A Series of Lanthanide Coordination Polymers as Luminescent Sensors for Selective Detection of Inorganic Ions and Nitrobenzene
Abstract
1. Introduction
2. Results and Discussion
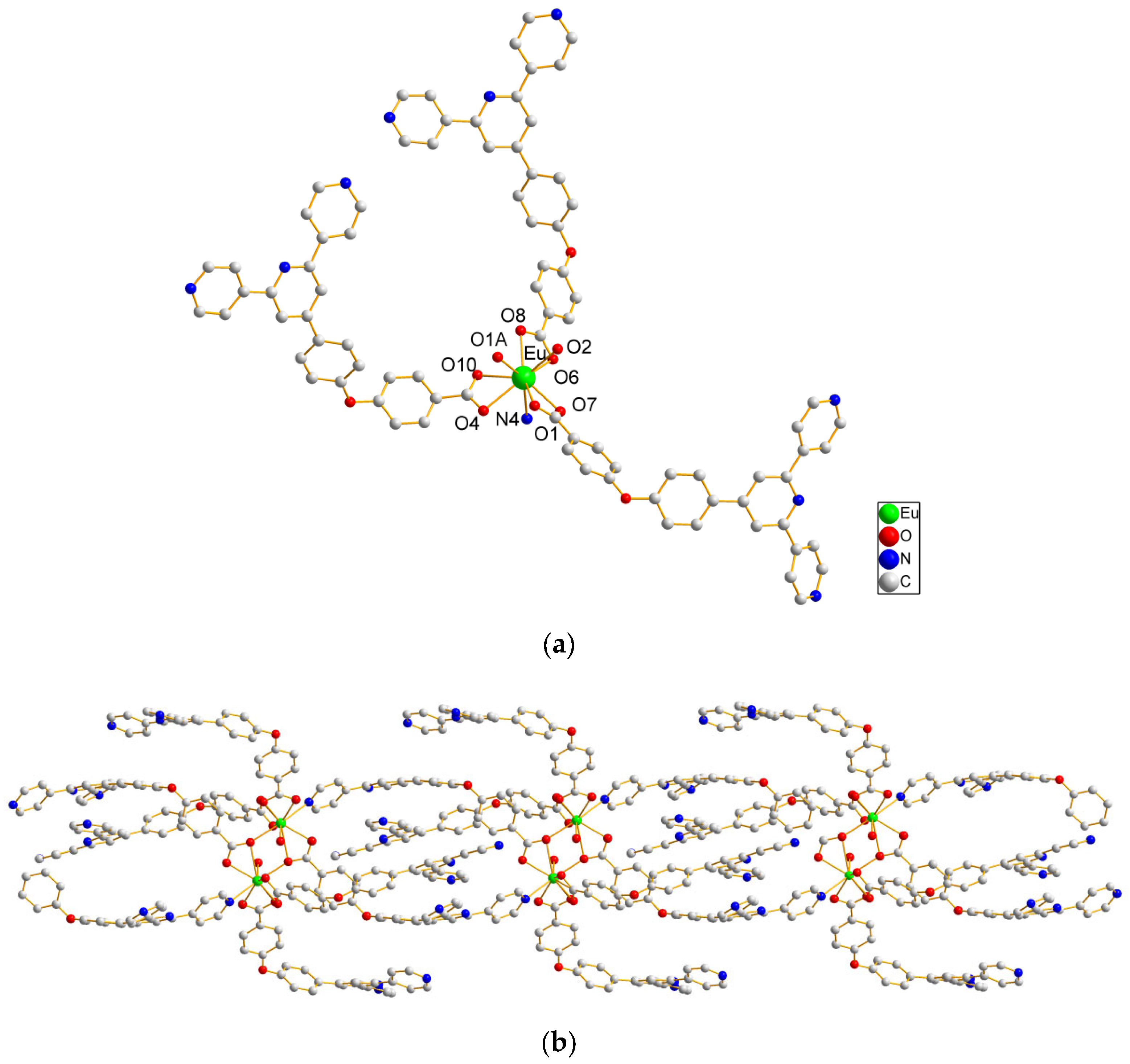
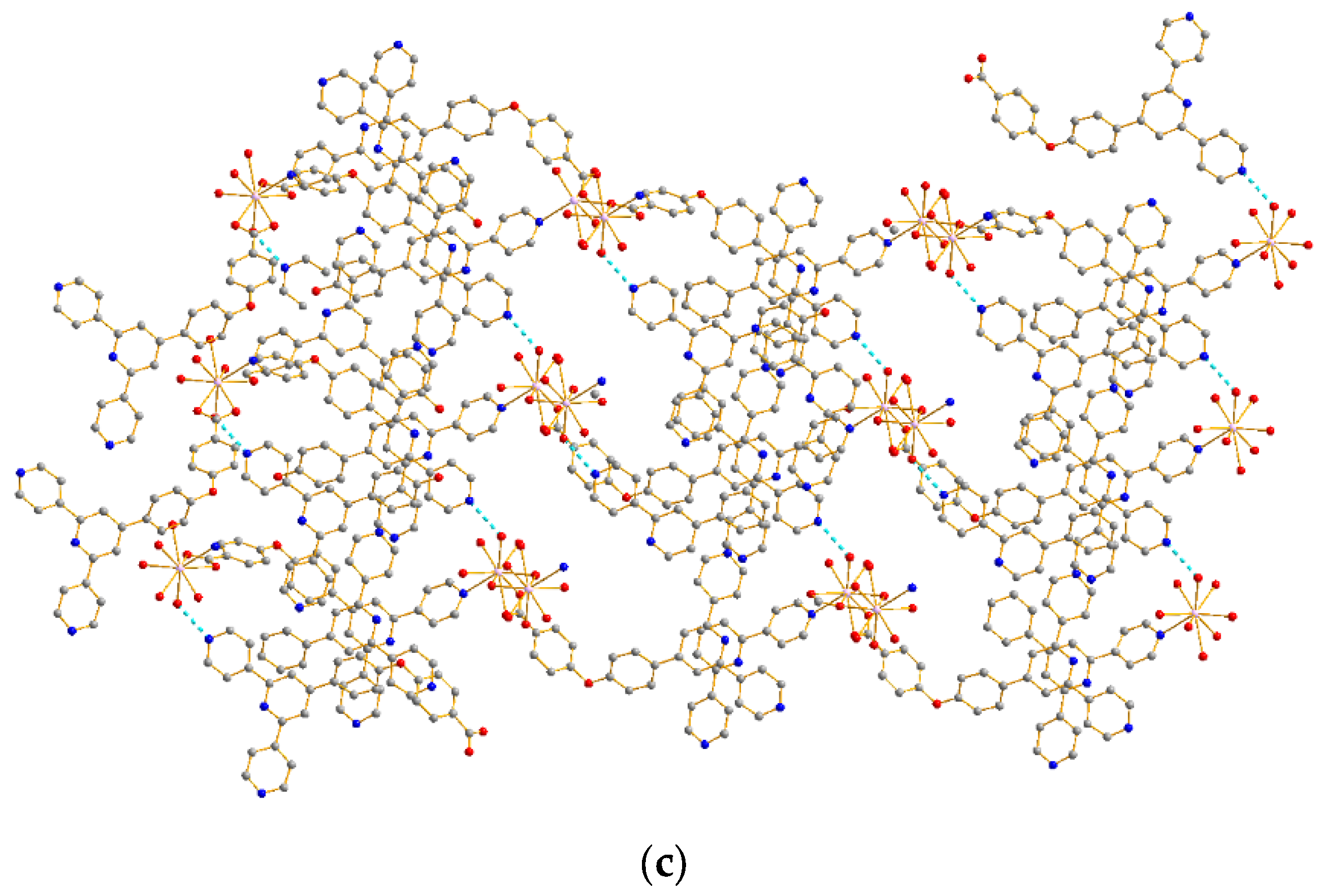
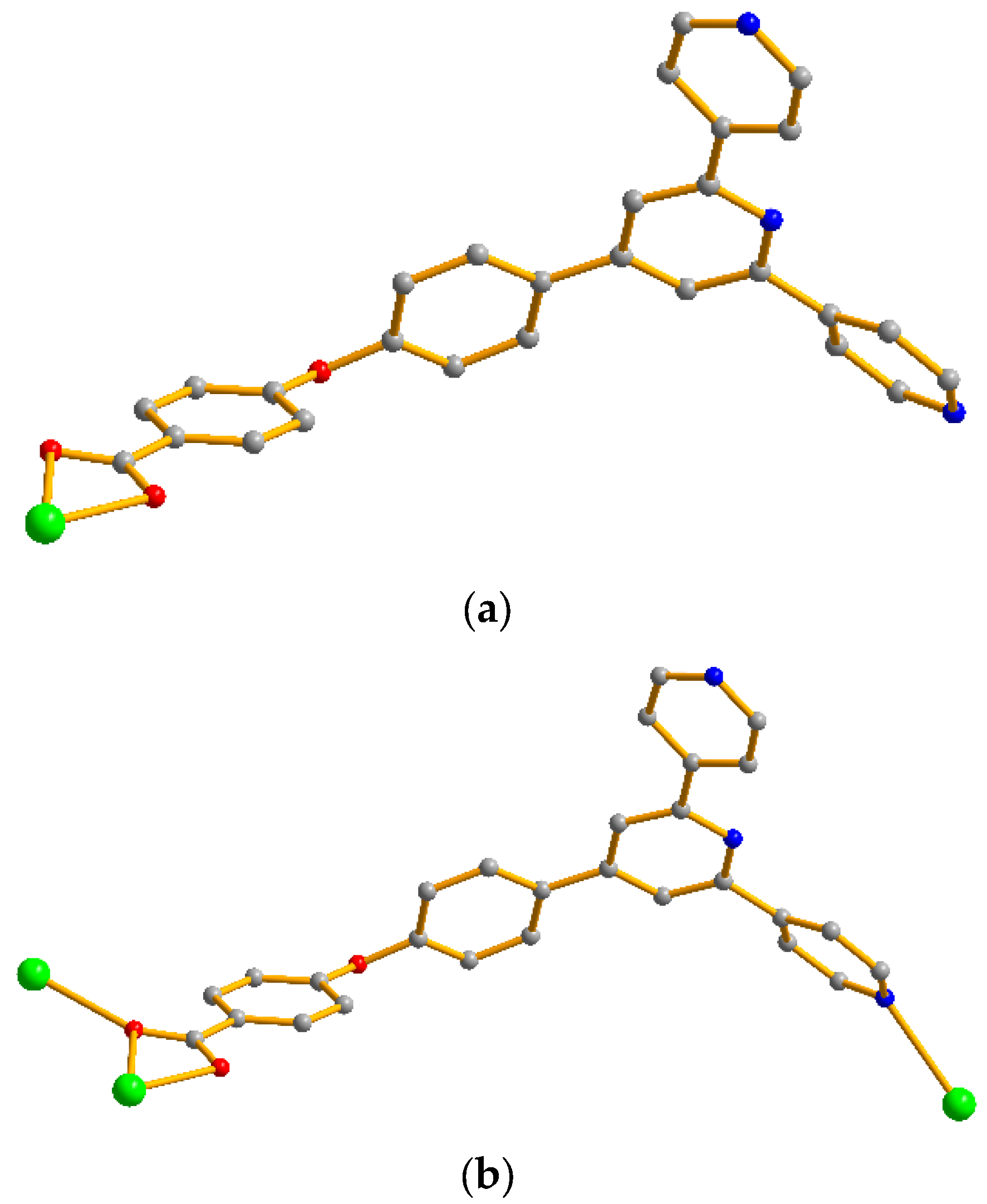
2.1. Structural Description of Complexes 1–7
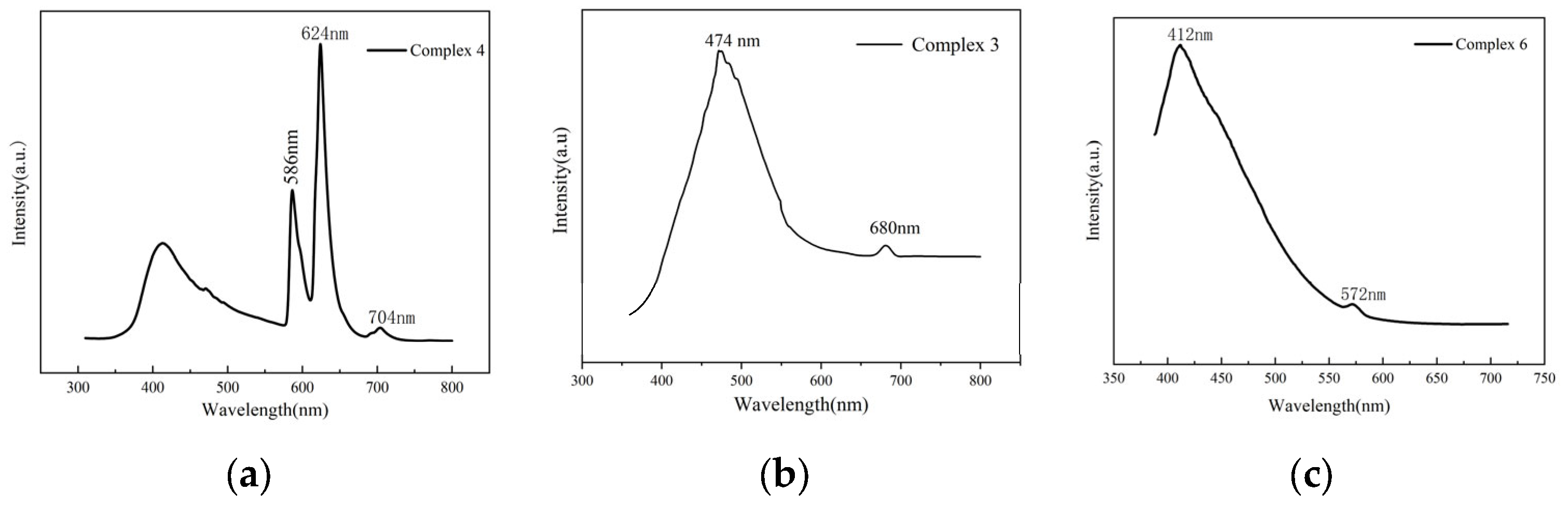
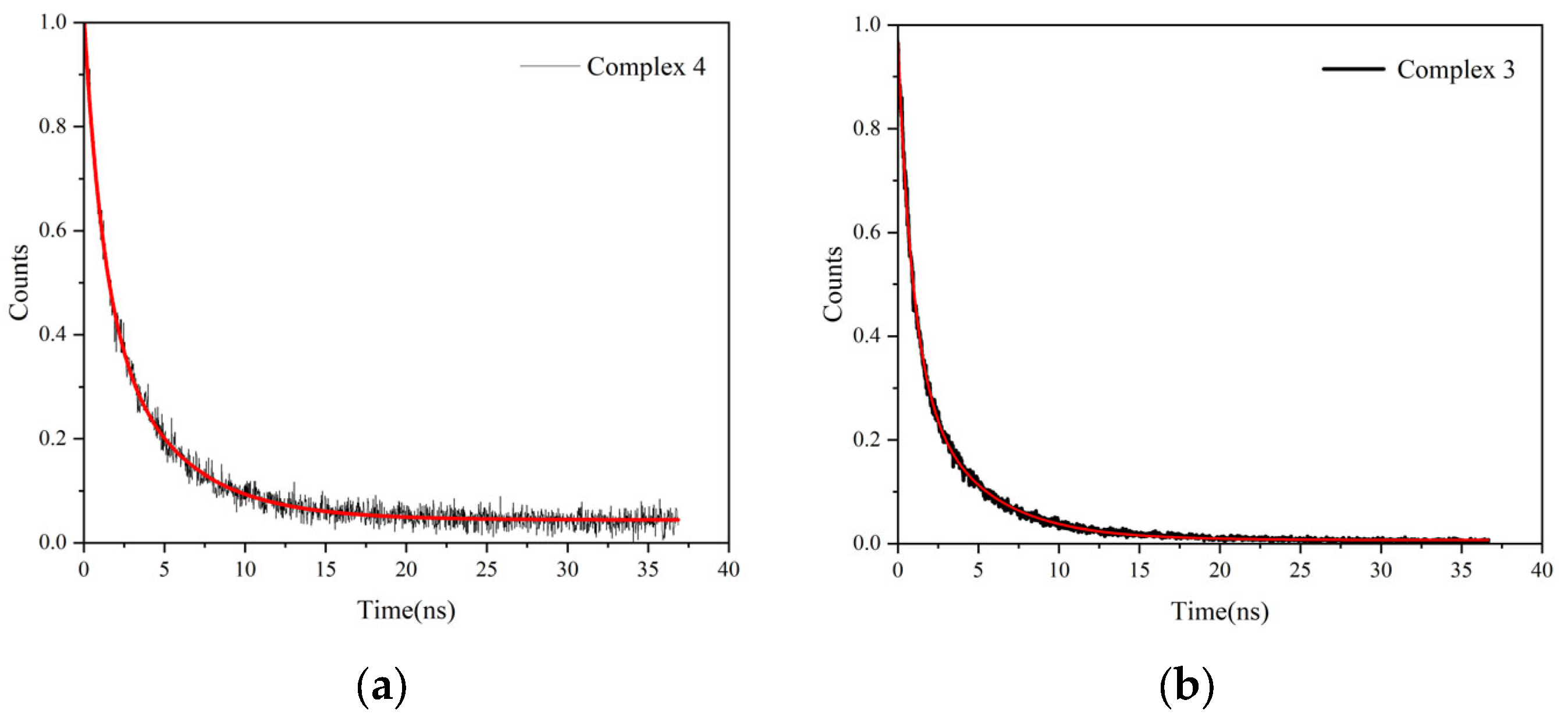
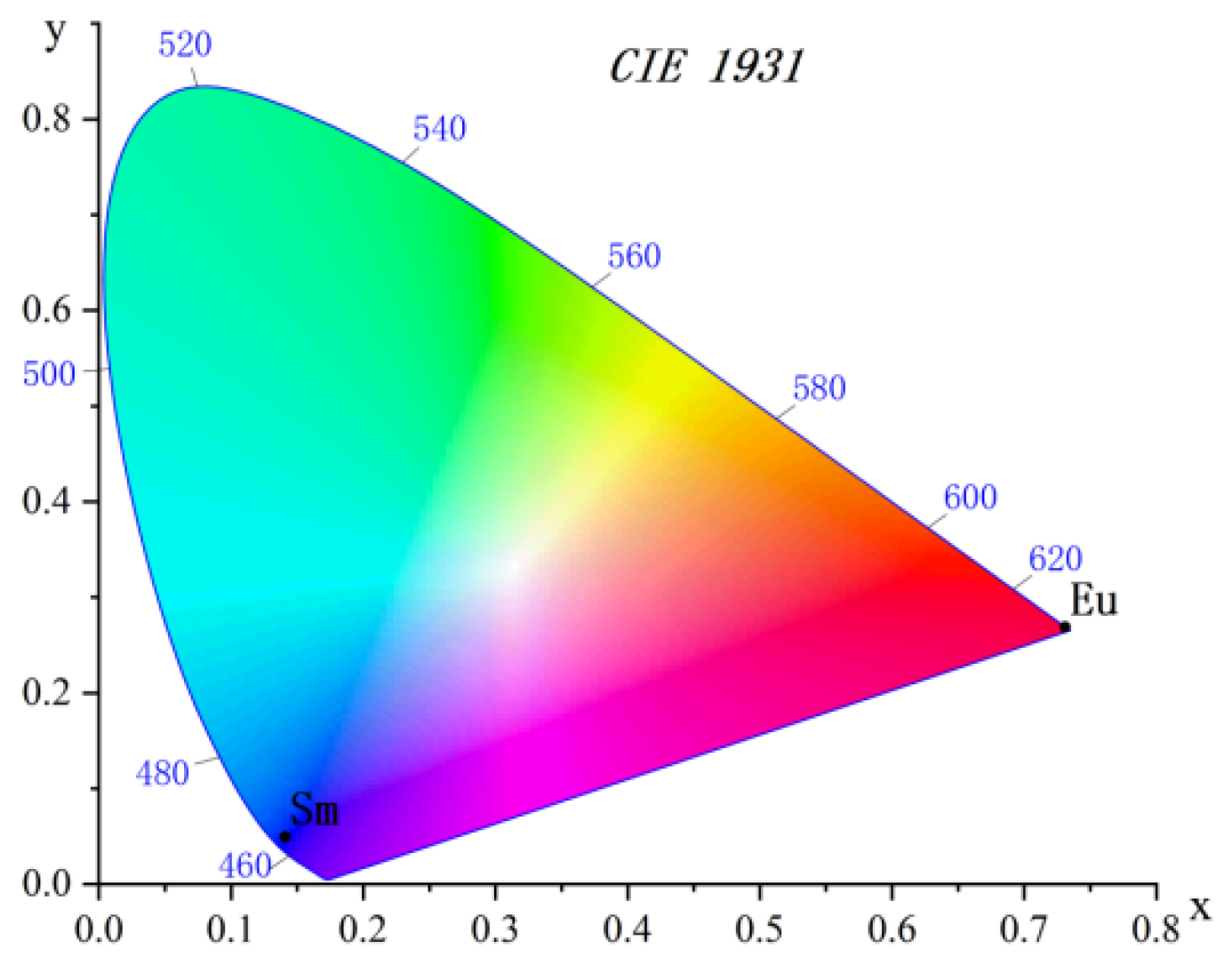
2.2. Luminescent Properties
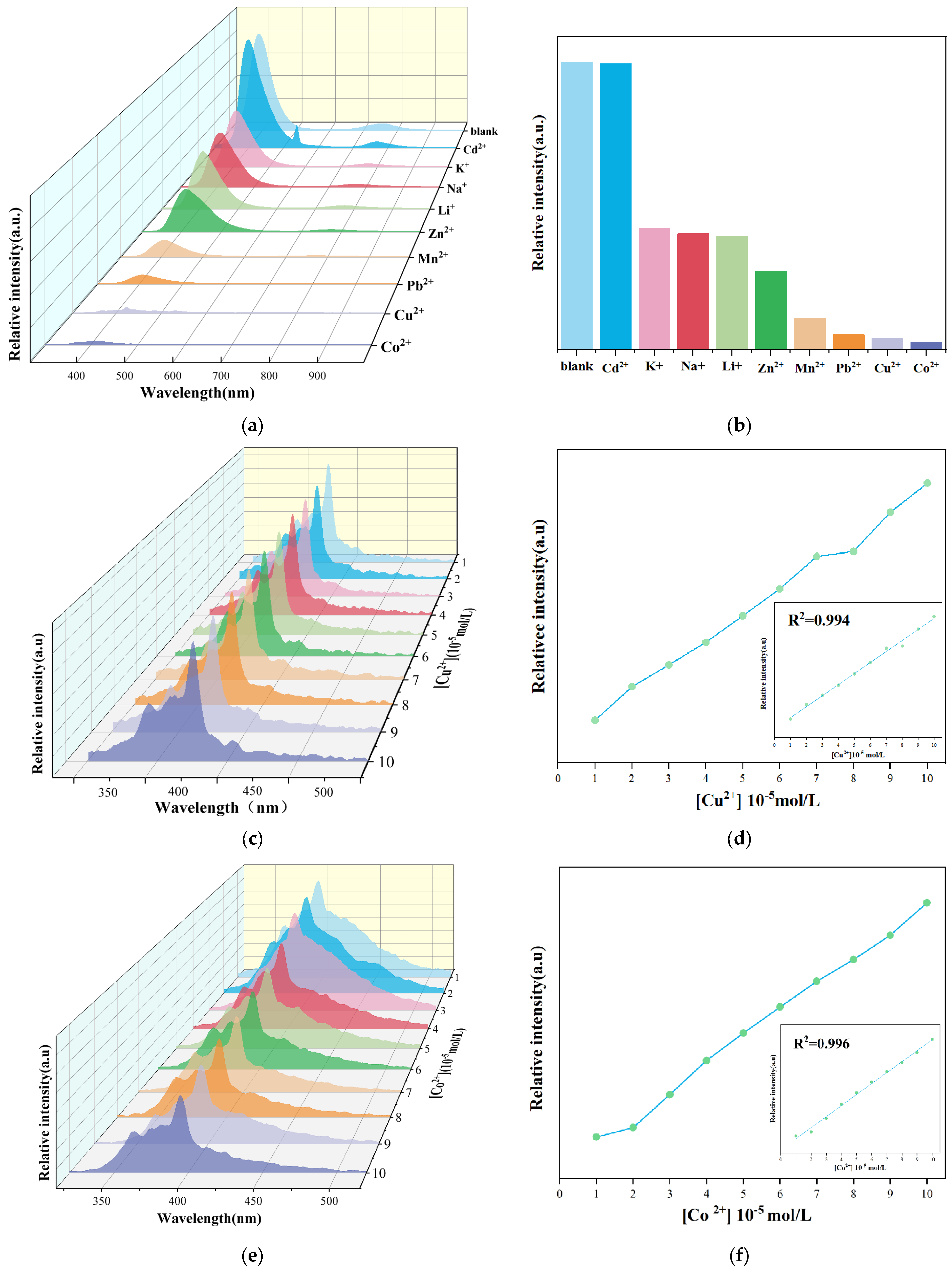
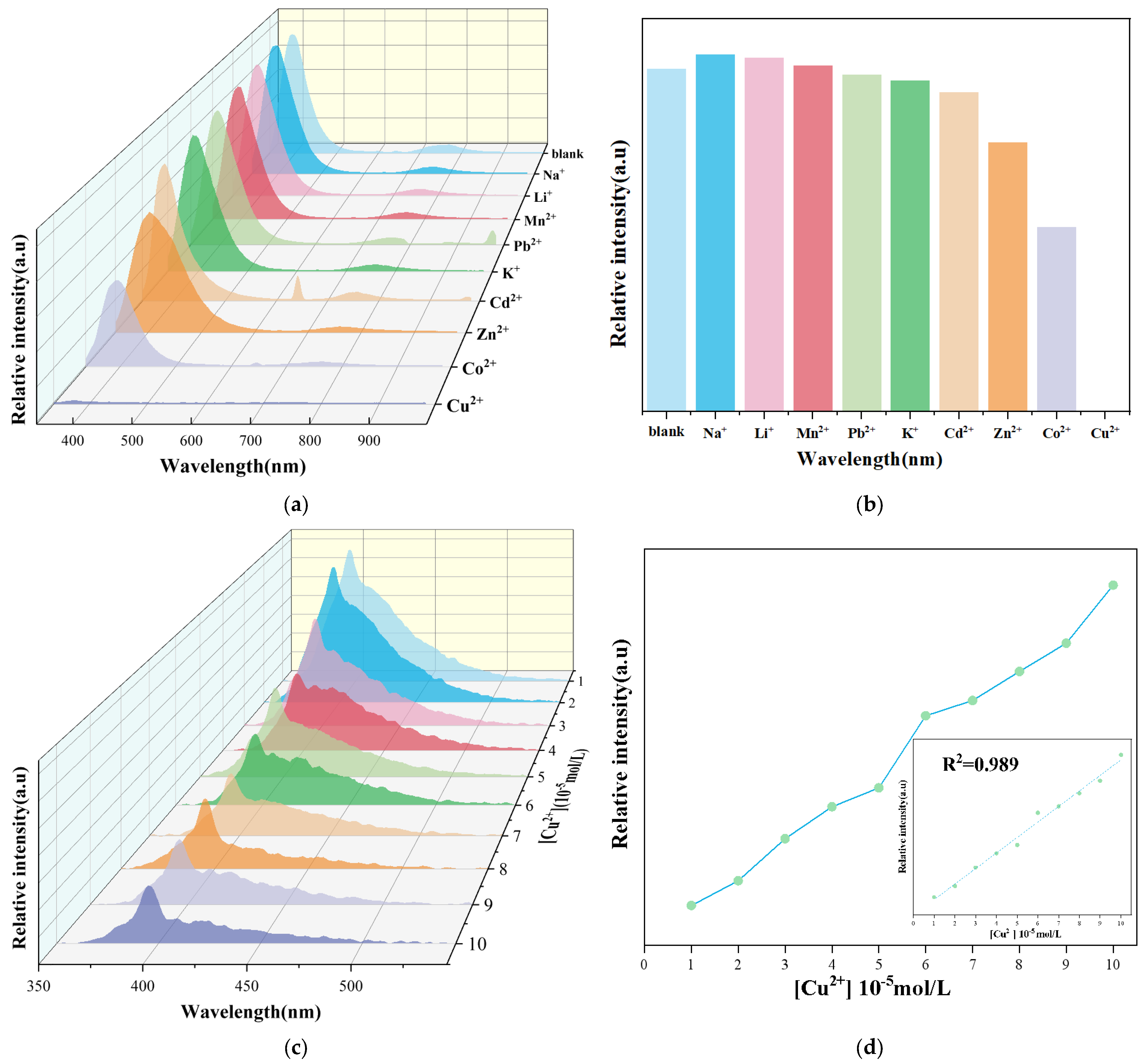
2.3. Detection of Metal Ions
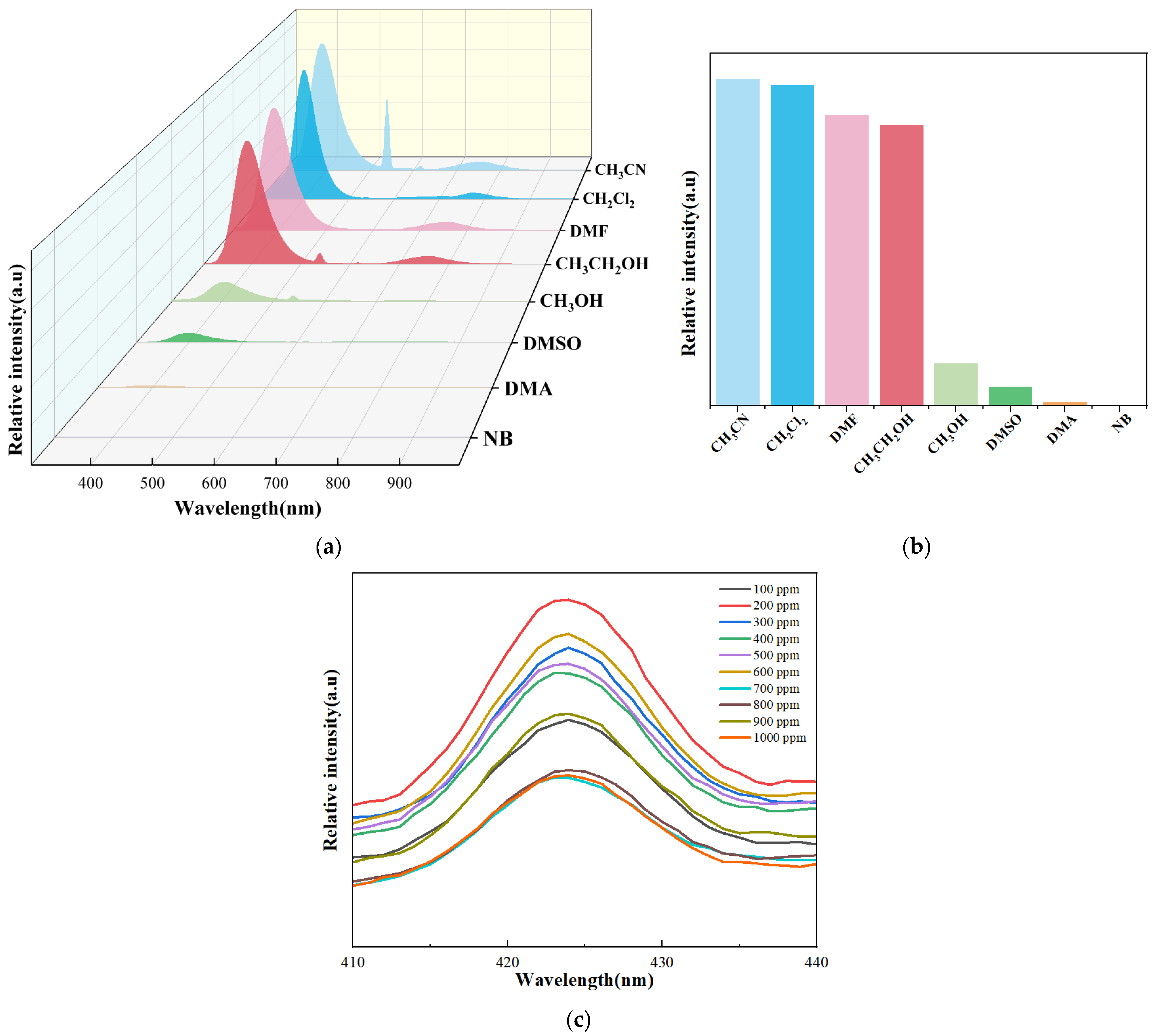
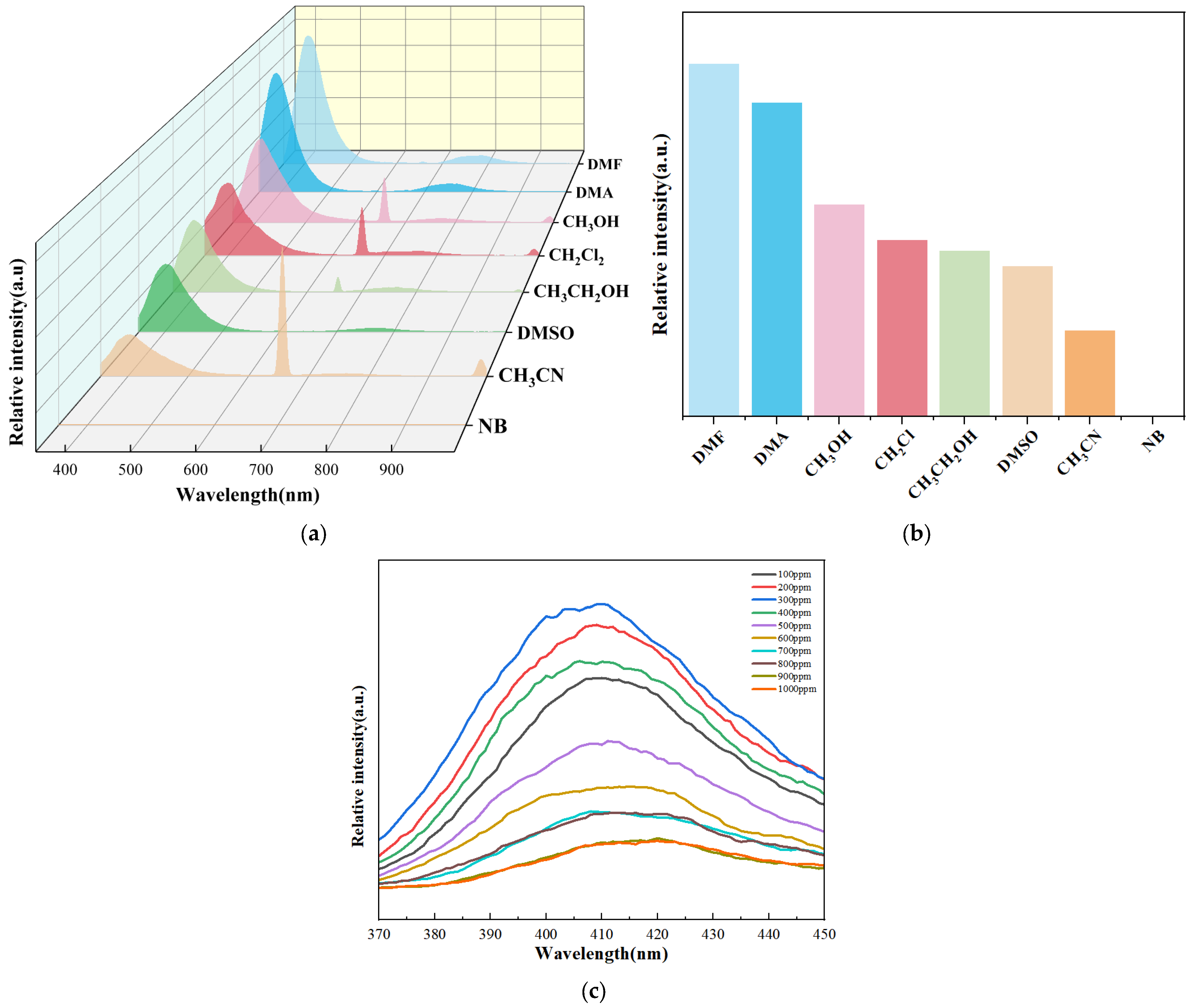
2.4. Sensing of Organic Small Molecules
2.5. PXRD and FT-IR
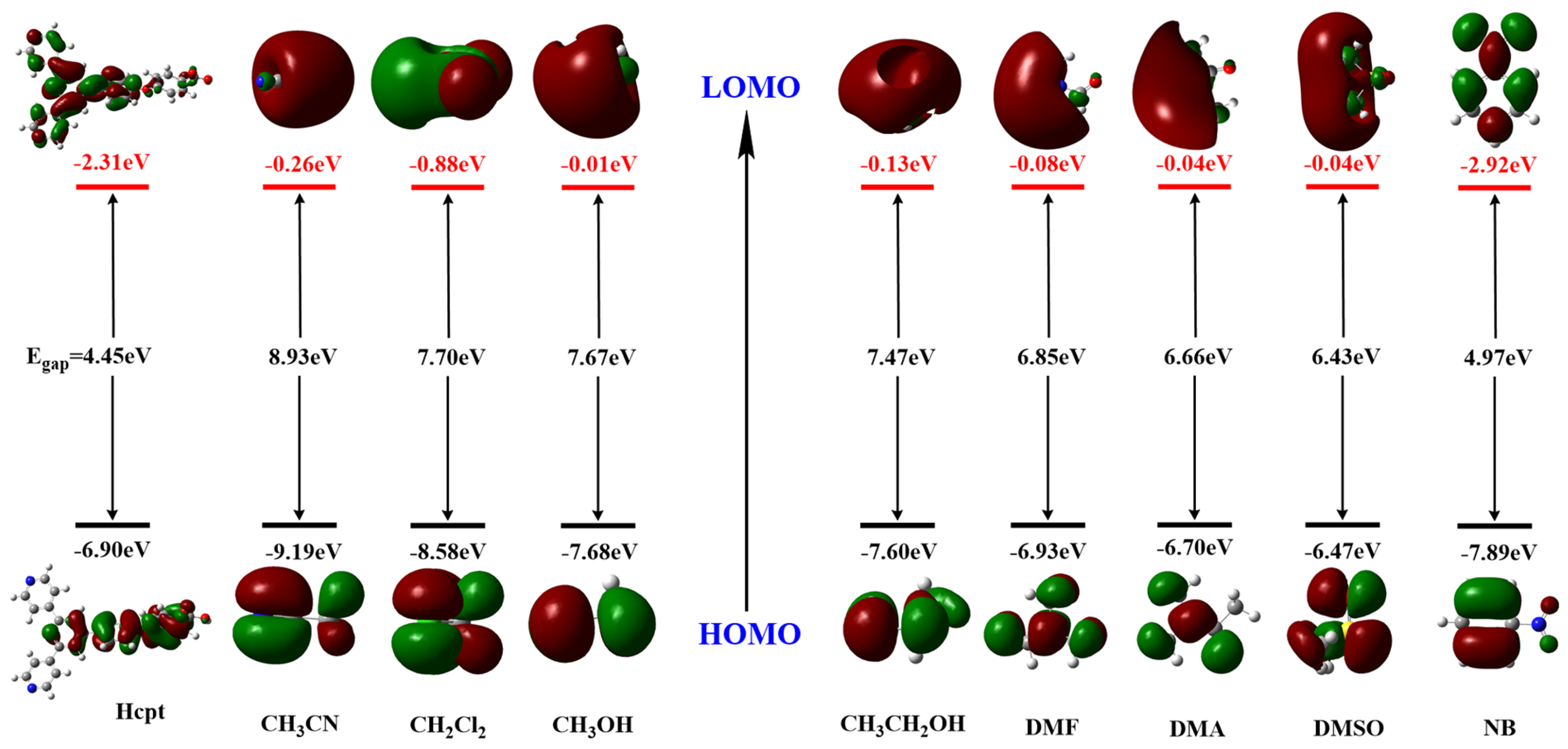
2.6. Theoretical Studies
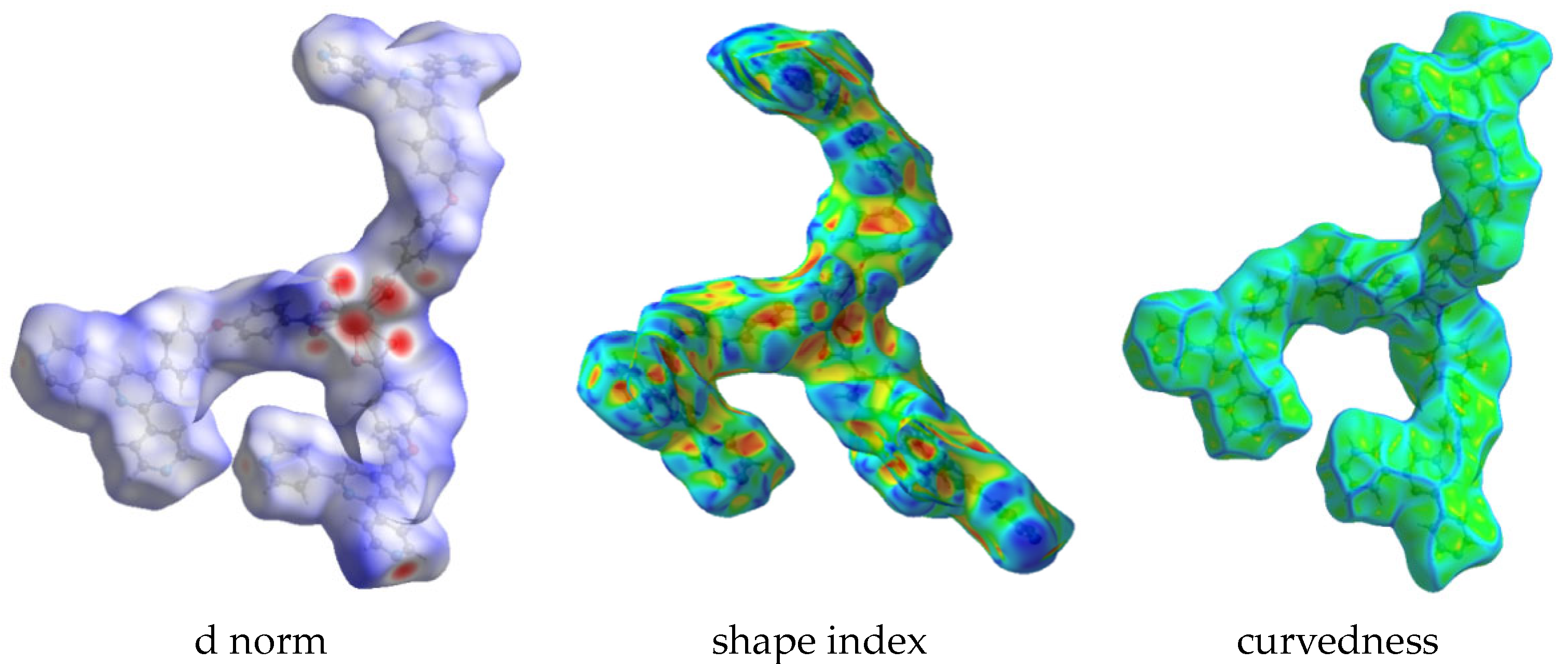
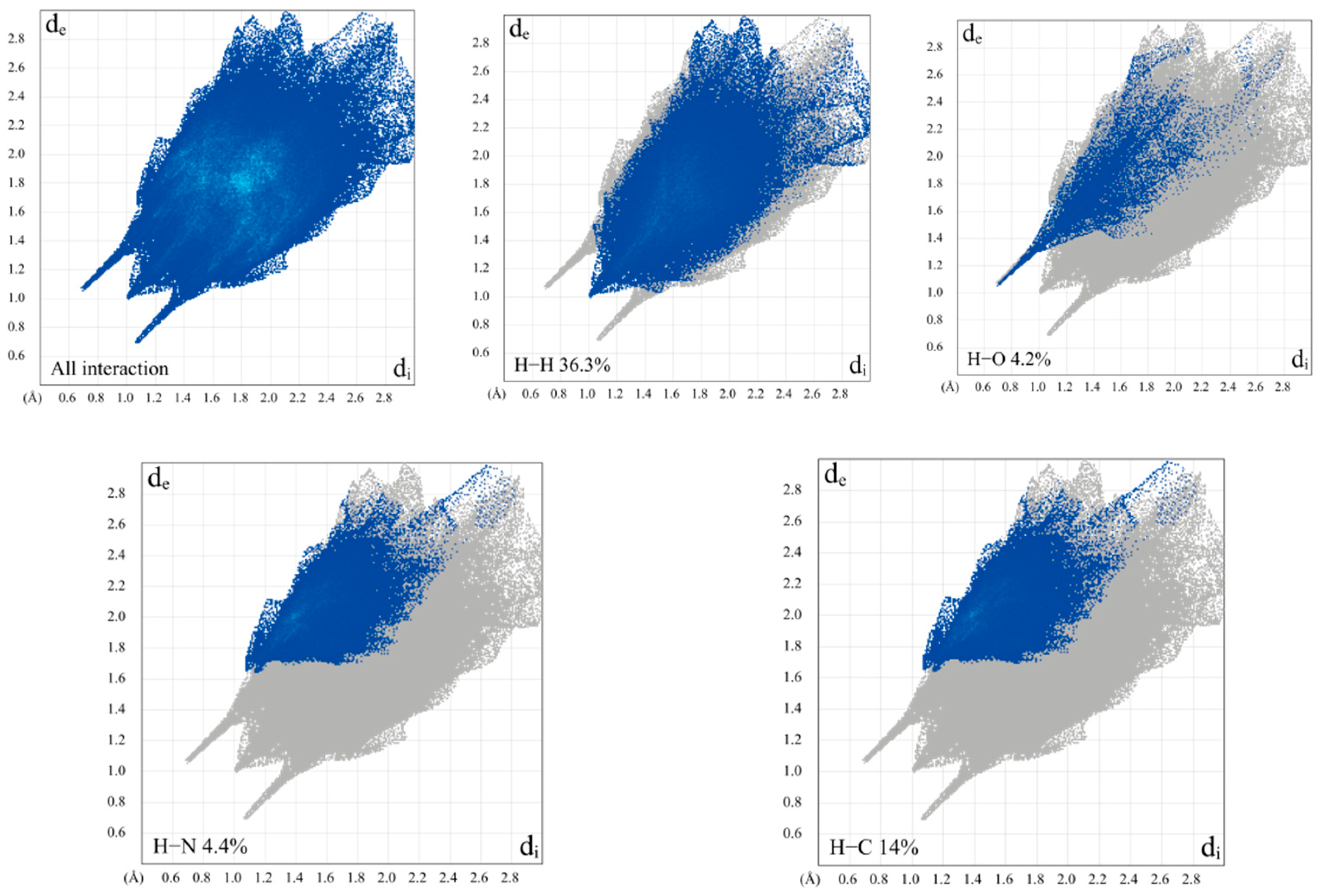
2.7. Hirshfeld Surface Analysis
3. Materials and Methods
3.1. Materials and General Method
3.2. Synthesis of Complexes
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Bu, X.; Wu, T.; Zheng, S.T.; Wang, L.; Feng, P. Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun. 2013, 4, 2344. [Google Scholar] [CrossRef]
- Boyd, S.A.; Sheng, G.; Teppen, B.J.; Johnston, C.T. Mechanisms for the Adsorption of Substituted Nitrobenzenes by Smectite Clays. Environ. Sci. Technol. 2001, 35, 4227–4234. [Google Scholar] [CrossRef]
