Preparation and Application of Co-Doped Zinc Oxide: A Review
Abstract
:1. Introduction
2. Preparation Methods of Co-Doped ZnO
2.1. Hydrothermal
2.2. Solvothermal
2.3. Sol-Gel
2.4. Other Methods
2.4.1. Combustion
2.4.2. Spray Pyrolysis
2.4.3. Wet Chemical “Liquid Ceramic”
2.4.4. Chemical
2.4.5. RF Magnetron Sputtering
2.4.6. Acoustic Chemical
2.4.7. Electron Beam Evaporation Deposition
3. Performance Study of ZnO
3.1. Optical Performance
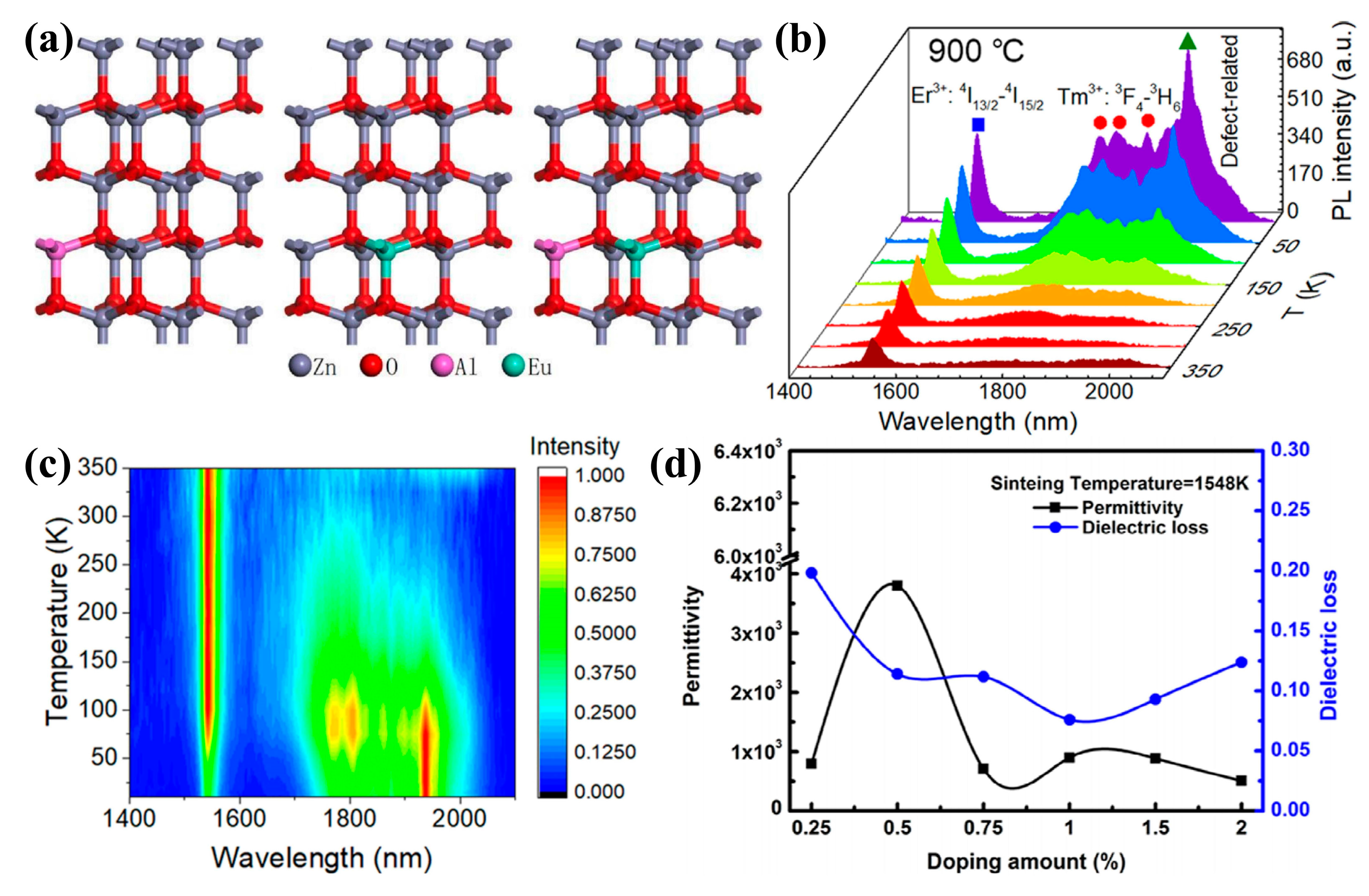
3.2. Electrical Performance
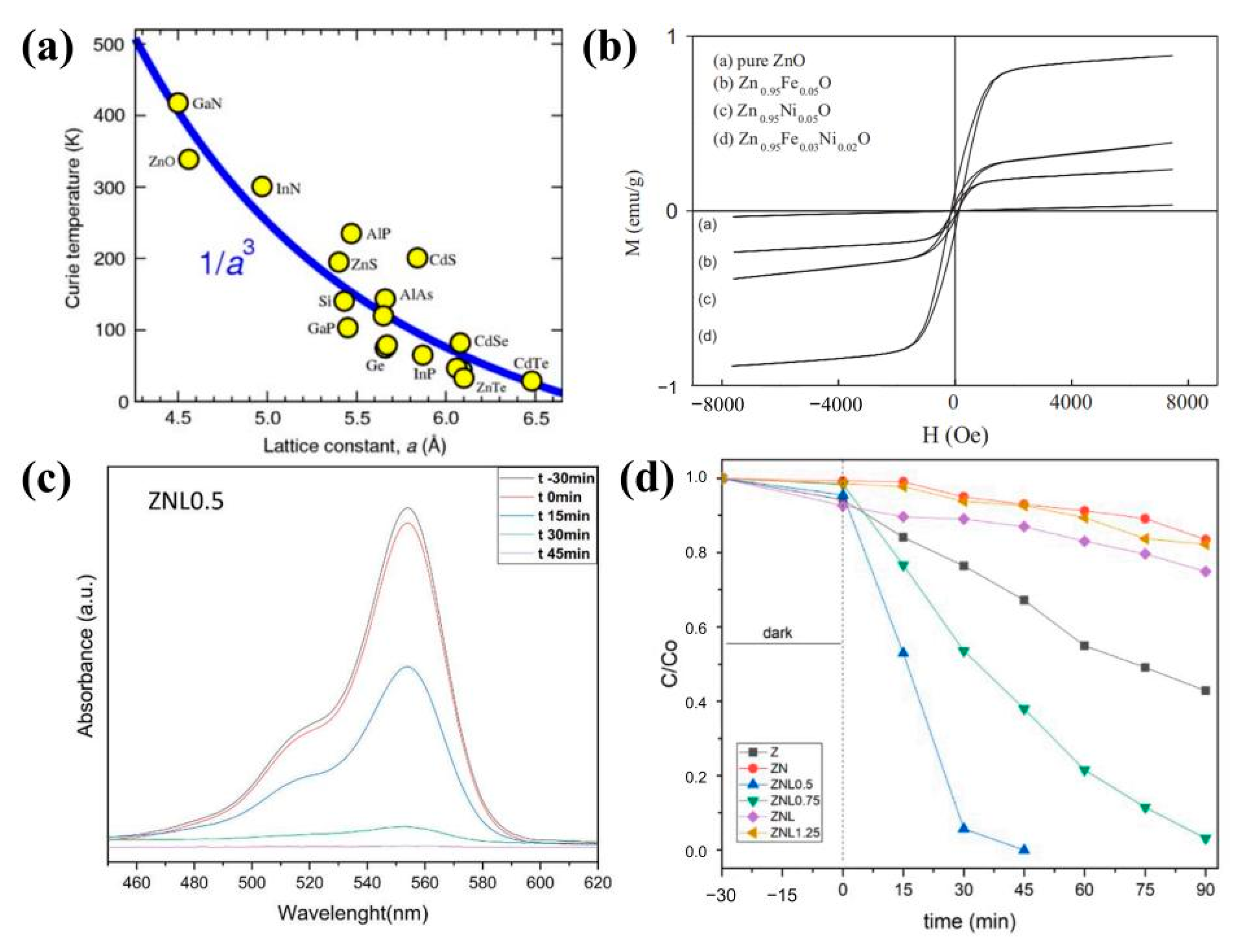
3.3. Magnetic Properties
| Composition | TM Content | Magnetism | TC (K) | Fabrication Method | References |
|---|---|---|---|---|---|
| Fe-Nd-ZnO | Fe: 2.00% Nd: 1.00~5.00% | 0.003 µB | 5–380 | hydrothermal | [18] |
| Co-Ga-ZnO | Co: 5.00% Ga: 1.00% | −0.800 emu/g | 500 | PLD | [113] |
| Mn-P-ZnO | Mn: 0.05% P: 0.02% | 0.050 emu/g | 300 | PLD | [114] |
| Co-Al-ZnO | Co: 0.04% Al: 0.01% | 0.830 µB/Co2+ | 5–350 | molecular beam epitaxy | [115] |
| Mn-Ni-ZnO | Mn: 0.02% Ni: 0.01% | 0.005 emu/g | 50–350 | hydrothermal | [19] |
| Na-Co-ZnO | Na: 0.03% Co: 0.05% | 0.023 emu/g | 300 | sol-gel | [116] |
| Mn-Fe-ZnO | Mn: 0.02% Fe: 1.00% | ~0.035 emu/g | 2–350 | in situ vapor-phase transport approach | [117] |
| Ag-N-ZnO | Ag: 3.00% N: 5.00% | 2.300 emu/cm3 | 4300 | RF sputtering | [118] |
| Fe-Mg-ZnO | Fe: 0.86% Mg: 0.04% | - | 5–400 | sol-gel | [119] |
| Mn-N-ZnO | Mn: 4.13% N: 1.88% | 0.120 and 0.170 kA·m−1 | 300 | sol-gel | [120] |
| Bi-Cu-ZnO | Bi: Below detection limit Cu: 0.60% | ~0.500 emu/cm3 | 10–300 | a vapor-phase transport | [121] |
| Cr-Co-ZnO | Cr: 0.09% Co: 0.03% | 0.010 emu/g | 10–300 | citric gel route | [122] |
| Fe-Co-ZnO | Fe: 0.05% Co: 0.05% | - | 5–300 | sol-gel | [123] |
| Al-Mn-ZnO | Al: 0.03% Mn: 0.03% | 0.019 emu/g | 300–503 | sol-gel | [124] |
| In-Mn-ZnO | In: 0.10% Mn: 0.10% | 0.080 emu/g | 2–300 | solvothermal | [125] |
| Mn-Ni-ZnO | Mn: 0.04% Ni: 0.03% | 0.015 × 10−9 emu/g | - | sol-gel | [126] |
| Na-F-ZnO | Na: 0.01% F: 0.01% | 3.020 × 10−4 emu/g | - | sol-gel | [127] |
| Cr-Ni-ZnO | Cr: 1.00% Ni: 1.00% | 0.010 emu/g | 20–300 | hydrothermal | [128] |
| Ni-Na-ZnO | Ni: 3.00% Na: 3.00% | 0.160 emu/g | - | pulsed laser deposition | [129] |
| Cu-Co-ZnO | Cu: 0.02% Co: 0.02% | - | - | RF magnetron sputtering technique | [130] |
| Mn-Sn-ZnO | Mn: 3.00% Sn: 5.00% | 6.000 × 10−5 emu | 250 | vapor transport | [131] |
| Nd-Mn-ZnO | Nd: 1.00% Mn: 1.00% | - | 5–290 | PLD | [132] |
| Mn-Na-ZnO | Mn: 0.05% Na: 0.05% | 1.520 µB | 300 | PLD | [133] |
| Co-Eu-ZnO | Co: 0.04% Eu: 0.04% | 65.000 emu/cm3 | 77 | ion implantation | [134] |
| F-Na-ZnO | F: 0.03% Na: 0.03% | 0.053 emu/g | 300 | MEMS | [135] |
| Fe-Co-ZnO | Fe: 2.00% Co: 2.00% | 0.960 emu/cm3 | 30–300 | CVD | [136] |
| Li-Co-ZnO | Li: 0.10% Co: 0.05% | ~0.480 µB/Co | 5–300 | soft chemical | [137] |
| Co-Ga-ZnO | Co: 5.00% Ga: 1.00% | 0.420 µB/Co | 300 | PLD | [138] |
| Ni-Li-ZnO | Ni: 0.03% Li: 0.03% | 0.800 emu/g | 25–290 | solvothermal | [139] |
| Cu-Al-ZnO | Cu: 0.02% Al: 3.00% | 6.800 emu/cm3 | 5–300 | PLD | [140] |
3.4. Photocatalytic
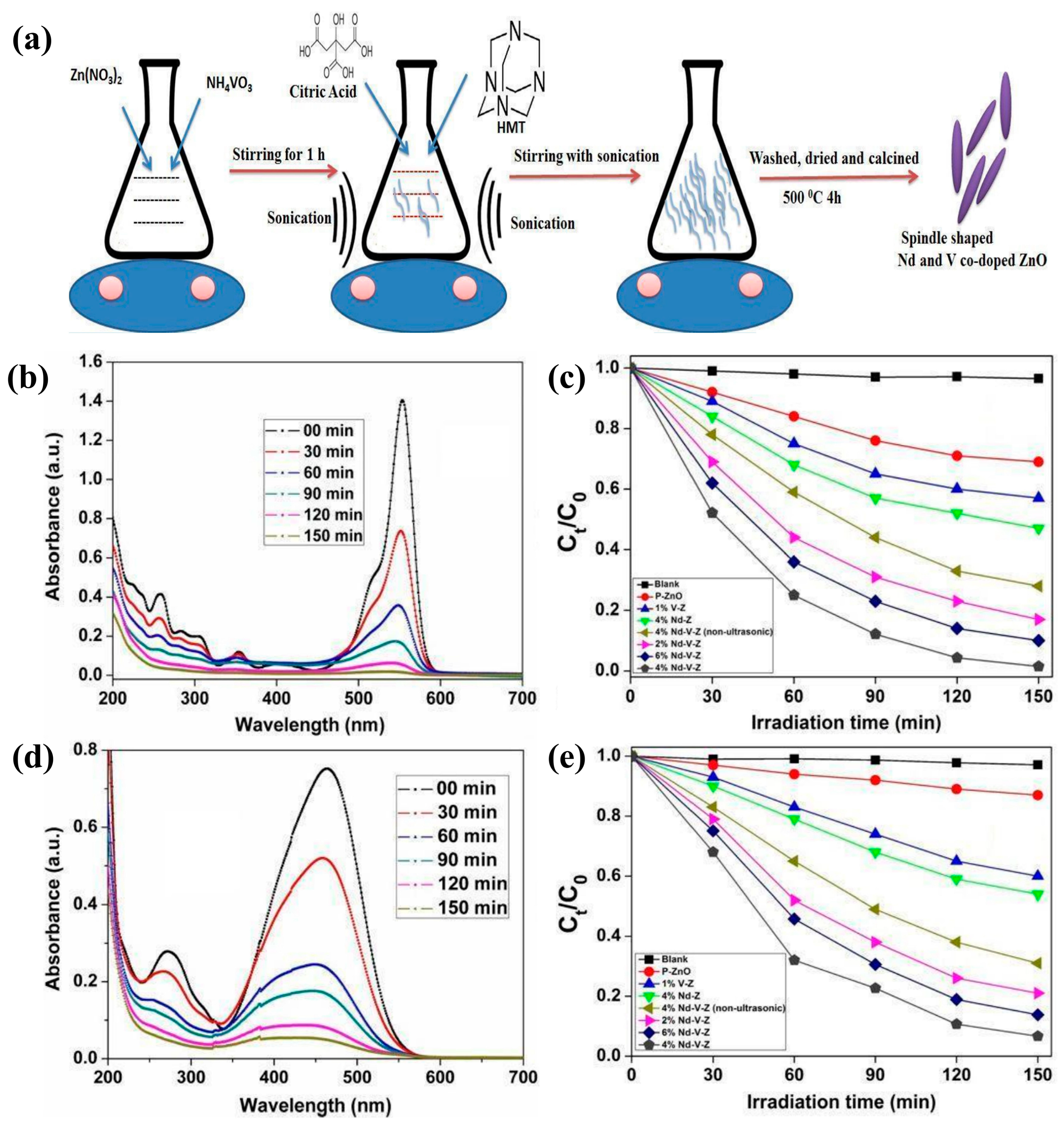
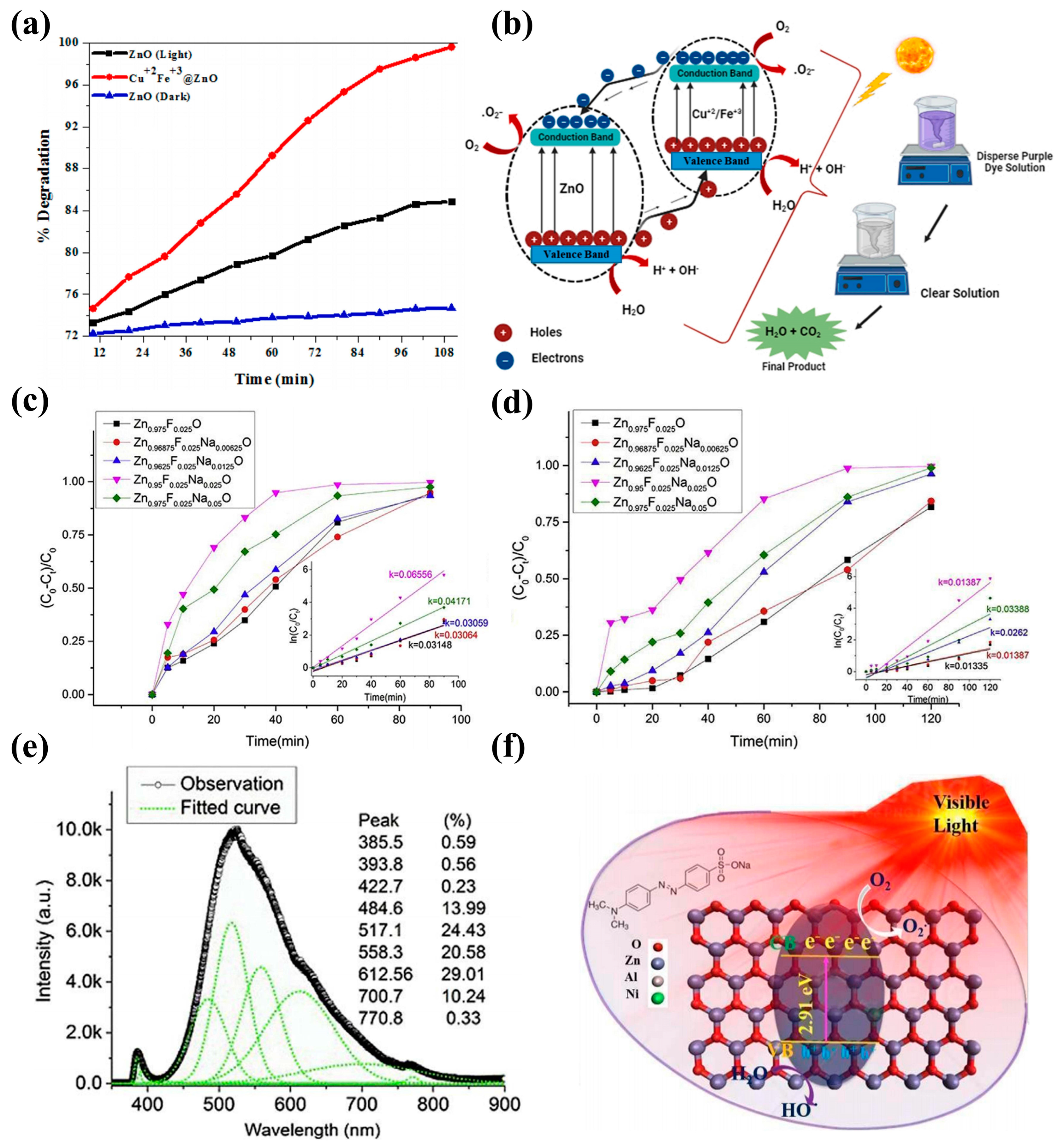
| Composition | Light Source | Pollutant | Experimental Conditions | PE | Fabrication Method | References |
|---|---|---|---|---|---|---|
| Fe-Cu-ZnO/GO | UV | Dark green dye | CL = 0.05 g·L−1 tr = 90 min | 99.28% | sol-gel | [156] |
| C-Ce-ZnO/ C-La-ZnO | visible | MB | CL = 0.01 g·L−1 tr = 80 min | 89%/ 99% | sol-gel | [99] |
| Fe-Pb-ZnO | UV | MB | tr = 90 min | reduced | microwave-assisted hydrothermal | [157] |
| Fe-Eu-ZnO | solar light | MO | CL = 0.001 g·L−1 tr = 120 min | 94% | co-precipitation | [158] |
| Al-Er-ZnO | 450 W Xe arc lamp | RhB | tr = 120 min | above 90% | hydrothermal | [159] |
| Ni-Co-ZnO | 100 W tungsten lamp | RhB | tr = 360 min | 42% | co-precipitation | [13] |
| Ag-Al-ZnO | UV | MB | tr = 120 min | 57% | microwave-assisted chemical synthesis technique | [150] |
| Mn-Cu-ZnO | UV | MB | tr = 30 min | - | hydrothermal | [160] |
| La-Ce-ZnO | UV | MB | CL = 0.01 g·L−1 tr = 120 min | 95.2% | Solvothermal route. | [161] |
| Cr-In-ZnO | visible | MB | tr = 180 min | 95% | Spray pyrolysis technique | [162] |
| In-Mg-ZnO | UV | OR-II | tr = 240 min | 88.57% | chemical co-precipitation | [28] |
| Ce-Ni-ZnO | UV | MB | tr = 120 min | 81.3% | sol-gel | [29] |
| Er-Al-ZnO | UV | RhB | tr = 120 min | 93% | hydrothermal | [163] |
| Eu-Tb-ZnO | UV | MB | tr = 50 min | 99.9% | combustion | [141] |
| Bi-N-ZnO | UV | RhB | tr = 180 min | 89% | hydrothermal | [164] |
| Gd-N-ZnO | UV | MB | tr = 60 min | 87% | wet chemical co-precipitation | [142] |
| Ag-N-ZnO | visible | MO | tr = 120 min | 98.82% | sol-gel | [36] |
4. Application of ZnO Nanomaterials
4.1. Photocatalyst
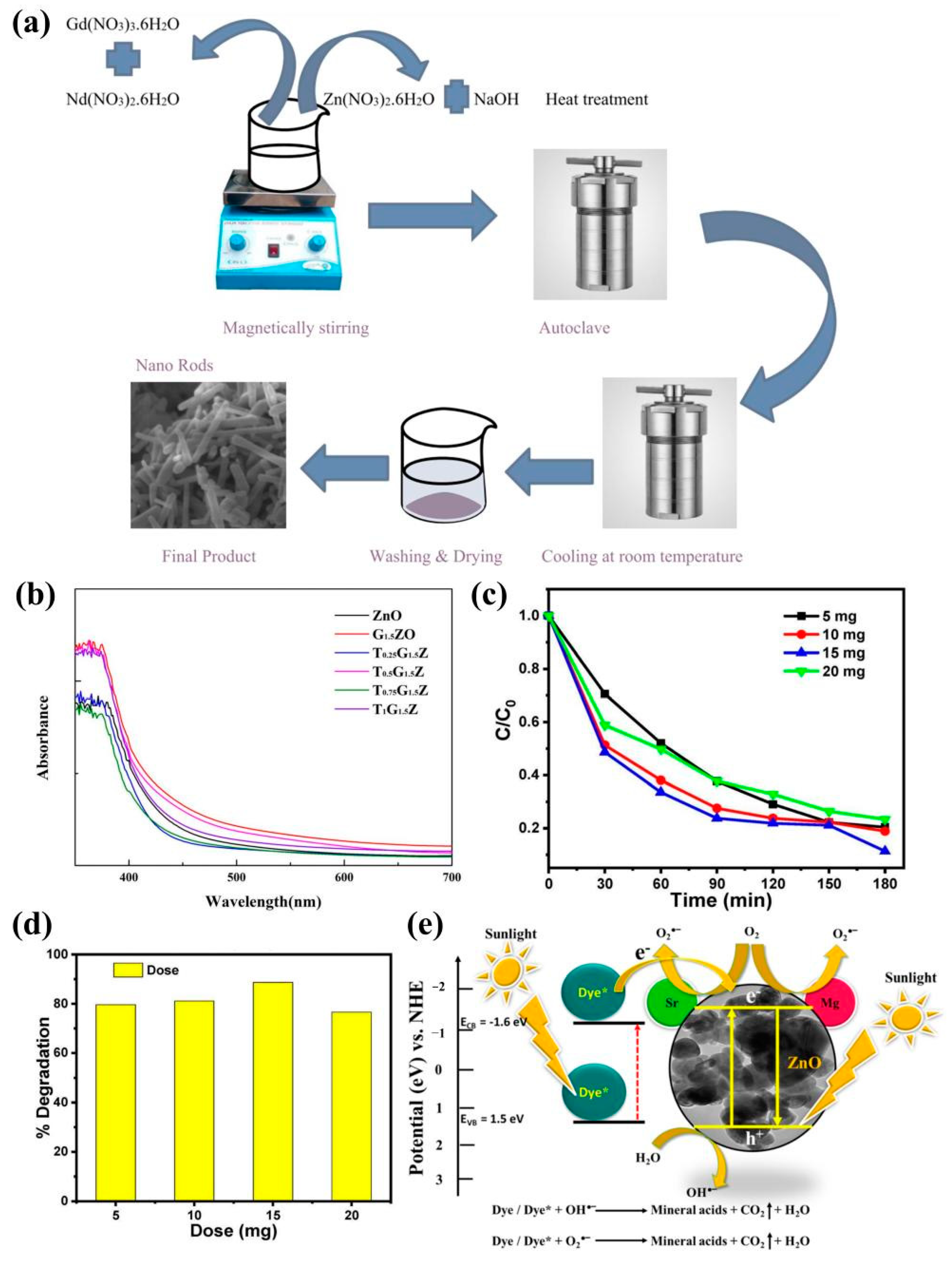
4.2. Solar Cells

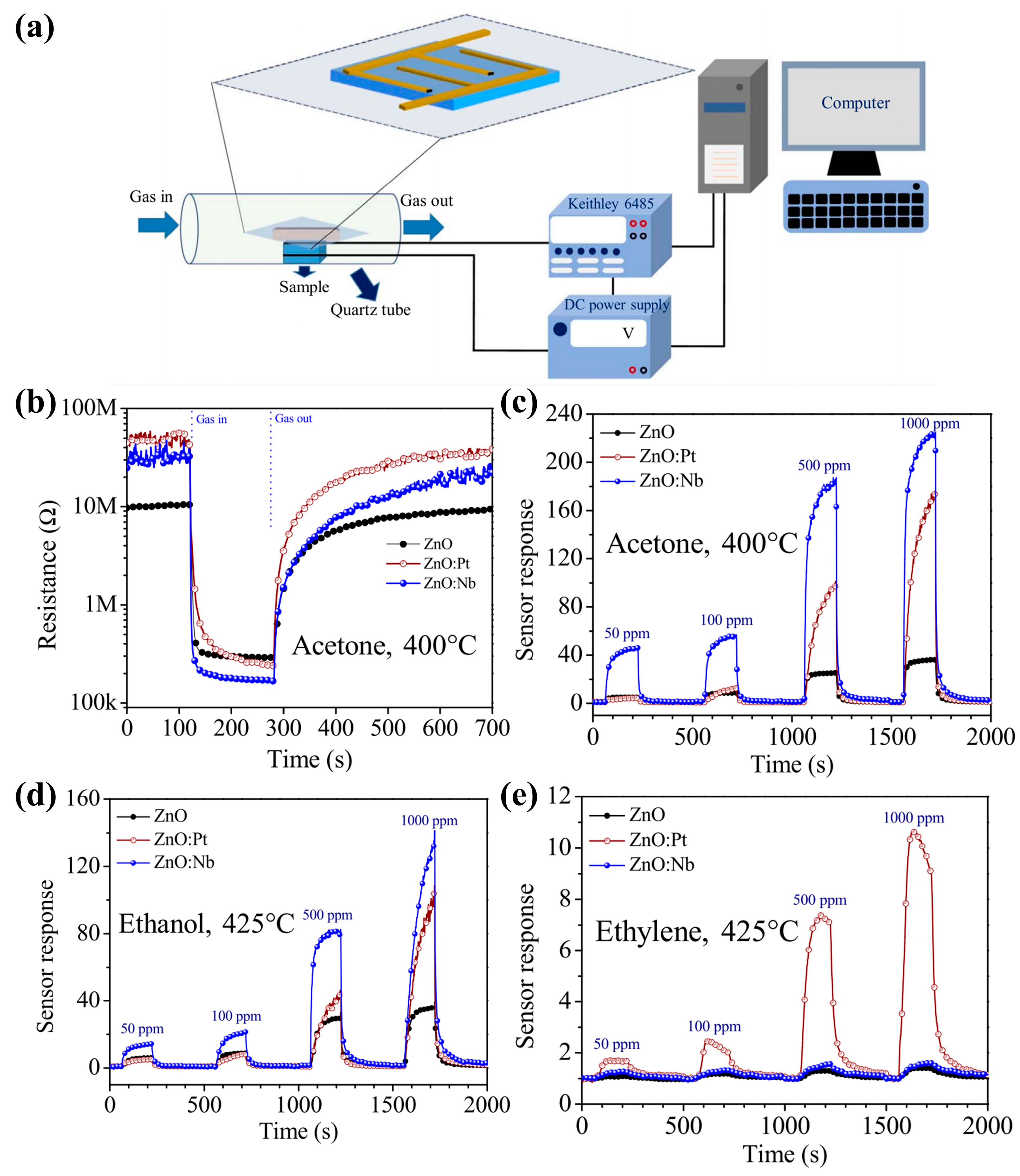
4.3. Gas Sensors
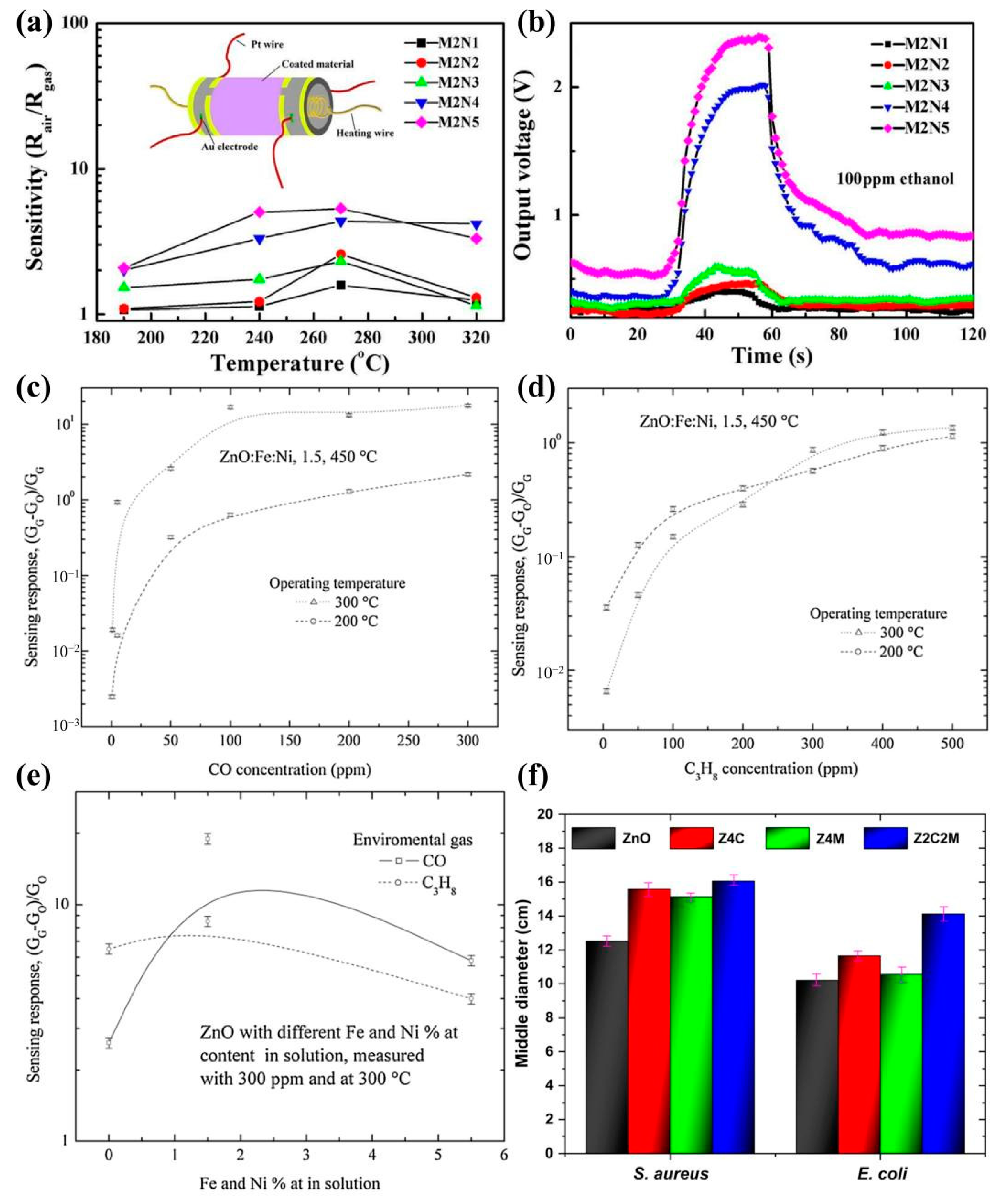
4.4. Biomedicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Q.; Lin, R.; Jiang, L.; Wan, J.; Chen, C. Fabrication and photocatalysis of ZnO nanotubes on transparent conductive graphene-based flexible substrates. Sci. China Mater. 2018, 61, 1007–1011. [Google Scholar] [CrossRef]
- Mathis, J.E.; Lieffers, J.J.; Mitra, C.; Reboredo, F.A.; Bi, Z.; Bridges, C.A.; Kidder, M.K.; Paranthaman, M.P. Increased photocatalytic activity of TiO2 mesoporous microspheres from codoping with transition metals and nitrogen. Ceram. Int. 2016, 42, 3556–3562. [Google Scholar] [CrossRef]
- Daideche, K.; Lahmar, H.; Lerari, D.; Azizi, A. Influence of deposition potential on the electrochemical growth and photocatalysis performance of SnO2 nanostructures. Inorg. Chem. Commun. 2023, 147, 110154. [Google Scholar] [CrossRef]
