Optimization of Enzyme-Assisted Extraction of Rosemary Essential Oil Using Response Surface Methodology and Its Antioxidant Activity by Activating Nrf2 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Extraction of Rosemary Essential Oil
2.3. Experimental Design
2.3.1. Single-Factor Experiments of Enzyme Amount, Enzyme Digestion pH, Enzyme Digestion Temperature, and Time
2.3.2. Box–Behnken Design
2.4. GC-MS Analysis of Rosemary Essential Oil
2.5. Antioxidant Activity of Rosemary Essential Oil
2.5.1. DPPH Radical Scavenging Activity of Rosemary Essential Oil
2.5.2. ABTS+ Radical Scavenging Activity of Rosemary Essential Oil
2.5.3. Hydroxyl Radical Scavenging Activity of Rosemary Essential Oil
2.5.4. Superoxide Anion Radical Scavenging Activity of Rosemary Essential Oil
2.6. Cell Culture
2.7. MTT Assay
2.8. ROS Scavenging Activity of Rosemary Essential Oil In Vitro
2.9. Assessment of Enzymatic and Non-Enzymatic Antioxidant Parameters In Vitro
2.10. Western Blot Analysis
2.11. Molecular Docking
2.12. Statistical Analysis
3. Results and Discussion
3.1. Results of Enzyme-Assisted Extraction Optimization by Single Factor and RSM
3.1.1. Single-Factor Results
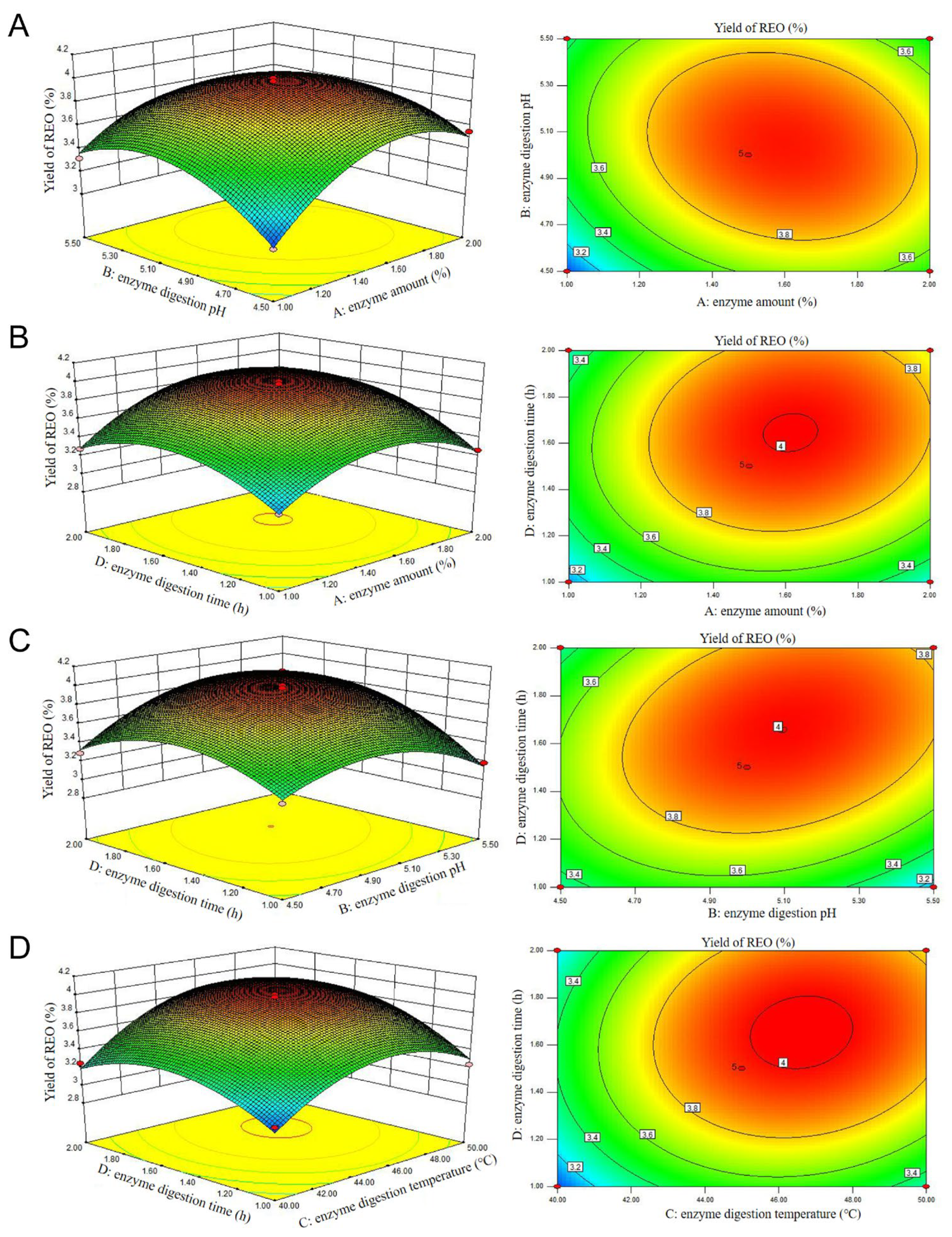
3.1.2. The RSM Results, Variance Analysis, and Verification of Enzyme-Assisted Extraction
3.2. Components of Rosemary Essential Oil
3.3. Rosemary Essential Oil Scavenged Free Radicals in DPPH, ABTS, OH−, and O2− Assay
3.4. Effect of Rosemary Essential Oil on A549 Cell Viability
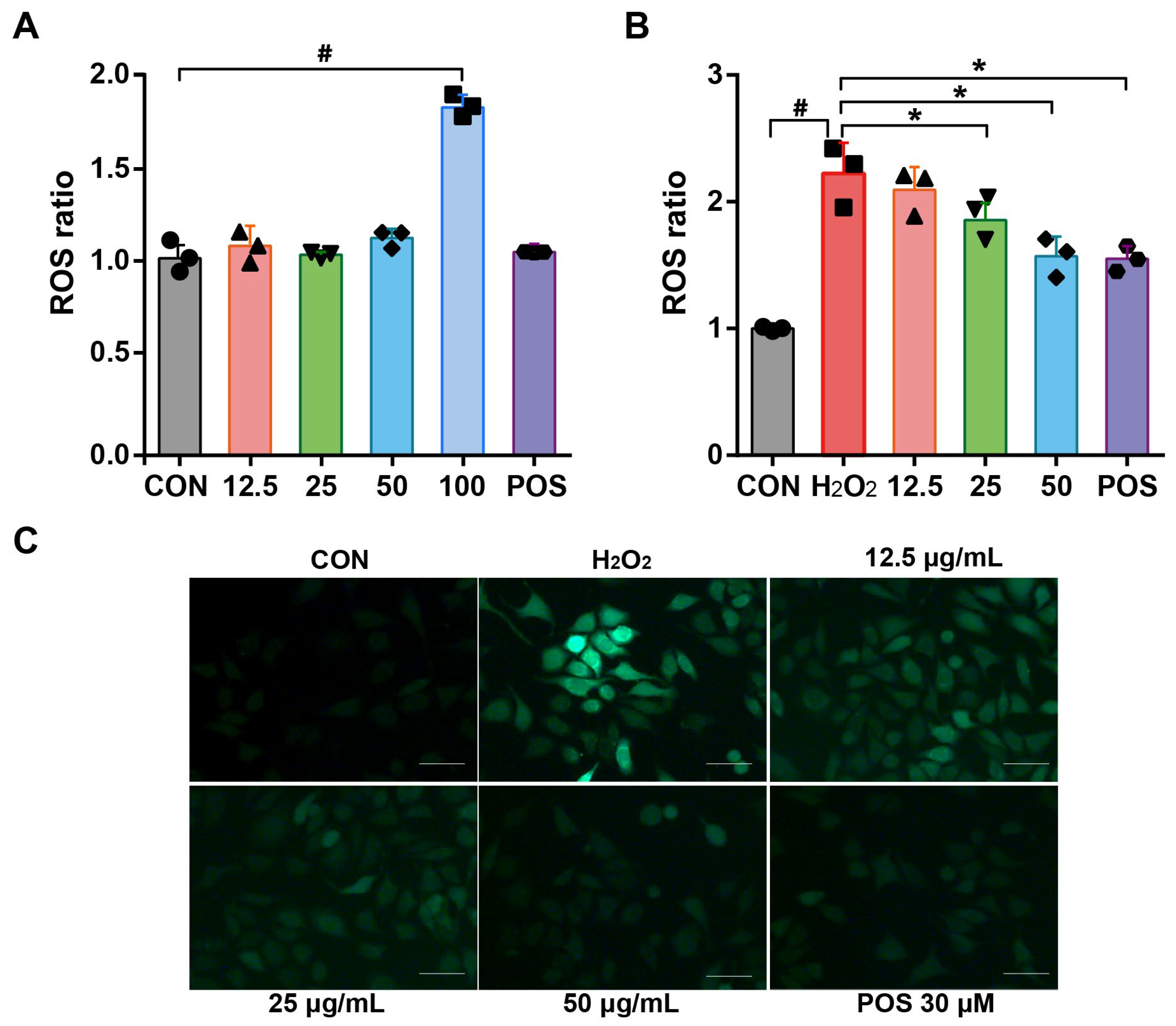
3.5. Effect of Rosemary Essential Oil on the Inhibition of ROS Production in H2O2-Induced A549 Cells
3.6. Effect of Rosemary Essential Oil on Enzymatic and Non-Enzymatic Antioxidant Parameters in H2O2-Induced A549 Cells
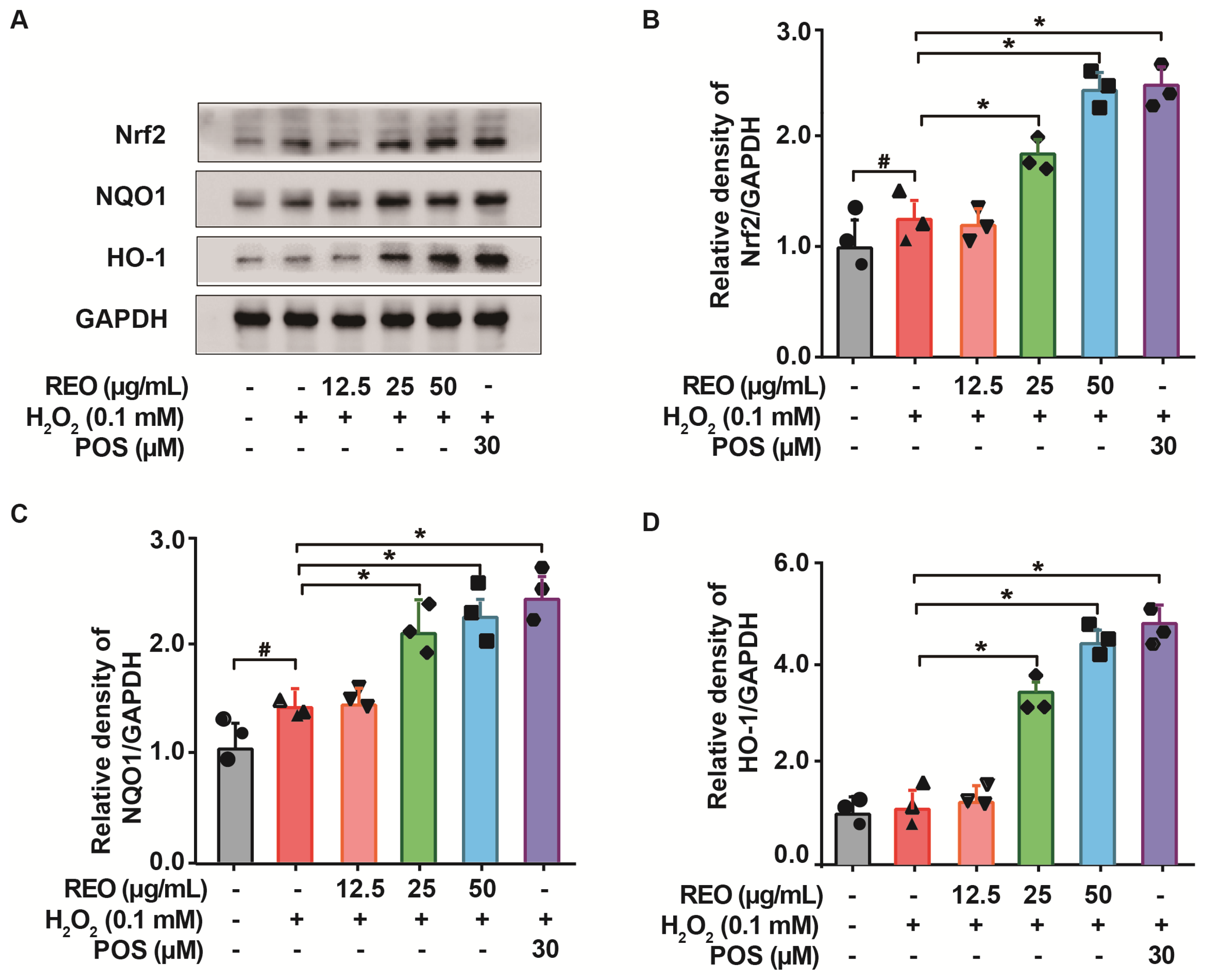
3.7. Effects of Rosemary Essential Oil on Nrf2 Signaling Pathway in A549 Cells
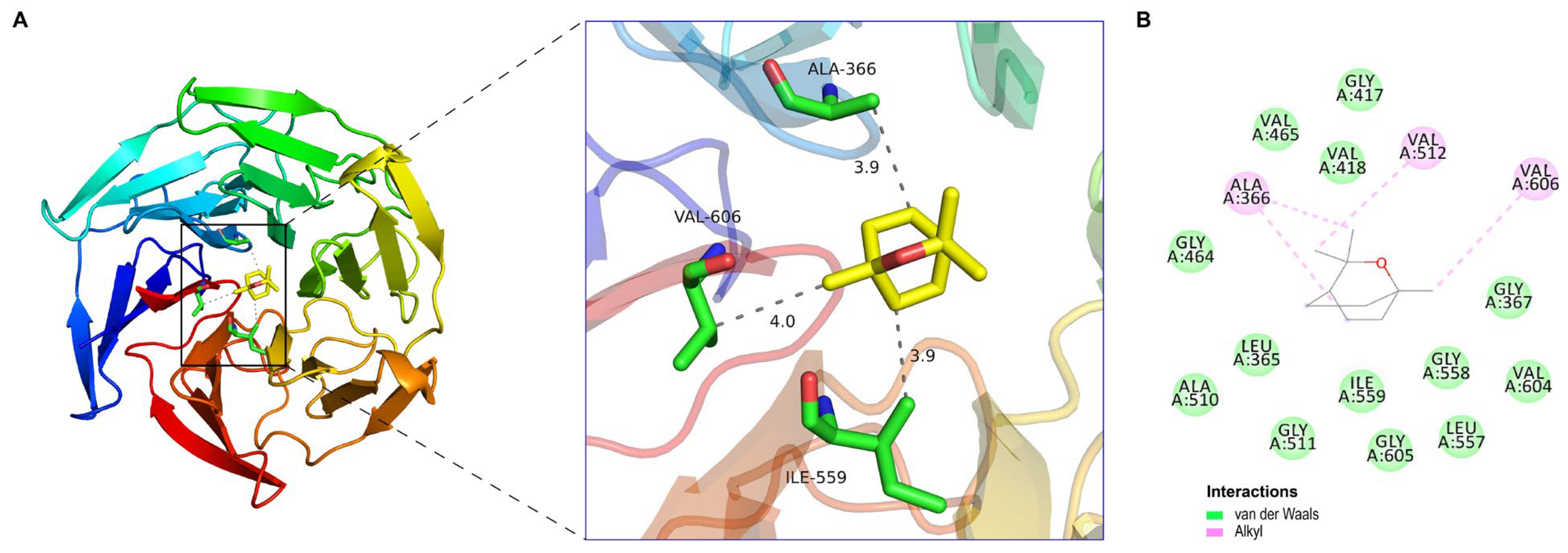