- Li, Z.; Zhan, Z.; Hu, M. A luminescent terbium coordination polymer as a multifunctional water-stable sensor for detection of Pb2+ ions, PO43− ions, Cr2O72− ions, and some amino acids. CrystEngComm 2020, 22, 6727. [Google Scholar] [CrossRef]
- Gahlaut, P.S.; Gautam, D.; Yadav, K.; Barun, J. Supramolecular Gels for the Sensing and Extraction of Heavy Metal Ions from Wastewater. J. Mol. Struct. 2023, 1272, 134152. [Google Scholar] [CrossRef]
- Fayed, T.A.; El-Nahass, M.N.; El-Daly, H.A.; Shokry, A.A. Development of nanomaterial chemosensors for toxic metal ions sensing. Appl. Organomet. Chem. 2019, 33, 4868. [Google Scholar] [CrossRef]
- Mandal, S.; Das, A.; Biswas, A.; Halder, A.; Mondal, D.; Mondal, T.K. Adaptable Biomolecule-Interactive Dual Colorimetric Chemosensor for Cu2+ and Pd2+: Insight from Crystal Structure, Photophysical Investigations, Real-Time Sampling, and Molecular Logic Circuits. Cryst. Growth Des. 2024, 24, 1051–1067. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Goswami, R.; Smith, V.J.; Bajaj, H.C.; Neogi, S. Pore wall-functionalized luminescent Cd (II) framework for selective CO2 adsorption, highly specific 2, 4, 6-trinitrophenol detection, and colorimetric sensing of Cu2+ ions. ACS Sustain. Chem. Eng. 2018, 6, 10295–10306. [Google Scholar] [CrossRef]