- Van Dao, D.; Ngoc Bich, T.T.; Thu Ha, N.T.; Wang, W.; Kim, T.; Kim, H.; Khanh Duy, P.H.; Ha, N.N.; Thuy Van, D.T.; Lee, I.-H. Hematite Fe2O3@nitrogen-doped graphene core-shell photocatalyst for efficient cephalexin degradation under visible light irradiation. Ceram. Int. 2022, 48, 34533–34542. [Google Scholar] [CrossRef]
- Zhao, L.; Xi, X.; Liu, Y.; Ma, L.; Nie, Z. Growth mechanism and visible-light-driven photocatalysis of organic solvent dependent WO3 and nonstoichiometric WO3-x nanostructures. J. Taiwan Inst. Chem. Eng. 2020, 115, 339–347. [Google Scholar] [CrossRef]
- Tseng, T.-T.; Uan, J.-Y.; Tseng, W.J. Synthesis, microstructure, and photocatalysis of In2O3 hollow particles. Ceram. Int. 2011, 37, 1775–1780. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, J.; Jiang, L.; Yang, Q.; Yan, N. Advances in green synthesis and applications of graphene. Nano Res. 2021, 14, 3724–3743. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Li, B.; Tang, K.; Ma, Y.; Cao, M.; Wang, L.; Wang, L. A boron and gallium co-doped ZnO intermediate layer for ZnO/Si heterojunction diodes. Appl. Surf. Sci. 2018, 428, 61–65. [Google Scholar] [CrossRef]
- Rong, P.; Jiang, Y.-F.; Wang, Q.; Gu, M.; Jiang, X.-L.; Yu, Q. Photocatalytic degradation of methylene blue (MB) with Cu1–ZnO single atom catalysts on graphene-coated flexible substrates. J. Mater. Chem. A 2022, 10, 6231–6241. [Google Scholar] [CrossRef]
- Kazeminezhad, I.; Saadatmand, S.; Yousefi, R. Effect of transition metal elements on the structural and optical properties of ZnO nanoparticles. Bull. Mater. Sci. 2016, 39, 719–724. [Google Scholar] [CrossRef]
- Azfar, A.K.; Kasim, M.F.; Lokman, I.M.; Rafaie, H.A.; Mastuli, M.S. Comparative study on photocatalytic activity of transition metals (Ag and Ni)-doped ZnO nanomaterials synthesized via sol-gel method. R. Soc. Open Sci. 2020, 7, 191590. [Google Scholar] [CrossRef] [PubMed]
- Sushama, S.; Murkute, P.; Ghadi, H.; Chakrabarti, S. Enhancing Acceptor-Based Optical Behavior in Phosphorus-Doped ZnO Thin Films Using Boron as Compensating Species. ACS Appl. Electron. Mater. 2019, 1, 325–339. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 2018, 448, 481–488. [Google Scholar] [CrossRef]
- Kolomys, O.F.; Strelchuk, V.V.; Rarata, S.V.; Hayn, R.; Savoyant, A.; Giovannelli, F.; Delorem, F.; Tkach, V. Optical and structural properties of individual Co-doped ZnO microwires. Superlattices Microstruct. 2018, 118, 7–15. [Google Scholar] [CrossRef]
- Hu, F.; Liu, Q.; Sun, Z.; Yao, T.; Pan, Z.; Li, Y.; He, J.; He, B.; Xie, Z.; Yan, W.; et al. Cu and Co codoping effects on room-temperature ferromagnetism of (Co,Cu):ZnO dilute magnetic semiconductors. J. Appl. Phys. 2011, 109, 103705. [Google Scholar] [CrossRef]
- Karmakar, L.; Das, D. Prominent c-axis oriented Si-doped ZnO thin film prepared at low substrate temperature in RF magnetron sputtering and its UV sensing in p-Si/n-SZO heterojunction structures. J. Phys. Chem. Solids 2021, 151, 109907. [Google Scholar] [CrossRef]
- Ramany, K.; Shankararajan, R.; Savarimuthu, K.; Gunasekaran, I.; Rajamanickam, G.; Narendhiran, S.; Perumalsamy, R. Experimental Analysis of Transition Metal (Ni-V) Codoped ZnO Nanorods for Piezoelectric Accelerometer Application. IEEE Trans. Nanotechnol. 2020, 19, 728–735. [Google Scholar] [CrossRef]
- Diamandescu, L.; Cernea, M.; Tolea, F.; Secu, E.C.; Trusca, R.; Secu, M.; Enculescu, M. (Fe, Nd) codoped ZnO micro–and nanostructures with multifunctional characteristics like photocatalytic activity, optical and ferromagnetic properties. Ceram. Int. 2018, 44, 21962–21975. [Google Scholar] [CrossRef]
- Gao, Q.; Dai, Y.; Han, B.; Zhu, W.; Li, X.; Li, C. Enhanced gas-sensitivity and ferromagnetism performances by the Ni-doping induced oxygen vacancies in (Mn, Ni) codoped ZnO nanorods. Appl. Surf. Sci. 2019, 490, 178–187. [Google Scholar]
- Arul Mary, J.; Judith Vijaya, J.; Dai, J.H.; Bououdina, M.; John Kennedy, L.; Song, Y. Experimental and first-principles DFT studies of electronic, optical and magnetic properties of cerium–manganese codoped zinc oxide nanostructures. Mater. Sci. Semicond. Process. 2015, 34, 27–38. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, V.; Kaur, P.; Lal, M.; Negi, P.; Sharma, R. Effect on the dielectric properties due to In–N co-doping in ZnO particles. J. Mater. Sci. Mater. Electron. 2021, 32, 8991–9004. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, Y.; Song, W. Effects of substrate temperature on the structural, morphological, electrical and optical properties of Al and Ga co-doped ZnO thin films grown by DC magnetron sputtering. Mater. Lett. 2015, 145, 279–282. [Google Scholar] [CrossRef]
- Ahsan, H.M.; Lal, K.; Saleem, M.; Mustafa, G.M.; Khan, M.A.; Haidyrah, A.S.; Atiq, S. Tuning the dielectric behavior and energy storage properties of Mn/Co co-doped ZnO. Mater. Sci. Semicond. Process. 2021, 134, 105977. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; Naveed ur Rahman, M.; Riaz, M.; Hussain, A.; Iqbal, F. Hydrothermally derived Co, Ni co-doped ZnO nanorods; structural, optical, and morphological study. Opt. Mater. 2021, 111, 110606. [Google Scholar] [CrossRef]
- Padmavathy, V.; Sankar, S. Influence of rare earth (La and Y) codoping on optical properties of ZnO:Ag nanograins. Optik 2020, 220, 165133. [Google Scholar] [CrossRef]
- Senol, S.D.; Ozugurlu, E.; Arda, L. Synthesis, structure and optical properties of (Mn/Cu) co-doped ZnO nanoparticles. J. Alloys Compd. 2020, 822, 153514. [Google Scholar] [CrossRef]
- Dib, K.; Trari, M.; Bessekhouad, Y. (S,C) co-doped ZnO properties and enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 505, 144541. [Google Scholar] [CrossRef]
- Benzitouni, S.; Zaabat, M.; Aida, M.S.; Ebothe, J.; Michel, J.; Boudine, B.; Mansouri, L.; Saidani, T. Morphology and photocatalytic activity of porous (In, Mg) co-doped ZnO nanoparticles. Optik 2018, 156, 949–960. [Google Scholar] [CrossRef]