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, S.; Duan, F.; Sang, Z.; Wei, S.; Liu, H.; Tan, H. Rosemary: Unrevealing an old aromatic crop as a new source of promising functional food additive—A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13273. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, S.D.; Androutsopoulou, C.; Hahalis, P.; Kotsalou, C.; Vantarakis, A.; Lamari, F.N. Rosemary extract and essential oil as drink ingredients: An evaluation of their chemical composition, genotoxicity, antimicrobial, antiviral, and antioxidant properties. Foods 2021, 10, 3143. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and biochemical characterization of essential oils and their corresponding hydrolats from six species of the Lamiaceae family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of Rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 2305–6320. [Google Scholar] [CrossRef] [PubMed]
- El Kharraf, S.; El-Guendouz, S.; Farah, A.; Bennani, B.; Mateus, M.C.; Miguel, M.G. Hydrodistillation and simultaneous hydrodistillation-steam distillation of Rosmarinus officinalis and Origanum compactum: Antioxidant, anti-inflammatory, and antibacterial effect of the essential oils. Ind. Crops Prod. 2021, 168, 113591. [Google Scholar] [CrossRef]
- Khruengsai, S.; Promhom, N.; Sripahco, T.; Siriwat, P.; Pripdeevech, P. Optimization of enzyme-assisted microwave extraction of Zanthoxylum limonella essential oil using response surface methodology. Sci. Rep. 2023, 13, 12872. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of Pectin Enzymatic Extraction from Malus domestica ‘Fălticeni’ Apple Pomace with Celluclast 1.5 L. Molecules 2019, 24, 2158. