Influence of Cheese Composition on Aroma Content, Release, and Perception
Abstract
:1. Introduction
2. Results
2.1. Gross Composition of Cheeses
2.2. Taste and Texture of Cheeses
2.3. Microstructure and Image Analysis
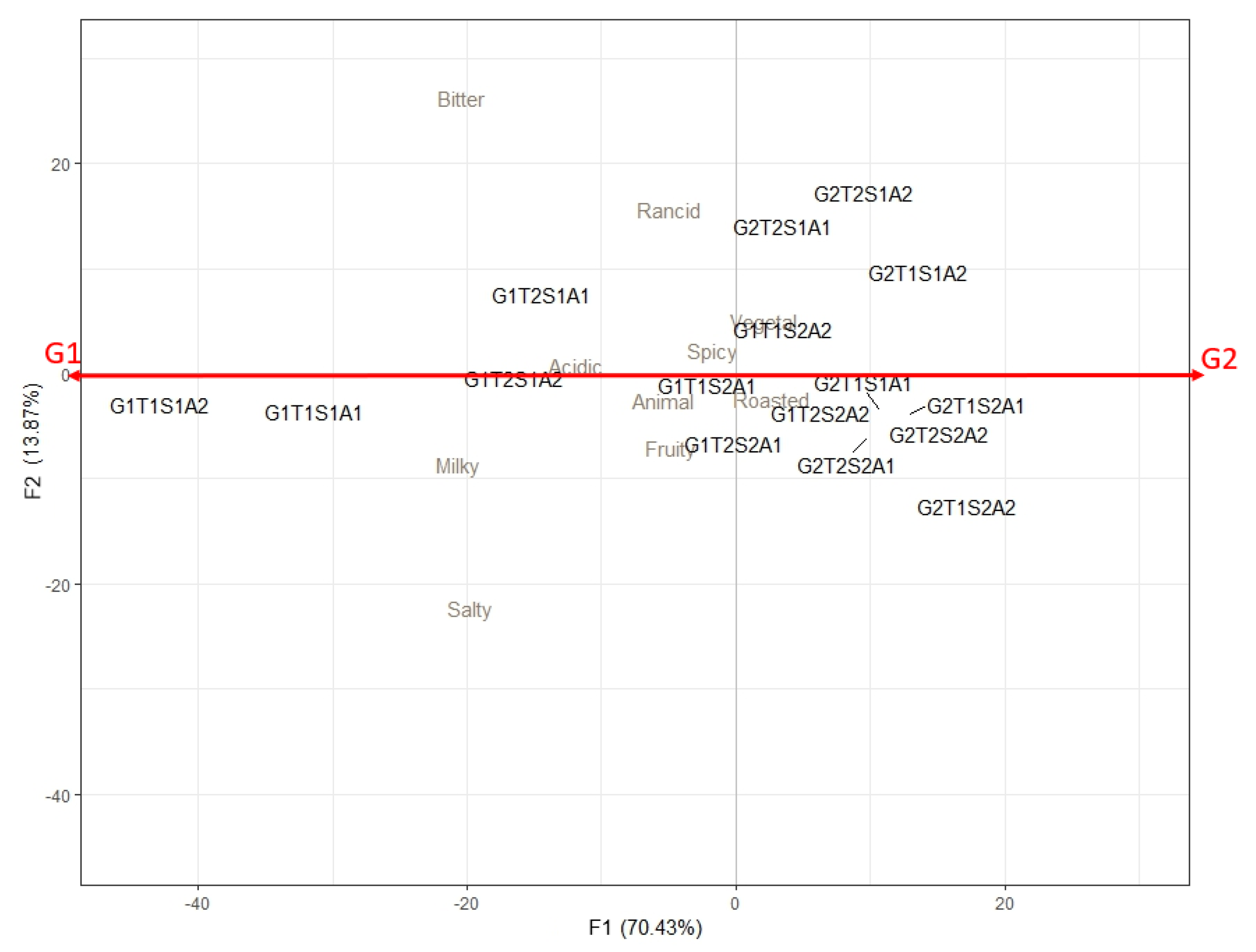
2.4. Sensory Evaluation of Cheese Aroma
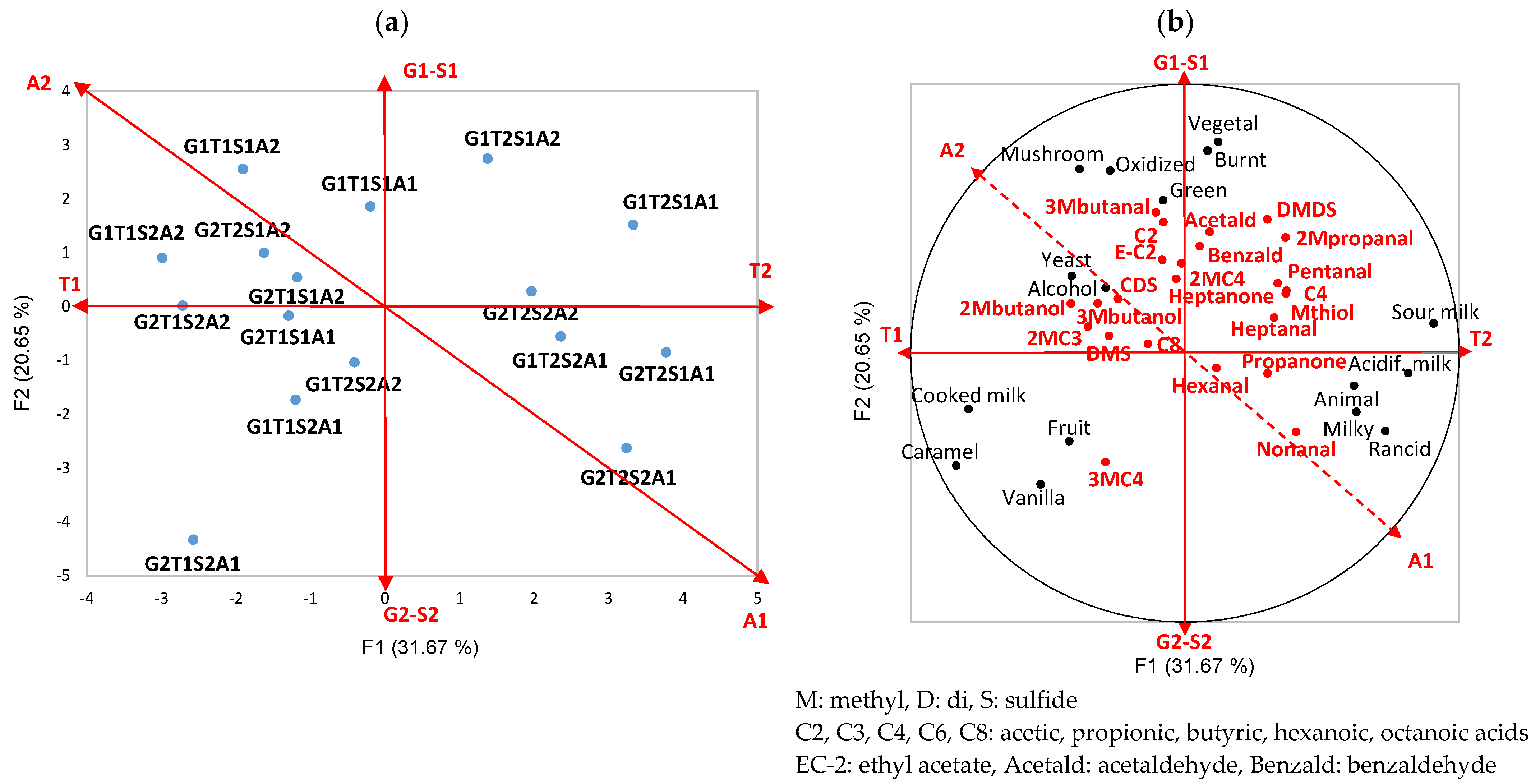
2.5. Identification of Aroma Compounds in Cheese by HS-SPME–GC–MS
| Correspondence between small code and large code for cheeses | ||||||||||||||||
| Small code | C-01 | C-02 | C-03 | C-04 | C-05 | C-06 | C-07 | C-08 | C-09 | C-10 | C-11 | C-12 | C-13 | C-14 | C-15 | C-16 |
| Large code | G1T1S1A1 | G1T1S1A2 | G1T2S1A1 | G1T2S1A2 | G1T1S2A1 | G1T1S2A2 | G1T2S2A1 | G1T2S2A2 | G2T1S1A1 | G2T1S1A2 | G2T2S2A1 | G2T2S2A2 | G2T2S1A1 | G2T2S1A2 | G2T1S2A1 | G2T1S2A2 |
| G: fat; T: lactose in whey; S: salt; 1: lower level; 2: higher level; A1 and A2: both adjunct strains. | ||||||||||||||||
2.6. Dynamic Aroma Release from Cheese
2.6.1. TCATA
2.6.2. PTR-ToF-MS Analyses
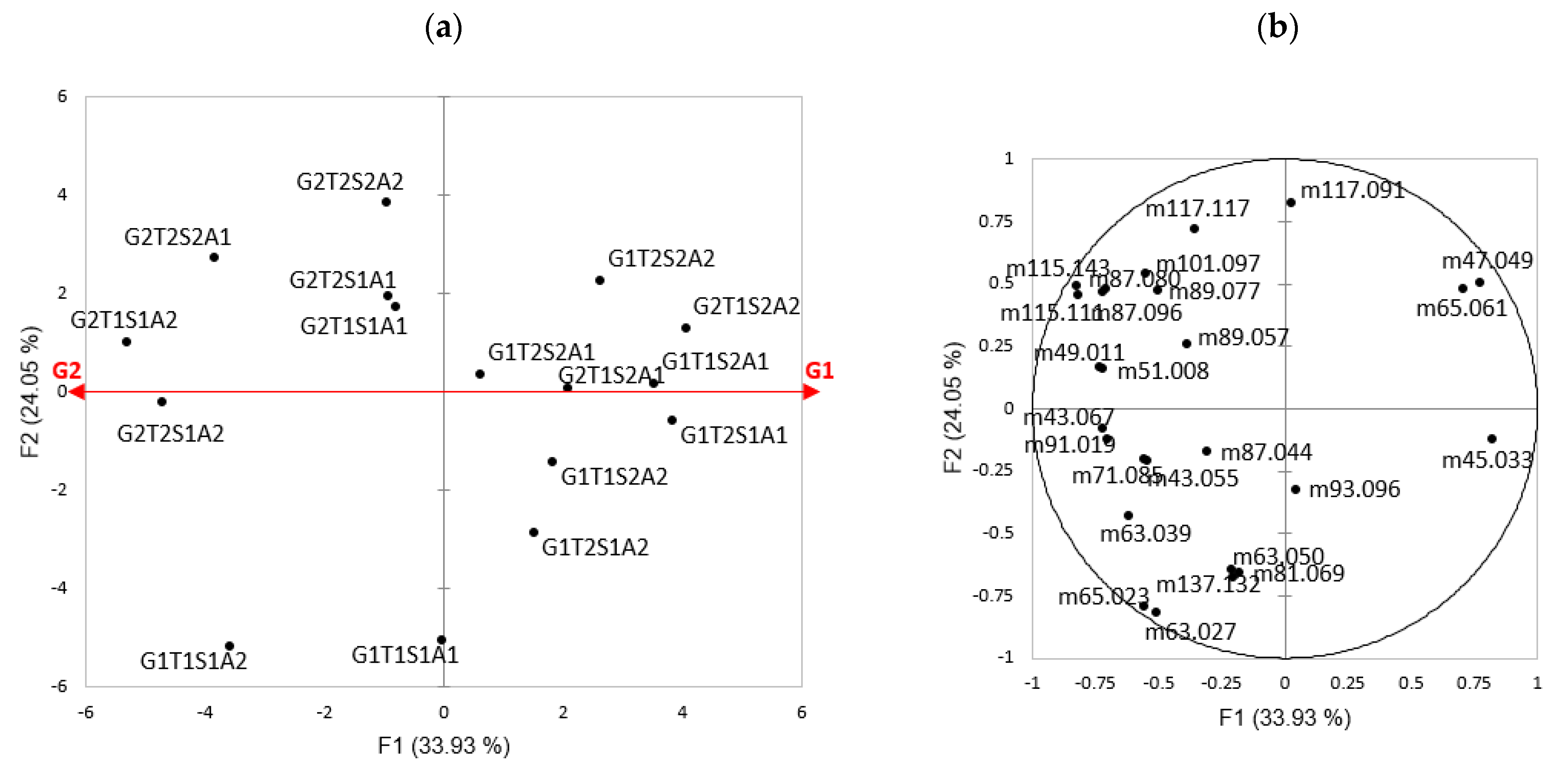
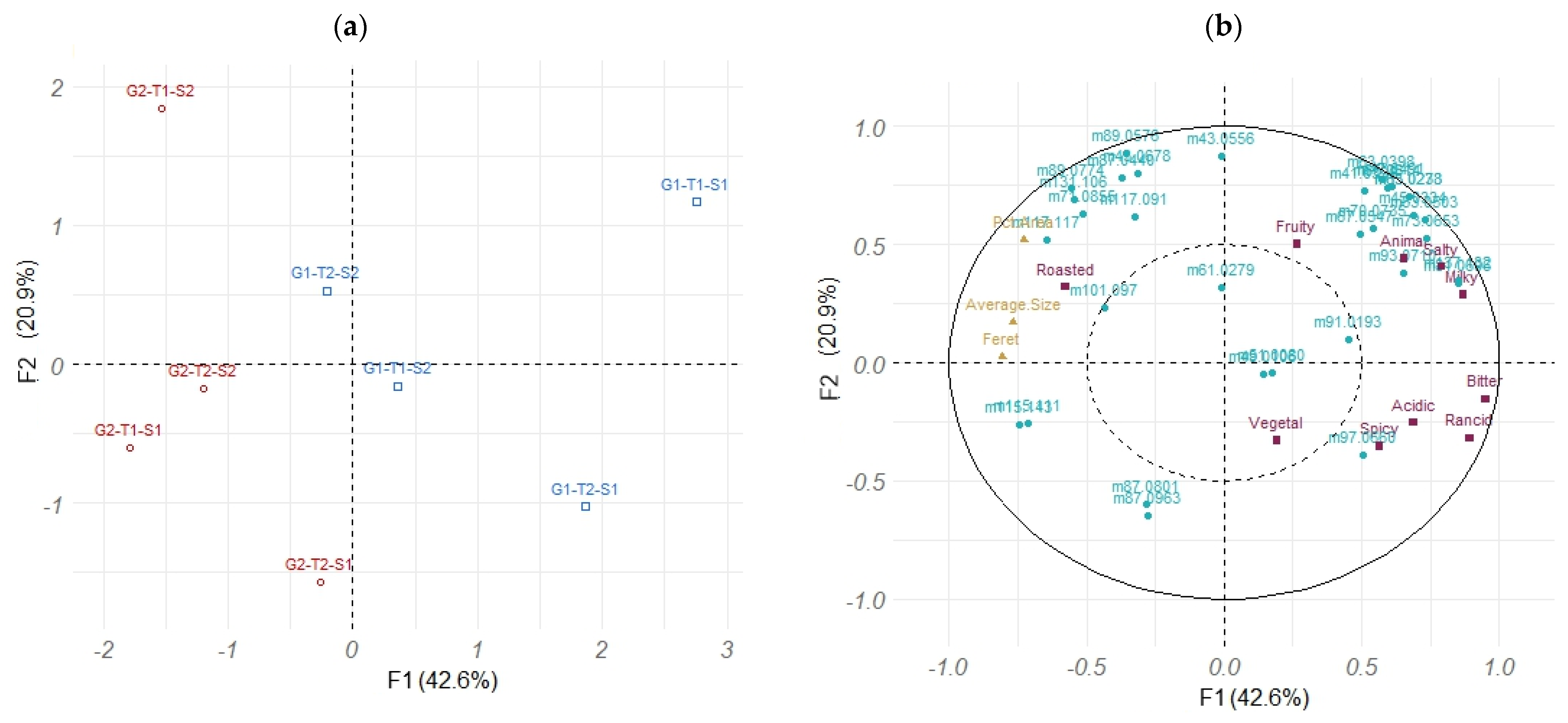
2.7. Multivariate Analyses
3. Discussion
4. Materials and Methods
4.1. Fabrication of the Cheeses
4.2. Description of Aroma, Texture and Tastes of Cheese by Sensory Analysis
4.3. Rheological and Microstructure Characteristics of the Cheeses
4.4. Identification of Aroma Compounds in Cheese by HS-SPME-GC-MS
4.5. Dynamic Aroma Release Study
4.5.1. TCATA
4.5.2. PTR-ToF-MS Analyses
4.5.3. Protocol of Dynamic Aroma Release Study
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouton, Y.; Buchin, S.; Duboz, G.; Pochet, S.; Beuvier, E. Effect of mesophilic lactobacilli and enterococci adjunct cultures on the final characteristics of a microfiltered milk Swiss-type cheese. Food Microbiol. 2009, 26, 183–191. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Di Cagno, R.; Buchin, S.; De Angelis, M.; Gobbetti, M. Use of autochthonous mesophilic lactic acid bacteria as starter cultures for making Pecorino Crotonese cheese: Effect on compositional, microbiological and biochemical attributes. Food Res. Int. 2019, 116, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.M.; Nieto-Arribas, P.; Seseña, S.; Chicón, R.; Castro, L.; Palop, L.; Cabezas, L. Volatile composition and improvement of the aroma of industrial Manchego cheese by using Lactobacillus paracasei subsp. paracasei adjunct and other autochthonous strains as starters. Eur. Food Res. Technol. 2014, 238, 485–494. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Influence of starter culture on flavor and headspace volatile profiles of fermented whey and whey produced from fermented milk. J. Dairy Sci. 2005, 88, 3745–3753. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, E.; Lazzi, C.; Tabanelli, G.; Gatti, M.; Neviani, E.; Gardini, F. Nonstarter lactic acid bacteria volatilomes produced using cheese components. J. Dairy Sci. 2013, 96, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Boisard, L.; Tournier, C.; Sémon, E.; Noirot, E.; Guichard, E.; Salles, C. Salt and fat contents influence the microstructure of model cheeses, chewing/swallowing and in vivo aroma release. Flavour Fragr. J. 2014, 29, 95–106. [Google Scholar] [CrossRef]