- Costa-Silva, M.; Araujo, F.P.; Guerra, Y.; Viana, B.C.; Silva-Filho, E.C.; Osajima, J.A.; Almeida, L.C.; Skovroinski, E.; Peña-Garcia, R. Photocatalytic, structural and optical properties of Ce–Ni co-doped ZnO nanodisks-like self-assembled structures. Mater. Chem. Phys. 2022, 292, 126814. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Rong, P.; Wu, K.; Yang, Q.; Yu, Q. Properties and Configurations of B-N Co-Doped ZnO Nanorods Fabricated on ITO/PET Substrate. Front. Energy Res. 2021, 9, 700901. [Google Scholar] [CrossRef]
- Jiaqi, Y.; Chunyan, Y.; Hailiang, D.; Tianbao, L.; Wei, J.; Fuhong, M.; Guangmei, Z.; Zhuxia, Z. Effect of copper and silver co-doping on growth behaviour and photoelectric properties of n-ZnO nanorods/p-GaN heterojunction light-emitting diodes. Philos. Mag. 2022, 102, 1247–1260. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Abdeltwab, E.; Atta, A.; Taher, F.A. Controlled growth of hexagonal nanocrystals Co and Gd co-doping ZnO by hydrothermal method. Emerg. Mater. Res. 2020, 9, 1032–1040. [Google Scholar] [CrossRef]
- Zhong, M.; Li, Y.; Hu, Y.; Zhu, M.; Li, W.; Jin, H.; Wang, S.; Li, Y.; Zhao, H. Enhancement of zinc vacancies in room-temperature ferromagnetic Cr–Mn codoped ZnO nanorods synthesized by hydrothermal method under high pulsed magnetic field. J. Alloys Compd. 2015, 647, 823–829. [Google Scholar] [CrossRef]
- Tang, J.-F.; Lu, Y.-M.; Tseng, Z.-L.; Chu, S.-Y. Effects of multilayer buffer on structural properties of ZnO nanostructures grown using a solvothermal method. CrystEngComm 2016, 18, 9357–9362. [Google Scholar] [CrossRef]
- Youssef, A.M.; Yakout, S.M. Superior sunlight photocatalytic of N/La codoped ZnO nanostructures synthesized using different chelating agents. Opt. Mater. 2020, 107, 110072. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Gao, Z.; Ye, B.C. Effect of Different Activated Carbon as Carrier on the Photocatalytic Activity of Ag-N-ZnO Photocatalyst for Methyl Orange Degradation under Visible Light Irradiation. Nanomaterials 2017, 7, 258. [Google Scholar] [CrossRef]
- Lingyan Gao, G.S. Preparation of Yb3+/Er3+ co-doped CeO2 nanopowder by combustion method and its upconversion luminescence properties. J. Hunan Univ. Technol. 2013, 27, 11–15. [Google Scholar]
- Paraguay, D.F.; Miki-Yoshida, M.; Espinosa-Magaña, F. EELS Studies of ZnO and ZnO:In Films Deposited By Spray Pyrolysis. Microsc. Microanal. 2020, 7, 1224–1225. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Straumal, P.B.; Myatiev, A.A.; Schütz, G.; Goering, E.J.; Tietze, T.; Baretzky, B. Grain boundaries as the controlling factor for the ferromagnetic behaviour of Co-doped ZnO. Philos. Mag. 2013, 93, 1371–1383. [Google Scholar] [CrossRef]
- Murugadoss, G.; Jayavel, R.; Rajesh Kumar, M. Structural and optical properties of highly crystalline Ce, Eu and co-doped ZnO nanorods. Superlattices Microstruct. 2015, 82, 538–550. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, G.; Liu, X.; Tang, Y.; Mo, Z.; Yang, D. Study on photoelectric properties of Al-Eu codoped ZnO. J. Alloys Compd. 2021, 882, 160606. [Google Scholar] [CrossRef]
- Andrade Neto, N.F.; Oliveira, Y.G.; Bomio, M.R.D.; Motta, F.V. Synthesis and Characterization of Co2+ and Mn2+ Codoped ZnO Nanoparticles Obtained by the Sonochemical Method: Photocatalytic and Antimicrobial Properties. J. Electron. Mater. 2019, 48, 5900–5905. [Google Scholar] [CrossRef]
- Li, A.; Bi, H.; Liu, Y.; Wu, M. Structure and luminescence properties of Co, Cu co-doped ZnO thin films. Chin. J. Lumin. 2008, 29, 289–293. [Google Scholar]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Wang, C.N.; Li, Y.L.; Gong, F.L.; Zhang, Y.H.; Fang, S.M.; Zhang, H.L. Advances in Doped ZnO Nanostructures for Gas Sensor. Chem. Rec. 2020, 20, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Tajik, S.; Garkani Nejad, F.; Safaei, M. Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Alev, O.; Sarıca, N.; Özdemir, O.; Arslan, L.Ç.; Büyükköse, S.; Öztürk, Z.Z. Cu-doped ZnO nanorods based QCM sensor for hazardous gases. J. Alloys Compd. 2020, 826, 154177. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354–376. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Fraleoni-Morgera, A.; Cesini, I.; Kumar, P.; Oddo, C.M. Hydrothermally Grown ZnO Nanorods as Promising Materials for Low Cost Electronic Skin. Chem. Nano Mat. 2019, 6, 15–31. [Google Scholar] [CrossRef]
- Qin, L.; Mawignon, F.J.; Hussain, M.; Ange, N.K.; Lu, S.; Hafezi, M.; Dong, G. Economic Friendly ZnO-Based UV Sensors Using Hydrothermal Growth: A Review. Materials 2021, 14, 4083. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, H.; Wang, Q.; Cheng, S.; Jiang, L.; Zhang, Y.; Ai, T.; Guo, C. Hydrothermal synthesis, characterization and properties of boron-doped ZnO sheets grown on p-diamond film. Mater. Lett. 2014, 128, 284–286. [Google Scholar] [CrossRef]
- Yu, Q.; Rong, P.; Ren, S.; Jiang, L.; Li, Y. Fabrication and electrochemical performance of Al-Doped ZnO nanosheets on graphene-based flexible substrates. Front. Mater. 2019, 6, 208. [Google Scholar] [CrossRef]
- Yang, N.Q.; Li, J.; Wang, Y.N.; Ma, J. Investigation of photocatalytic properties based on Fe and Ce Co-doped ZnO via hydrothermal method and first principles. Mater. Sci. Semicond. Process. 2021, 131, 105835. [Google Scholar] [CrossRef]
- Das, S.; Bandyopadhyay, A.; Saha, P.; Das, S.; Sutradhar, S. Enhancement of room-temperature ferromagnetism and dielectric response in nanocrystalline ZnO co-doped with Co and Cu. J. Alloys Compd. 2018, 749, 1–9. [Google Scholar] [CrossRef]
- Achouri, F.; Corbel, S.; Balan, L.; Mozet, K.; Girot, E.; Medjahdi, G.; Said, M.B.; Ghrabi, A.; Schneider, R. Porous Mn-doped ZnO nanoparticles for enhanced solar and visible light photocatalysis. Mater. Des. 2016, 101, 309–316. [Google Scholar] [CrossRef]
