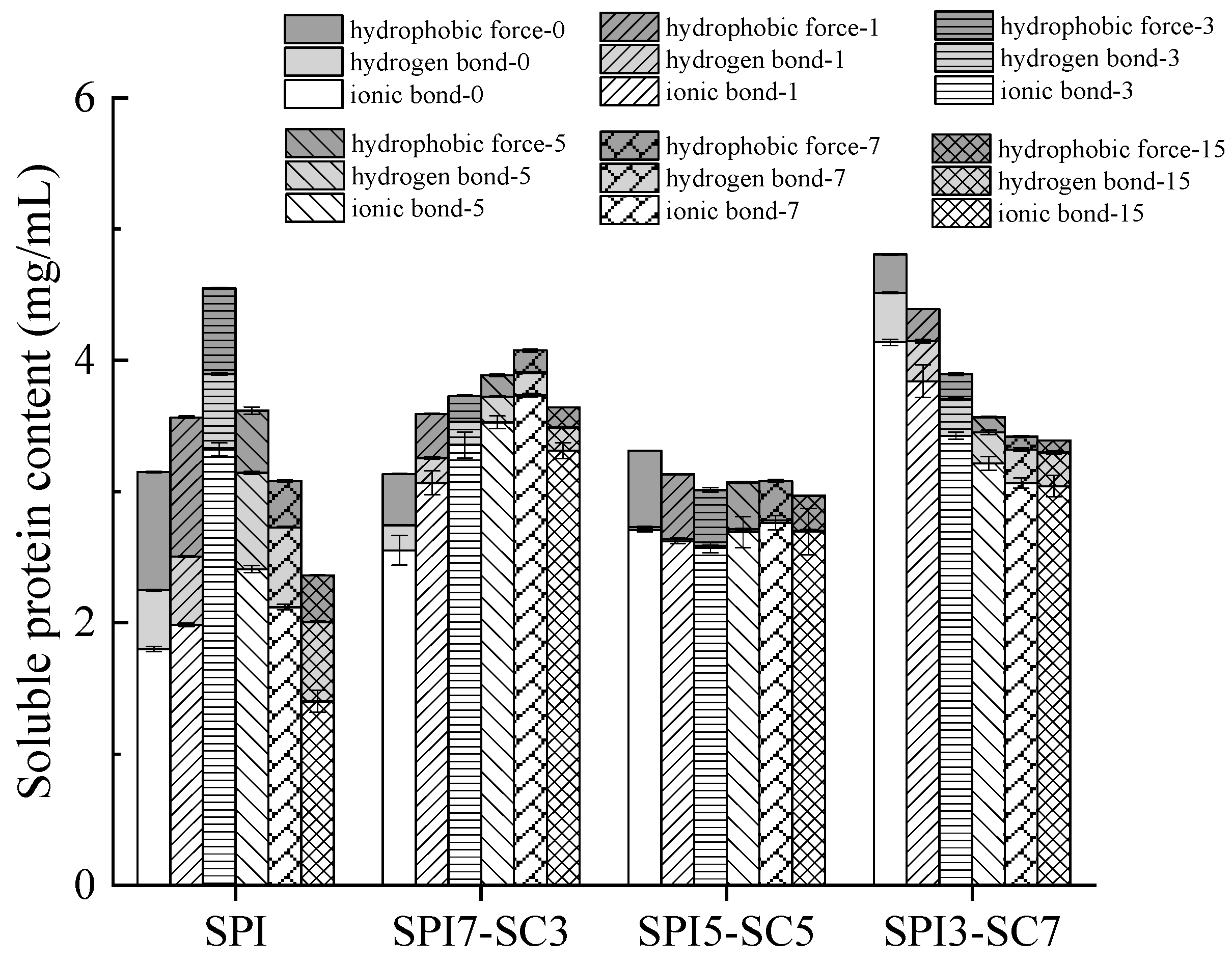
2.6. Interactions between Proteins
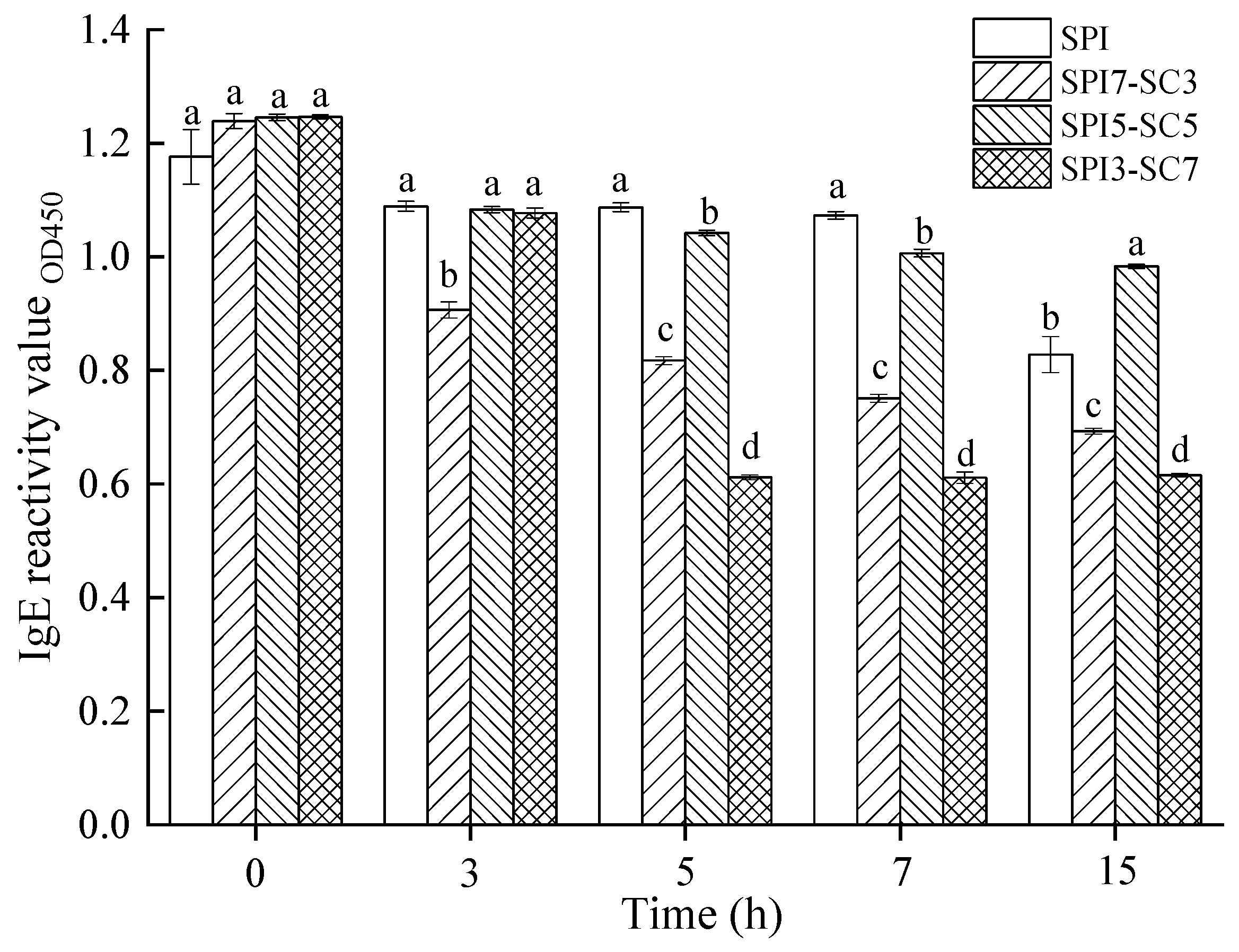
2.7. IgE-Binding Capacity of Soy Proteins in Dual-Protein System
3. Materials and Methods
3.1. Materials
3.2. Crosslinked Dual-Protein Samples Preparation
3.3. Analysis of Protein Composition via Electrophoresis
3.4. Spectrophotometric Analysis of Ultraviolet (UV) Absorption
3.5. Analysis of Fluorescence Spectra in Dual-Protein Samples
3.6. Assessment of Surface Hydrophobicity
3.7. Determination of Free Sulfhydryl (-SH) Group Content
3.8. Elucidation of Intermolecular Interactions
3.9. Immunoassay for Assessing IgE Reactivity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guyomarc’h, F.; Arvisenet, G.; Bouhallab, S.; Canon, F.; Deutsch, S.M.; Drigon, V.; Gagnaire, V. Mixing milk, egg and plant resources to obtain safe and tasty foods with environmental and health benefits. Trends Food Sci. Technol. 2021, 108, 119–132. [Google Scholar] [CrossRef]
- Wu, C.; Wang, T.; Ren, C.; Ma, W.; Wu, D.; Xu, X.; Wang, L.; Du, M. Advancement of food-derived mixed protein systems: Interactions, aggregations, and functional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 627–651. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Silva, D.D.; Singh, C.; Arasi, S.; Muraro, A.; Zuberbier, T.; Ebisawa, M.; Lozano, M.A.; Roberts, G. Systematic review of monotherapy with biologicals for children and adults with IgE-mediated food allergy. Clin. Transl. Allergy 2022, 12, e12123. [Google Scholar] [CrossRef]
- Rui, X.; Huang, J.; Xing, G.; Zhang, Q.; Li, W.; Dong, M. Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains. LWT-Food Sci. Technol. 2019, 99, 156–165. [Google Scholar] [CrossRef]
- Do, A.B.; Williams, K.; Toomer, O.T. In vitro digestibility and immunoreactivity of bovine milk proteins. Food Chem. 2016, 190, 581–587. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Fu, G.; Wu, Z.; Cheng, J. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.; Mafra, I. Bovine milk allergens: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, H.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Effect of microbial transglutaminase cross-linking on the quality characteristics and potential allergenicity of tofu. Food Funct. 2019, 10, 5485–5497. [Google Scholar] [CrossRef]
