Evolution of the Structure and Morphology of Dual-Linker ZIF-301-eIm
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of ZIF-301-eIm Derivatives via Two Crystallization Pathways
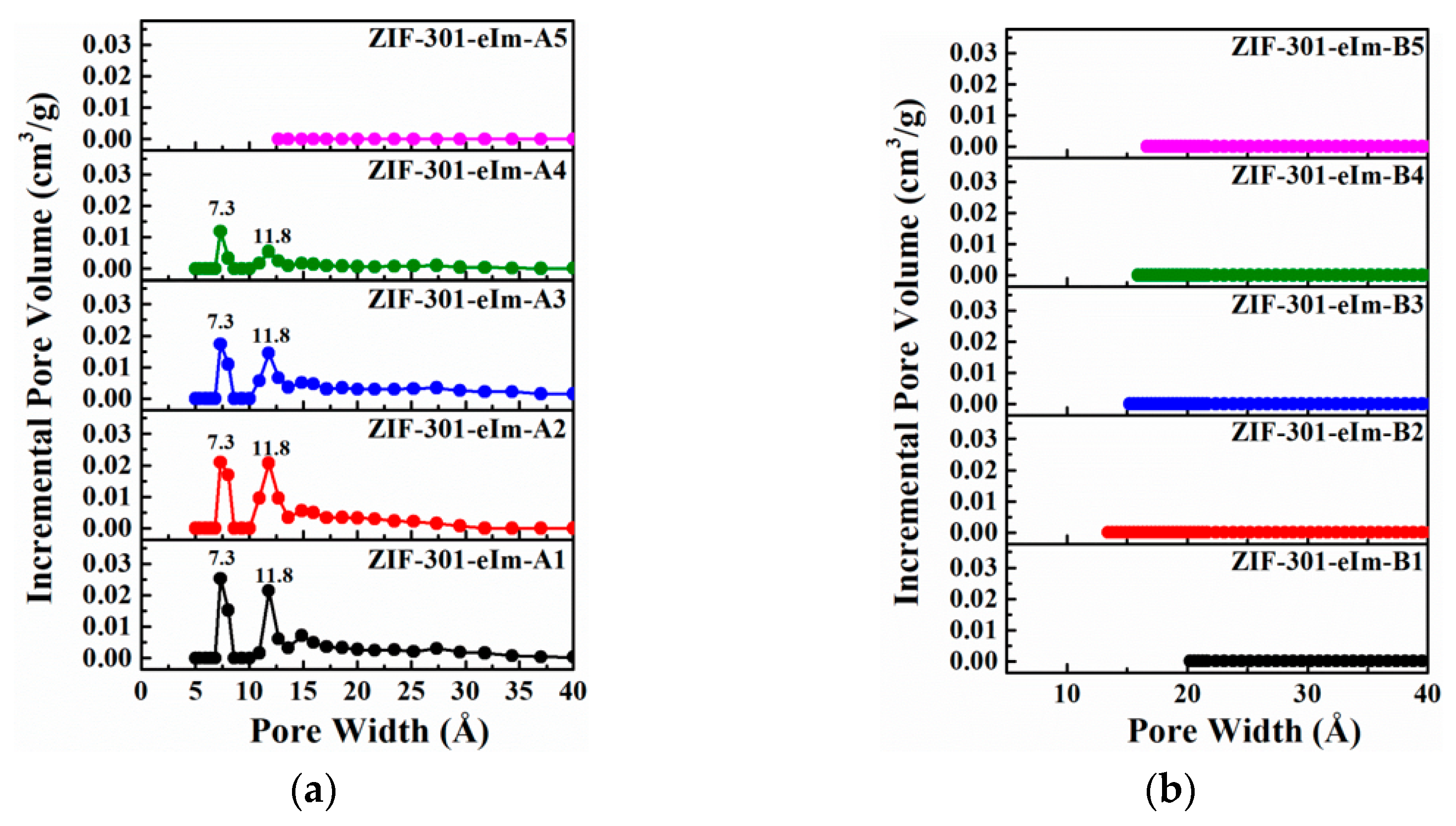
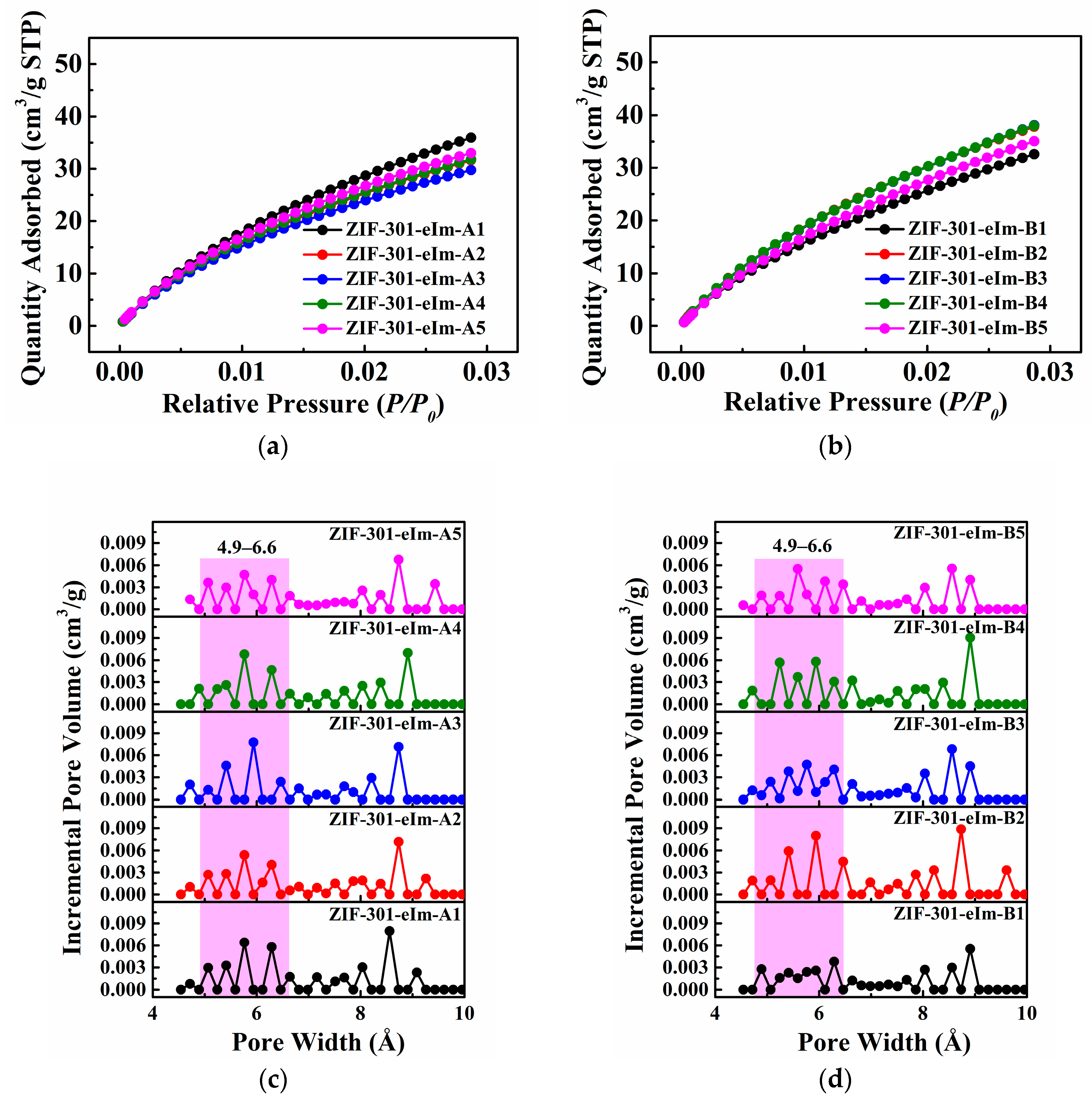
2.2. Pore Structure Analysis of the ZIF-301-eIm Derivatives Prepared via Two Crystallization Pathways
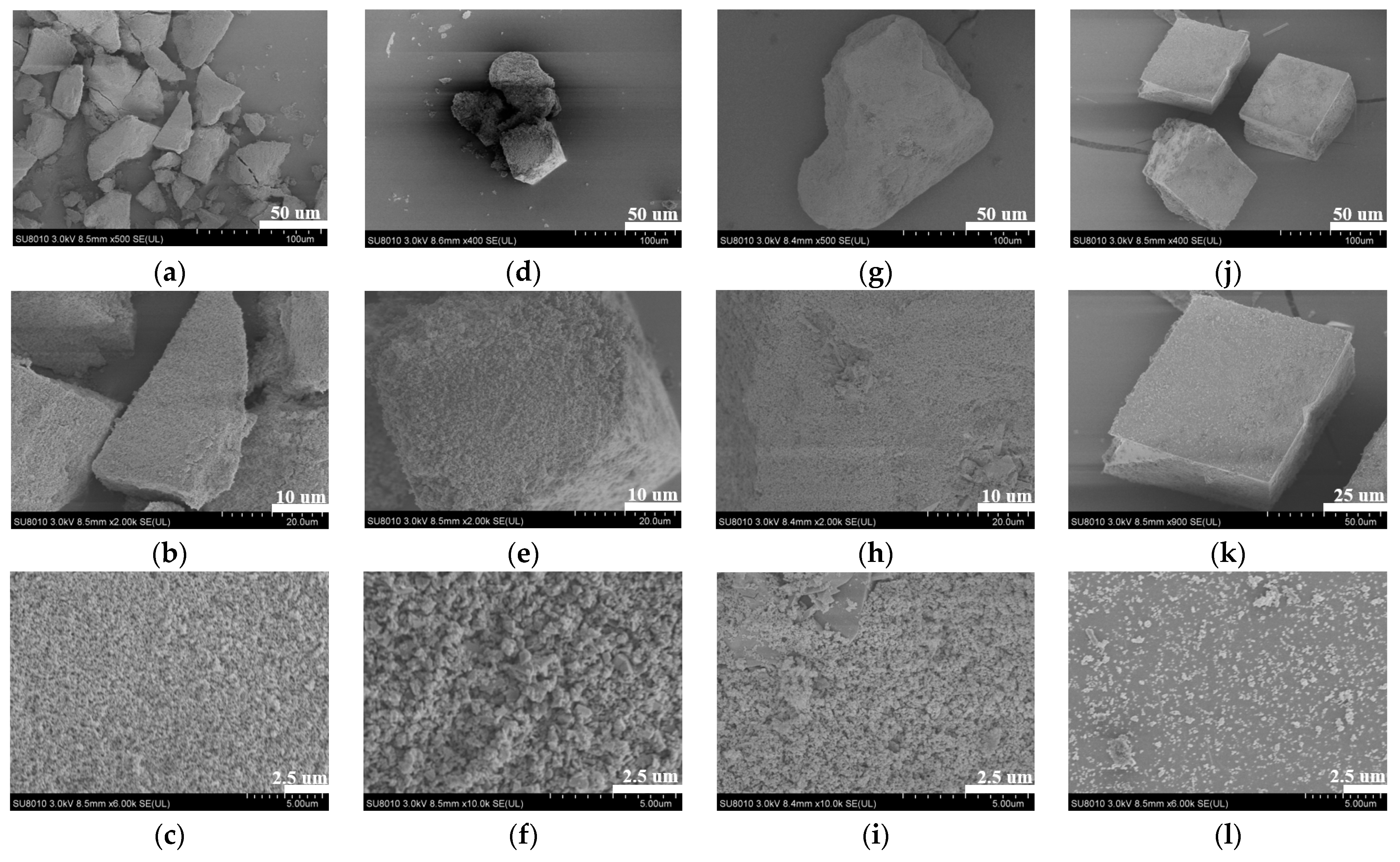
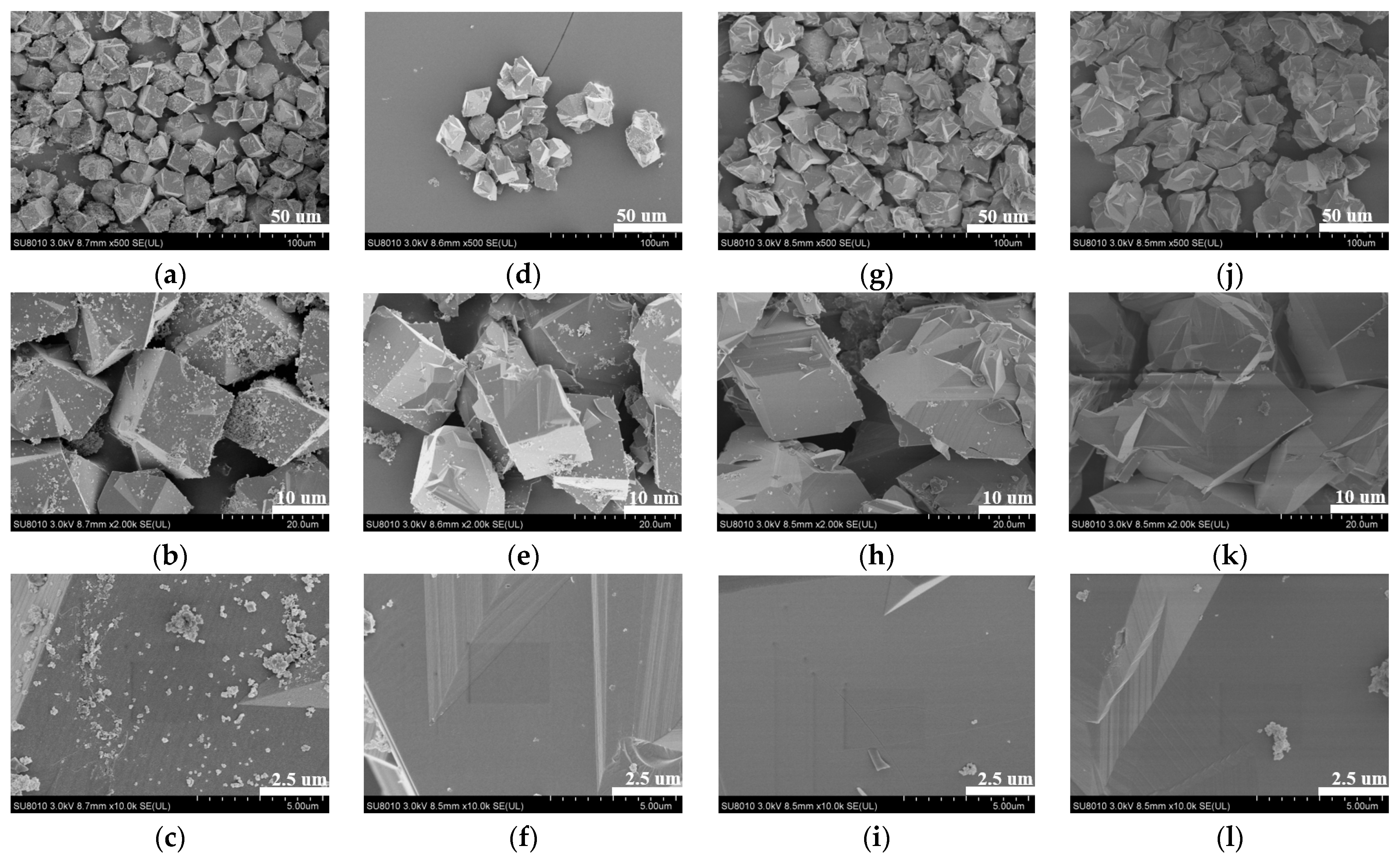
2.3. Morphological Analysis of the ZIF-301-eIm Derivatives Prepared via Two Crystallization Pathways
2.4. Hydrophobicity of the ZIF-301-eIm Derivatives
2.5. Static Adsorption and Dynamic Column Adsorption of ZIF-301-eIm Derivatives toward Acetone/Butanol
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of ZIF-301-eIm Derivatives via Two Crystallization Pathways
3.3. Characterization
3.4. Static Batch Adsorption
3.5. Dynamic Column Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Rong, Z.; Nguyen, H.L.; Yaghi, O.M. Structural chemistry of zeolitic imidazolate frameworks. Inorg. Chem. 2023, 62, 20861–20873. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Dutta, S.; Hossain, M.S.A.; Shiddiky, M.J.A.; Tung, K.L.; Shieh, F.K.; Tsung, C.K.; Wu, K.C.; Yamauchi, Y. Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications. Adv. Mater. 