- Huang, L.; Ran, Z.; Liu, X.; Huang, C.M.; Qin, Q.P.; Zhou, J. One Luminescent Cadmium Iodide with Free Bifunctional Azole Sites as a Triple Sensor for Cu2+, Fe3+, and Cr2O72− Ions. Inorg. Chem. 2022, 61, 14156. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Naha, S.; Velmathi, S. Colorimetric and fluorescent chemosensors for Cu 2+. A comprehensive review from the years. Anal. Methods-UK. 2017, 9, 552. [Google Scholar] [CrossRef]
- Hao, Z.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Su, S.; Zhang, H. One-dimensional channel-structured Eu-MOF for sensing small organic molecules and Cu2+ ion. J. Mater Chem. A. 2013, 1, 11043. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Cheng, H.; Chen, X.; Song, M.; Tang, M.; Wu, Y. Syntheses, structures of N-(substituted)-2-aza-[3]-ferrocenophanes and their application as redox sensor for Cu2+ ion. Appl. Organomet. Chem. 2012, 26, 449. [Google Scholar] [CrossRef]
- Mohandoss, S.; Stalin, T. A new fluorescent PET sensor probe for Co2+ ion detection: Computational, logic device and living cell imaging applications. RSC Adv. 2017, 7, 16581. [Google Scholar] [CrossRef]
- Khan, I.M.; Shakya, S. Exploring Colorimetric Real-Time Sensing Behavior of a Newly Designed CT Complex toward Nitrobenzene and Co2+: Spectrophotometric, DFT/TD-DFT, and Mechanistic Insights. ACS Omega 2019, 4, 9983. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Highly selective visible and near-IR sensing of Cu2+ based on thiourea-salicylaldehyde coordination in aqueous media. Chemistry 2011, 17, 1410. [Google Scholar] [CrossRef]
