Abstract
The chemical valorization of widespread molecules in renewable sources is a field of research widely investigated in the last decades. In this context, we envisaged that indole-3-carbinol, present in different Cruciferae plants, could be a readily available building block for the synthesis of various classes of indoles through a palladium-catalyzed Tsuji–Trost-type reaction with O and S soft nucleophiles. The regiochemical outcome of this high-yielding functionalization shows that the nucleophilic substitution occurs only at the benzylic position. Interestingly, with this protocol, the sulfonyl unit could be appended to the indole nucleus, providing convenient access to new classes of molecules with potential bioactivity.
1. Introduction
Nowadays, the valorization of bioactive compounds found in natural sources is gaining high interest due to the growing trend of promoting the use of renewable resources following a circular biobased approach towards natural product-based drug discovery [1]. Significant efforts have been made to establish sustainable processes in which natural scaffolds are extracted and chemically modified using efficient catalytic methods, aiming to create molecules with improved biological properties [2].
One of the most common kinds of natural products of biological relevance is represented by indole-containing alkaloids, with more than 4100 examples. A large number of them have been deeply examined for their remarkable anticancer, antibacterial, antiviral, antifungal, and antiplasmodial activities, attracting significant attention as possible leads for novel therapies [3].
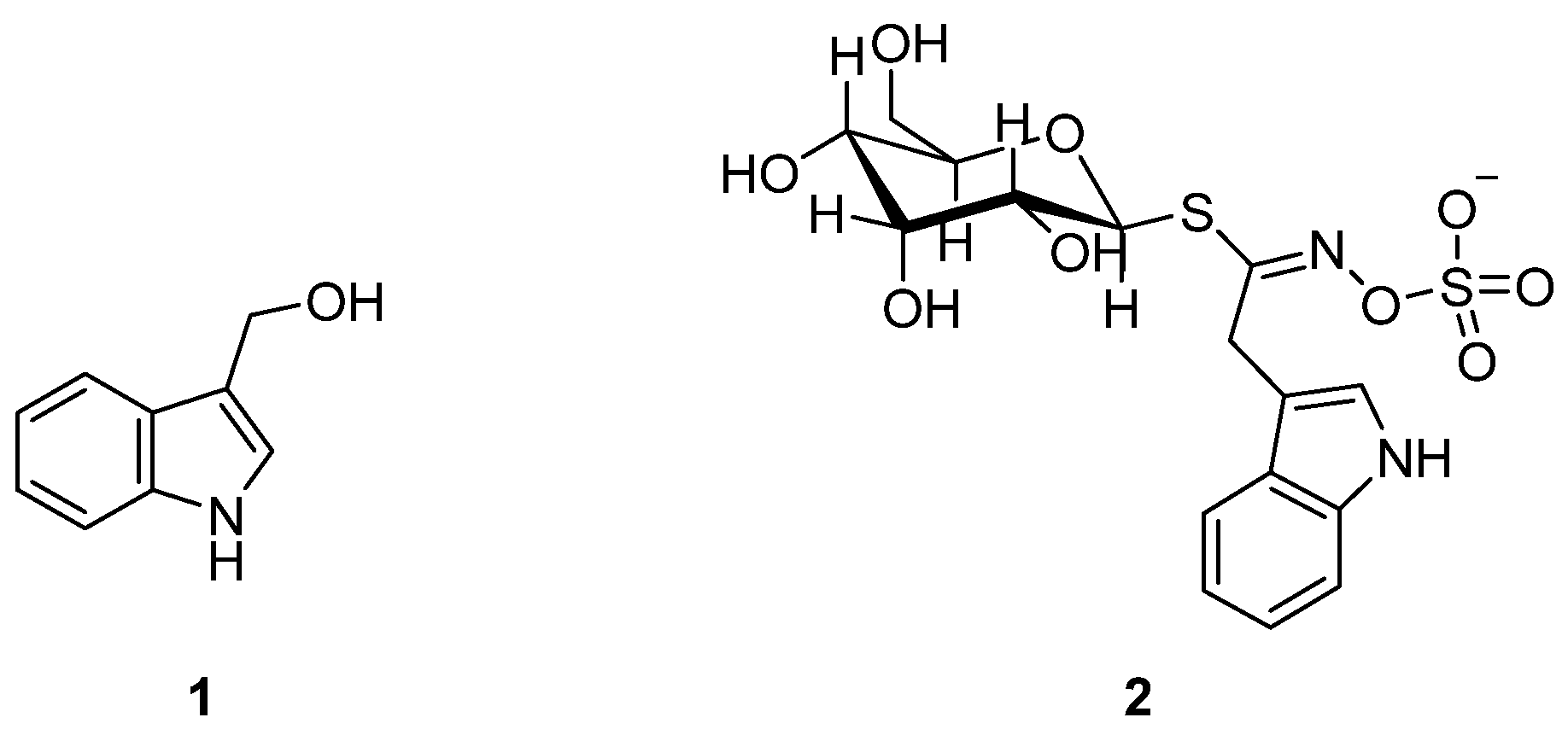
In this regard, an excellent instance is represented by the indole-3-carbinol (I3C) 1 (Figure 1).
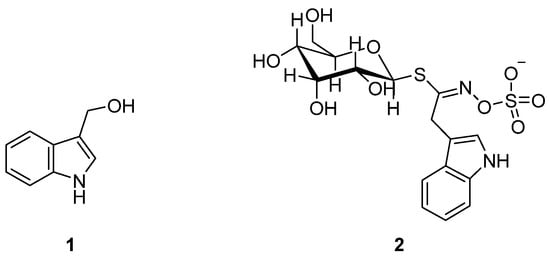
Figure 1.
Indole-3-carbinol (I3C) 1 and glycobrassicin 2 structures.
Particularly, it is a naturally occurring phytochemical found in the Brassicaceae species of cruciferous vegetables derived from the myrosinase-catalyzed hydrolysis of glycobrassicin 2 (Figure 1) [4]. I3C has been found to exhibit antioxidative, anti-inflammatory, antiatherogenic, antiviral, antithrombotic, and, most notably, anticarcinogenic activity [5,6,7,8,9,10,11,12,13,14,15,16].
Despite this broad spectrum of activities and its high therapeutic potential, many drawbacks, notably its metabolic instability, limit the I3C development in drug discovery; therefore, structural modifications of this nucleus remain a challenging and demanding research subject [17].
Synthetic transformations for the functionalization of the indole nucleus based on indole carbinole and its derivatives are described and widely employed. Among them, two main approaches are explored:
- in situ generation under acidic or basic reaction conditions of highly unstable and reactive transient indole methides followed by the regioselective conjugated addition of nucleophiles [18,19]. This approach has been strictly correlated to in situ aza-o-QMs generation/nucleophile Michael-type [20,21];
- Tsuji–Trost-type reaction. Indeed, analogous to electrophilic systems of heteroaromatics with extended π conjugation featuring carboxylate and carbonate leaving groups, activated carbinols can generate π-(η3 indolyl)–palladium electrophilic intermediates in the presence of Pd(0) in equilibrium with cationic π-(η1-indolyl)-palladium complexes [22].
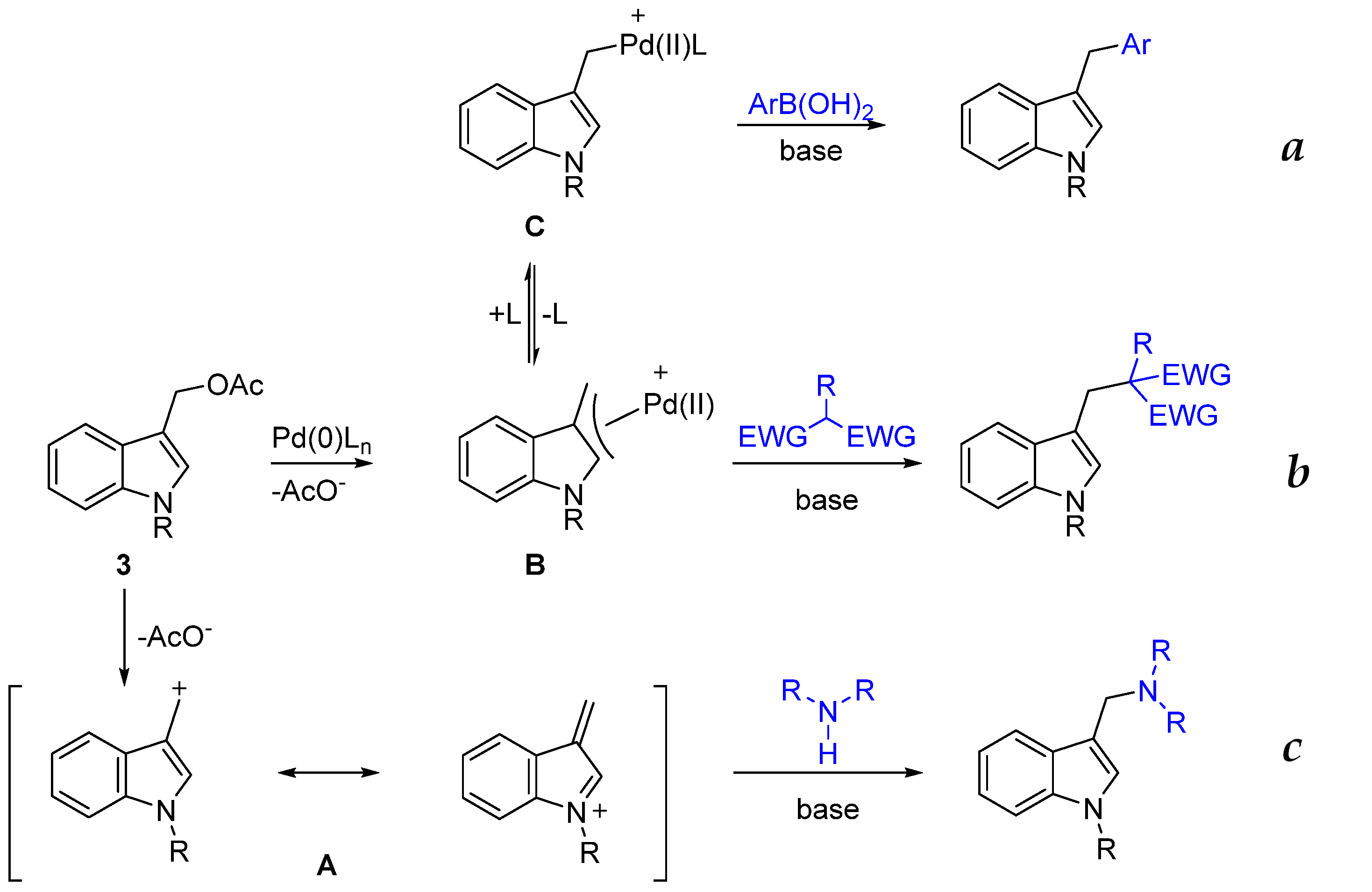
Recently, part of our studies focused on the functionalization of indoles using activated carbinols as precursors of transient indole methides [18] or as substrates for palladium-catalyzed Tsuji–Trost-type reactions with different classes of carbon soft nucleophiles [23] and Suzuki–Miyaura cross-coupling with aryl boronic acids [24]. These synthetic approaches are summarized in Scheme 1.