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M.; Boylston, T.D.; Clark, S. Effects of pro-oxidants and antioxidants on the total antioxidant capacity and lipid oxidation products of milk during refrigerated storage. J. Food Sci. 2018, 83, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; An, Q.; Ma, H.; Mu, Y.; Qiao, W.; Zhang, Z.; Zhang, J.; Huang, X.; Li, L. New acylated phenolic glycosides with ROS-scavenging activity from Psidium guajava leaves. J. Agric. Food Chem. 2019, 67, 11089–11098. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.D.; de Moraes, A.A.B.; Santana da Costa, K.; Pereira Galúcio, J.M.; Taube, P.S.; Leal Costa, C.M.; Cruz, J.N.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in Vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 2006, 99, 149–157. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Selvan, G.T.; Ashok, A.K.; Rao, S.J.A.; Gollapalli, P.; Kumari, S.K.; Chaudhury, N.K. Nrf2-regulated antioxidant response ameliorating ionizing radiation-induced damages explored through in vitro and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 41, 8472–8484. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’amato, S.; Sterzo, C.L.; Paparella, A. Characterization of essential oils obtained from Abruzzo Autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 2304–8158. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effect of the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L. essential oil and its polyphenolic extract. Ind. Crops Prod. 2013, 48, 144–152. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.; Zhang, W.; Xia, F.; Yang, E.; Li, H.; Bai, H.; Shi, L. Label-free quantitative proteomics reveals the antibacterial mechanism of rosemary essential oil against Salmonella enterica serovar Typhimurium. Ind. Crops Prod. 2022, 189, 115757. [Google Scholar] [CrossRef]