2017, 29, 1700213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Furukawa, H.; Gandara, F.; Nguyen, H.T.; Cordova, K.E.; Yaghi, O.M. Selective capture of carbon dioxide under humid conditions by hydrophobic chabazite-type zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. Engl. 2014, 53, 10645–10648. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Z.; Zhou, X.; Lin, Y.; Hou, J. Fabrication of an Fe-doped ZIF-67 derived magnetic Fe/Co/C composite for effective removal of congo red. Molecules 2024, 29, 2078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xu, Y.; Yao, X.; Wang, C.; Liu, P.; Zhao, H.; Lu, J.; Ju, J. Efficient removal of tetracycline by metal–organic framework ZIF-67 and its mechanism. Separations 2024, 11, 63. [Google Scholar] [CrossRef]
- Xiao, C.; Tian, J.; Chen, Q.; Hong, M. Water-stable metal-organic frameworks (MOFs): Rational construction and carbon dioxide capture. Chem. Sci. 2024, 15, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Letwaba, J.; Uyor, U.O.; Mavhungu, M.L.; Achuka, N.O.; Popoola, P.A. A review on MOFs synthesis and effect of their structural characteristics for hydrogen adsorption. RSC Adv. 2024, 14, 14233–14253. [Google Scholar] [CrossRef] [PubMed]