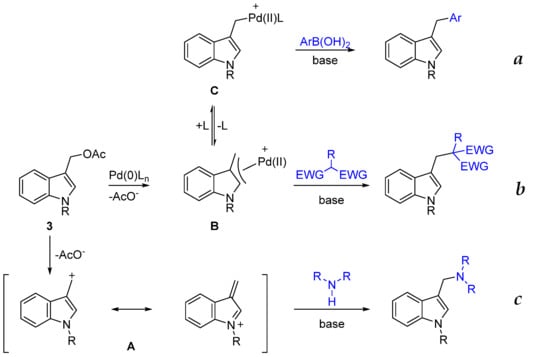
Scheme 1.
Our previous works on the reactivity of activated indole3carbinol (I3C). (a) [24] (b) [23] (c) [18].
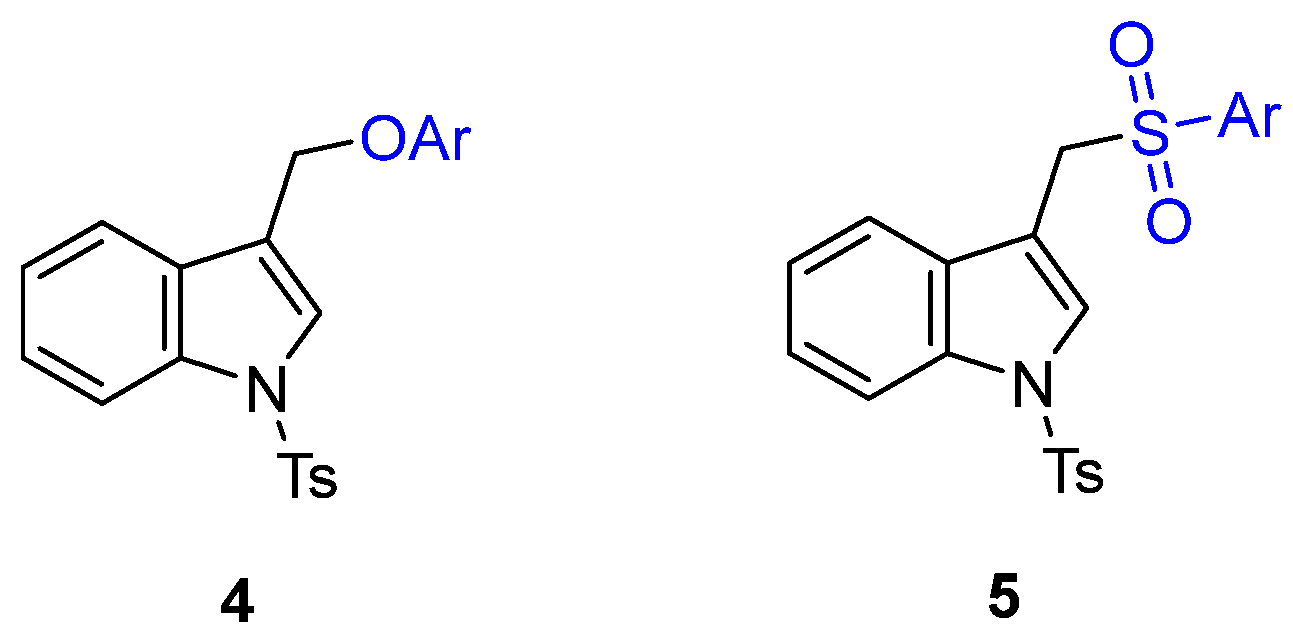
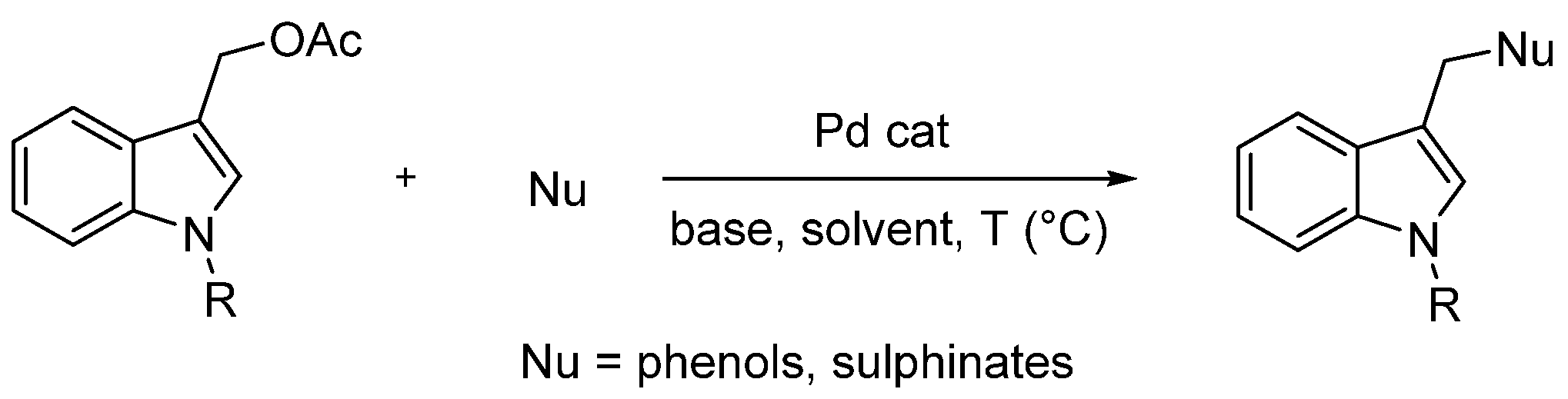
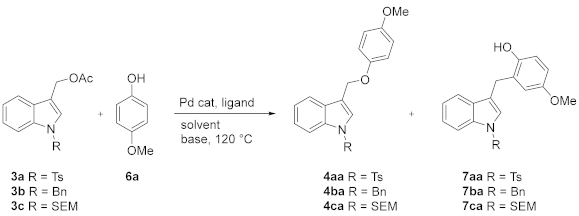
However, since the functionalization of indole-3-carbinol with O and S soft nucleophiles was not explored, based on our background, we hypothesized that (1-substituted-indol-3-yl)methyl acetates 3 could be readily available precursors of two classes of indole-containing derivatives: 1-substituted 3-(aryloxymethyl)-1H-indole 4 and 3-((arylsulfonyl)methyl)-1H-indole 5 (Figure 2) through palladium-catalyzed Tsuji–Trost-type reactions with phenols or aryl sulfinates as soft nucleophiles, respectively (Scheme 2).
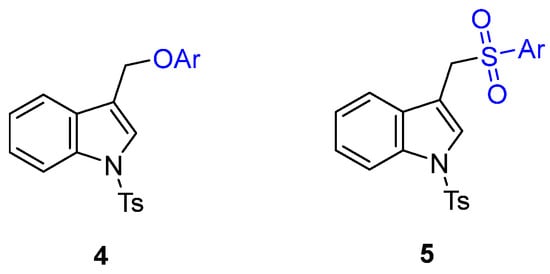
Figure 2.
Structures of 1-substituted 3-(aryloxymethyl)-1H-indole 4 and 3-((arylsulfonyl)methyl)-1H-indole 5.
Scheme 2.
Working hypothesis.
The aryloxy and arylsulfonyl groups attached to the indole scaffold appeared interesting to us allowing for structural modifications that can fine-tune pharmacological properties. This versatility is crucial for optimizing drug potency, selectivity, and pharmacokinetic properties.
To the best of our knowledge, the compounds 4 were synthesized for the first time by Yongxiang Liu from (1-tosyl-1H-indol-3-yl)methanol and phenols via Mitsunobu reaction with an approximate yield of 50% [25].
Hereafter we report the results of our investigation.
2. Results
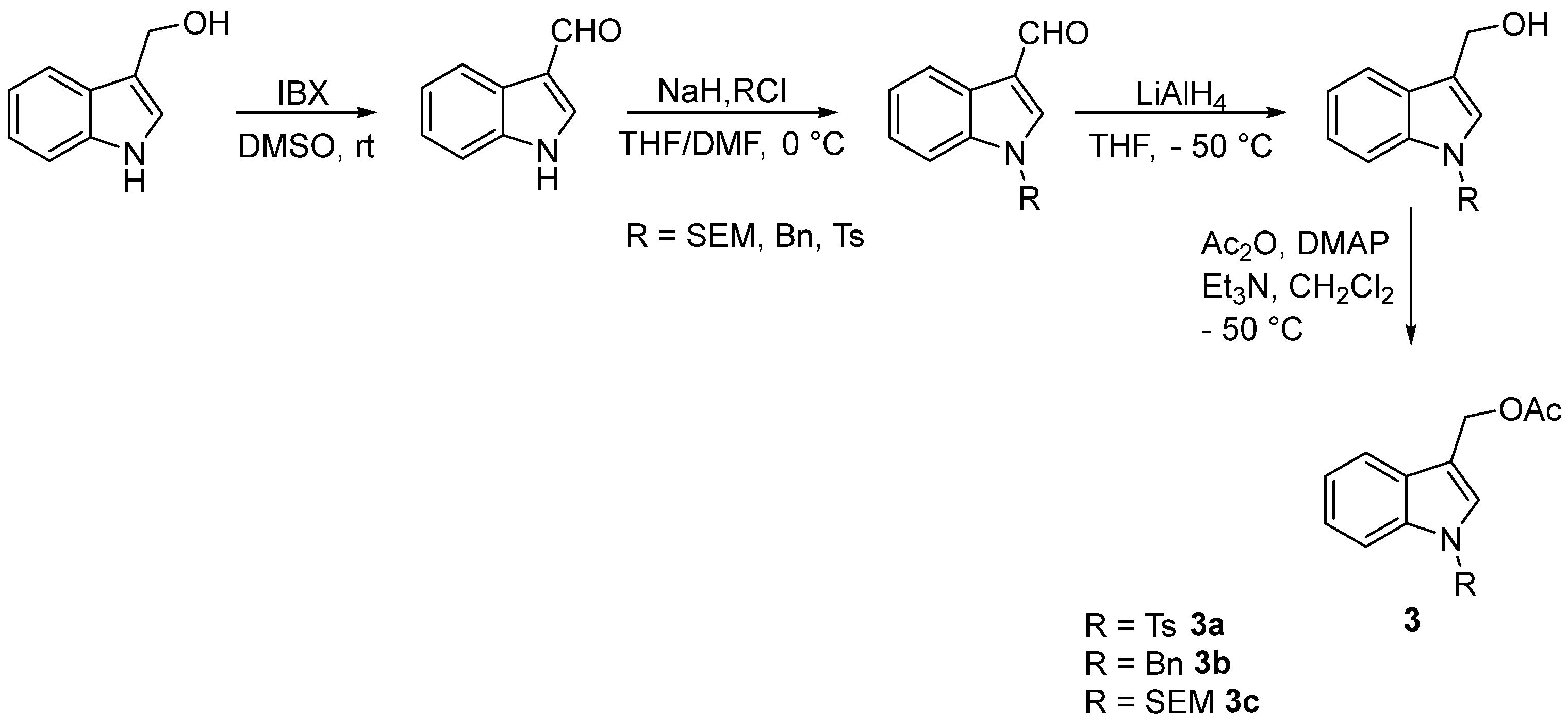
The choice of (1-substituted-1H-indol-3-yl)methyl acetates 3 instead of (1H-indol-3-yl)methyl acetate as precursors for the functionalization of activated I3C with O and S soft nucleophiles is due to the low selectivity in N/O acetylation of the N-free I3C under different reaction conditions [18,23].