- Vellingiri, K.; Boukhvalov, D.W.; Pandey, S.K.; Deep, A.; Kim, K.H. Luminescent metal-organic frameworks for the detection of nitrobenzene in aqueous media. Sens. Actuators B Chem. 2017, 245, 305–313. [Google Scholar] [CrossRef]
- Li, X.Y.; Zeng, H.; Hu, H.M.; Sun, L.J.; Zhang, J.L.; Wang, X.F. Multiterpyridyl Ligand/Cadmium(II) Coordination Polymer Nanosheets for Recoverable Luminescent Sensors. ACS Appl. Nano Mater. 2022, 5, 7113. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, M.M.; Silva-Carrillo, C.; Hinostroza-Mojarro, J.J.; Rivera-Lugo, Y.Y.; Valle-Trujillo, P.; Trujillo-Navarrete, B. Electrochemical sensor for determination of nitrobenzene in aqueous solution based on nanostructures of TiO2/GO. Fuel 2021, 283, 119326. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Wang, S.; Yang, J.; Hu, S. A calixarene-based coordination cage as an efficient luminescent sensor for Fe3+, MnO4−, NB and 2, 4-DNP in aqueous medium. CrystEngComm 2023, 25, 1495. [Google Scholar] [CrossRef]
- Karmakar, A.; Samanta, P.; Dutta, S.; Ghosh, S.K. Fluorescent “turn-on” sensing based on metal–organic frameworks (MOFs). Chem.-Asian J. 2019, 14, 4506–4519. [Google Scholar] [CrossRef]
- Wu, J.X.; Yan, B. Photofunctional hybrid based lanthanide functionalized metal-organic frameworks by ion exchange and coordination modification for luminescent sensing. Inorg. Chem. Commun. 2016, 70, 189. [Google Scholar] [CrossRef]