- Banglani, T.H.; Chandio, I.; Ali, A.; Memon, A.A.; Yang, J.; Kazi, M.; Thebo, K.H. Recent progress in 2D and 3D metal–organic framework-based membranes for water sustainability. Environ. Sci. Water Res. Technol. 2024, 10, 1061–1096. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, X.; Li, M.Z.; Li, J.R. Non-CO2 greenhouse gas separation using advanced porous materials. Chem. Soc. Rev. 2024, 53, 2056–2098. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Lan, X.; Wang, J.; Liu, X.-H.; Muddassir, M.; Srivastava, D.; Kushwaha, A.; Kumar, A. A new Cd(II)-based coordination polymer as a luminescent sensor and adsorbent for dichromate ions. CrystEngComm 2024, 26, 2353–2360. [Google Scholar] [CrossRef]
- Tuo, K.; Li, J.; Li, Y.; Liang, C.; Shao, C.; Hou, W.; Li, Z.; Pu, S.; Deng, C. Construction of hierarchical porous and polydopamine/salicylaldoxime functionalized zeolitic imidazolate framework-8 via controlled etching for uranium adsorption. Mater. Horiz. 2024, 11, 3364–3374. [Google Scholar] [CrossRef]
- Kato, T.; Akiyama, I.; Mori, F.; Shinohara, A.; Ogura, Y.; Ito, A.; Ohtani, M. Boron-imidazolate coordination networks with 3d transition metals for enhanced CO2 adsorption capability. Mater. Adv. 2024, 5, 4151–4158. [Google Scholar] [CrossRef]
- Lal, S.; Singh, P.; Singhal, A.; Kumar, S.; Singh Gahlot, A.P.; Gandhi, N.; Kumari, P. Advances in metal-organic frameworks for water remediation applications. RSC Adv. 2024, 14, 3413–3446. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Zhang, Z.-H.; Fang, H.; Guo, X.