- Gierczynski, I.; Laboure, H.; Guichard, E. In vivo aroma release of milk gels of different hardnesses: Inter-individual differences and their consequences on aroma perception. J. Agric. Food Chem. 2008, 56, 1697–1703. [Google Scholar] [CrossRef]
- Lethuaut, L.; Weel, K.G.C.; Boelrijk, A.E.M.; Brossard, C.D. Flavor perception and aroma release from model dairy desserts. J. Agric. Food Chem. 2004, 52, 3478–3485. [Google Scholar] [CrossRef]
- Landy, P.; Pollien, P.; Rytz, A.; Leser, M.E.; Sagalowicz, L.; Blank, I.; Spadone, J.C. Model studies on the release of aroma compounds from structured and nonstructured oil systems using proton-transfer reaction mass spectrometry. J. Agric. Food Chem. 2007, 55, 1915–1922. [Google Scholar] [CrossRef]
- Buettner, A.; Beer, A.; Hannig, C.; Settles, M.; Schieberle, P. Physiological and analytical studies on flavor perception dynamics as induced by the eating and swallowing process. Food. Qual. Prefer. 2002, 13, 497–504. [Google Scholar] [CrossRef]
- Buettner, A.; Beauchamp, J. Chemical input—Sensory output: Diverse modes of physiology-flavour interaction. Food. Qual. Prefer. 2010, 21, 915–924. [Google Scholar] [CrossRef]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and flavor perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef]
- Harrison, M. Effect of breathing and saliva flow on flavor release from liquid foods. J. Agric. Food Chem. 1998, 46, 2727–2735. [Google Scholar] [CrossRef]
- Labouré, H.; Repoux, M.; Courcoux, P.; Feron, G.; Guichard, E. Inter-individual retronasal aroma release variability during cheese consumption: Role of food oral processing. Food Res. Int. 2014, 64, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Mestres, M.; Kieffer, R.; Buettner, A. Release and Perception of Ethyl Butanoate during and after Consumption of Whey Protein Gels: Relation between Textural and Physiological Parameters. J. Agric. Food Chem. 2006, 54, 1814–1821. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Feron, G.; Guichard, E.; Rodriguez-Bencomo, J.J.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayon, M.A. Understanding the Role of Saliva in Aroma Release from Wine by Using Static and Dynamic Headspace Conditions. J. Agric. Food Chem. 2014, 62, 8274–8288. [Google Scholar] [CrossRef] [PubMed]
- Repoux, M.; Semon, E.; Feron, G.; Guichard, E.; Laboure, H. Inter-individual variability in aroma release during sweet mint consumption. Flavour Fragr. J. 2012, 27, 40–46. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Roozen, J.P. Influence of mastication and saliva on aroma release in a model mouth system. Food Chem. 2000, 71, 339–345. [Google Scholar] [CrossRef]