- Li, Q.; Qian, J.; Zhou, J.; Du, L.; Zhao, Q. Highly chemically and thermally stable lanthanide coordination polymers for luminescent probes and white light emitting diodes. CrystEngComm 2020, 22, 2667. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Liu, D.; Liu, M.; Qian, J. Two-dimensional Cd(II) coordination polymer encapsulated by Tb3+ as a reversible luminescent probe for Fe3+. RSC Adv. 2019, 9, 34949. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, P.; Tu, X.; Min, H.; Yu, X.; Li, X.; Cheng, P. A bifunctional luminescent metal–organic framework for the sensing of paraquat and Fe3+ ions in water. Chem.-Asian J. 2019, 14, 3611. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Y.; Ma, Y.; Huang, L.; Zhao, Y.; Wang, L. Luminescent lanthanide complex sensor for Acac and Cd2+. Photochem. Photobiol. 2021, 97, 664. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Huang, P.P.; Zhang, Z.J.; Tian, F.W.; Ge, J.; Cao, X.Y.; Liu, J.; Wang, D.; Zheng, N.; Lu, J.F.; et al. A new 3D Cd-MOF with 2fold interpenetrated as “turn-on/turn-off” fluorescent sensor for selective and sensitive detection of Cu2+, Al3+ and Fe3+ ions. J. Mol. Struct. 2024, 1299, 137162. [Google Scholar] [CrossRef]