- Serralutzu, F.; Stangoni, A.; Amadou, B.; Tijan, D.; Re, G.A.; Marceddu, S.; Dore, A.; Bullitta, S. Essential oil composition and yield of a Rosmarinus officinalis L. natural population with an extended flowering season in a coastal Mediterranean environment and perspectives for exploitations. Genet. Resour. Crop Evol. 2020, 67, 1777–1793. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Camargo, S.E.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Augustus, A.R.; Jana, S.; Samsudeen, M.B.; Nagaiah, H.P.; Shunmugiah, K.P. In vitro and in vivo evaluation of the anti-infective potential of the essential oil extracted from the leaves of Plectranthus amboinicus (lour.) spreng against Klebsiella pneumoniae and elucidation of its mechanism of action through proteomics approach. J. Ethnopharmacol. 2024, 330, 118202. [Google Scholar] [CrossRef] [PubMed]
- Najibullah, S.N.M.; Ahamad, J.; Sultana, S.; Zafar, A. Chemical Characterization and Evaluation of Anticancer Activity of Pistacia terebinthus Linn. Fruits Essential Oil. J. Essent. Oil Bear. Plants 2022, 25, 180–187. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Azarkhiavi, K.; Behravan, J.; Mosaffa, F.; Sehatbakhsh, S.; Shirani, K.; Karimi, G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J. Complement. Integr. Med. 2014, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-K.; Hsu, C.-H.; Tsai, M.-L.; Chen, R.-H.; Drummen, G.P.C. Inhibition of oxidative stress by low-molecular-weight polysaccharides with various functional groups in skin fibroblasts. Int. J. Mol. Sci. 2013, 14, 19399–19415. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, W.; Wu, Y.; Zhang, M.; Wang, L.; Wang, Y.; Wang, Y.; Liu, W. Cyclophilin A inhibits A549 cell oxidative stress and apoptosis by modulating the PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2021, 41, BSR20203219. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 2227–9059. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Job, J.T.; Narayanankutty, V. Glutathione an antioxidant tripeptide: Dual roles in carcinogenesis and chemoprevention. Curr. Protein Pept. Sci. 2019, 20, 907–917. [Google Scholar] [CrossRef] [PubMed]