- Zukuls, A.; Eglītis, R.; Käämbre, T.; Kook, M.; Kisand, V.; Maiorov, M.; Ignatans, R.; Duarte, R.F.; Järvekülg, M.; Šutka, A. Magnetic and optical properties in degenerated transition metal and Ga co-substituted ZnO nanocrystals. J. Alloys Compd. 2019, 805, 1191–1199. [Google Scholar] [CrossRef]
- Šutka, A.; Käämbre, T.; Joost, U.; Kooser, K.; Kook, M.; Duarte, R.F.; Kisand, V.; Maiorov, M.; Döbelin, N.; Smits, K. Solvothermal synthesis derived Co-Ga codoped ZnO diluted magnetic degenerated semiconductor nanocrystals. J. Alloys Compd. 2018, 763, 164–172. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Girish Kumar, S.; Kavitha, R. Lanthanide ions doped ZnO based photocatalysts. Sep. Purif. Technol. 2021, 274, 118853. [Google Scholar] [CrossRef]
- Yuan, H. Preparation of High Concentration Co, Cu Co-Doped ZnO Thin Films and Their Optical, Magnetic Properties; Southwest University for Nationalities: Chengdu, China, 2013. [Google Scholar]
- Alshammari, A.S.; Khan, Z.R.; Gandouzi, M.; Mohamed, M.; Bouzidi, M.; Shkir, M.; Alshammari, H.M. Tailoring the optical properties and the UV detection performance of sol-gel deposited ZnO nanostructured thin films via Cd and Na co-doping. Opt. Mater. 2022, 126, 112146. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Chiu, W.-Y. Enhanced Electrical Properties and Stability of P-Type Conduction in ZnO Transparent Semiconductor Thin Films by Co-Doping Ga and N. Coatings 2020, 10, 1069. [Google Scholar] [CrossRef]
- Li, D.; Miu, C.; Liu, L.; Luo, X.; Wei, K.; Xiao, Z. Synthesis of novel blue silicate long afterglow materials by combustion method and their luminescent properties. Chin. J. Rare Met. 2004, 28, 662–665. [Google Scholar]
- Miu, C.; Li, D.; Luo, X.; Lin, G.; Liu, J.; Wei, K. Rapid synthesis of novel blue silicate long afterglow materials by combustion method. Chin. Ceram. 2003, 39, 27–29. [Google Scholar]
- Sathish, P.; Ravichandran, K.; Sakthivel, B.; Panneerselvam, A. Enhancing the Antibacterial Efficiency of ZnO Nanopowders Synthesized by Combustion Method Through Ag + Fe Co-doping. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1407–1413. [Google Scholar] [CrossRef]
- Compton, J.S.; Peterson, C.A.; Dervishogullari, D.; Sharpe, L.R. Spray Pyrolysis as a Combinatorial Method for the Generation of Photocatalyst Libraries. ACS Comb. Sci. 2019, 21, 489–499. [Google Scholar] [CrossRef]
- Lanfredi, S.; Storti, F.; Simões, L.P.M.; Djurado, E.; Nobre, M.A.L. Synthesis and structural characterization of calcium titanate by spray pyrolysis method. Mater. Lett. 2017, 201, 148–151. [Google Scholar] [CrossRef]
- Zamudio-Hernández, A.; Sánchez-Cuevas, J.J.; Mercado-Zúñiga, C.; Zárate-Medina, J.; Rosas, G. Synthesis of Multi-walled Carbon Nanotubes by Spray Pyrolysis Method. Microsc. Microanal. 2020, 26, 2436–2438. [Google Scholar] [CrossRef]
- Karaköse, E.; Çolak, H. Structural and optical properties of ZnO nanorods prepared by spray pyrolysis method. Energy 2017, 140, 92–97. [Google Scholar] [CrossRef]
- Askri, B.; Riahi, I.; Mimouni, R.; Amlouk, M. Photoluminescence and dielectric properties of (Al/Cu) and (In/Cu) co-doped ZnO sprayed thin films under the oxygen deficiency conditions. Superlattices Microstruct. 2021, 150, 106731. [Google Scholar] [CrossRef]
- Lee, Y.; Fujimoto, T.; Yamanaka, S.; Yamanaka, K. Evaluation of photocatalysis of Au supported ZnO prepared by the spray pyrolysis method. Adv. Powder Technol. 2021, 32, 1619–1626. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Protasova, S.G.; Myatiev, A.A.; Straumal, P.B.; Goering, E.; Baretzky, B. Amorphous grain boundary layers in the ferromagnetic nanograined ZnO films. Thin Solid Films 2011, 520, 1192–1194. [Google Scholar] [CrossRef]
- Ali, R.N.; Naz, H.; Li, J.; Zhu, X.; Liu, P.; Xiang, B. Band gap engineering of transition metal (Ni/Co) codoped in zinc oxide (ZnO) nanoparticles. J. Alloys Compd. 2018, 744, 90–95. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R. Analysis of structural, optical and magnetic properties of Fe/Co co-doped ZnO nanocrystals. Ceram. Int. 2017, 43, 8488–8496. [Google Scholar] [CrossRef]
- Ghanbari Shohany, B.; Khorsand Zak, A. Doped ZnO nanostructures with selected elements—Structural, morphology and optical properties: A review. Ceram. Int. 2020, 46, 5507–5520. [Google Scholar] [CrossRef]
- Qi Yu, T.A. Liyun Jiang, Yingtang Zhang, Chuang Li and Xinqiang Yuan, Efficient energy transfer in Eu-doped ZnO on diamond film. RSC Adv. 2014, 4, 53946–53949. [Google Scholar]
- Mustafa, L.; Anjum, S.; Waseem, S.; Javed, S.; Ramay, S.M.; Atiq, S. Effect of Co and Ni codoping on the structural, magnetic, electrical and optical properties of ZnO. Mater. Res. Bull. 2016, 84, 32–38. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Yang, P. Electronic and Optical Properties of Al, Eu Single-Doped and Al-Eu Co-Doped ZnO. JOM 2020, 73, 373–379. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Ghobadi, N.; Yahyavi, F. Enhanced optical and hydrophilic properties of V and La co-doped ZnO thin films. Opt. Quantum Electron. 2017, 49, 39. [Google Scholar] [CrossRef]
- Liu, W.-W.; Liu, C.-L.; Chen, X.-B.; Lu, J.-H.; Chen, H.-X.; Miao, Z.-Z. First-principles study on electronic and optical properties of S, N single-doped and S-N co-doped ZnO. Phys. Lett. A 2020, 384, 126172. [Google Scholar] [CrossRef]
- Bouziani, I.; Kibbou, M.; Haman, Z.; Benhouria, Y.; Essaoudi, I.; Ainane, A.; Ahuja, R. Electronic and optical properties of ZnO nanosheet doped and codoped with Be and/or Mg for ultraviolet optoelectronic technologies: Density functional calculations. Phys. Scr. 2020, 95, 015804. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Peluso, M.A.; Thomas, H.; Damonte, L.C.; Sambeth, J.E. Structural and optical properties of ZnO and manganese-doped ZnO. J. Alloys Compd. 2016, 689, 416–424. [Google Scholar] [CrossRef]