- Wang, L.; Tu, B.; Xu, W.; Fu, Y.; Zheng, Y. Uranyl Organic Framework as a Highly Selective and Sensitive Turn-on and Turn-off Luminescent Sensor for Dual Functional Detection Arginine and MnO. Inorg. Chem. 2020, 59, 5004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Q.W.; Wei, M.M.; Chen, J.Y.; Wang, H.H.; Li, X. Lanthanide ternary mixed-ligand coordination polymers as fluorescent sensors for the sensitive and selective detection of chlorogenic acid. CrystEngComm 2022, 24, 6367. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, B. Eu(III)-Functionalized MIL-124 as Fluorescent Probe for Highly Selectively Sensing Ions and Organic Small Molecules Especially for Fe(III) and Fe(II). ACS Appl. Mater. Interfaces 2015, 7, 721. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Z.; Zhang, R.; Li, Y.Z.; Guo, Z.; Zheng, H.G. Solvatochromic Behavior of a Nanotubular Metal-Organic Framework for Sensing Small Molecules. J. Am. Chem. Soc. 2011, 133, 4172. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Bierbaumer, F.; Fischer, R.C.; Vicente, R.; Tubau, À.; Ferran, A.; Massoud, S.S. Structural Characterization, Magnetic and Luminescent Properties of Praseodymium(III)-4,4,4-Trifluoro-1-(2-Naphthyl)Butane-1,3-Dionato(1-) Complexes. Crystals 2021, 11, 179. [Google Scholar] [CrossRef]