- More, G.K.; Makola, R.T. In-vitro analysis of free radical scavenging activities and suppression of LPS-induced ROS production in macrophage cells by Solanum sisymbriifolium extracts. Sci. Rep. 2020, 10, 6493. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Li, D.; Ma, H.; Mu, Y.; Zheng, D.; Huang, X.; Li, L. Chemical Characterization and Hepatoprotective Effects of a Standardized Triterpenoid-Enriched Guava Leaf Extract. J. Agric. Food Chem. 2021, 69, 3626–3637. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Activation of Nrf2 by natural bioactive compounds: A promising approach for stroke. Int. J. Mol. Sci. 2020, 21, 4875. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Abdul-Hammed, M.; Idris, M.O.; Oyedele, Q.K.; Adedotun, I.O. Molecular dynamics, quantum mechanics and docking studies of some Keap1 inhibitors–An insight into the atomistic mechanisms of their antioxidant potential. Heliyon 2021, 7, e07317. [Google Scholar] [CrossRef] [PubMed]

| Variable | Low Level (−1) | Central Level (0) | High Level (+1) |
|---|---|---|---|
| A (enzyme amount/%) | 1.5 | 2.0 | 2.5 |
| B (enzyme digestion pH) | 4.5 | 5.0 | 5.5 |
| C (enzyme digestion temperature/°C) | 40 | 45 | 50 |
| D (enzyme digestion time/h) | 1.5 | 2.0 | 2.5 |
| RUN | A | B | C | D | Actual Yield (%) |
|---|---|---|---|---|---|
| 1 | −1 | 0 | 0 | 1 | 3.28 |
| 2 | −1 | −1 | 0 | 0 | 3.03 |
| 3 | 0 | 0 | 1 | 1 | 3.71 |
| 4 | −1 | 1 | 0 | 0 | 3.32 |
| 5 | 0 | 0 | 1 | −1 | 3.24 |
| 6 | 0 | 0 | 0 | 0 | 3.97 |
| 7 | 0 | 0 | 0 | 0 | 3.98 |
| 8 | 1 | −1 | 0 | 0 | 3.55 |
| 9 | 0 | 0 | −1 | −1 | 3.09 |
| 10 | 0 | 0 | −1 | 1 | 3.25 |
| 11 | 1 | 0 | 1 | 0 | 3.56 |
| 12 | 0 | −1 | 0 | −1 | 3.29 |
| 13 | 1 | 0 | 0 | −1 | 3.26 |
| 14 | −1 | 0 | 1 | 0 | 3.39 |
| 15 | 1 | 0 | −1 | 0 | 3.17 |
| 16 | 0 | 0 | 0 | 0 | 3.93 |
| 17 | 1 | 0 | 0 | 1 | 3.71 |
| 18 | 0 | 0 | 0 | 0 | 3.98 |
| 19 | −1 | 0 | 0 | −1 | 3.12 |