The (1-substituted-indol-3-yl)methyl acetates 3 were obtained with excellent overall yield from renewable sources I3C 1 according to the four-step sequence outlined in Scheme 3. Interestingly, the oxidation 1 to the corresponding 3-formylindole derivative was performed using IBX [26], a nonmetallic green oxidant with excellent recyclability [27]. Moreover, the reduction and acetylation reactions did not require any purification.
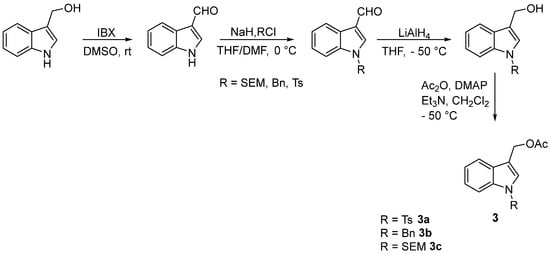
Scheme 3.
Synthesis of (1–substituted–indol–3−yl)methyl acetates 3.
The reaction of 3a–c with 4-methoxyphenol 6a was initially explored. Part of our optimization study using different ligands, solvents, and bases is summarized in Table 1.

Table 1.
Optimization studies for the reaction of 3 with 4-methoxyphenol 6a a.
Based on our previous results in analogs of gramine synthesis [18], we started our investigation by reacting the acetates 3a–b with 6a in the absence of palladium catalyst. No evidence of the substitution products 4aa was observed (Table 1, entries 1–3). Slightly better results, but still unsatisfactory from a synthetic point of view, were obtained by switching to (1-SEM-1H-indol-3-yl)methyl acetate 3c (Table 1, entries 4–5). These results suggested that the approach based on the sequential in situ generation under basic condition of indole-based iminium methide (A)/aza-Michael-type addition (Scheme 1) could not be a good strategy for synthesizing the target compounds. We then continued our screening in the presence of palladium catalysis, assuming that the Tsuji–Trost-type reaction could be a suitable protocol for converting 3c into the corresponding 4ca.
Taking advantage of our results in palladium-catalyzed benzylic-like nucleophilic substitution of benzofuran-2-ylmethyl acetates with S, O, and C soft nucleophiles [28], we thought that the neutral Pd(ally)LCl complexes [29,30] containing Buchwald dialkylmonophosphine ligands [31] could be highly effective precatalysts also in the conversion of 3c to 4ca. As reported by us, the active palladium Pd(ally)LCl complex could be generated in situ by dissolving [Pd(η3-C3H5)Cl]2 and Buchwald-type ligand in THF at room temperature. Initial attempts were based on the use of [Pd(η3-C3H5)Cl]2 as a source of palladium, XPhos as the ligand, MeCN as a solvent in the presence of different bases (Table 1, entries 6, 9–12) at 120 °C. The best results were obtained with K2CO3, having isolated 4ca in 75% yield (Table 1, entry 6). Substitution of [Pd(η3-C3H5)Cl]2 with other sources of palladium [Pd2dba3 and Pd(PPh3)4] and XPhos with other ligands, keeping all other parameters the same, led to worse outcomes (Table 1, entries 7–8, 14–18). A poor yield of 4ca was obtained by carrying out the reaction in DMF or DMSO instead of MeCN (Table 1, entries 19–20). In approximately all tests starting from 3c, the C-alkylated compound 7ca was isolated together with the expected O-alkylated main product 4ca. Experimental results suggested that the O/C-alkylation ratio, usually affected by the degree of aggregation with bidentate anions, was influenced by several parameters such as the M+ size, solvation, and nature of the ligand. Eventually, with the optimized reaction conditions in hand ([Pd(C3H5)Cl]2, XPhos, MeCN/THF, K2CO3, 120 °C), we compared the reactivity of 3c with 3b and 3a, and we were pleased to find that the desired final product was isolated in 97% yield starting from the N-Ts substrate (Table 1, entry 19).
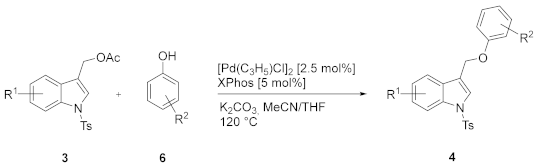
We next explored the scope and generality of the reaction (Table 2).

Table 2.
N-Ts-1-methyl-3-(aryloxymethyl)-1H-indole 4 from N-substituted-indol-3-ylmethyl acetates 3 and phenols 6 a.
Good to excellent results were usually obtained with various indoles and phenols. Phenols bearing neutral, electron-releasing, and electron-withdrawing substituents except for the nitro group (Table 2, entries 7 and 8) can be used. Furthermore, the presence of groups close to the C-OH bond does not hamper the reaction (Table 2, entries 3 and 13). Among tested indoles, (6-chloro-1-tosyl-1H-indol-3-yl)methyl acetate 3f leads to a complex reaction mixture, probably for competitive cross-coupling reactions.
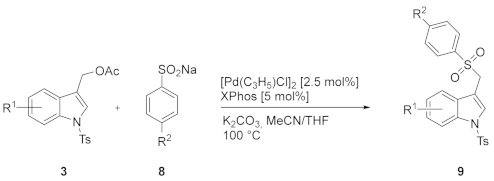
The potential of the developed strategy is further highlighted by the investigation of the palladium-catalyzed regioselective sulfonylation of (1-tosyl-1H-indol-3-yl)methyl acetates 3 with sulfinic acid salt 8.
Even if the aryl sulfone fragment is present in several compounds exhibiting important biological activities [32,33,34], and many protocols have been developed for their synthesis [35,36,37,38,39,40,41,42,43], less attention has been devoted to the formation of 3-((arylsulfonyl)methyl)-1H-indole [44,45]. The sulfonylation of 3a with commercially available sodium 4-methylbenzenesulfinate 8a was attempted under the reaction conditions successfully employed with phenols. Lowering the reaction temperature to 100 °C, the indole 9aa was isolated in 98% yield (Table 3, entry 2). Also with this class of soft nucleophiles, the formation of product 9 by a base-promoted reaction can be ruled out by recovering almost quantitatively the starting indole acetate 3a under metal-free conditions (Table 3, entry 1). Subsequently, the protocol was extended to include functionalized indoles and arylsulfinates 8 (Table 3).

Table 3.
N-Ts-1-methyl-3-((arylsulfonyl)methyl)-1H-indole 9 from N-substituted-indol-3-ylmethyl acetates 3 and sodium arylsulfinates 8 a.
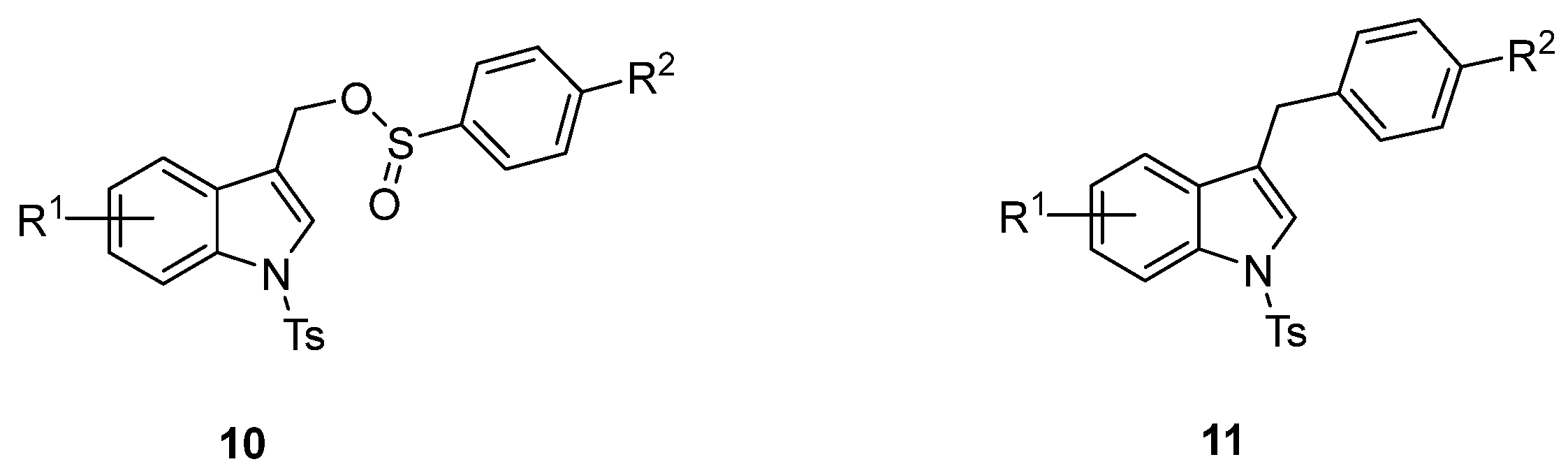
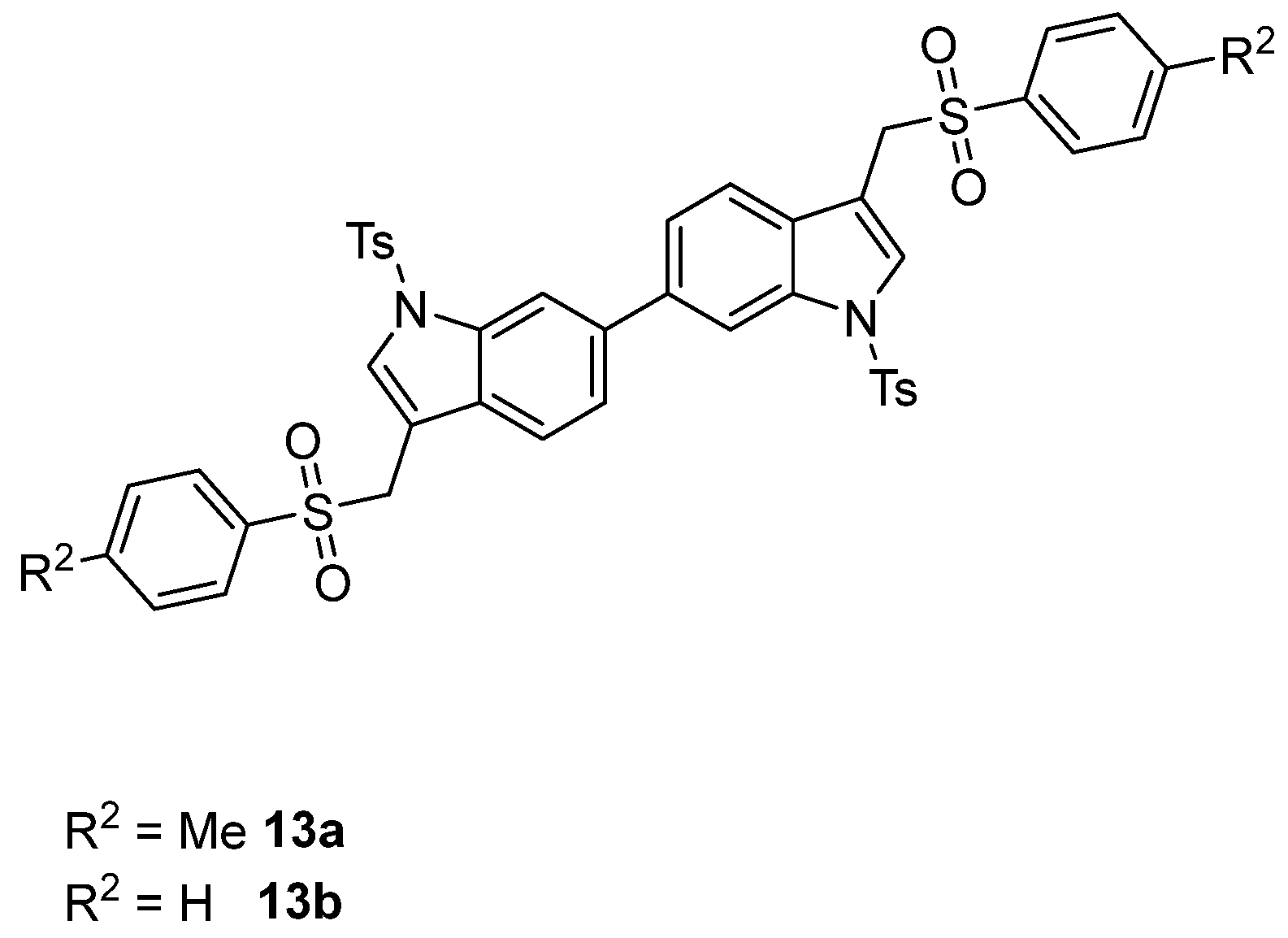
The formation of the (1-tosyl-1H-indol-3-yl)methyl arylsulfinate 10 resulting from the competitive O-attack of the ambident sulfinate anion as well as the desulfination product 11 (Figure 3) was never observed [46,47,48,49,50]. Interestingly, good yields were also obtained by using the (6-chloro-1-tosyl-1H-indol-3-yl)methyl acetate 3f (Table 3, entries 8 and 9) along with a small amount of homocoupling byproduct 13 (Figure 4). Furthermore, the presence of nitro and methoxy on the indole nucleus as substituents was well tolerated (Table 3, entries 4, 5, 10, 11).