- El Ghoul, J.; Al-Harbi, F.F. Synthesis, Structural and Optical Properties of Er and V Codoping ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 31, 272–278. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, Z.; Wang, J.; Bao, F.; Zhang, J.; Ma, Y.; Sham, T.-K.; Sun, X. Synthesis and Structure-Dependent Optical Properties of ZnO Nanocomb and ZnO Nanoflag. J. Phys. Chem. C 2017, 121, 26076–26085. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhao, H.; Wang, Z.L.; Yang, Y. Dual-polarity response in self-powered ZnO NWs/Sb2Se3 film heterojunction photodetector array for optical communication. Nano Energy 2020, 68, 104312. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, B.; Zheng, L.; Hu, Z.; Ma, Z.; Xu, R.; Hong, F.; Zhao, L.; Li, S.; Wang, L.; et al. Experimental Realization of 1400–2100 nm Broadband Emission for Wide-Bandwidth Optical Communication in Er–Tm Codoped ZnO Films and Devices. J. Phys. Chem. C 2020, 124, 3747–3755. [Google Scholar] [CrossRef]
- Pan, X.; Wang, X.; Tian, B.; Wang, C.; Zhang, H.; Guizani, M. Machine-Learning-Aided Optical Fiber Communication System. IEEE Netw. 2021, 35, 136–142. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Z.; Li, Y.; Liu, Y. Colossal permittivity and dielectric relaxation of (Li, In) Co-doped ZnO ceramics. J. Alloys Compd. 2017, 698, 200–206. [Google Scholar] [CrossRef]
- Zankat, A.; Boricha, H.; Shrimali, V.G.; Gadani, K.; Sagapariya, K.; Rajyaguru, B.; Gal, M.; Pandya, D.D.; Solanki, P.S.; Shah, N.A. Electrical properties of ZnO:ZnAlO nanoparticle matrix composites. J. Alloys Compd. 2019, 788, 623–631. [Google Scholar] [CrossRef]
- Alagha, S.; Heedt, S.; Vakulov, D.; Mohammadbeigi, F.; Kumar, E.S.; Schäpers, T.; Isheim, D.; Watkins, S.P.; Kavanagh, K.L. Electrical properties of lightly Ga-doped ZnO nanowires. Semicond. Sci. Technol. 2017, 32, 125010. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Zhang, M.; Cheng, W.; Liao, B.; Ying, M. Structural, electrical and magnetic properties of Gd-doped and (Al, Gd) codoped ZnO films. J. Alloys Compd. 2023, 933, 167744. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.H.; Elhouichet, H. Effect of Sb doping on the electrical and dielectric properties of ZnO nanocrystals. Ceram. Int. 2019, 45, 8000–8007. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Ali, M.A.; Afifi, G.; Imam, H.; Al-Assiri, M.S. Effects of Annealing Temperatures on the Structural and Dielectric Properties of ZnO Nanoparticles. Silicon 2016, 10, 301–307. [Google Scholar] [CrossRef]
- Jayachandraiah, C.; Krishnaiah, G. Influence of cerium dopant on magnetic and dielectric properties of ZnO nanoparticles. J. Mater. Sci. 2017, 52, 7058–7066. [Google Scholar] [CrossRef]
- Youssef, A.M.; Yakout, S.M. Enhanced visible light photocatalytic activity of C/La or Ce codoped ZnO nanostructures: Morphological, optical, magnetic and electrical properties studies. J. Environ. Chem. Eng. 2020, 8, 103565. [Google Scholar] [CrossRef]
- Mahdhi, H.; Ayadi, Z.B.; Djessas, K. Physical properties of metal-doped ZnO thin films prepared by RF magnetron sputtering at room temperature. J. Solid State Electrochem. 2019, 23, 3217–3224. [Google Scholar] [CrossRef]
- Cheon, D.; Son, M.; Ham, M.-H.; Lee, W. Resistive switching in an amorphous ZnO dielectric film prepared on a Ga-doped ZnO transparent electrode. RSC Adv. 2016, 6, 103864–103871. [Google Scholar] [CrossRef]
- El hat, A.; Chaki, I.; Essajai, R.; Mzerd, A.; Schmerber, G.; Regragui, M.; Belayachi, A.; Sekkat, Z.; Dinia, A.; Slaoui, A.; et al. Growth and Characterization of (Tb,Yb) Co-Doping Sprayed ZnO Thin Films. Crystals 2020, 10, 169. [Google Scholar] [CrossRef]
- Das, A.; Guha Roy, P.; Dutta, A.; Sen, S.; Pramanik, P.; Das, D.; Banerjee, A.; Bhattacharyya, A. Mg and Al co-doping of ZnO thin films: Effect on ultraviolet photoconductivity. Mater. Sci. Semicond. Process. 2016, 54, 36–41. [Google Scholar] [CrossRef]
- Alqadi, M.K.; Migdadi, A.B.; Alzoubi, F.Y.; Al-Khateeb, H.M.; Almasri, A.A. Influence of (Ag–Cu) co-doping on the optical, structural, electrical, and morphological properties of ZnO thin films. J. Sol-Gel Sci. Technol. 2022, 103, 319–334. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, L.; Ai, T. Fabrication and characterization of Au-doped ZnO nanocandles synthesized on diamond film. Mater. Lett. 2015, 152, 142–144. [Google Scholar] [CrossRef]
- Parvez Ahmad, M.D.; Venkateswara Rao, A.; Suresh Babu, K.; Narsinga Rao, G. Particle size effect on the dielectric properties of ZnO nanoparticles. Mater. Chem. Phys. 2019, 224, 79–84. [Google Scholar] [CrossRef]
- Gao, W.; Li, D.; Gao, J.; Yang, P. Investigation on electronic and optical properties of Ga-Eu codoped ZnO. Chem. Phys. 2020, 536, 10826. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yu, Z.; Li, W.; Zhu, M.; Jin, H.; Liu, Y.; Li, Y.; Skotnicova, K. Study on the high magnetic field processed ZnO based diluted magnetic semiconductors. Ceram. Int. 2019, 45, 19583–19595. [Google Scholar] [CrossRef]
- Straumal, B.B.; Protasova, S.G.; Mazilkin, A.A.; Goering, E.; Schutz, G.; Straumal, P.B.; Baretzky, B. Ferromagnetic behaviour of ZnO: The role of grain boundaries. Beilstein J. Nanotechnol. 2016, 7, 1936–1947. [Google Scholar] [CrossRef]
- Pan, F.; Song, C.; Liu, X.J.; Yang, Y.C.; Zeng, F. Ferromagnetism and possible application in spintronics of transition-metal-doped ZnO films. Mat. Sci. Eng. R 2008, 62, 1–35. [Google Scholar] [CrossRef]
- Pearton, S.J.; Norton, D.P.; Ivill, M.P.; Hebard, A.F.; Zavada, J.M.; Chen, W.M.; Buyanova, I.A. Ferromagnetism in Transition-Metal Doped ZnO. J. Electron. Mater. 2006, 36, 462–471. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Z.; Zhang, L.; Zhang, C.; Yang, H.; Jiang, J. Synthesis and characterization of Fe and Ni co-doped ZnO nanorods synthesized by a hydrothermal method. Ceram. Int. 2014, 40, 14635–14640. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Li, W.; Zhang, W.; Ye, Y.; Zhang, L.; Ye, Z. Co–Ga codoping effect on preferred growth orientations and properties of ferromagnetic ZnO thin films. J. Magn. Magn. Mater. 2015, 374, 278–282. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Ye, Z.Z.; Lu, B.; Lu, J.G.; Huang, J.Y.; Zhang, Y.Z.; Xie, Z. Defect-induced ferromagnetism in insulating Mn–P codoped ZnO grown in oxygen-rich environment. Solid State Commun. 2013, 155, 16–20. [Google Scholar] [CrossRef]