| 20 | 0 | 1 | 0 | 1 | 3.80 |
| 21 | 0 | 0 | 0 | 0 | 4.00 |
| 22 | 0 | −1 | −1 | 0 | 3.03 |
| 23 | −1 | 0 | −1 | 0 | 2.95 |
| 24 | 0 | 1 | 0 | −1 | 3.19 |
| 25 | 1 | 1 | 0 | 0 | 3.45 |
| 26 | 0 | 1 | 1 | 0 | 3.64 |
| 27 | 0 | 1 | −1 | 0 | 3.09 |
| 28 | 0 | −1 | 1 | 0 | 3.54 |
| 29 | 0 | −1 | 0 | 1 | 3.29 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value | Inference |
|---|---|---|---|---|---|---|
| Model | 2.96 | 14 | 0.21 | 63.16 | <0.0001 | ** |
| A | 0.22 | 1 | 0.22 | 64.69 | <0.0001 | ** |
| B | 0.047 | 1 | 0.047 | 14.15 | 0.0021 | ** |
| C | 0.51 | 1 | 0.51 | 153.49 | <0.0001 | ** |
| D | 0.28 | 1 | 0.28 | 83.65 | <0.0001 | ** |
| AB | 0.038 | 1 | 0.038 | 11.36 | 0.0046 | ** |
| AC | 6.76 × 10−4 | 1 | 6.76 × 10−4 | 0.2 | 0.66 | NS |
| AD | 0.02 | 1 | 0.02 | 6.11 | 0.0269 | * |
| BC | 3.80 × 10−4 | 1 | 3.80 × 10−4 | 0.11 | 0.7411 | NS |
| BD | 0.093 | 1 | 0.093 | 27.88 | 0.0001 | ** |
| CD | 0.024 | 1 | 0.024 | 7.27 | 0.0174 | * |
| A2 | 0.76 | 1 | 0.76 | 227.68 | <0.0001 | ** |
| B2 | 0.55 | 1 | 0.55 | 163.84 | <0.0001 | ** |
| C2 | 0.83 | 1 | 0.83 | 247.52 | <0.0001 | ** |
| D2 | 0.54 | 1 | 0.54 | 162.01 | <0.0001 | ** |
| Residual | 0.047 | 14 | 3.35 × 10−3 | |||
| Lack of fit | 0.044 | 10 | 4.36 × 10−3 | 5.38 | 0.0596 | NS |
| Pure error | 3.25 × 10−3 | 4 | 8.11 × 10−4 | |||
| Cor total | 3.01 | 28 | ||||
| Std. Dev. = 0.058 | Mean = 3.44 | C.V.% = 1.68 | R2 = 0.9844 | R2 Adj = 0.9688 | R2 Pred = 0.9148 | |
| NO a. | Rt (Min) | Name | CAS Number | RI b (NIST) | RI c (C5~C40) | Content d (%) |
|---|---|---|---|---|---|---|
| 1 | 7.38 | Thujene | 2867-05-2 | 911 | 912 | 0.28 ± 0.01 |
| 2 | 8.40 | α-Pinene | 7785-70-8 | 917 | 916 | 0.97 ± 0.28 |
| 3 | 8.72 | Tricyclene | 508-32-7 | 919 | 922 | 0.03 ± 0.01 |
| 4 | 9.72 | Camphene | 79-92-5 | 933 | 936 | 0.18 ± 0.05 |
| 5 | 10.26 | 1,5-Heptadiene, 2,5-dimethyl-3-methylene- | 124-19-6 | 949 | 953 | 0.28 ± 0.08 |