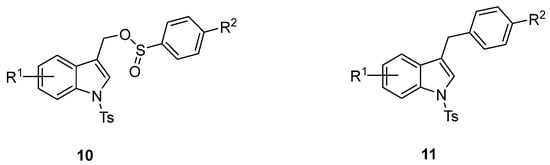
Figure 3.
Structures of expected byproducts 10 and 11.
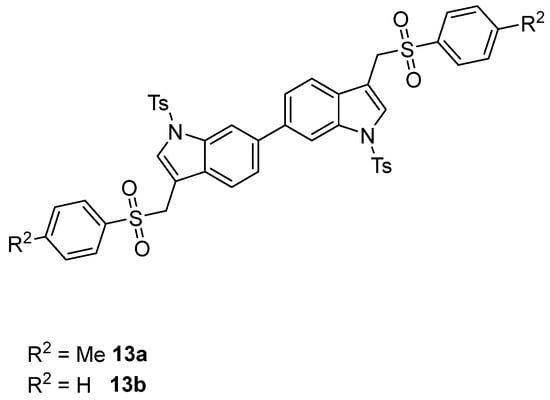
Figure 4.
Structures of homocoupling byproducts 13a and 13b.
3. Discussion
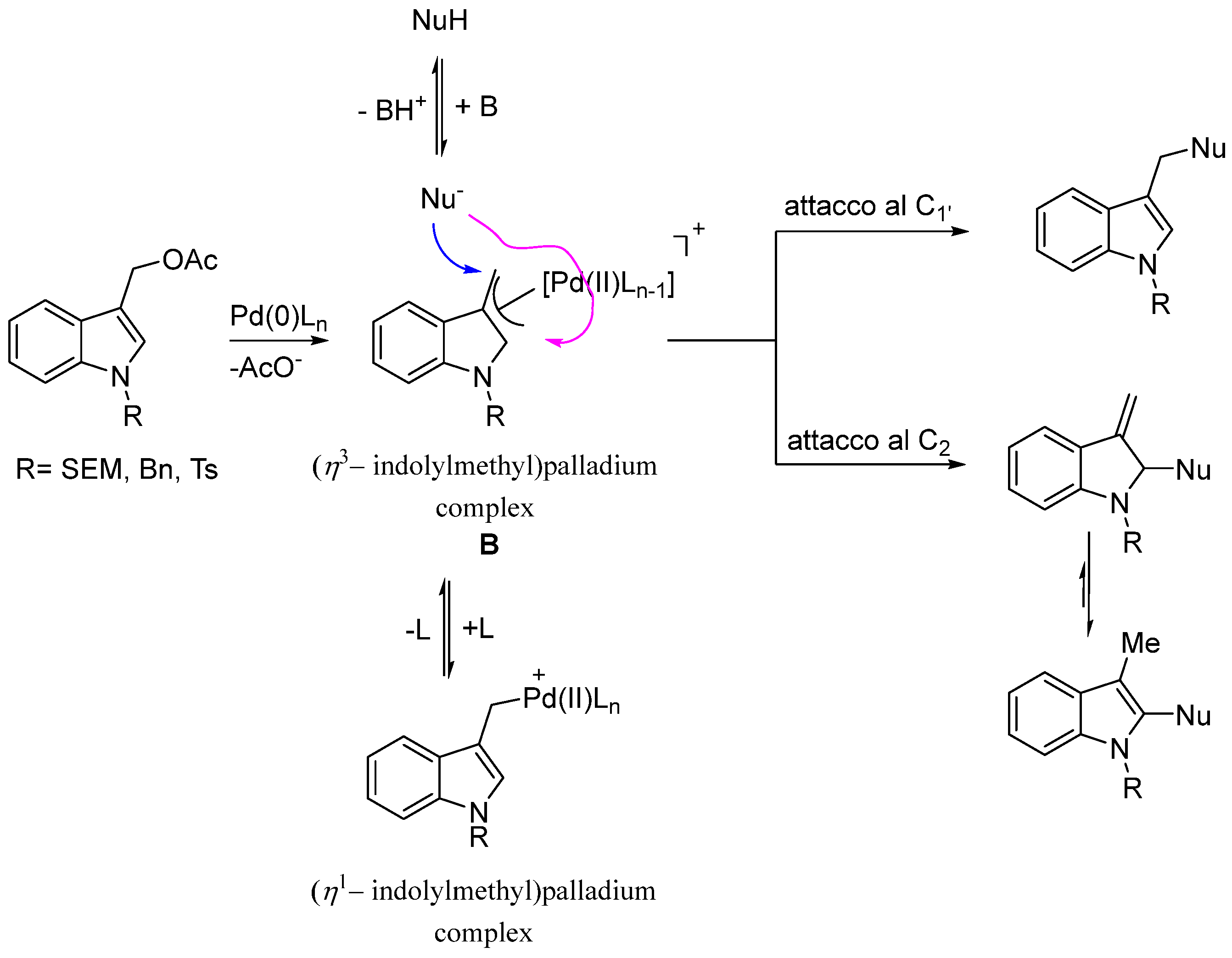
The regioselective outcome of the functionalization of the 1-substituted-indol-3-yl)methyl acetates 3 with S and O soft nucleophiles represents the principal goal of our investigation. It is known that this type of substrate could generate the (η3-indolylmethyl)palladium complex B which undergoes the nucleophilic attack of the added nucleophile at the benzylic carbon C1′ or C2 position of the indole ring (Scheme 4).
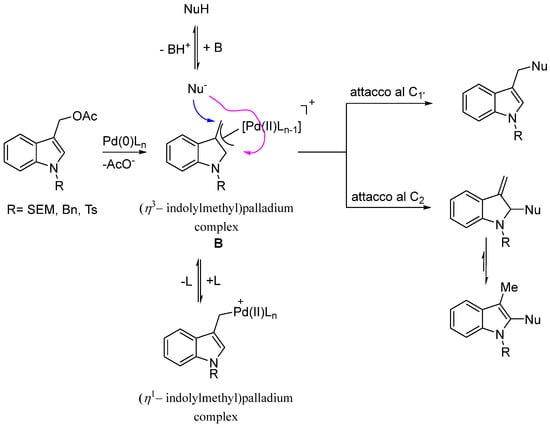
Scheme 4.
Formation of the (η3–indolylmethyl)palladium complex B and its reaction with nucleophiles.
In all the tested cases, under our reaction conditions, regardless of the nature of the nucleophiles, the reaction led only to the formation of the C1′ substituted products in high overall yield.
4. Conclusions
In conclusion, I3C represents a readily available building block for synthesizing highly desirable biologically active indole-containing sulfone and aryloxy fragments. The procedure is of wide scope, tolerates a variety of functional groups, and proceeds in yields ranging from good to excellent in a regioselective manner.
In addition, given the highlighted efficiency and atom economy, the proposed method may represent a reasonable valorization route for the exploitation of I3C and/or its derivative obtained from renewable sources.
5. Materials and Methods
5.1. General Information
All of the commercially available reagents, catalysts, bases, and solvents were used as purchased, without further purification. Starting materials and reaction products were purified by flash chromatography using SiO2 as stationary phase, eluting with n-hexane/ethyl acetate (EtOAc) mixtures. 1H NMR (400.13 MHz), 13C NMR (100.6 MHz), and 19F spectra (376.5 MHz) were recorded with a Bruker Avance 400 spectrometer. Splitting patterns were designed as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), or bs (broad singlet). HRMS of samples were recorded using a MALDI-TOF spectrometer AB SCIEX TOF/TOF 5800 using matrix in combination with KI for the ionization, with an Orbitrap Exactive (Thermo Fisher, Norristown, PA, USA) mass spectrometer with ESI source positive as well negative. Melting points were determined with a Büchi B-545 apparatus and are uncorrected.
5.2. General Experimental Procedures
Starting materials 3a–c were prepared according to the literature procedures [18,19,20,22], through the four-step sequence of reactions depicted in Scheme 2. Starting materials 3d, f, g were obtained from the corresponding commercially available 3-formylindole. Experimental procedures for 3e are detailed in Supplementary Materials.
5.2.1. Typical Procedure for the Preparation of 1-Substituted 3-(aryloxymethyl)-1H-indole 4: 3-((4-methoxyphenoxy)methyl)-1-tosyl-1H-indole 4aa
In a 50 mL Carousel Tube Reactor (Radely Discovery Technology) containing a magnetic stirring bar, [Pd(η3-C3H5)Cl]2 (2.6 mg, 0.007 mmol, 0.025 equiv.) and XPhos (6.7 mg, 0.014 mmol, 0.05 equiv.) were dissolved at room temperature with 0.5 mL of anhydrous THF under argon. Then, (1-tosyl-1H-indol-3-yl)methyl acetate 3a (100.0 mg, 0.290 mmol, 1.00 equiv.), 4-methoxyphenol 6a (71.9 mg, 0.580 mmol, 2.00 equiv.), K2CO3 (80.3 mg, 0.580 mmol, 2.00 equiv.), and 2.0 mL of anhydrous MeCN were added, and the resulting mixture was stirred for 1.5 h at 120 °C under argon. After this time, the reaction mixture was cooled to room temperature, diluted with Et2O, extracted twice with NaOH 2.0 N, and then washed with brine. The organic layer was dried over Na2SO4, iltered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with 80/20 (v/v) n-hexane/AcOEt mixture (Rf = 0.24) to obtain 3-((4-methoxyphenoxy)methyl)-1-tosyl-1H-indole 4aa (97% yield, 114.5 mg).