-A.; Du, G.-T.; Wang, Q.; Xue, D.-X. A (4,6)-c copper–organic framework constructed from triazole-inserted dicarboxylate linker with CO2 selective adsorption. CrystEngComm 2024, 26, 1204–1208. [Google Scholar] [CrossRef]
- Noh, K.; Lee, J.; Kim, J. Compositions and structures of zeolitic imidazolate frameworks. Isr. J. Chem. 2018, 58, 1075–1088. [Google Scholar] [CrossRef]
- Hayashi, H.; Cote, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef]
- Biswal, B.P.; Panda, T.; Banerjee, R. Solution mediated phase transformation (RHO to SOD) in porous Co-imidazolate based zeolitic frameworks with high water stability. Chem. Commun. 2012, 48, 11868–11870. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A combined experimental−computational investigation of carbon dioxide capture in a series of isoreticular zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Panda, T.; Gupta, K.M.; Jiang, J.; Banerjee, R. Enhancement of CO2 uptake in iso-reticular Co based zeolitic imidazolate frameworks via metal replacement. CrystEngComm 2014, 16, 4677–4680. [Google Scholar] [CrossRef]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.B.; Liu, Q.; Trickett, C.A.; Gutierrez-Puebla, E.; Monge, M.A.; Cong, H.; Aldossary, A.; Deng, H.; Yaghi, O.M. Principles of designing extra-large pore openings and cages in zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2017, 139, 6448–6455. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Huang, R.K.; Zheng, B.; Wang, P.; Shi, Q.; Zhang, W.X.; Dong, J. Large breathing effect in ZIF-65(Zn) with expansion and contraction of the SOD cage. Nat. Commun. 2022, 13, 4569. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Blad, C.R.; Brunelli, N.A.; Lydon, M.E.; Lively, R.P.; Jones, C.W.; Nair, S. Hybrid zeolitic imidazolate frameworks: Controlling framework porosity and functionality by mixed-linker synthesis. Chem. Mater. 2012, 24, 1930–1936. [Google Scholar] [CrossRef]
- Song, J.; Meng, Q.; Wang, J.; Guo, X.; Wei, P.; Dong, J.; Shi, Q. Length exclusion separation of acetone/butanol using ZIF-302 derivatives with adjustable ellipsoidal cage sizes. Sep. Purif. Technol. 2023, 312, 123371. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Gao, M.; Ge, L.; Wang, M.; Dong, J.; Shi, Q. Introduction of hydrogen bond recognition sites in ZIF-71 for effective separation of bio-diols in aqueous solutions. AIChE J. 2023, 70, e18239. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, H.; Sun, J.; Mao, Y.; Liu, G.; Jin, W. Novel ZIF-300 mixed-matrix membranes for efficient CO2 capture. ACS Appl. Mater. Interfaces 2017, 9, 38575–38583. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.-S.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Liu, G.; Lee, K.