| 6 | 10.96 | Aniline | 124-13-0 | 954 | 958 | 1.04 ± 0.12 |
| 7 | 13.45 | Sabinene | 3387-41-5 | 961 | 964 | 0.12 ± 0.09 |
| 8 | 13.74 | β-Pinene | 127-91-3 | 964 | 965 | 20.23 ± 2.36 |
| 9 | 14.02 | Myrcene | 123-35-3 | 981 | 980 | 0.13 ± 0.06 |
| 10 | 14.19 | 3-Octanol | 589-98-0 | 994 | 997 | 0.17 ± 0.08 |
| 11 | 14.35 | p-Cymene | 99-87-6 | 1021 | 1025 | 0.15 ± 0.01 |
| 12 | 14.57 | 1,8-Cineole | 470-82-6 | 1028 | 1030 | 53.48 ± 4.37 |
| 13 | 14.69 | D-Limonene | 5989-27-5 | 1030 | 1034 | 3.25 ± 1.35 |
| 14 | 14.75 | 1-Hexanol, 2-ethyl- | 104-76-7 | 1031 | 1035 | 0.25 ± 0.13 |
| 15 | 15.17 | 1,3,6-Octatriene, 3,7-dimethyl-, (Z)- | 3338-55-4 | 1039 | 1042 | 0.07 ± 0.01 |
| 16 | 15.49 | Linalool | 78-70-6 | 1104 | 1108 | 0.08 ± 0.01 |
| 17 | 15.56 | Bicyclo[2.2.1]heptane, 1,7,7-trimethyl- | 464-15-3 | 1140 | 1144 | 4.25 ± 0.89 |
| 18 | 15.63 | D-Camphor | 464-49-3 | 1144 | 1149 | 1.53 ± 0.45 |
| 19 | 15.78 | Pinocarvone | 24-41-3 | 1164 | 1161 | 0.36 ± 0.09 |
| 20 | 16.18 | Borneol | 464-43-7 | 1166 | 1166 | 5.21 ± 0.26 |
| 21 | 16.32 | Benzofuran, 4,5,6,7-tetrahydro-3,6-dimethyl- | 494-90-6 | 1169 | 1170 | 0.20 ± 0.01 |
| 22 | 16.44 | dl-Menthol | 89-78-1 | 1173 | 1174 | 3.15 ± 0.97 |
| 23 | 16.69 | α-Terpineol | 98-55-5 | 1191 | 1196 | 0.10 ± 0.01 |
| 24 | 16.82 | D-Verbenone | 80-57-9 | 1200 | 1202 | 0.14 ± 0.10 |
| 25 | 16.87 | Terpinen-4-ol | 562-74-3 | 1206 | 1205 | 0.17 ± 0.01 |
| 26 | 17.17 | 4-Acetylbenzoic acid | 586-89-0 | 1236 | 1236 | 0.34 ± 0.21 |
| 27 | 17.42 | Pulegone | 89-82-7 | 1244 | 1246 | 1.23 ± 0.12 |
| 28 | 17.55 | Piperitone | 89-81-6 | 1252 | 1258 | 0.68 ± 0.05 |
| 29 | 19.88 | (+)-Cyclosativene | 22469-52-9 | 1364 | 1369 | 0.05 ± 0.01 |
| 30 | 19.94 | α-ylangene | 14912-44-8 | 1406 | 1408 | 0.21 ± 0.08 |
| 31 | 20.35 | β- Caryophyllene | 87-44-5 | 1417 | 1420 | 0.07 ± 0.01 |
| 32 | 21.20 | Elemene | 11029-06-4 | 1445 | 1449 | 0.32 ± 0.13 |