3-((4-methoxyphenoxy)methyl)-1-tosyl-1H-indole 4aa: 97% yield; yellow solid; mp: 132–133 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2919, 1694, 1447, 1357, 1176 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.95 (d, J = 8.3 Hz, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.58 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.31 (td, J1 = 7.8 Hz, J2 = 0.8 Hz, 1H), 7.23 (td, J1 = 7.8 Hz, J2 = 0.6 Hz, 1H), 7.17 (d, J = 8.2 Hz, 2H), 6.88 (d, J = 9.1 Hz, 2H), 6.80 (d, J = 9.1 Hz, 2H), 5.10 (s, 2H), 3.75 (s, 3H), 2.31 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H21KNO4S: [M+K]+ 446.0828, found: 446.0833.
5.2.2. Typical Procedure for the Preparation of 1-tosyl 3-((arylsulfonyl)methyl)-1-tosyl-1H-indole 9: Synthesis of 1-tosyl-3-(tosylmethyl)-1H-indole 9aa
In a 50 mL Carousel Tube Reactor (Radely Discovery Technology) containing a magnetic stirring bar, [Pd(η3-C3H5)Cl]2 (2.6 mg, 0.007 mmol, 0.025 equiv.) and XPhos (6.7 mg, 0.014 mmol, 0.05 equiv.) were dissolved at room temperature with 0.5 mL of anhydrous THF under argon. Then, (1-tosyl-1H-indol-3-yl)methyl acetate 3a (100.0 mg, 0.290 mmol, 1.00 equiv.), sodium 4-tolylsulfinate 8a (103.3 mg, 0.580 mmol, 2.00 equiv.), K2CO3 (80.3 mg, 0.580 mmol, 2.00 equiv.), and 2.0 mL of anhydrous MeCN were added, and the mixture was stirred for 1.5 h at 100 °C. After this time, the reaction mixture was cooled to room temperature, diluted with Et2O, and washed with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with 80/20 (v/v) n-hexane/AcOEt mixture (Rf = 0.24) to obtain 1-tosyl-3-(tosylmethyl)-1H-indole 9aa.
1-tosyl-3-(tosylmethyl)-1H-indole 9aa: 98% yield; red solid; mp: 207–208 °C; Rf = 0.24 (n-hexane/AcOEt, 80:20); IR (neat): 2927, 1598, 1309, 1163, 659 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.95 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.39 (s, 1H), 7.32 (d, J = 7.7 Hz, 2H), 7.29–7.26 (m, 2H), 7.19–7.15 (m, 1H), 7.13 (d, J = 8.2 Hz, 2H), 4.43 (s, 2H), 2.40 (s, 3H), 2.39 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 145.3 (C), 144.9 (C), 135.0 (C), 134.7 (C), 130.0 (overlapping) (CH), 129.7 (C), 129.6 (CH), 128.5 (CH), 127.3 (CH), 126.9 (CH), 125.1 (CH), 123.5 (CH), 119.6 (CH), 113.5 (CH), 110.1 (C), 53.6 (CH2), 21.6 (overlapping) (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H21KNO4S2: [M+K]+ 478.0549, found: 478.0554.
5.3. Characterization Data of Synthesized Compounds
Characterization data of starting materials 3a–g are reported in Supplementary Materials.
Characterization Data of Final Compounds 4aa–ed and 9aa–gb
1-benzyl-3-((4-methoxyphenoxy)methyl)-1H-indole 4ba: 57% yield; yellow oil; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2879, 1678, 1435, 1267, 1098, 895 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.62 (d, J = 7.7 Hz, 1H), 7.20–6.98 (m, 10H), 6.86 (d, J = 9.2 Hz, 2H), 6.75 (d, J = 9.2 Hz, 2H), 6.80 (d, J = 9.1 Hz, 2H), 5.16 (s, 2H), 5.09 (s, 2H), 3.66 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.0 (C), 153.3 (C), 137.3 (C), 137.0 (C), 128.9 (CH), 128.0 (CH), 127.7 (CH), 127.0 (CH), 122.3 (CH), 119.9 (CH), 119.5 (CH), 116.2 (CH), 114.7 (CH), 114.7 (CH), 110. 0 (CH), 63.3 (CH2), 55.8 (CH3), 50.1 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H21NNaO2: [M+Na]+ 366.1470, found: 366.1472.
2-((1-benzyl-1H-indol-3-yl)methyl)-4-methoxyphenol 7ba: 13% yield; brown oil; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2919, 1596, 1347, 1256, 1177, 989 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.49 (d, J = 8.2 Hz, 1H), 7.23–7.17 (m, 4H), 7.10 (td, J1 = 7.5 Hz, J2 = 0.8 Hz, 1H), 7.03–7.00 (m, 3H), 6.84 (s, 2H), 6.75 (d, J = 2.9 Hz, 1H), 6.80 (d, J = 8.7 Hz, 1H), 6.62 (dd, J1 = 8.7 Hz, J2 = 2.9 Hz, 1H), 5.16 (s, 2H), 5.18 (s, 2H), 4.64 (bs, 1H), 4.02 (s, 2H), 3.67 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 153.8 (C), 148.4 (C), 137.6 (C), 137.2 (C), 128.9 (CH), 128.0 (CH), 127.7 (CH), 127.6 (C), 126.8 (C), 126.6 (CH), 122.4 (CH), 119.54 (CH), 119.49 (CH), 116.8 (CH), 116.3 (CH), 112.6 (CH), 112.5 (C), 110. 0 (CH), 55.8 (CH3), 50.1 (CH2), 27.4 (CH2); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H21NNaO2: [M+Na]+ 366.1470, found: 366.1473.
3-((4-methoxyphenoxy)methyl)-1-((2-(trimethylsilyl)ethoxy)methyl)-1-H-indole 4ca: 97% yield; yellow solid; mp: 111–113 °C; Rf = 0.23 (n-hexane/AcOEt, 80:20); IR (neat): 3030, 2789, 1447, 1327, 1145 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.75 (d, J = 7.9 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.31 (td, J1 = 7.0 Hz, J2 = 0.9 Hz, 1H), 7.28–7.21 (m, 2H), 7.03–6.99 (m, 2H), 6.91–6.87 (m, 2H), 5.49 (s, 2H), 5.24 (s, 2H), 3.81 (m, 3H), 3.52 (t, J = 8.1 Hz, 2H), 0.93 (t, J = 8.1 Hz, 1H), 0.00 (s, 9H); 13C NMR (100.6 MHz) (CDCl3): δ 154.0 (C), 153.3 (C), 137.0 (C), 128.0 (C), 127.5 (CH), 122.7 (CH), 120.5 (CH), 119.5 (CH), 116.1 (CH), 114.7 (CH), 112.2 (C), 110.2 (CH), 75.7 (CH2), 66.0 (CH2), 63.3 (CH2), 55.8 (CH3), 17.8 (CH2), −1.33 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H29KNO3Si: [M+K]+ 422.1554, found: 422.1554.
4-methoxy-2-((1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indol-3-yl)methyl)phenol 7ca: 97% yield; yellow oil; Rf = 0.21 (n-hexane/AcOEt, 80:20); IR (neat): 2988, 1567, 1347, 1278, 1165, 982 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.57 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.25 (dd, J1 = 8.1 Hz, J2 = 0.9 Hz, 1H), 7.15 (dd, J1 = 7.5 Hz, J2 = 0.7 Hz, 1H), 6.99 (s, 1H), 6.82 (d, J = 2.9 Hz, 1H), 6.78 (d, J = 8.7 Hz, 1H), 6.72 (dd, J1 = 8.7 Hz, J2 = 2.9 Hz, 1H), 5.43 (s, 2H), 4.65 (bs, 1H), 4.09 (s, 2H), 3.76 (m, 3H), 3.45 (t, J = 8.1 Hz, 2H), 0.88 (t, J = 8.1 Hz, 1H), 0.00 (s, 9H); 13C NMR (100.6 MHz) (CDCl3): δ 148.0 (C), 137.2 (C), 128.4 (C), 127.8 (CH), 126. 3 (CH), 122.7 (CH), 120.5 (C), 120.1 (CH), 119.4 (CH), 116.7 (CH), 116.4 (CH), 113.6 (C), 112.6 (CH), 110 (CH), 75.4 (CH2), 66.0 (CH2), 63.3 (CH2), 55.8 (CH3), 26.9 (CH) 17.8 (CH2), −1.39 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H29KNO3Si: [M+K]+ 422.1554, found: 422.1554.
3-((4-(tert-butyl)phenoxy)methyl)-1-tosyl-1H-indole 4ab: 83% yield; yellow solid; mp: 126–128 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2957, 1596, 1361, 1215, 1100 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.98 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 8.3 Hz, 2H), 7.62 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.36–7.28 (m, 3H), 7.27–7.24 (m, 1H), 7.23–7.18 (m, 2H), 6.92 (d, J = 8.8 Hz, 2H), 5.15 (s, 2H), 2.33 (s, 3H), 1.31 (s, 9H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C26H27KNO3S: [M+K]+ 472.1349, found: 472.1349.
3-((4-fluorophenoxy)methyl)-1-tosyl-1H-indole 4ac: 81% yield; white solid; mp: 126–127 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2957, 1596, 1474, 1366, 1172 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.98 (d, J = 8.3 Hz, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.60 (s, 1H), 7.59 (d, J = 7.4 Hz, 1H), 7.34 (td, J1 = 7.7 Hz, J2 = 0.9 Hz, 1H), 7.25 (td, J1 = 8.4 Hz, J2 = 0.8 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 6.98–6.87 (m, 4H), 5.14 (s, 2H), 2.34 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 157.6 (d, JCF = 240.0 Hz, C), 154.60 (d, JCF = 2.2 Hz, C), 145.2 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.6 (C), 126.9 (CH), 125.1 (d, JCF = 20.4 Hz, CH), 123.6 (CH), 120.0 (CH), 118.3 (C), 116.3 (d, JCF = 8.0 Hz, CH), 116.1 (CH), 115.9 (CH), 113.9 (CH), 63.2 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H19FNO3S: [M+H]+ 396.1070, found: 396.1071.
3-(([1,1′-biphenyl]-2-yloxy)methyl)-1-tosyl-1H-indole 4ad: 80% yield; white solid; mp: 125–127 °C; Rf = 0.22 (n-hexane/AcOEt, 90:10); IR (neat): 2957, 1596, 1443, 1281, 1118, 1053, cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.96 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 7.1 Hz, 2H), 7.47 (s, 1H), 7.43–7.27 (m, 7H), 7.20–7.15 (m, 3H), 7.12–7.06 (m, 2H), 5.15 (s, 2H), 2.32 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 155.4 (C), 145.0 (C), 138.5 (C), 135.4 (C), 135.3 (C), 132.0 (C), 131.1 (CH), 130.0 (CH), 129.7 (CH), 129.4 (C), 128.7 (CH), 128.1 (CH), 127.1 (CH), 126.9 (CH), 125.0 (CH), 124.8 (CH), 123.4 (CH), 121.9 (CH), 119.9 (CH), 118.9 (C), 113.9 (CH), 113.8 (CH), 63.5 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C28H23KNO3S: [M+K]+ 492.1036, found: 492.1038.
methyl 3-((1-tosyl-1H-indol-3-yl)methoxy)benzoate 4ae: 83% yield; pale-yellow solid; mp: 128–130 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2957, 1714, 1509, 1367, 1099, 998 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.95 (d, J = 8.3 Hz, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.58 (s, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.31 (td, J1 = 7.8 Hz, J2 = 0.9 Hz, 1H), 7.23 (td, J1 = 7.8 Hz, J2 = 0.6 Hz, 1H), 7.17 (d, J = 8.3 Hz, 2H), 6.88 (d, J = 9.1 Hz, 2H), 6.80 (d, J = 9.1 Hz, 2H), 5.10 (s, 2H), 3.75 (s, 3H), 2.31 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C24H21KNO5S: [M+K]+ 474.0778, found: 474.0779.