-R.; Jin, W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Membr. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Li, R.; Zhu, H.; Duan, J.; Guo, Y.; Liu, G.; Jin, W. ZIF-301 MOF/6FDA-DAM polyimide mixed-matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 264, 118431. [Google Scholar] [CrossRef]
- van Essen, M.; Thür, R.; Houben, M.; Vankelecom, I.F.J.; Borneman, Z.; Nijmeijer, K. Tortuous mixed matrix membranes: A subtle balance between microporosity and compatibility. J. Membr. Sci. 2021, 635, 119517. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of adding graphene oxide and ZIF-301 to polysulfone to develop high performance mixed matrix membranes for selective carbon dioxide separation from post combustion flue gas. J. Membr. Sci. 2016, 514, 35–43. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. A novel zeolitic imidazolate framework based mixed-matrix membrane for efficient CO2 separation under wet conditions. J. Taiwan Inst. Chem. Eng. 2016, 65, 427–436. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of incorporating ZIF-302 and graphene oxide to polysulfone to develop highly selective mixed-matrix membranes for carbon dioxide separation from wet post-combustion flue gases. J. Ind. Eng. Chem. 2016, 36, 154–162. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Combined effect of cnts with ZIF-302 into polysulfone to fabricate MMMs for enhanced CO2 separation from flue gases. Arab. J. Sci. Eng. 2016, 41, 2573–2582. [Google Scholar] [CrossRef]