- Mautner, F.A.; Bierbaumer, F.; Vicente, R.; Speed, S.; Tubau, Á.; Font-Bardía, M.; Fischer, R.C.; Massoud, S.S. Magnetic and luminescence properties of 8-coordinate holmium (iii) complexes containing 4, 4, 4-trifluoro-1-phenyl-and 1-(naphthalen-2-yl)-1, 3-butanedionates. Molecules 2022, 27, 1129. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Kong, Y.J.; Zhang, X.M.; Hou, G.Z.; Chen, H.C.; Zheng, H.G. Fabrication of a robust lanthanide metal–organic framework as a multifunctional material for Fe (III) detection, CO2 capture, and utilization. J. Mater. Chem. C 2021, 9, 6051. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, M.Y.; Zhang, X.N.; Li, G.M.; Zhang, X.D.; Han, Z.B. Two series of solvent-dependent lanthanide coordination polymers demonstrating tunable luminescence and catalysis properties. Cryst. Growth Des. 2014, 14, 3002–3009. [Google Scholar] [CrossRef]
- Tarlton, M.L.; Skanthakumar, S.; Hutchison, D.; Gremillion, A.J.; Oliver, A.G.; Wilson, R.E. Synthesis of an isostructural series of 12-coordinate lanthanide nitrate hybrid double perovskites with cubic symmetry. Inorg. Chem. 2022, 61, 17101–17108. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.T.; Zhou, Y.F.; Huang, D.C.; Wei, X.; Su, M.Y.; Wang, K.; Han, S.; Hong, M.C. Crystal structure, multiplex photoluminescence, and magnetic properties of a series of lanthanide coordination polymers based on quinoline carboxylate ligand. Cryst. Growth Des. 2013, 13, 5420–5432. [Google Scholar] [CrossRef]