4-((1-tosyl-1H-indol-3-yl)methoxy)benzonitrile 4af: 75% yield; yellow solid; mp: 142–144 °C; Rf = 0.25 (n-hexane/AcOEt, 90:10); IR (neat): 2957, 1509, 1216, 1083, 1032, 971 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.01 -7.99 (m, 2H), 7.75 (d, J = 8.2 Hz, 2H), 7.64 (s, 1H), 7.60–7.56 (m, 3H), 7.30–7.22 (m, 3H), 7.38–7.34 (m, 1H), 7.29–7.25 (m, 1H), 7.22 (d, J = 8.2 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H) 5.23 (s, 2H), 2.35 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 161.8 (C), 145.4 (C), 135.4 (C), 135.2 (C), 134.2 (CH), 130.1 (CH), 129.3 (C), 127.0 (CH), 125.4 (CH), 125.2 (CH), 123.7 (CH), 119.9 (CH), 119.2 (C), 117.2 (C), 115.7 (CH), 113.9 (CH), 104.6 (C), 62.8 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H19N2O3S: [M+H]+ 403.1116, found: 403.1119.
5-methoxy-3-((4-methoxyphenoxy)methyl)-1-tosyl-1H-indole 4da: 83% yield; yellow solid; mp: 108–110 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2955, 1596, 1366, 1082, 972 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.88 (d, J = 9.0 Hz, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.56 (s, 1H), 7.18 (d, J = 8.2 Hz, 2H), 7.03 (d, J = 2.3 Hz, 1H), 6.95 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 6.91 (d, J = 9.0 Hz, 2H), 6.83 (d, J = 9.1 Hz, 2H), 5.09 (s, 2H), 3.80 (s, 3H), 3.78 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C24H23KNO5S: [M+K]+ 476.0934, found: 476.0931.
3-((4-(tert-butyl)phenoxy)methyl)-5-methoxy-1-tosyl-1H-indole 4db: 97% yield; orange solid; mp: 122–124 °C; Rf = 0.22 (n-hexane/AcOEt, 90:10); IR (neat): 2965, 2865, 1509, 1215, 1032, 973 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.87 (d, J = 9.0 Hz, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.59 (s, 1H), 7.33 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 7.02 (d, J = 2.3 Hz, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.98–6.91 (m, 3H), 5.12 (s, 2H), 3.80 (s, 3H), 2.34 (s, 3H), 1.32 (s, 9H); 13C NMR (100.6 MHz) (CDCl3): 156.6 (C), 156.4 (C), 145.0 (C), 144.1 (C), 135.3 (C), 130.8 (C), 130.1 (C), 130.0 (CH), 126.9 (CH), 126.4 (CH), 125.7 (CH), 118.7 (C), 114.7 (CH), 114.4 (CH), 114.4 (CH), 102.4 (CH), 62.5 (CH2), 55.8 (CH3), 34.2 (C), 31.6 (CH3), 21.7 (CH3). HRMS: m/z (MALDI-TOF) positive ion, calculated for C27H29KNO4S: [M+K]+ 502.1454, found: 502.1452.
3-(([1,1′-biphenyl]-2-yloxy)methyl)-5-methoxy-1-tosyl-1H-indole 4dc: 83% yield; yellow solid; mp: 132–135 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2975, 1596, 1475, 1294, 1055, 895 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.83 (d, J = 9.1 Hz, 1H), 7.64 (d, J = 8.3 Hz, 2H), 7.49 (dd, J1 = 8.3 Hz, J2 = 1.4 Hz, 2H), 7.43 (s, 1H), 7.38–7.27 (m, 5H), 7.16 (d, J = 8.1 Hz, 2H), 7.08 (dd, J1 = 7.8 Hz, J2 = 5.4 Hz, 2H), 6.89 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 6.82 (d, J = 2.4 Hz, 1H), 5.12 (s, 2H), 3.68 (s, 3H), 2.32 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C29H25KNO4S: [M+K]+ 522.1141, found: 522.1138.
Methyl 3-((5-methoxy-1-tosyl-1H-indol-3-yl)methoxy)benzoate 4de: 83% yield; yellow solid; mp: 136–138 °C; Rf = 0.19 (n-hexane/AcOEt, 90:10); IR (neat): 2956, 1716, 1595, 1475, 1032, 754 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.80 (d, J = 9.0 Hz, 1H), 7.63 (d, J = 8.3 Hz, 2H), 7.61–7.57 (m, 2H), 7.53 (s, 1H), 7.27 (t, J = 8.1 Hz, 1H), 7.13–7.06 (m, 3H), 6.93 (d, J = 2.4 Hz, 1H), 6.87 (d, J1 = 9.1 Hz, J2 = 2.4 Hz, 2H), 5.11 (s, 2H), 3.84 (s, 3H), 3.73 (s, 3H), 2.25 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 154.3 (C), 152.7 (C), 145.1 (C), 135.4 (C), 135.2 (C), 130.0 (CH), 129.7 (C), 127.0 (CH), 125.1 (CH), 125.0 (CH), 123.5 (CH), 120.1 (CH), 118.7 (C), 116.2 (CH), 114.8 (CH), 113.8 (CH), 63.3 (CH2), 55.8 (CH3), 27.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C25H23KNO6S: [M+K]+ 504.0883, found: 504.0880.
3-(([1,1′-biphenyl]-2-yloxy)methyl)-5-phenyl-1-tosyl-1H-indole 4ed: 89 yield%; gray solid; mp: 130–132 °C d; yellow solid; mp: 110–112 °C; Rf = 0.22 n-hexane/AcOEt 90:10); IR (neat): 2965, 2372, 1594, 1374, 1054, 891 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.99 (d, J = 8.7 Hz, 1H), 7.70 (d, J = 8.1 Hz, 2H), 7.59 (d, J = 0.9 Hz, 1H), 7.53–7.46 (m, 6H), 7.42–7.38 (m, 2H), 7.34–7.20 (m, 6H), 7.17 (d, J = 8.1 Hz, 2H), 7.09–7.04 (m, 2H), 5.18 (s, 2H), 2.31 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 155.3 (C), 145.1 (C), 141.2 (C), 138.4 (C), 136.9 (C), 135.3 (C), 134.8 (C), 131.9 (C), 131.2 (CH), 130.0 (CH), 129.6 (CH), 128.8 (CH), 128.7 (CH), 128.0 (CH), 127.5 (CH), 127.1 (CH), 127.0 (CH), 126.9 (CH), 125.3 (CH), 124.6 (CH), 121.8 (CH), 119.0 (C), 118.6 (CH), 113.9 (CH), 113.7 (CH), 63.3 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C34H27KNO3S: [M+K]+ 568.1349, found: 568.1347.
3-((phenylsulfonyl)methyl)-1-tosyl-1H-indole 9ab: 98% yield; yellow solid; mp: 185–187 °C; Rf = 0.20 (n-hexane/AcOEt, 87:13); IR (neat): 3000, 2919, 1653, 1454, 1337, 1008 cm−1; 1H NMR (400.13 MHz) (DMSO-d6): δ 7.85 (d, J = 8.3 Hz, 1H), 7.72–7.66 (m, 5H), 7.55–7.49 (m, 4H), 7.41 (d, J = 8.3 Hz, 2H), 7.31 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 4.92 (s, 2H), 2.34 (s, 3H); 13C NMR (100.6 MHz) (DMSO-d6): δ 146.3 (C), 138.3 (C), 134.34 (CH), 134.26 (C), 134.2 (C), 130.8 (CH), 130.1 (C), 129.6 (CH), 128.4 (CH), 128.0 (CH), 127.1 (CH), 125.5 (CH), 123.7 (CH), 121.1 (CH), 113.4 (CH), 111.1 (C), 52.0 (CH2), 21.5 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H19KNO4S2: [M+K]+ 464.0393, found: 464.0389.
5-methoxy-1-tosyl-3-(tosylmethyl)-1H-indole 9da: 84% yield; brown solid; mp: 159–160 °C; Rf = 0.21 (n-hexane/AcOEt, 80:20); IR (neat): 2925, 1597, 1167, 802, 535 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.73 (d, J = 9.0 Hz, 1H), 7.61 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 8.1 Hz, 2H), 7.25 (s, 1H), 7.16 (d, J = 8.1 Hz, 2H), 7.02 (d, J = 8.1 Hz, 2H), 6.80 (dd, J1 = 9.0 Hz, J2 = 2.1 Hz, 1H), 6.56 (d, J = 2.1 Hz, 1H), 4.29 (s, 2H), 3.64 (s, 3H), 2.29 (s, 3H), 2.28 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 156.7 (C), 145.3 (C), 145.0 (C), 134.9.7 (C), 134.7 (C), 130.8 (C), 130.0 (CH), 129.6 (CH), 129.4 (C), 128.7 (CH), 128.0 (CH), 126.9 (CH), 114.7 (CH), 114.6 (CH), 110.3 (C), 101.5 (CH), 55.6 (CH3), 53.8 (CH2), 21.70 (CH3), 21.69 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C24H23KNO5S2: [M+K]+ 508.0655, found: 508.0652.
5-methoxy-3-((phenylsulfonyl)methyl)-1-tosyl-1H-indole 9db: 76% yield; brown solid; mp: 173–174 °C; Rf = 0.23 (n-hexane/AcOEt, 78:22); IR (neat): 2925, 1594, 1167, 812, 593 cm−1; 1H NMR (400.13 MHz) (DMSO-d6): δ 7.72 (d, J = 9.0 Hz, 1H), 7.69–7.65 (m, 3H), 7.63 (d, J = 7.3 Hz, 2H), 7.49–7.45 (m, 3H), 7.40 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 2.4 Hz, 1H), 6.89 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 4.89 (s, 2H), 3.66 (s, 3H), 2.34 (s, 3H); 13C NMR (100.6 MHz) (DMSO-d6): δ 156.4 (C), 146.1 (C), 138.4 (C), 134.30 (CH), 134.28 (C), 131.3 (C), 130.7 (CH), 129.5 (CH), 128.8 (C), 128.7 (CH), 128.5 (CH), 127.0 (CH), 114.5 (CH), 114.3 (CH), 111.4 (C), 103.3 (CH), 55.8 (CH3), 52.0 (CH2), 21.5 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H21KNO5S2: [M+K]+ 494.0498, found: 494.0498.