- Benjamin, O.; Silcock, P.; Beauchamp, J.; Buettner, A.; Everett, D.W. Tongue Pressure and Oral Conditions Affect Volatile Release from Liquid Systems in a Model Mouth. J. Agric. Food Chem. 2012, 60, 9918–9927. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E.; Repoux, M.; Qannari, E.M.; Labouré, H.; Feron, G. Model cheese aroma perception is explained not only by in vivo aroma release but also by salivary composition and oral processing parameters. Food Funct. 2017, 8, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Syarifuddin, A.; Septier, C.; Salles, C.; Thomas-Danguin, T. Reducing salt and fat while maintaining taste: An approach on a model food system. Food. Qual. Prefer. 2016, 48, 59–69. [Google Scholar] [CrossRef]
- Guichard, E.; Salles, C.; Morzel, M.; Le Bon, A.-M. Flavour: From Food to Perception; John Wiley & Sons, Inc.: Chichester, UK, 2017; p. 424. [Google Scholar]
- Tarrega, A.; Yven, C.; Semon, E.; Mielle, P.; Salles, C. Effect of oral physiology parameters on in-mouth aroma compound release using lipoprotein matrices: An in vitro approach. Foods 2019, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Repoux, M.; Labouré, H.; Courcoux, P.; Andriot, I.; Sémon, E.; Yven, C.; Feron, G.; Guichard, E. Combined effect of cheese characteristics and food oral processing on in vivo aroma release. Flavour Fragr. J. 2012, 27, 414–423. [Google Scholar] [CrossRef]
- Chen, L.; Yan, R.Y.; Zhao, Y.H.; Sun, J.Y.; Zhang, Y.Y.; Li, H.H.; Zhao, D.R.; Wang, B.W.; Ye, X.Q.; Sun, B.G. Characterization of the aroma release from retronasal cavity and flavor perception during baijiu consumption by Vocus-PTR-MS, GCxGC-MS, and TCATA analysis. LWT-Food Sci. Technol. 2023, 11, 174. [Google Scholar] [CrossRef]
- Le Quéré, J.L.; Schoumacker, R. Dynamic Instrumental and Sensory Methods Used to Link Aroma Release and Aroma Perception: A Review. Molecules 2023, 28, 33. [Google Scholar] [CrossRef]
- Wendin, K.; Langton, M.; Caous, L.; Hall, C. Dynamic analyses of sensory and microstructural properties of cream cheese. Food Chem. 2000, 71, 363–378. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Soukoulis, C.; Aprea, E.; Biasioli, F.; Cappellin, L.; Schuhfried, E.; Märk, T.D.; Gasperi, F. Proton transfer reaction time-of-flight mass spectrometry monitoring of the evolution of volatile compounds during lactic acid fermentation of milk. Rapid Commun. Mass Spectrom. 2010, 24, 2127–2134. [Google Scholar] [CrossRef]
- Pineau, N.; de Bouille, A.G.; Lepage, M.; Lenfant, F.; Schlich, P.; Martin, N.; Rytz, A. Temporal dominance of sensations: What is a good attribute list? Food. Qual. Prefer. 2012, 26, 159–165. [Google Scholar] [CrossRef]