- Lu, Z.L.; Miao, W.; Zou, W.Q.; Xu, M.X.; Zhang, F.M. Enhanced ferromagnetism in single crystalline Co-doped ZnO thin films by Al codoping. J. Alloys Compd. 2010, 494, 392–395. [Google Scholar] [CrossRef]
- Gu, H.; Jiang, Y.; Xu, Y.; Yan, M. Evidence of the defect-induced ferromagnetism in Na and Co codoped ZnO. Appl. Phys. Lett. 2011, 98, 012502. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Wang, J. Mediated ferromagnetism in ZnO nanorods with heavily codoped MnFe. AIP Adv. 2020, 10, 055019. [Google Scholar] [CrossRef]
- Li, W.; Fang, L.; Ruan, H.; Qin, G.; Zhang, P.; Zhang, H.; Ye, L.; Kong, C. Oxygen vacancies induced ferromagnetism in Ag–N codoped ZnO thin films. Mater. Lett. 2015, 143, 128–130. [Google Scholar] [CrossRef]
- Laiho, R.; Ojala, I.; Vlasenko, L. Percolation of ferromagnetism in ZnO codoped with Fe and Mg. J. Appl. Phys. 2010, 108, 053915. [Google Scholar] [CrossRef]
- Shanthi, S.; Muthukumarasamy, N.; Agilan, S.; Balasundaraprabhu, R. Room temperature ferromagnetism in Mn, N codoped ZnO thin films prepared by hydrothermal synthesis. Mater. Technol. 2013, 29, 52–56. [Google Scholar] [CrossRef]
- Xu, C.; Chun, J.; Kim, D.; Chon, B.; Joo, T. Structural characterization and low temperature growth of ferromagnetic Bi–Cu codoped ZnO bicrystal nanowires. Appl. Phys. Lett. 2007, 91, 153104. [Google Scholar] [CrossRef]
- Aljawfi, R.N.; Rahman, F.; Batoo, K.M. Surface defect mediated magnetic interactions and ferromagnetism in Cr/Co Co-doped ZnO nanoparticles. J. Magn. Magn. Mater. 2013, 332, 130–136. [Google Scholar] [CrossRef]
- Beltrán, J.J.; Barrero, C.A.; Punnoose, A. Evidence of Ferromagnetic Signal Enhancement in Fe and Co Codoped ZnO Nanoparticles by Increasing Superficial Co3+ Content. J. Phys. Chem. C 2014, 118, 13203–13217. [Google Scholar] [CrossRef]
- Sivagamasundari, A.; Chandrasekar, S.; Pugaze, R.; Rajagopan, S.; Kannan, R. Thermal ionization induced metal-semiconductor transition and room temperature ferromagnetism in trivalent doped ZnO codoped with lithium. J. Appl. Phys. 2014, 115, 093902. [Google Scholar] [CrossRef]
- Paul, S.; Dalal, B.; Das, M.; Mandal, P.; De, S.K. Enhanced Magnetic Properties of In–Mn-Codoped Plasmonic ZnO Nanoflowers: Evidence of Delocalized Charge Carrier-Mediated Ferromagnetic Coupling. Chem. Mater. 2019, 31, 8191–8204. [Google Scholar] [CrossRef]
- Pugaze, R.; Sivagamasundari, A.; Vanidha, D.; Chandrasekar, S.; Arunkumar, A.; Rajagopan, S.; Kannan, R. Room Temperature Ferromagnetism in Dual Doped (Mn2+, Ni2+) ZnO Codoped with Li1+ Prepared Using EDTA Sintered at Low Temperature. J. Mater. Sci. Technol. 2014, 30, 275–279. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, M. Influence of Na and F doping on microstructures, optical and magnetic properties of ZnO films synthesized by sol-gel method. Ceram. Int. 2018, 44, 15531–15534. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, S.; Li, Y.; Hu, Y.; Zhu, M.; Jin, H.; Li, Y.; Zhang, H.; Zhao, H. Room temperature ferromagnetic Cr–Ni codoped ZnO diluted magnetic. Ceram. Int. 2015, 41, 451–457. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Zhu, L.; Zhang, Y.; Ye, Z.; He, B. The effects of Ni–Na codoping on structure and properties of ZnO films by pulsed. J. Alloys Compd. 2012, 513, 445–448. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Yang, J.; Gao, M.; Liu, X.; Wei, M. Comparative studies of the structural and magnetic properties in Cu, Co codoped ZnO multilayer films sputtered on different substrates. J. Mater. Sci. Mater. Electron. 2016, 28, 2949–2953. [Google Scholar] [CrossRef]
- Norton, D.P.; Pearton, S.J.; Hebard, A.F.; Theodoropoulou, N.; Boatner, L.A.; Wilson, R.G. Ferromagnetism in Mn-implanted ZnO Sn single crystals. Appl. Phys. Lett. 2003, 82, 239–241. [Google Scholar]
- Xu, Q.; Schmidt, H.; Hochmuth, H.; Lorenz, M.; Setzer, A.; Esquinazi, P.; Meinecke, C.; Grundmann, M. Room temperature ferromagnetism in Nd- and Mn-codoped ZnO films. J. Phys. D Appl. Phys. 2008, 41, 105012. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, L.Q.; Lu, Y.H.; Ye, Z.Z.; Lu, J.G.; Pan, X.H.; Huang, J.Y. Ferromagnetic enhancement and magnetic anisotropy in nonpolar-oriented (Mn, Na)-codoped ZnO thin films. Appl. Phys. Lett. 2012, 101, 242401. [Google Scholar] [CrossRef]
- Photongkam, P.; Zhang, Y.B.; Assadi, M.H.N.; Li, S.; Yu, D.; Ionescu, M.; Pan, A.V. Enhancement of Co substitution induced by Eu codoping in ZnO-based diluted magnetic semiconducting thin films. J. Appl. Phys. 2010, 107, 033909. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, M.; Dong, C.; Ma, J.; Wang, X. Mechanistic insights into magnetic and gas sensing properties of (F, Na)-codoped ZnO nanocrystals by room-temperature photoluminescence. Appl. Surf. Sci. 2019, 496, 143511. [Google Scholar] [CrossRef]
- Liu, L.Q.; Xiang, B.; Zhang, X.Z.; Zhang, Y.; Yu, D.P. Synthesis and room temperature ferromagnetism of Fe Co-codoped ZnO nanowires. Appl. Phys. Lett. 2006, 88, 063104. [Google Scholar] [CrossRef]
- Jayakumar, O.D.; Sudakar, C.; Persson, C.; Sudarsan, V.; Sakuntala, T.; Naik, R.; Tyagi, A.K. 1D Morphology Stabilization and Enhanced Magnetic Properties of Co:ZnO Nanostructures on Codoping with Li: A Template-Free Synthesis. Cryst. Growth Des. 2009, 9, 4450–4455. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, Z.; Lu, B.; Lu, J.; Zhang, Y.; Zhu, L.; Huang, J.; Zhang, W.; Huang, J.; Zhang, J.; et al. Ferromagnetism induced by donor-related defects in Co-doped ZnO thin films. J. Alloys Compd. 2011, 509, 2149–2153. [Google Scholar] [CrossRef]