5-phenyl-1-tosyl-3-(tosylmethyl)-1H-indole 9ea: 80% yield; white solid; mp: 162–164 °C; Rf = 0.22 (n-hexane/AcOEt 90:10); IR (neat): 2966, 2371, 2260, 1595, 1372, 1014 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.96 (d, J = 8.3 Hz, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 7.5 Hz, 2H), 7.47 (s, 1H), 7.42–7.28 (m, 7H), 7.20–7.15 (m, 3H), 7.12–7.06 (m, 2H), 5.15 (s, 2H), 2.32 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): 155.4 (C), 145.0 (C), 138.5 (C), 135.4 (C), 135.3 (C), 132.0 (C), 131.1 (CH), 130.0 (CH), 129.7 (CH), 129.4 (C), 128.7 (CH), 128.1 (CH), 127.1 (CH), 126.9 (CH), 125.0 (CH), 124.8 (CH), 123.4 (CH), 121.9 (CH), 119.9 (CH), 118.9 (C), 113.9 (CH), 113.8 (CH), 63.5 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C29H25KNO4S2: [M+K]+ 554.0862, found: 554.0859.
5-phenyl-3-((phenylsulfonyl)methyl)-1-tosyl-1H-indole 9eb: 97% yield; yellow solid; mp: 158–160 °C; Rf = 0.19 n-hexane/AcOEt 90:10); IR (neat): 2967, 1450, 1301, 1171, 1032, 887 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.98 (d, J = 8.7 Hz, 1H), 7.76 (d, J = 8.1 Hz, 2H), 7.56 (d, J = 7.7 Hz, 2H), 7.53–7.36 (m, 9H), 7.33 (d, J = 7.1 Hz, 1H), 7.31–7.23 (m, 3H), 4.46 (s, 2H), 2.37 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 145.5 (C), 140.9 (C), 137.7 (C), 137.2 (C), 135.0 (C), 134.1 (C), 134.0 (CH), 130.2 (C), 130.1 (CH), 129.0 (CH), 128.8 (CH), 128.7 (CH), 128.0 (CH), 127.4 (CH), 127.3 (CH), 127.1 (CH), 124.8 (CH), 117.9 (CH), 113.9 (CH), 110.3 (C), 53.6 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C28H23KNO4S2: [M+K]+ 540.0706, found: 540.0710
6-chloro-1-tosyl-3-(tosylmethyl)-1H-indole 9fa: 75% yield; white solid; mp: 177–178 °C; Rf = 0.23 (n-hexane/AcOEt, 85:15); IR (neat): 2922, 2860, 1591, 1137, 673 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 7.88 (d, J = 1.6 Hz, 1H), 7.65 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.2 Hz, 2H), 7.28 (s, 1H), 7.23–7.17 (m, 3H), 7.06 (d, J = 8.4 Hz, 3H), 4.30 (s, 2H), 2.32 (s, 6H); 13C NMR (100.6 MHz) (CDCl3): δ 145.8 (C), 145.2 (C), 135.1 (C), 134.8 (C), 134.7 (C), 131.3 (C), 130.3 (CH), 129.8 (CH), 128.6 (CH), 128.3 (C), 127.9 (CH), 127.0 (CH), 124.4 (CH), 120.7 (CH), 113.8 (CH), 110.0 (C), 53.6 (CH2), 21.79 (CH3), 21.77 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H20ClKNO4S2: [M+K]+ 512.0159, found: 512.0157.
6-chloro-3-((phenylsulfonyl)methyl)-1-tosyl-1H-indole 9fb: 62% yield; white solid; mp: 210–211 °C; Rf = 0.21 (n-hexane/AcOEt, 85:15); IR (neat): 2928, 2794, 1597, 1302, 1173, 668 cm−1; 1H NMR (400.13 MHz) (DMSO-d6): δ 7.84 (s, 1H), 7.75 (d, J = 8.3 Hz, 2H), 7.69 (t, J = 7.1 Hz, 1H), 7.64 (d, J = 8.0 Hz, 2H), 7.60–7.54 (m, 2H), 7.50 (t, J = 7.5 Hz, 2H), 7.45 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.6 Hz, 1H), 4.36 (s, 2H), 2.30 (s, 3H); 13C NMR (100.6 MHz) (DMSO-d6): δ 145.8 (C), 137.6 (C), 135.1 (C), 134.9 (C), 134.1 (CH), 131.5 (C), 130.3 (CH), 129.1 (CH), 128.6 (CH), 128.2 (C), 127.9 (CH), 127.1 (CH), 124.5 (CH), 120.6 (CH), 113.9 (CH), 109.9 (C), 53.6 (CH2), 21.8 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H18ClKNO4S2: [M+K]+ 498.0003, found: 497.9998.
5-nitro-1-tosyl-3-(tosylmethyl)-1H-indole 9ga: 92% yield; white solid; mp: 230–231 °C; Rf = 0.23 (n-hexane/AcOEt, 70:30); IR (neat): 2912, 1508,1336, 1122, 532 cm−1; 1H NMR (400.13 MHz) (DMSO- d6): δ 8.36 (d, J = 1.8 Hz, 1H), 8.18 (dd, J1 = 9.2 Hz, J2 = 1.8 Hz, 1H), 8.11 (d, J = 9.2 Hz, 1H), 7.84–7.82 (m, 3H), 7.50 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.27 (d, J = 8.0 Hz, 2H), 5.00 (s, 2H), 2.37 (s, 3H), 2.33 (s, 3H); 13C NMR (100.6 MHz) (DMSO-d6): δ 147.0 (C), 145.1 (C), 144.1 (C), 137.2 (C), 135.4 (C), 133.8 (C), 131.2 (CH), 131.1 (CH), 130.1 (C), 130.0 (CH), 128.6 (CH), 127.3 (CH), 120.5 (CH), 117.6 (CH), 114.3 (CH), 112.3 (C), 51.9 (CH2), 21.6 (CH3), 21.4 (CH3). HRMS: m/z (MALDI-TOF) positive ion, calculated for C23H20KN2O6S2: [M+K]+ 523.0400, found: 523.0400.
5-nitro-3-((phenylsulfonyl)methyl)-1-tosyl-1H-indole 9gb: 83% yield; white solid; mp: 229–230 °C; Rf = 0.20 (n-hexane/AcOEt, 70:30); IR (neat): 3100, 1522, 1345, 1125, 535 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.16 (dd, J1 = 8.3 Hz, J2 = 2.2 Hz, 1H), 8.07–8.00 (m, 2H), 7.77 (d, J = 8.3 Hz, 2H), 7.68 (s, 1H), 7.63–7.58 (m, 2H), 7.57–7.50 (m, 1H), 7.39–7.28 (m, 4H), 4.45 (s, 2H), 2.40 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 146.4 (C), 144.4 (C), 137.7 (C), 137.5 (C), 134.4 (CH), 134.3 (CH), 130.5 (CH), 130.4 (CH), 129.7 (C), 129.3 (CH), 128.6 (CH), 127.2 (CH), 120.4 (CH), 116.0 (CH), 114.0 (CH), 110.7 (C), 53.3 (CH2), 21.8 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C22H18KN2O6S2: [M+K]+ 509.0243, found: 509.0241.
1,1′-ditosyl-3,3′-bis(tosylmethyl)-1H,1′H-6,6′-biindole 13a: 12% yield; white solid; mp: 228–230 °C; Rf = 0.23 (n-hexane/AcOEt, 70:30); IR (neat): 2943, 1600, 1283, 1159, 535 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.04 (s, 1H), 7.72 (d, 2H, J = 8.3 Hz), 7.45 (d, 2H, J = 8.2 Hz), 7.42–7.33 (m, 2H), 7.32 (s, 1H), 7.25 (d, J = 8.2 Hz, 2H), 7.18 (s, 1H), 7.12 (d, J = 8.2 Hz, 2H), 4.38 (s, 2H), 3.64 (s, 3H), 2.35 (s, 3H), 2.31 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 145.7 (C), 145.1 (C), 138.7 (C), 135.4 (C), 134.9 (C), 134.8 (C), 130.3 (CH), 129.8 (CH), 129.1 (C), 128.7 (CH), 127.8 (CH), 127.2 (CH), 123.5 (CH), 120.2 (CH), 112.6 (CH), 110.0 (C), 53.8 (CH2), 21.82 (CH3), 21.79 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C46H40KN2O8S4: [M+K]+ 915.1305, found: 915.1306.
3,3′-bis((phenylsulfonyl)methyl)-1,1′-ditosyl-1H,1′H-6,6′-biindole 13b: 8% yield; white solid; mp: 230–231 °C; Rf = 0.23 (n-hexane/AcOEt, 70:30); IR (neat): 2922, 1597, 1294, 1170, 583 cm−1; 1H NMR (400.13 MHz) (CDCl3): δ 8.04 (s, 1H), 7.65–7.49 (m, 3H), 7.41–7.28 (m, 5H), 7.26 (d, 2H, J = 8.2 Hz), 4.41 (s, 2H), 2.31 (s, 3H); 13C NMR (100.6 MHz) (CDCl3): δ 145.6 (C), 138.6 (C), 137.6 (C), 135.2 (C), 134.8 (C), 134.0 (CH), 130.2 (CH), 129.1 (CH), 128.9 (C), 128.5 (CH), 127.7 (CH), 127.0 (CH), 123.4 (CH), 119.9 (CH), 112.5 (CH), 109.7 (C), 53.7 (CH2), 21.7 (CH3); HRMS: m/z (MALDI-TOF) positive ion, calculated for C44H36KN2O8S4: [M+K]+ 887.0992, found: 887.0991.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29143434/s1. Reference [51] is cited in Supplementary Materials.
Author Contributions
Conceptualization, G.F. and A.I.; methodology, A.I. and A.G.; formal analysis, G.F. and A.G.; investigation, M.C., F.M., A.S. and R.Z.; writing—original draft preparation, A.I. and A.G.; writing—review and editing, G.F. and A.A.; supervision, A.G. and A.I.; project administration, G.F.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Sapienza University of Rome under Grant “Progetti Ateneo 2023”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained within the article or Supplementary Materials.