- Fotschki, J.; Wróblewska, B.; Fotschki, B.; Kalicki, B.; Rigby, N.; Mackie, A. Microbial transglutaminase alters the immunogenic potential and cross-reactivity of horse and cow milk proteins. J. Dairy Sci. 2020, 103, 2153–2166. [Google Scholar] [CrossRef]
- Ahmed, I.; Chen, H.; Li, J.; Wang, B.; Li, Z.; Huang, G. Enzymatic crosslinking and food allergenicity: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5856–5879. [Google Scholar] [CrossRef]
- Velazquez-Dominguez, A.; Hiolle, M.; Abdallah, M.; Delaplace, G.; Peixoto, P.P. Transglutaminase cross-linking on dairy proteins: Functionalities, patents, and commercial uses. Int. Dairy J. 2023, 143, 105688. [Google Scholar] [CrossRef]
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and functional analysis of various globulin proteins from soy seed. Crit. Rev. Food Sci. 2015, 55, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, X.; Jiang, S.; Xie, Q.; Mu, G.; Wu, X. Formulation of infant formula with different casein fractions and their effects on physical properties and digestion characteristics. Food Funct. 2022, 13, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, X.; Liu, H.; Liu, Z.; Zhao, K.; Ma, Y.; Zhang, K. Mechanical properties and water sensitivity of soybean protein isolate film improved by incorporation of sodium caseinate and transglutaminase. Prog. Org. Coat. 2021, 153, 106154. [Google Scholar] [CrossRef]
- Hsieh, J.F.; Yu, C.J.; Chang, J.Y.; Chen, S.T.; Tsai, H.Y. Microbial transglutaminase-induced polymerization of β-conglycinin and glycinin in soymilk: A proteomics approach. Food Hydrocoll. 2014, 35, 678–685. [Google Scholar] [CrossRef]
- Tang, T.; Shi, Y.; Yang, S.; Li, J.; Chen, J.; Wu, X.; Xing, G. A comparative study on the physicochemical characteristics and protein digestibility of tofu induced by magnesium chloride or combined with transglutaminase. Int. J. Food Sci. Technol. 2023, 58, 4666–4674. [Google Scholar] [CrossRef]
- Chanyongvorakul, Y.; Matsumura, Y.; Sakamoto, H.; Motoki, M.; Ikura, K.; Mori, T. Gelation of bean 11S globulins by Ca+-independent transglutaminase. Biosci. Biotechnol. Biochem. 1994, 58, 864–869. [Google Scholar] [CrossRef]