- Jayakumar, O.D.; Sudakar, C.; Persson, C.; Sudarsan, V.; Naik, R.; Tyagi, A.K. Tunable Ferromagnetism accompanied by Morphology Control in Li-doped Zn0.97Ni0.03O. J. Phys. Chem. C 2010, 114, 17428–17433. [Google Scholar] [CrossRef]
- Chakraborti, D.; Trichy, G.R.; Prater, J.T.; Narayan, J. The effect of oxygen annealing on ZnO:Cu and ZnO:(Cu,Al) diluted magnetic semiconductors. J. Phys. D Appl. Phys. 2007, 40, 7606–7613. [Google Scholar] [CrossRef]
- Ahmad, I.; Shoaib Akhtar, M.; Ahmed, E.; Ahmad, M.; Keller, V.; Qamar Khan, W.; Khalid, N.R. Rare earth co-doped ZnO photocatalysts: Solution combustion synthesis and environmental applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Alanazi, H.S.; Ahmad, N.; Alharthi, F.A. Synthesis of Gd/N co-doped ZnO for enhanced UV-vis and direct solar-light-driven photocatalytic degradation. RSC Adv 2021, 11, 10194–10202. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Luo, Z.; Jiang, L.; Li, X.; Bai, C.; Yu, Q. Fabrication and photocatalytic properties of Co doped ZnO nanomaterials. Mater. Lett. 2023, 350, 134952. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with Reduced TiO(2): From Black TiO(2) to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jiang, L.; Gao, S.; Zhang, S.; Ai, T.; Feng, X.; Wang, W. The highly efficient photocatalysts of B-doped ZnO microspheres synthesized on PET-ITO flexible substrate. Ceram. Int. 2017, 43, 2864–2866. [Google Scholar] [CrossRef]
- Miller, D.R.; Williams, R.E.; Akbar, S.A.; Morris, P.A.; McComb, D.W. STEM-Cathodoluminescence of SnO2 nanowires and powders. Sens. Actuators B 2017, 240, 193–203. [Google Scholar] [CrossRef]
- Lin, Z.; Du, C.; Yan, B.; Yang, G. Amorphous Fe2O3 for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 5582–5592. [Google Scholar] [CrossRef]
- Yan, Y.G.; Li, C.Y.; Zhou, L.X.; Xiong, W.; Zhang, J. Regulation of size and uniformity of In2O3 nanooctahedra. Nanoscale 2016, 8, 13708–13713. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, Q.; Wei, W.; Chen, Z.; Zhu, Y.; Zhang, P.; Zhang, Z.; Gao, Y. WO3 quantum-dots electrochromism. Nano Energy 2020, 68, 104350. [Google Scholar] [CrossRef]
- Sridhar, A.; Sakthivel, P.; Saravanakumar, K.; Sankaranarayanan, R.K. Dual doping effect of Ag+ & Al3+ on the structural, optical, photocatalytic properties of ZnO nanoparticles. Appl. Surf. Sci. Adv. 2023, 13, 100382. [Google Scholar]
- Ferreiro, A.; Flores-Carrasco, G.; Quevedo-López, M.; Urbieta, A.; Fernández, P.; Rabanal, M.E. Effect of lithium codoping on the structural, morphological and photocatalytic properties of Nd-doped ZnO. Ceram. Int. 2023, 49, 33513–33524. [Google Scholar] [CrossRef]
- Alam, U.; Shah, T.A.; Khan, A.; Muneer, M. One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2019, 212, 427–437. [Google Scholar] [CrossRef]
- Mubeen Tahir, M.; Dawood, A.; Hisham Alnasir, M.; Rashid Khan, H.; Zidan, A.M.; Asad Khan, M.; Jabeen, Z. Photocatalytic evaluation of disperse purple dye using Polyvinylpyrrolidone capped bare and Cu+2/Fe+3 codoped ZnO nano catalysts. Inorg. Chem. Commun. 2024, 161, 112031. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, M.; Luo, K.; Hu, W. Relationships between defect-related photoluminescence and photocatalytic activity of (F, Na)-codoped ZnO nanocrystals. Ceram. Int. 2019, 45, 16694–16697. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Duan, M.; Liu, M.; Zou, P.; Zhou, M. Enhanced visible photocatalytic activity of Fe-Cu-ZnO/graphene oxide photocatalysts for the degradation of organic dyes. Can. J. Chem. Eng. 2018, 96, 1479–1488. [Google Scholar] [CrossRef]
- Andrade Neto, N.F.; Matsui, K.N.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Study of the photocatalysis and increase of antimicrobial properties of Fe3+ and Pb2+ co-doped ZnO nanoparticles obtained by microwave-assisted hydrothermal method. Mater. Sci. Semicond. Process. 2019, 93, 123–133. [Google Scholar] [CrossRef]
- Yin, D.; Zhang, L.; Song, K.; Ou, Y.; Wang, C.; Liu, B.; Wu, M. ZnO nanoparticles co-doped with Fe3+ and Eu3+ ions for solar assisted photocatalysis. J. Nanosci. Nanotechnol. 2014, 14, 6077–6083. [Google Scholar] [CrossRef] [PubMed]
- Ghomri, R.; Shaikh, M.N.; Ahmed, M.I.; Bououdina, M.; Ghers, M. (Al, Er) co-doped ZnO nanoparticles for photodegradation of rhodamine blue. Appl. Phys. A 2016, 122, 895. [Google Scholar] [CrossRef]
- Tosun, M.; Senol, S.D.; Arda, L. Effect of Mn/Cu co-doping on the structural, optical and photocatalytic properties of ZnO nanorods. J. Mol. Struct. 2020, 1212, 128071. [Google Scholar] [CrossRef]
- Azmal Zaid, E.H.; Sin, J.-C.; Lam, S.-M.; Mohamed, A.R. Fabrication of La, Ce co-doped ZnO nanorods for improving photodegradation of methylene blue. J. Rare Earths 2023, 42, 76–83. [Google Scholar] [CrossRef]
- Mimouni, R.; Souissi, A.; Madouri, A.; Boubaker, K.; Amlouk, M. High photocatalytic efficiency and stability of chromium-indium codoped ZnO thin films under sunlight irradiation for water purification development purposes. Curr. Appl. Phys. 2017, 17, 1058–1065. [Google Scholar] [CrossRef]
- Ghomri, R.; Shaikh, M.N.; Ahmed, M.I.; Song, W.; Cai, W.; Bououdina, M.; Ghers, M. Pure and (Er, Al) co-doped ZnO nanoparticles: Synthesis, characterization, magnetic and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 10677–10685. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ishtiaq, H.; Ali, F.; Tahir, M.B.; Naeem, S.; Ul-Hamid, A.; Ikram, M.; Iqbal, T.; Kamal, M.R.; Alrobei, H.; et al. Synergistic effects of Bi and N doped on ZnO nanorods for efficient photocatalysis. Mater. Chem. Phys. 2022, 289, 126423. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Dong, Z.-C.; Liao, S.-H.; Chang, S.H. Optimizing luminescent properties of ZnO: Er3+ through temperature and dopant variation: XRD and emission spectroscopy studies. Int. J. Mod. Phys. B 2024, 2540026. [Google Scholar] [CrossRef]