- Deuscher, Z.; Andriot, I.; Sémon, E.; Repoux, M.; Preys, S.; Roger, J.-M.; Boulanger, R.; Labouré, H.; Le Quéré, J.-L. Volatile compounds profiling by using Proton Transfer Reaction—Time of Flight—Mass Spectrometry (PTR-ToF-MS). The case study of dark chocolates organoleptic differences. J. Mass Spectrom. 2019, 54, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, E.; Van Ruth, S.; Biasioli, G.; Gasperi, F.; Märk, T.D. Gas chromatography-olfactometry (GC-O) and proton transfer reaction-mass spectrometry (PTR-MS) analysis of the flavor profile of Grana Padano, Parmigiano Reggiano, and Grana Trentino cheeses. J. Agric. Food Chem. 2003, 51, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.; Buchin, S.; Achilleos, C.; Berodier, F.; Septier, C.; Courcoux, P.; Salles, C. In vivo sodium release and saltiness perception in solid lipoprotein matrices. 1. Effect of composition and texture. J. Agric. Food Chem. 2012, 60, 5287–5298. [Google Scholar] [CrossRef] [PubMed]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Guichard, E.; Ayed, C.; Salles, C. 1—Retention and release of aroma and taste compounds, influence on perception. In Flavor: From Food to Behaviors, Wellbeing and Health, 2nd ed.; Guichard, E., Salles, C., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2023; pp. 3–27. [Google Scholar] [CrossRef]
- Guichard, E.; Salles, C. Flavor: From Food to Behaviors, Wellbeing and Health, 2nd ed.; Elsevier Ltd.: Cambridge, MA, USA, 2022; p. 487. [Google Scholar] [CrossRef]
- Tarrega, A.; Yven, C.; Sémon, E.; Salles, C. Aroma release and chewing activity during eating different model cheeses. Int. Dairy J. 2008, 18, 849–857. [Google Scholar] [CrossRef]
- Lawrence, G.; Salles, C.; Palicki, O.; Septier, C.; Busch, J.; Thomas-Danguin, T. Using cross-modal interactions to counterbalance salt reduction in solid foods. Int. Dairy J. 2011, 21, 103–110. [Google Scholar] [CrossRef]
- Lawrence, G.; Salles, C.; Septier, C.; Busch, J.; Thomas-Danguin, T. Odour-taste interactions: A way to enhance saltiness in low-salt content solutions. Food. Qual. Prefer. 2009, 20, 241–248. [Google Scholar] [CrossRef]
- Peltier, C.; Visalli, M.; Labouré, H.; Hélard, C.; Andriot, I.; Cordelle, S.; Le Quéré, J.L.; Schlich, P. Automatic pretreatment and multiblock analysis of flavor release and sensory temporal data simultaneously collected in vivo. J. Chemometr. 2024, 38, e3450. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Tenenhaus, A. Regularized Generalized Canonical Correlation Analysis. Psychometrika 2011, 76, 257–284. [Google Scholar] [CrossRef]

| Product | Average Size (µm2) | Particle Area (%) | Circularity | Feret Diameter |
|---|---|---|---|---|
| G1T1S1 | 16.55 | 28.29 | 0.864 | 5.11 |
| G1T1S2 | 16.15 | 29.45 | 0.867 | 5.05 |
| G1T2S1 | 14.4 | 19.49 | 0.829 | 4.99 |
| G1T2S2 | 17.68 | 27.62 | 0.833 | 5.34 |
| G2T1S1 | 24.35 | 32.43 | 0.805 | 6.24 |
| G2T1S2 | 20.77 | 38.18 | 0.848 | 5.73 |
| G2T2S1 | 17.47 | 29.20 | 0.820 | 5.48 |
| G2T2S2 | 17.97 | 32.64 | 0.828 | 5.46 |
| Statistical results | ||||
| Mean-G1 | 15.96 | 25.53 | 0.848 | 5.1 |
| Mean-G2 | 19.89 | 32.69 | 0.825 | 5.7 |
| p-value (G) | *** | *** | *** | *** |
| Mean-T1 | 19.93 | 32.31 | 0.843 | 5.6 |
| Mean-T2 | 16.89 | 27.63 | 0.827 | 5.33 |