- Xing, G.; Giosafatto, C.V.L.; Rui, X.; Dong, M.; Mariniello, L. Microbial transglutaminase-mediated polymerization in the presence of lactic acid bacteria affects antigenicity of soy protein component present in bio-tofu. J. Funct. Foods. 2019, 53, 292–298. [Google Scholar] [CrossRef]
- Lavrinenko, I.A.; Holyavka, M.G.; Chernov, V.E.; Artyukhov, V.G. Second derivative analysis of synthesized spectra for resolution and identification of overlapped absorption bands of amino acid residues in proteins: Bromelain and ficin spectra in the 240–320 nm range. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117722. [Google Scholar] [CrossRef]
- Yang, A.; Xia, J.; Gong, Y.; Deng, H.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Changes in the structure, digestibility and immunoreactivities of glycinin induced by the cross-linking of microbial transglutaminase following heat denaturation. Int. J. Food Sci. Technol. 2017, 52, 2265–2273. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Jin, B. Fluorescence of tryptophan in aqueous solution. Spectrochim. Acta A 2013, 106, 54–59. [Google Scholar] [CrossRef]
- Yan, J.; Yin, L.; Qu, Y.; Yan, W.; Zhang, M.; Su, J.; Jia, X. Effect of calcium ions concentration on the properties and microstructures of doubly induced sorghum arabinoxylan/soy protein isolate mixed gels. Food Hydrocoll. 2022, 133, 107997. [Google Scholar] [CrossRef]
- Babiker, E.E. Effect of transglutaminase treatment on the functional properties of native and chymotrypsin-digested soy protein. Food Chem. 2000, 70, 139–145. [Google Scholar] [CrossRef]
- Meng, Y.; Li, C. Conformational changes and functional properties of whey protein isolate-polyphenol complexes formed by non-covalent interaction. Food Chem. 2021, 364, 129622. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yuan, J.; Wang, P.; Sun, H.; Fan, X.; Wang, Q. Facilitation of α-polylysine in TGase-mediated crosslinking modification for gluten and its effect on properties of gluten films. J. Cereal. Sci. 2017, 73, 108–115. [Google Scholar] [CrossRef]