- Seydioglu, T.; Kurnaz, S.; Tokeşer, E.A.; Yildirim, G.; Ozturk, O. Effect of foreign impurity and growth temperatures on hexagonal structure and fundamental properties of ZnO nanorods. Microsc. Res. Tech. 2024. Early View. [Google Scholar] [CrossRef]
- Martins, E.; Jerônimo, A.G.; Barbosa, R.; Neves, L.; Santos, E.; Meira, T.; Osajima, J.A.; Trigueiro, P.; Soares, A.S.; Peña-Garcia, R.R. Influence of Al cations insertion on the structural, morphological, optical properties, and methyl orange photocatalytic remotion of Pr-doped ZnO system. Mater. Chem. Phys. 2024, 318, 129300. [Google Scholar] [CrossRef]
- Rizwana Begum, S.; Anitha, A.G.; Thirumurugan, A.; Chidhambaram, N. Insights into the compositional and temperature-mediated magnetic characteristics of chromium-doped ZnO nanoparticles. J. Phys. Condens. Matter 2024, 36, 385805. [Google Scholar] [CrossRef]
- Rong, P.; Ren, S.; Yu, Q. Fabrications and Applications of ZnO Nanomaterials in Flexible Functional Devices-A Review. Crit. Rev. Anal. Chem. 2019, 49, 336–349. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Kalpana, S.; Senthil, T.S.; Kang, M. Electrical behavior and enhanced photocatalytic activity of (Ag, Ni) co-doped ZnO nanoparticles synthesized from co-precipitation technique. Water Sci. Technol. 2020, 81, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Tahir, M.B.; Sagir, M.; Bamufleh, H.S. Improved photocatalytic performance of Gd and Nd co-doped ZnO nanorods for the degradation of methylene blue. Ceram. Int. 2020, 46, 11955–11961. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Y.; Guo, J.; Zhang, J.; Fang, L. Effect of excessive doping of Ti on photocatalytic properties of Ti and Ga co-doped ZnO nanopowders. J. Sol-Gel Sci. Technol. 2022, 106, 158–172. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.C.; Shikandar, D.B.; Viswanatha, C.C.; Hosamani, G.; Prakash, K.; Manjunatha, N.K. Dual catalytic activity of hexagonal Mg–Sr codoped ZnO nanocrystals for the degradation of an industrial levafix olive reactive dye under sunlight and biosensing applications. React. Eng. 2023, 6, 188. [Google Scholar]
- Zhu, L.; Wang, Z.L. Recent Progress in Piezo-Phototronic Effect Enhanced Solar Cells. Adv. Funct. Mater. 2018, 29, 1808214. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Schaefer, M.; Yaghoubi, S.; Jun, D.; Schlaf, R.; Beatty, J.T.; Takshi, A. A ZnO nanowire bio-hybrid solar cell. Nanotechnology 2017, 28, 054006. [Google Scholar] [CrossRef] [PubMed]
- Chala, S.; Sengouga, N.; Yakuphanoğlu, F.; Rahmane, S.; Bdirina, M.; Karteri, İ. Extraction of ZnO thin film parameters for modeling a ZnO/Si solar cell. Energy 2018, 164, 871–880. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Wang, T. Recent progress and prospects of integrated perovskite/organic solar cells. Appl. Phys. Rev. 2020, 7, 031303. [Google Scholar] [CrossRef]
- Karunakaran, S.K.; Arumugam, G.M.; Yang, W.; Ge, S.; Khan, S.N.; Lin, X.; Yang, G. Research Progress on the Application of Lanthanide-Ion-Doped Phosphor Materials in Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 1035–1060. [Google Scholar] [CrossRef]
- Kumari, M.; Kundu, V.S.; Kumar, S.; Siwatch, S.; Chauhan, N. Nitrogen and silver codoped one-dimensional ZnO nanostructure for optoelectronic application. J. Sol-Gel Sci. Technol. 2019, 93, 302–308. [Google Scholar] [CrossRef]
- Amjad, M.; Khan, M.I.; Alwadai, N.; Irfan, M.; Ikram Ul, H.; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Photovoltaic Properties of ZnO Films Co-Doped with Mn and La to Enhance Solar Cell Efficiency. Nanomaterials 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Naeem, M.; Mustafa, G.M.; Abubshait, S.A.; Mahmood, A.; Al-Masry, W.; Al-Garadi, N.Y.A.; Ramay, S.M. Synthesis and characterization of Co and Ga co-doped ZnO thin films as an electrode for dye sensitized solar cells. Ceram. Int. 2020, 46, 26590–26597. [Google Scholar] [CrossRef]
- Derouiche, M.; Salhi, R.; Baklouti, S. Efficient down-conversion ZnO codoped (Er, Yb) nanopowders synthesized via sol-gel process for Si solar cell applications. J. Radiat. Res. Appl. Sci. 2023, 16, 100497. [Google Scholar] [CrossRef]
- Zhao, F.; Liang, Y.; Lee, J.B.; Hwang, S.J. Applications of rare earth Tb3+-Yb3+ co-doped down-conversion materials for solar cells. Mater. Sci. Eng. B 2019, 248, 114404. [Google Scholar] [CrossRef]
- Pramothkumar, A.; Senthilkumar, N.; Pitchaiya, S.; Eswaramoorthy, N.; Ramakrishnan, V.M.; Potheher, I.V. Perovskite solar cells: Investigation of structural, optical and device performance analysis of Al–Sn co-doped ZnO electron transport layer. J. Mater. Sci. Mater. Electron. 2023, 34, 627. [Google Scholar] [CrossRef]
- Jayaraman, V.K.; Biswal, R.R.; Hernandez, A.G.; Maldonado, A.; Gomez-Pozos, H. Synthesis and characterization of chemically sprayed ZnO:Fe:Ni thin films: Effect of codoping concentration and response as gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 7423–7433. [Google Scholar] [CrossRef]
- Medina Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys-Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Rong, P.; Yang, Z.; Zhang, J.; Zou, X.; Yu, Q. Preparation and Application of Co-Doped Zinc Oxide: A Review. Molecules 2024, 29, 3373. https://doi.org/10.3390/molecules29143373
Luo Z, Rong P, Yang Z, Zhang J, Zou X, Yu Q. Preparation and Application of Co-Doped Zinc Oxide: A Review. Molecules. 2024; 29(14):3373. https://doi.org/10.3390/molecules29143373
Chicago/Turabian StyleLuo, Zhaoyu, Ping Rong, Zhiyuan Yang, Jianhua Zhang, Xiangyu Zou, and Qi Yu. 2024. "Preparation and Application of Co-Doped Zinc Oxide: A Review" Molecules 29, no. 14: 3373. https://doi.org/10.3390/molecules29143373