| p-value (T) | *** | * | *** | - |
| Mean-S1 | 18.26 | 27.53 | 0.828 | 5.48 |
| Mean-S2 | 18.34 | 32.73 | 0.842 | 5.44 |
| p-value (S) | - | *** | * | - |
| Cheese Code | Main Sensory Attributes |
|---|---|
| G1T1S1A1 | Nut |
| G1T1S1A2 | Burnt |
| G1T2S1A1 | Roasted |
| G1T2S1A2 | Milky |
| G1T1S2A1 | Cooked milk, citrus fruit |
| G1T1S2A2 | Oxidized |
| G1T2S2A1 | Sulfur, animal |
| G1T2S2A2 | Alcohol, citrus fruit |
| G2T1S1A1 | Caramel, mushroom |
| G2T1S1A2 | Cooked milk, fresh milk, vanilla |
| G2T2S2A1 | Toasted, roasted, sulfur, rancid |
| G2T2S2A2 | Yeast, alcohol |
| G2T2S1A1 | Acidified milk, sour milk, nut, sweat |
| G2T2S1A2 | Citrus fruit, green vegetal, yeast, alcohol |
| G2T1S2A1 | Cooked milk, fresh milk, vanilla |
| G2T1S2A2 | Fruity, caramel, vegetable, sulfur |
| No. | Volatile Compounds | CAS | KIexp | KIlit | C-01 | C-02 | C-03 | C-04 | C-05 | C-06 | C-07 | C-08 | C-09 | C-10 | C-11 | C-12 | C-13 | C-14 | C-15 | C-16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids | ||||||||||||||||||||
| 1 | Acetic acid | 64-19-7 | - | 6.26 | 6.62 | 6.15 | 6.49 | 6.38 | 6.51 | 6.39 | 6.54 | 6.08 | 6.34 | 6.36 | 6.54 | 6.23 | 6.45 | 5.94 | 6.43 | |
| 2 | Propanoic acid | 79-09-4 | - | 3.37 | 3.63 | 3.18 | 3.44 | 3.91 | 3.59 | 3.92 | 3.52 | 3.22 | 3.53 | 3.71 | 3.54 | 3.71 | 3.50 | 2.49 | 3.07 | |
| 3 | Butanoic acid | 107-92-6 | - | 5.88 | 5.94 | 5.94 | 5.94 | 5.89 | 5.92 | 5.94 | 5.92 | 5.98 | 6.07 | 5.94 | 5.98 | 6.04 | 6.28 | 5.99 | 6.00 | |
| 4 | Hexanoic acid | 142-62-1 | - | 5.52 | 5.61 | 5.47 | 5.56 | 5.58 | 5.67 | 5.58 | 5.57 | 5.58 | 5.67 | 5.57 | 5.54 | 5.60 | 6.03 | 5.58 | 5.57 | |
| 5 | Octanoic acid | 124-07-2 | - | 5.00 | 5.13 | 5.05 | 5.16 | 5.03 | 5.20 | 5.15 | 5.19 | 5.00 | 5.08 | 5.09 | 5.05 | 5.07 | 5.27 | 4.98 | 5.00 | |
| 6 | 2-Methylpropanoic acid | 79-31-2 | - | 3.22 | 3.70 | 1.82 | 3.24 | 4.05 | 4.11 | 3.98 | 3.81 | 3.67 | 3.73 | 4.16 | 3.91 | 3.90 | 3.70 | 3.61 | 3.98 | |
| 7 | 2-Methylbutanoic acid | 116-53-0 | - | 3.87 | 4.26 | 3.27 | 3.87 | 4.46 | 4.60 | 4.25 | 4.29 | 4.13 | 4.19 | 4.52 | 4.25 | 4.24 | 4.37 | 4.18 | 4.48 | |
| 8 | 3-Methylbutanoic acid | 503-74-2 | - | 5.66 | 5.86 | 5.26 | 5.55 | 6.08 | 5.93 | 5.94 | 5.79 | 5.71 | 5.65 | 6.02 | 5.72 | 5.74 | 5.61 | 5.57 | 5.73 | |
| Aldehydes | ||||||||||||||||||||
| 9 | Acetaldehyde | 75-07-0 | <500 | 391 | 5.50 | 5.44 | 5.36 | 5.30 | 5.36 | 5.35 | 5.52 | 5.52 | 5.27 | 5.26 | 5.09 | 5.55 | 5.28 | 5.31 | 5.15 | 5.26 |
| 10 | Butanal | 123-72-8 | 602 | 585 | 3.93 | 5.44 | 5.06 | 5.17 | 4.77 | 6.16 | 6.48 | 5.24 | 4.61 | 4.59 | 5.26 | 5.91 | 5.12 | 5.69 | 3.81 | 3.30 |
| 11 | Pentanal | 110-62-3 | 698 | 698 | 4.44 | 5.41 | 5.07 | 5.11 | 4.88 | 4.85 | 5.04 | 6.43 | 4.88 | 5.06 | 5.12 | 4.99 | 4.88 | 5.05 | 3.50 | 3.15 |
| 12 | Hexanal | 66-25-1 | 800 | 797 | 4.40 | 5.73 | 4.91 | 5.53 | 5.74 | 5.71 | 6.68 | 6.65 | 5.01 | 5.27 | 5.29 | 5.69 | 5.33 | 5.60 | 4.90 | 4.80 |