Acknowledgments
We gratefully acknowledge Sapienza University of Rome, Catholic University of Sacred Heart, Rome, University of Teramo, and University of L’ Aquila.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xue, X. Medicinal Chemistry Strategies for the Modification of Bioactive Natural Products. Molecules 2024, 29, 689. [Google Scholar] [CrossRef] [PubMed]
- Umer, S.M.; Solangi, M.; Khan, K.M.; Saleem, R.S.Z. Indole-Containing Natural Products 2019–2022: Isolations, Reappraisals, Syntheses, and Biological Activities. Molecules 2022, 27, 7586. [Google Scholar] [CrossRef]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.-N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Antioxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef]
- Caruso, J.A.; Campana, R.; Wei, C.; Su, C.-H.; Hanks, A.M.; Bornmann, W.G.; Keyomarsi, K. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERα-positive breast cancer cells. Cell Cycle 2014, 13, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Cram, E.J.; Liu, B.D.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol Inhibits CDK6 Expression in Human MCF-7 Breast Cancer Cells by Disrupting Sp1 Transcription Factor Interactions with a Composite Element in the CDK6 Gene Promoter. J. Biol. Chem. 2001, 26, 22332–22340. [Google Scholar] [CrossRef]
- Moiseeva, E.P.; Heukers, R. Indole-3-carbinol-induced modulation of NF-κB signalling is breast cancer cell-specific and does not correlate with cell death. Breast Cancer Res. Treat. 2008, 109, 451–462. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Zhang, L.; Dai, Q.; Si, H.; Zhang, L.; Eltom, S.E.; Si, H. Combined Luteolin and Indole-3-Carbinol Synergistically Constrains ERα-Positive Breast Cancer by Dual Inhibiting Estrogen Receptor Alpha and Cyclin-Dependent Kinase 4/6 Pathway in Cultured Cells and Xenograft Mice. Cancers 2021, 13, 2116. [Google Scholar] [CrossRef]
- Centofanti, F.; Buono, A.; Verboni, M.; Tomino, C.; Lucarini, S.; Duranti, A.; Pandolfi, P.P.; Novelli, G. Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives. Pharmaceuticals 2023, 16, 240. [Google Scholar] [CrossRef]
- Megna, B.W.; Carney, P.R.; Nukaya, M.; Geiger, P.; Kennedy, G.D. Indole-3-carbinol induces tumor cell death: Function follows form. J. Surg. Res. 2016, 204, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fadlalla, K.; Elgendy, R.; Gilbreath, E.; Pondugula, S.R.; Yehualaeshet, T.; Mansour, M.; Serbessa, T.; Manne, U.; Samuel, T. 3-(2-Bromoethyl)-Indole Inhibits the Growth of Cancer Cells and NF-KB Activation. Oncol. Rep. 2015, 34, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Rhee, Y.-H.; Lee, H.-J.; Lee, E.-O.; Kim, K.-H.; Park, M.-J.; Jeon, B.-H.; Shim, B.-S.; Jung, C.-H.; Choi, S.-H.; et al. Antiplatelet and antithrombotic activity of indole-3-carbinol in vitro and in vivo. Phytother. Res. 2008, 22, 58–64. [Google Scholar] [CrossRef]
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Seyedhosseini, F.S.; Behnampour, N.; Yazdani, Y. Indole-3-carbinol induces G1 cell cycle arrest and apoptosis through aryl hydrocarbon receptor in THP-1 monocytic cell line. J. Recept. Signal Transduct. 2017, 37, 506–514. [Google Scholar] [CrossRef]
- Mohammadi, S.; Memarian, A.; Sedighi, S.; Behnampour, N.; Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: A crucial role for aryl hydrocarbon receptor. Autoimmunity 2018, 51, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Omar, H.A.; Kulp, S.K.; Chen, C.S. Pharmacological exploitation of indole-3-carbinol to develop potent antitumor agents. Mini Rev. Med. Chem. 2010, 10, 398–404. [Google Scholar] [CrossRef]
- Arcadi, A.; Berden, G.; Ciogli, A.; Corinti, D.; Crestoni, M.E.; De Angelis, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; et al. Reactivity of Indolylmethylacetates with N, O, and S Soft Nucleophiles: Evidence of 2-Alkylideneindolenines and 3-Alkylideneindoleninium Generation by ESI-MS and IRMPD Spectroscopy. Eur. J. Org. Chem. 2022, 2022, e202201166. [Google Scholar] [CrossRef]
- Luparello, C.; Cruciata, I.; Joerger, A.C.; Ocasio, C.A.; Jones, R.; Tareque, R.K.; Bagley, M.C.; Spencer, J.; Walker, M.; Austin, C.; et al. Genotoxicity and Epigenotoxicity of Carbazole-Derived Molecules on MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 3410. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, C.-J.; Feng, Q.-Z.; Zhai, J.-J.; Qi, S.-S.; Zhong, A.-G.; Chu, M.-M.; Xu, D.-Q. Copper-catalyzed asymmetric 1, 6-conjugate addition of in situ generated para-quinone methides with β-ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef]
- Arcadi, A.; Calcaterra, A.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Mazzoccanti, G.; Serraiocco, A. One-pot synthesis of dihydroquinolones by sequential reactions of o-aminobenzyl alcohol derivatives with Meldrum’s acids. Org. Biomol. Chem. 2022, 20, 3160–3173. [Google Scholar] [CrossRef] [PubMed]
- Primault, G.; Legros, J.-Y.; Fiaud, J.-C. Palladium-catalyzed benzylic-like nucleophilic substitution of benzofuran-, benzothiophene- and indole-based substrates by dimethyl malonate anion. J. Organomet. Chem. 2003, 687, 353–364. [Google Scholar] [CrossRef]
- Arcadi, A.; Aschi, M.; Chiarini, M.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iavarone, F.; Iazzetti, A.; Serraiocco, A.; Zoppoli, R. Experimental Results and Mechanistic Insights on the Reactions of Indolylmethyl Acetates with Soft Carbon Pronucleophiles. ACS Omega 2024, 9, 28450–28462. [Google Scholar] [CrossRef]
- Arcadi, A.; Calcaterra, A.; Chiarini, M.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Marsicano, V.; Serraiocco, A. Synthesis of Indole/Benzofuran-Containing Diarylmethanes through Palladium-Catalyzed Reaction of Indolylmethyl or Benzofuranylmethyl Acetates with Boronic Acids. Synthesis 2021, 54, 741–753. [Google Scholar] [CrossRef]
- Tang, Y.; Zhuang, K.; Zhang, X.; Xie, F.; Yang, L.; Lin, B.; Cheng, M.; Li, D.; Liu, Y. Ferric Chloride Catalyzed 1,3-Rearrangement of (Phenoxymethyl)heteroarenes to (Heteroarylmethyl)phenols. Eur. J. Org. Chem. 2020, 23, 3441–3451. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S.; Palmisano, G. Oxidation of Alcohols with o-Iodoxybenzoic Acid in DMSO: A New Insight into an Old Hypervalent Iodine Reagent. J. Org. Chem. 1995, 60, 7272–7276. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Fabrizi, G.; Fochetti, A.; Ghirga, F.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Mazzoccanti, G.; Serraiocco, A. Palladium-catalyzed Tsuji–Trost-type reaction of benzofuran-2-ylmethyl acetates with nucleophiles. RSC Adv. 2021, 11, 909–917. [Google Scholar] [CrossRef]
- DeAngelis, A.J.; Gildner, P.G.; Chow, R.; Colacot, T.J. Generating Active “L-Pd(0)” via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure–Activity Studies in Challenging Cross-Coupling Reactions. J. Org. Chem. 2015, 80, 6794–6813. [Google Scholar] [CrossRef]
- McGarry, K.R.; McDaniel, M.; Chan, B.C.; O’Connor, A.R.J.P. Synthesis and characterization of (π-allyl) palladium (II) complexes containing dialkylbiaryl phosphine ligands. Polyhedron 2016, 114, 101–109. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361. [Google Scholar] [CrossRef]
- Meadows, D.C.; Gervay-Hague, J. Vinyl sulfones: Synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 2006, 26, 793–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hussain, S.; Parveen, S.; Zhang, S.; Yang, Y.; Zhu, C. Sulfonyl group-containing compounds in the design of potential drugs for the treatment of diabetes and its complications. Curr. Med. Chem. 2012, 19, 3578–3604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.-Y.; Qin, H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef]
- Simpkins, N.S. Sulfones in Organic Synthesis; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Le Duc, G.; Bernoud, E.; Prestat, G.; Cacchi, S.; Fabrizi, G.; Iazzetti, A.; Madec, D.; Poli, G. Palladium-Catalyzed Aromatic Sulfonylation: A New Catalytic Domino Process Exploiting in situ Generated Sulfinate Anions. Synlett 2011, 2011, 2943–2946. [Google Scholar] [CrossRef]
- Shavnya, A.; Hesp, K.D.; Mascitti, V.; Smith, A.C. Palladium-Catalyzed Synthesis of (Hetero)Aryl Alkyl Sulfones from (Hetero)Aryl Boronic Acids, Unactivated Alkyl Halides, and Potassium Metabisulfite. Angew. Chem. Int. Ed. 2015, 54, 13571–13575. [Google Scholar] [CrossRef]
- Johnson, M.W.; Bagley, S.W.; Mankad, N.P.; Bergman, R.G.; Mascitti, V.; Toste, F.D. Application of Fundamental Organometallic Chemistry to the Development of a Gold-Catalyzed Synthesis of Sulfinate Derivatives. Angew. Chem. Int. Ed. 2014, 53, 4404–4407. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Menon, R.S. Recent developments in the chemistry of allenyl sulfones. Org. Biomol. Chem. 2020, 8, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, L. Recent Advances in Transition-Metal-Mediated Chelation- Assisted Sulfonylation of Unactivated C−H Bonds. Adv. Synth. Catal. 2019, 361, 1710–1732. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.-C.; Wang, X.-d.; Wu, L. Photoredox Catalysis in C–S Bond Construction: Recent Progress in Photo-Catalyzed Formation of Sulfones and Sulfoxides. Adv. Synth. Catal. 2018, 360, 386–400. [Google Scholar] [CrossRef]
- Higham, J.I.; Bull, J.A. Copper catalysed oxidative α-sulfonylation of branched aldehydes using the acid enhanced reactivity of manganese (IV) oxide. Chem. Commun. 2020, 56, 4587–4590. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Zhu, H.; Liu, Y.; Li, Q.; Yang, L.; Wu, G.; Fan, Q.; Xie, Z.; Le, Z. Palladium-catalyzed sulfonylative coupling of benzyl(allyl) carbonates with arylsulfonyl hydrazides. Green Synth. Catal. 2022, 3, 110–115. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Li, L.; Wen, K.; Yao, X.; Pang, J.; Wu, T.; Tang, X. Copper-Mediated Decarboxylative Sulfonylation of Arylacetic Acids with Sodium Sulfinates. Org. Lett. 2020, 22, 7164–7168. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhu, Y.-S.; Yan, K.-X.; Cui, T.-W.; Zhu, X.; Hao, X.-Q.; Song, M.-P. Iron-Catalyzed C–H Sulfonylmethylation of Indoles in Water–PEG400. Synlett 2019, 30, 1924–1928. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Parisi, L.M.; Bernini, R. Unsymmetrical Diaryl Sulfones and Aryl Vinyl Sulfones through Palladium-Catalyzed Coupling of Aryl and Vinyl Halides or Triflates with Sulfinic Acid Salts. J. Org. Chem. 2004, 69, 5608–5614. [Google Scholar] [CrossRef] [PubMed]
- Modha, S.G.; Mehta, V.P.; Van der Eycken, E.V. Transition metal-catalyzed C–C bond formation via C–S bond cleavage: An overview. Chem. Soc. Rev. 2013, 42, 5042–5055. [Google Scholar] [CrossRef] [PubMed]
- Ortgies, D.H.; Hassanpour, A.; Chen, F.; Woo, S.; Forgione, P. Desulfination as an Emerging Strategy in Palladium-Catalyzed C–C Coupling Reactions. Eur. J. Org. Chem. 2016, 2016, 408–425. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, J.; Liu, J.; Peng, S.; Deng, G.-J. Pd-Catalyzed Desulfitative Heck Coupling with Dioxygen as the Terminal Oxidant. Org. Lett. 2011, 13, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, Q.; Cheng, Y.; Lan, J.; You, J. Palladium-Catalyzed Desulfitative C-H Arylation of Heteroarenes with Sodium Sulfinates. Chem. Eur. J. 2011, 48, 13415–13419. [Google Scholar] [CrossRef]
- Moseley, J.D.; Murray, P.M.; Turp, E.R.; Tyler, S.N.G.; Burn, R.T. A mild robust generic protocol for the Suzuki reactionusing an air stable catalyst. Tetrahedron 2012, 68, 6010–6017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).