- Wen, P.; Xia, C.; Zhang, L.; Chen, Y.; Xu, H.; Cui, G.; Wang, J. Effects of different dry heating temperatures on the spatial structure and amino acid residue side-chain oxidative modification of soybean isolated proteins. Food Chem. 2023, 405, 134795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.; Tian, X.; Liu, J.; Ye, H.; Shen, X. Effect of ultrasound pretreatment on structural, physicochemical, rheological and gelation properties of transglutaminase cross-linked whey protein soluble aggregates. Ultrason. Sonochem. 2021, 74, 105553. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, J.H.; Ng, P.K.W. Functional and thermal properties of wheat, barley, and soy flours and their blends treated with a microbial transglutaminase. J. Food Sci. 2005, 70, c380–c386. [Google Scholar] [CrossRef]
- Qin, X.S.; Chen, S.S.; Li, X.J.; Luo, S.Z.; Zhong, X.Y.; Jiang, S.T.; Zhao, Y.Y.; Zheng, Z. Gelation properties of transglutaminase-induced soy protein isolate and wheat gluten mixture with ultrahigh pressure pretreatment. Food Bioprocess Technol. 2017, 10, 866–874. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Mu, D.; Zhao, Y.; Luo, S.; Zhong, X.; Jiang, S.; Li, X.; Zheng, Z. Effect of partial hydrolysis with papain on the characteristics of transglutaminase-crosslinked tofu gel. J. Food Sci. 2018, 83, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.R.; Fan, F.F.; Lv, C.J.; Wang, H.P.; Li, Y.; Hu, S.; Zhao, W.R.; Chen, H.B.; Huang, J.; Mei, L.H. Improving the thermostability and activity of transaminase from Aspergillus terreus by charge-charge interaction. Front. Chem. 2021, 9, 664156. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, L.; Zhou, W.; Pan, J.; Gong, D.; Zhang, G. Effects of baicalein and chrysin on the structure and functional properties of β-lactoglobulin. Foods 2022, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Damodaran, S. In vitro digestibility and IgE reactivity of enzymatically cross-linked heterologous protein polymers. Food Chem. 2017, 221, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bu, G.; Xi, G. Effects of heat treatment on the antigenicity, antigen epitopes, and structural properties of β-conglycinin. Food Chem. 2021, 346, 128962. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, T.; Hui, T.; Chang, Y.; Chen, X.; Xing, G. Effect of transglutaminase crosslinking on the protein structure and potential allergenicity of dual-protein system, with a focus on β-conglycinin. LWT-Food Sci. Technol. 2023, 188, 115417. [Google Scholar] [CrossRef]