| 13 | Heptanal | 111-71-7 | 901 | 897 | 3.86 | 5.07 | 4.35 | 4.48 | 4.35 | 4.40 | 5.18 | 6.03 | 3.67 | 4.35 | 4.61 | 4.59 | 4.49 | 4.55 | 3.47 | 3.26 |
| 14 | Nonanal | 124-19-6 | 1100 | 1103 | 3.71 | 4.42 | 4.43 | 4.47 | 4.29 | 4.16 | 4.58 | 4.80 | 4.22 | 4.40 | 4.71 | 4.44 | 4.61 | 4.56 | 4.35 | 4.32 |
| 15 | 2-Methylpropanal | 78-84-2 | 546 | 552 | 4.52 | 5.11 | 4.55 | 4.70 | 5.04 | 4.71 | 4.76 | 4.50 | 3.98 | 3.55 | 4.34 | 5.05 | 4.57 | 4.73 | 3.12 | 3.15 |
| 16 | 3-Methylbutanal | 590-86-3 | 653 | 652 | 5.77 | 6.33 | 5.12 | 5.46 | 5.33 | 5.56 | 5.22 | 5.28 | 5.64 | 5.37 | 5.35 | 5.21 | 5.43 | 5.18 | 4.95 | 4.90 |
| 17 | 2-Methylbutanal | 96-17-3 | 663 | 660 | 4.61 | 4.66 | 4.61 | 4.61 | 4.83 | 4.61 | 4.61 | 4.61 | 4.89 | 4.25 | 4.76 | 4.70 | 4.96 | 4.25 | 4.24 | 3.09 |
| 18 | Benzaldehyde | 100-52-7 | 996 | 964 | 4.09 | 5.57 | 4.61 | 4.17 | 5.49 | 4.40 | 4.82 | 4.48 | 3.26 | 3.59 | 3.39 | 5.13 | 3.44 | 4.68 | 3.06 | 3.00 |
| Alcohols | ||||||||||||||||||||
| 19 | Ethanol | 64-17-5 | <500 | 412 | 6.85 | 7.28 | 7.02 | 7.07 | 7.01 | 6.94 | 7.26 | 7.28 | 7.06 | 6.90 | 7.04 | 6.90 | 6.94 | 6.98 | 6.94 | 6.98 |
| 20 | Butanol | 71-36-3 | 672 | 657 | 5.12 | 6.40 | 5.07 | 5.24 | 5.71 | 6.37 | 5.12 | 5.12 | 5.19 | 4.77 | 5.61 | 5.50 | 5.27 | 5.31 | 4.76 | 4.38 |
| 21 | 3-Methylbutan-1-ol | 123-51-3 | 737 | 738 | 5.89 | 7.34 | 5.77 | 6.76 | 6.68 | 7.39 | 6.66 | 7.45 | 6.70 | 7.25 | 6.62 | 7.30 | 6.53 | 7.39 | 6.11 | 6.53 |
| 22 | 2-Methylbutan-1-ol | 137-32-6 | 739 | 740 | 5.10 | 6.89 | 4.46 | 5.87 | 5.90 | 6.89 | 5.69 | 6.62 | 5.62 | 6.05 | 5.66 | 6.16 | 5.56 | 6.25 | 5.24 | 5.46 |
| Ketones | ||||||||||||||||||||
| 23 | Propan-2-one | 67-64-1 | <500 | 479 | 5.15 | 5.53 | 5.70 | 5.56 | 5.42 | 5.61 | 6.05 | 5.94 | 5.58 | 5.52 | 5.53 | 5.77 | 5.66 | 5.87 | 5.51 | 5.44 |
| 24 | Butan-2-one | 78-93-3 | 610 | 587 | 5.17 | 6.37 | 5.14 | 5.63 | 5.82 | 5.41 | 5.57 | 4.88 | 4.64 | 4.75 | 4.90 | 5.87 | 4.90 | 5.44 | 3.47 | 4.50 |
| 25 | Pentan-2-one | 107-87-9 | 700 | 679 | 3.72 | 5.09 | 6.04 | 5.51 | 4.68 | 4.14 | 5.13 | 5.22 | 5.77 | 5.82 | 5.07 | 5.17 | 5.46 | 5.99 | 4.79 | 4.72 |
| 26 | Heptan-2-one | 110-43-0 | 888 | 888 | 4.66 | 5.95 | 5.47 | 5.71 | 5.44 | 5.55 | 5.62 | 5.77 | 5.51 | 5.74 | 5.36 | 5.49 | 5.42 | 5.66 | 5.12 | 5.34 |
| 27 | Nonan-2-one | 821-55-6 | 1091 | 1085 | 3.75 | 5.29 | 4.52 | 4.84 | 4.67 | 4.79 | 4.85 | 5.10 | 4.68 | 4.76 | 4.30 | 4.41 | 4.29 | 4.42 | 3.97 | 4.44 |
| 28 | Butane-2,3-dione | 431-03-8 | 602 | 596 | 6.00 | 6.58 | 4.07 | 5.38 | 5.74 | 5.17 | 5.02 | 4.66 | 5.93 | 5.77 | 6.17 | 4.98 | 4.64 | 4.95 | 4.44 | 4.99 |
| 29 | Acetoin | 513-86-0 | 719 | 713 | 6.16 | 6.63 | 6.53 | 6.63 | 6.97 | 6.67 | 7.07 | 6.44 | 6.58 | 6.85 | 6.86 | 5.49 | 6.51 | 5.66 | 5.81 | 6.00 |
| Esters | ||||||||||||||||||||
| 30 | Ethyl acetate | 141-78-6 | 615 | 609 | 4.66 | 6.28 | 4.11 | 5.18 | 5.69 | 5.41 | 5.33 | 5.37 | 4.94 | 4.61 | 4.95 | 4.99 | 4.57 | 4.98 | 3.69 | 4.08 |