- Gustafsson, M.; Zou, X. Crystal formation and size control of zeolitic imidazolate frameworks with mixed imidazolate linkers. J. Porous Mater. 2013, 20, 55–56. [Google Scholar] [CrossRef]
- Self, K.; Telfer, M.; Greer, H.; Zhou, W. Reversed crystal growth of RHO zeolitic imidazolate framework (ZIF). Chem.–A Eur. J. 2015, 21, 19090–19095. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, T.; Bae, Y. Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous Mesoporous Mater. 2016, 224, 294–301. [Google Scholar] [CrossRef]
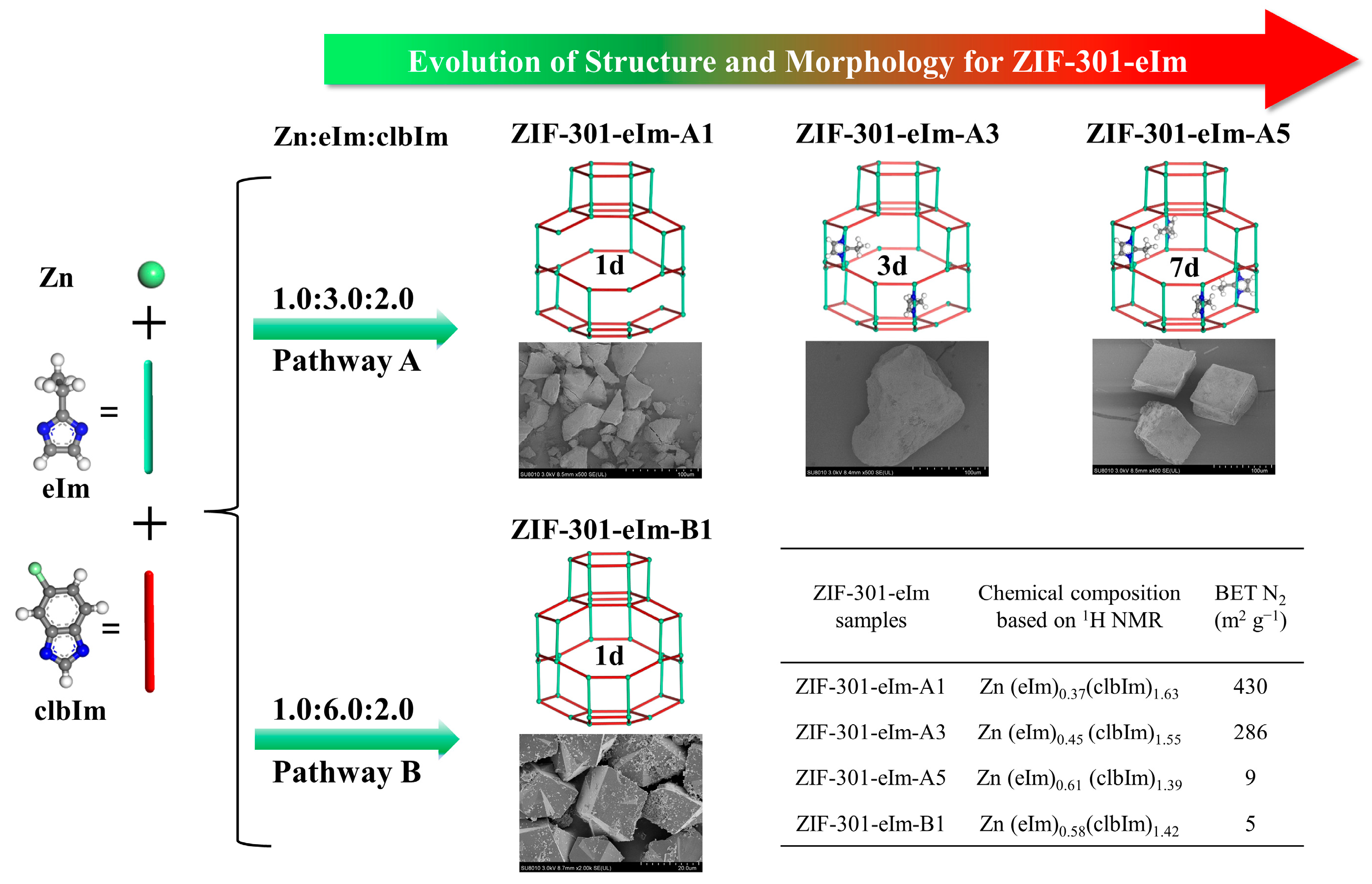
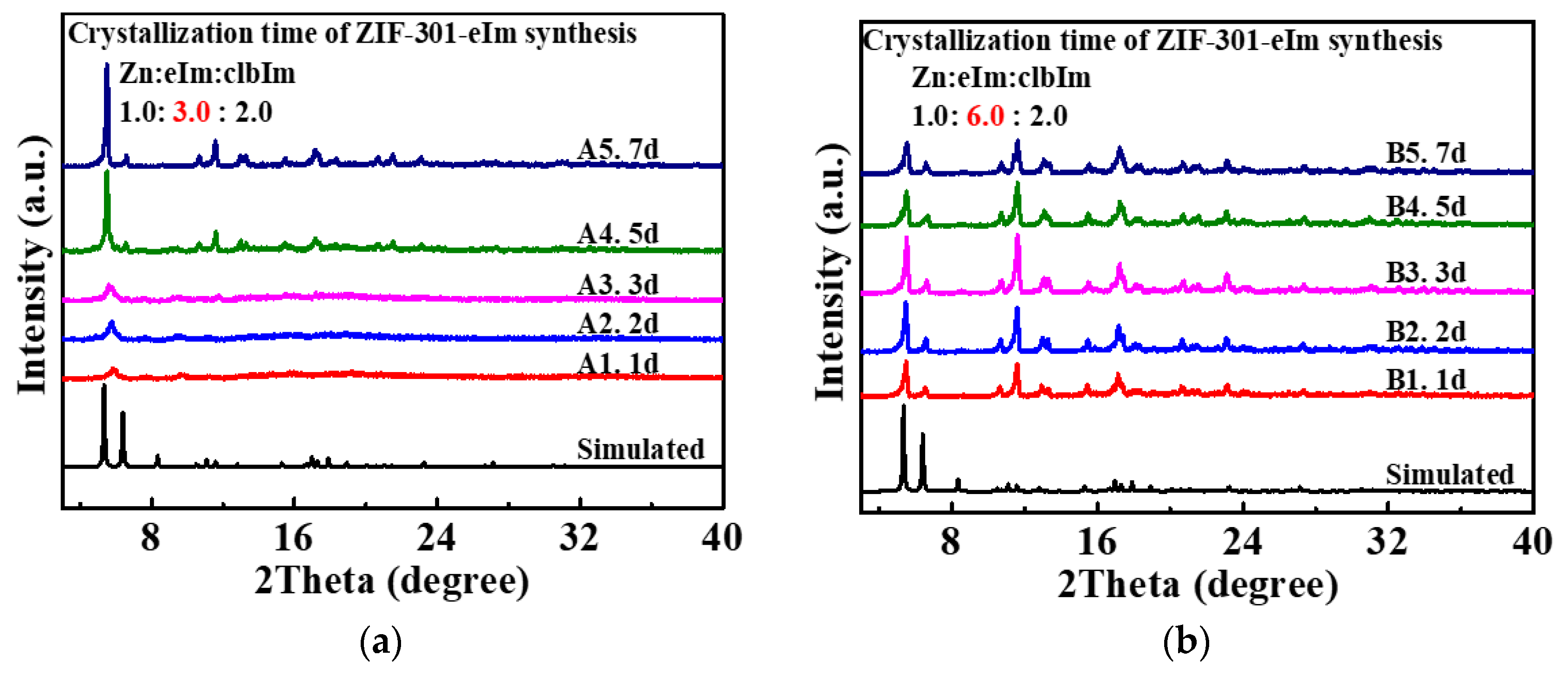

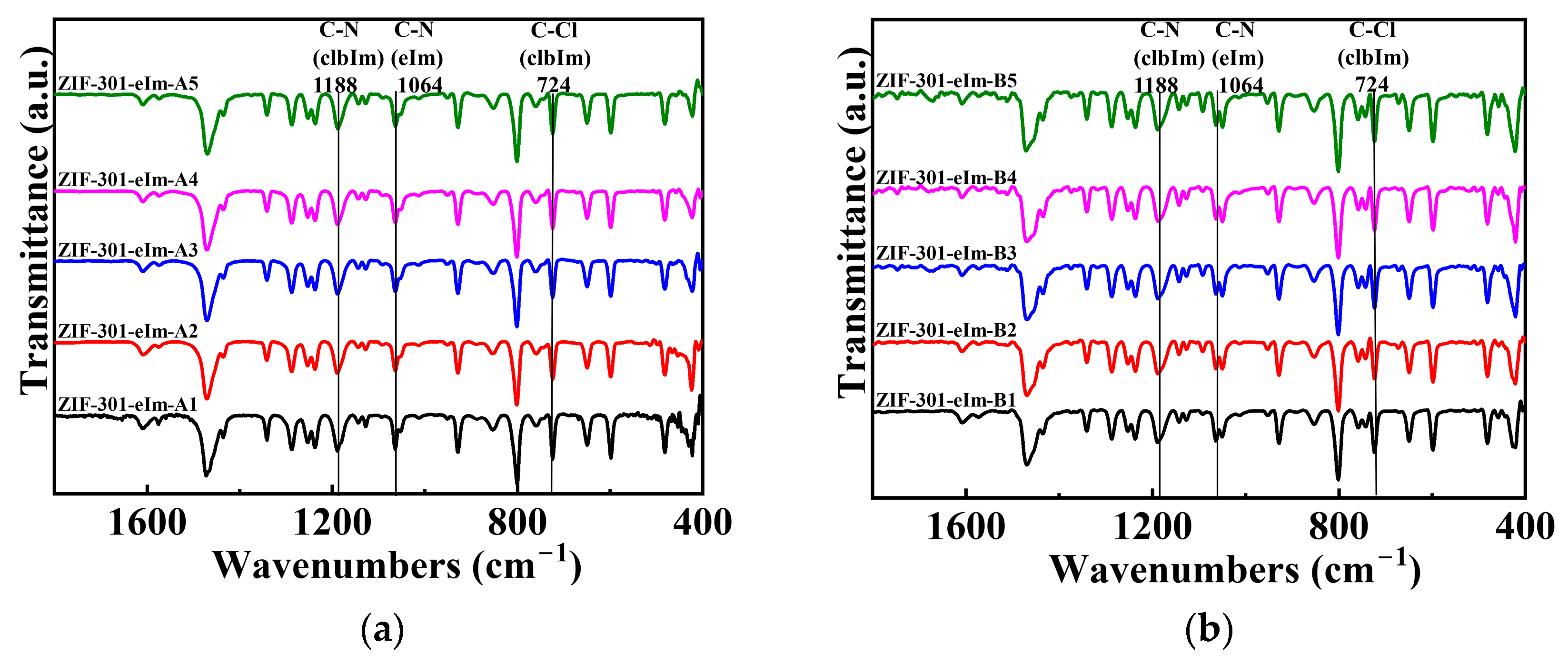
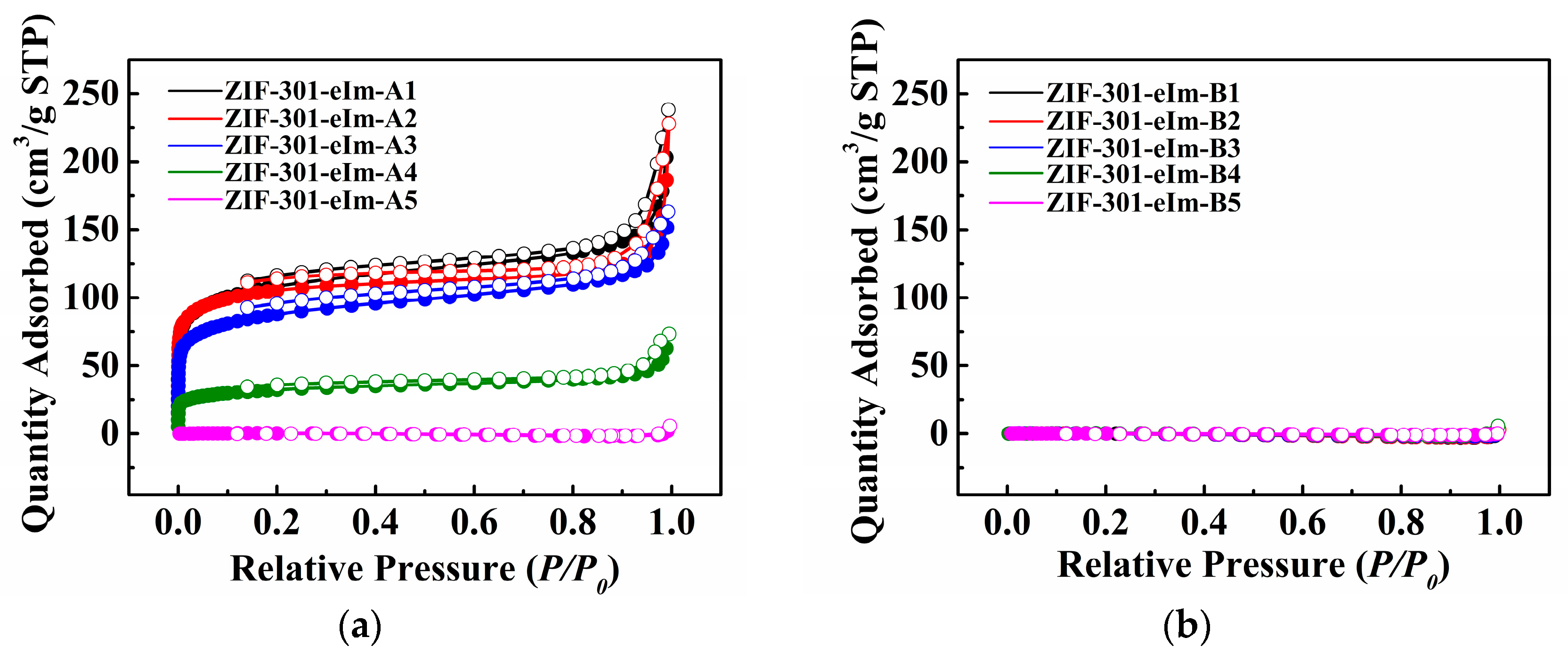




| ZIF-301-eIm Derivative | Crystallization Time | Chemical Composition Based on 1H NMR Spectroscopy a | BET Surface Area (m2 g−1) b | Pore Volume | |
|---|---|---|---|---|---|
| N2 | CO2 | (cm3 g−1) c | |||
| Zn:eIm:clbIm (synthesis) = 1.0:3.0:2.0 | |||||
| ZIF-301-eIm-A1 | 1d | Zn(eIm)0.37(clbIm)1.63 | 430 | 273 | 0.369 |
| ZIF-301-eIm-A2 | 2d | Zn(eIm)0.40(clbIm)1.60 | 363 | 235 | 0.353 |
| ZIF-301-eIm-A3 | 3d | Zn(eIm)0.45(clbIm)1.55 | 286 | 210 | 0.253 |