- Ni, K.; Gao, Y.; Ye, X. Study on the structure and formation mechanism of 15S globulin of soybeans. Food Hydrocoll. 2021, 113, 106461. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Liu, Y.; Zhang, Q.; Li, W.; Dong, M.; Rui, X. The conformational structural change of soy glycinin via lactic acid bacteria fermentation reduced immunoglobulin E reactivity. Foods 2021, 10, 2969. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, A.; Gao, J.; Chen, H. Characterization of physicochemical properties and IgE-binding of soybean proteins derived from the HHP-treated seeds. J. Food Sci. 2014, 79, C2157–C2163. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, P.; Zou, M.; Yang, R.; Tian, M.; Gu, Z. Microbial transglutaminase-modified protein network and its importance in enhancing the quality of high-fiber tofu with okara. Food Chem. 2019, 289, 169–176. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, G.; Hui, T.; Liu, J.; Yang, S. Impact of Transglutaminase-Mediated Crosslinking on the Conformational Changes in a Dual-Protein System and IgE Reactivity of Soy Protein. Molecules 2024, 29, 3371. https://doi.org/10.3390/molecules29143371
Xing G, Hui T, Liu J, Yang S. Impact of Transglutaminase-Mediated Crosslinking on the Conformational Changes in a Dual-Protein System and IgE Reactivity of Soy Protein. Molecules. 2024; 29(14):3371. https://doi.org/10.3390/molecules29143371
Chicago/Turabian StyleXing, Guangliang, Tianran Hui, Jia Liu, and Siran Yang. 2024. "Impact of Transglutaminase-Mediated Crosslinking on the Conformational Changes in a Dual-Protein System and IgE Reactivity of Soy Protein" Molecules 29, no. 14: 3371. https://doi.org/10.3390/molecules29143371
APA StyleXing, G., Hui, T., Liu, J., & Yang, S. (2024). Impact of Transglutaminase-Mediated Crosslinking on the Conformational Changes in a Dual-Protein System and IgE Reactivity of Soy Protein. Molecules, 29(14), 3371. https://doi.org/10.3390/molecules29143371