| 31 | Ethyl butanoate | 105-54-4 | 799 | 798 | 3.87 | 5.17 | 5.01 | 5.32 | 5.18 | 5.21 | 6.02 | 5.80 | 5.21 | 5.13 | 5.07 | 5.17 | 5.05 | 5.21 | 4.97 | 4.90 |
| Sulfurs | ||||||||||||||||||||
| 32 | Methanethiol | 74-93-1 | <500 | 422 | 3.76 | 3.97 | 4.08 | 4.13 | 3.97 | 4.00 | 4.73 | 4.60 | 4.05 | 4.21 | 4.28 | 4.44 | 3.93 | 4.49 | 3.26 | 3.94 |
| 33 | Carbon disulfide | 75-15-0 | 529 | 549 | 5.75 | 5.75 | 5.48 | 5.41 | 5.43 | 5.37 | 5.19 | 5.18 | 5.28 | 5.20 | 5.41 | 5.66 | 5.20 | 5.38 | 5.62 | 5.60 |
| 34 | Dimethylsulfide | 75-18-3 | 511 | 521 | 4.46 | 5.04 | 4.81 | 4.92 | 4.54 | 4.91 | 4.71 | 5.08 | 5.13 | 5.43 | 4.72 | 5.27 | 5.15 | 5.57 | 5.08 | 5.25 |
| 35 | Dimethyldisulfide | 624-92-0 | 747 | 747 | 4.57 | 4.57 | 4.63 | 4.63 | 4.63 | 4.57 | 4.63 | 4.63 | 3.72 | 4.57 | 4.68 | 4.87 | 4.80 | 5.23 | 3.11 | 4.87 |
| Hydrocarbon | ||||||||||||||||||||
| 36 | Benzene | 71-43-2 | 661 | 657 | 5.84 | 5.84 | 5.59 | 5.66 | 4.99 | 5.02 | 4.16 | 4.99 | 4.18 | 4.12 | 5.38 | 6.06 | 5.22 | 5.55 | 4.10 | 4.67 |
| Experimental Mass (m/z, g·mol−1) | Chemical Formula [MH]+ | Expected Mass (m/z, g·mol−1) | Tentative Identification 1 or Identified with HS-SPME–GC–MS 2 | CAS | Ref. |
|---|---|---|---|---|---|
| 43.055 | C3H7+ | 43.055 | Alkyl fragment 1 | [33] | |
| 45.033 | C2H5O+ | 45.033 | Acetaldehyde 2 | 75-07-0 | [33,34] |
| 47.049 | C2H6OH+ | 47.049 | Ethanol 2 | 64-17-5 | [34] |
| 49.010 | CH5S+ | 49.011 | Methanethiol 2 | 74-93-1 | [33,34] |
| 63.027 | C2H7S+ | 63.026 | Dimethyldisulfide 2 | 624-92-0 | [33,34] |
| 63.039 | C2H7O2+ | 63.044 | Acetaldehyde water fragment 1 | [33] | |
| 65.023 | C5H5+ | 65.038 | Fragment 1 | [33] | |
| 65.061 | C2H9O2+ | 65.060 | Ethanol-water cluster 1 | [33] | |
| 71.085 | C5H11+ | 71.086 | Fragment (terpene, ester) 1 | [33] | |
| 81.069 | C6H9+ | 81.070 | Terpene fragment 1 | [33] | |
| 87.044 | C4H7O2+ | 87.044 | Butane-2,3-dione 2 | 431-03-8 | [33] |
| 87.080 | C5H11O+ | 87.080 | 3-Methylbutanal 2 2-Methylbutanal 2 Pentanal 2 | 590-86-3 96-17-3 110-62-3 | [33] |
| 87.096 | C4H11N2+ | 87.092 | Piperazine 1 | ||
| 89.057 | C4H9O2+ | 89.060 | Acetoin 2 2-Methylpropanoic acid 2 Butanoic acid 2 Ethyl acetate 2 | 513-86-0 79-31-2 107-92-6 141-78-6 | [33] |
| 89.077 | C5H13O+ | 89.096 | 3-Methylbutan-1-ol 2 2-Methylbutan-1-ol 2 | 123-51-3 137-32-6 | [33] |
| 91.019 | C3H7OS+ | 91.021 | Methyl thioacetate 1 | ||
| 93.071 | C7H9+ | 93.069 | Toluene 1 Terpene fragment 1 | 108-88-3 | [33] |
| 101.097 | C6H13O+ | 101.096 | Hexanal 2 | 66-25-1 | [33] |
| 115.111 | C7H15O+ | 115.112 | Heptanal 2 Heptan-2-one 2 | 111-71-7 110-43-0 | [33] [33] |
| 115.143 | C8H19+ | 115.148 | Octane 1 | ||
| 117.091 | C6H13O+ | 117.091 | Hexanoic acid 2 | 142-62-1 | [33] |
| 117.117 | C7H17O+ | 117.127 | Heptan-2-ol 1 Heptan-1-ol 1 | 543-49-7 111-70-6 | [33] |
| 137.132 | C10H17+ | 137.132 | beta-Myrcene 1 Limonene 1 beta-Ocimene 1 3-Carene 1 alpha-pinene 1 | 123-35-3 138-86-3 13877-91-3 13466-78-9 80-56-8 | [33] |
| Cheese Code | Fat (G, %) | Whey Lactose (T, g·L−1) | Salt (S, %) | Lactic Acid Bacteria (A) |