| ZIF-301-eIm-A4 | 5d | Zn(eIm)0.56(clbIm)1.44 | 108 | 229 | 0.113 |
| ZIF-301-eIm-A5 | 7d | Zn(eIm)0.61(clbIm)1.39 | 9 | 240 | 0.005 |
| Zn:eIm:clbIm (synthesis) = 1.0:6.0:2.0 | |||||
| ZIF-301-eIm-B1 | 1d | Zn(eIm)0.58(clbIm)1.42 | 5 | 242 | 0.003 |
| ZIF-301-eIm-B2 | 2d | Zn(eIm)0.62(clbIm)1.38 | 0 | 293 | 0 |
| ZIF-301-eIm-B3 | 3d | Zn(eIm)0.63(clbIm)1.37 | 0 | 297 | 0 |
| ZIF-301-eIm-B4 | 5d | Zn(eIm)0.64(clbIm)1.36 | 0 | 285 | 0 |
| ZIF-301-eIm-B5 | 7d | Zn(eIm)0.64(clbIm)1.36 | 0 | 284 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Xie, B.; Wang, J.; Wu, Y.; Shi, Q.; Dong, J. Evolution of the Structure and Morphology of Dual-Linker ZIF-301-eIm. Molecules 2024, 29, 3395. https://doi.org/10.3390/molecules29143395
Wei P, Xie B, Wang J, Wu Y, Shi Q, Dong J. Evolution of the Structure and Morphology of Dual-Linker ZIF-301-eIm. Molecules. 2024; 29(14):3395. https://doi.org/10.3390/molecules29143395
Chicago/Turabian StyleWei, Ping, Boyao Xie, Jiang Wang, Yanjun Wu, Qi Shi, and Jinxiang Dong. 2024. "Evolution of the Structure and Morphology of Dual-Linker ZIF-301-eIm" Molecules 29, no. 14: 3395. https://doi.org/10.3390/molecules29143395
APA StyleWei, P., Xie, B., Wang, J., Wu, Y., Shi, Q., & Dong, J. (2024). Evolution of the Structure and Morphology of Dual-Linker ZIF-301-eIm. Molecules, 29(14), 3395. https://doi.org/10.3390/molecules29143395