| 33 | 22.10 | α-Caryophyllene | 6753-98-6 | 1452 | 1455 | 0.18 ± 0.05 |
| 34 | 22.39 | γ-Muurolene | 30021-74-0 | 1474 | 1478 | 0.05 ± 0.01 |
| 35 | 22.58 | Espatulenol | 6750-60-3 | 1571 | 1576 | 0.18 ± 0.11 |
| 36 | 22.69 | Globulol | 51371-47-2 | 1575 | 1577 | 0.15 ± 0.08 |
| 37 | 22.99 | Caryophyllene oxide | 1139-30-6 | 1578 | 1581 | 0.09 ± 0.01 |
| Sample | DPPH | ABTS | O2− | OH− |
|---|---|---|---|---|
| REO | 33.61 ± 0.24 | 25.04 ± 1.33 | 50.86 ± 3.66 | 43.13 ± 6.32 |
| VC | 17.93 ± 0.30 | 15.52 ± 1.45 | 30.27 ± 3.89 | 23.95 ± 3.45 |
| BHA | 37.24 ± 0.45 | 29.31 ± 2.56 | 58.50 ± 4.27 | 45.57 ± 5.71 |
| BHT | 48.35 ± 0.67 | 35.75 ± 2.78 | 67.63 ± 5.89 | 47.25 ± 5.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, L.; Xu, Y.; Cheng, B.; Zhao, M. Optimization of Enzyme-Assisted Extraction of Rosemary Essential Oil Using Response Surface Methodology and Its Antioxidant Activity by Activating Nrf2 Signaling Pathway. Molecules 2024, 29, 3382. https://doi.org/10.3390/molecules29143382
Li Y, Huang L, Xu Y, Cheng B, Zhao M. Optimization of Enzyme-Assisted Extraction of Rosemary Essential Oil Using Response Surface Methodology and Its Antioxidant Activity by Activating Nrf2 Signaling Pathway. Molecules. 2024; 29(14):3382. https://doi.org/10.3390/molecules29143382
Chicago/Turabian StyleLi, Yuanyuan, Lei Huang, Yongfang Xu, Biao Cheng, and Mingqin Zhao. 2024. "Optimization of Enzyme-Assisted Extraction of Rosemary Essential Oil Using Response Surface Methodology and Its Antioxidant Activity by Activating Nrf2 Signaling Pathway" Molecules 29, no. 14: 3382. https://doi.org/10.3390/molecules29143382
APA StyleLi, Y., Huang, L., Xu, Y., Cheng, B., & Zhao, M. (2024). Optimization of Enzyme-Assisted Extraction of Rosemary Essential Oil Using Response Surface Methodology and Its Antioxidant Activity by Activating Nrf2 Signaling Pathway. Molecules, 29(14), 3382. https://doi.org/10.3390/molecules29143382