|---|---|---|---|---|
| G1T1S1A1 | 40 | 33 | 2.5 | A1 |
| G1T1S1A2 | 40 | 33 | 2.5 | A2 |
| G1T2S1A1 | 40 | 42 | 2.5 | A1 |
| G1T2S1A2 | 40 | 42 | 2.5 | A2 |
| G1T1S2A1 | 40 | 33 | 4 | A1 |
| G1T1S2A2 | 40 | 33 | 4 | A2 |
| G1T2S2A1 | 40 | 42 | 4 | A1 |
| G1T2S2A2 | 40 | 42 | 4 | A2 |
| G2T1S1A1 | 50 | 33 | 2.5 | A1 |
| G2T1S1A2 | 50 | 33 | 2.5 | A2 |
| G2T2S1A1 | 50 | 42 | 2.5 | A1 |
| G2T2S1A2 | 50 | 42 | 2.5 | A2 |
| G2T1S2A1 | 50 | 33 | 4 | A1 |
| G2T1S2A2 | 50 | 33 | 4 | A2 |
| G2T2S2A1 | 50 | 42 | 4 | A1 |
| G2T2S2A2 | 50 | 42 | 4 | A2 |
| List of Descriptors | ||
|---|---|---|
| Fresh lactic | Cooked milk | Milky |
| Acidified milk | Sour milk | Animal |
| Fruity | Citrus fruit | Jam |
| Caramel | Toasted | Burnt |
| Mushroom | Vegetables | Green |
| Vegetal | Broth | Yeast |
| Nut | Soap | Tyre |
| Oxidized | Alcohol | Chemical |
| Sulfur | Rancid | Rust |
| Sweat | Mild roasted | Strong Roasted |
| TCATA Attributes | List of Different Aromas Regrouped in the TCATA Attributes | Sensory References |
|---|---|---|
| Acid | Lactic acid solution at 1.5 mg·L−1 in Evian water | |
| Bitter | Caffeine solution at 0.5 mg·L−1 in Evian water | |
| Milky | Milk, cream, yogurt, cottage cheese, boiled milk, melted butter | Cottage cheese |
| Fruity | Apricot jam | |
| Mild roasted | Hazelnut, vanilla, caramel | Crumbled French biscuit named “Petit Beurre” |
| Vegetable | Vegetable broth, cut grass, leek, mushroom, earthy | Green vegetable soup |
| Animal | Leather, horse, sweat | Leather pieces macerated in water |
| Rancid, soap, sour | Marseille soap shavings |
| Taste Attributes | Aroma Attributes |
|---|---|
| Salty | Animal |
| Bitter | Vegetable |
| Acid | Milky |
| Mild roasted | |
| Spicy | |
| Rancid, soap, sour | |
| Fruity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriot, I.; Septier, C.; Peltier, C.; Noirot, E.; Barbet, P.; Palme, R.; Arnould, C.; Buchin, S.; Salles, C. Influence of Cheese Composition on Aroma Content, Release, and Perception. Molecules 2024, 29, 3412. https://doi.org/10.3390/molecules29143412
Andriot I, Septier C, Peltier C, Noirot E, Barbet P, Palme R, Arnould C, Buchin S, Salles C. Influence of Cheese Composition on Aroma Content, Release, and Perception. Molecules. 2024; 29(14):3412. https://doi.org/10.3390/molecules29143412
Chicago/Turabian StyleAndriot, Isabelle, Chantal Septier, Caroline Peltier, Elodie Noirot, Pascal Barbet, Romain Palme, Céline Arnould, Solange Buchin, and Christian Salles. 2024. "Influence of Cheese Composition on Aroma Content, Release, and Perception" Molecules 29, no. 14: 3412. https://doi.org/10.3390/molecules29143412
APA StyleAndriot, I., Septier, C., Peltier, C., Noirot, E., Barbet, P., Palme, R., Arnould, C., Buchin, S., & Salles, C. (2024). Influence of Cheese Composition on Aroma Content, Release, and Perception. Molecules, 29(14), 3412. https://doi.org/10.3390/molecules29143412