- Jing, T.; Chen, L.; Jiang, F.; Yang, Y.; Zhou, K.; Yu, M.; Cao, Z.; Li, S.; Hong, M. Fabrication of a Robust Lanthanide Metal–Organic Framework as a Multifunctional Material for Fe(III) Detection, CO2 Capture, and Utilization. Cryst. Growth Des. 2018, 18, 2956. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, F.; Xu, C.; Feng, Y.; Zhang, G.; Liu, W.A. A multifunctional Eu-CP as a recyclable luminescent probe for the highly sensitive detection of Fe3+/Fe2+, Cr2O72−, and nitroaromatic explosives. Dalton Trans. 2018, 47, 7480. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Singh-Wilmot, M.A.; Carter, K.P.; Cahill, C.L.; Ridenour, J.A. Lanthanide-2, 3, 5, 6-tetrabromoterephthalic acid metal–organic frameworks: Evolution of halogen···halogen interactions across the lanthanide series and their potential as selective bifunctional sensors for the detection of Fe3+, Cu2+, and nitroaromatics. Cryst. Growth Des. 2018, 19, 305. [Google Scholar] [CrossRef]
- Zhong, X.F.; Ma, Z.C.; Lin, J.J.; Wu, Y.; Liang, G.; Zhang, Y.Y.; Chen, D.J.; Xie, K.P.; Mo, Z.W.; Chen, X.M. Metal–Organic Frameworks with Triazine and Amine Functional Groups for Iodine Removal and Sensitive Detection of Cu2+ and Fe3+ Ions. Cryst. Growth Des. 2023, 23, 8793. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; VanDuyne, R.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105. [Google Scholar] [CrossRef]
- Liu, B. Metal–organic framework-based devices: Separation and sensors. J. Mater. Chem. 2012, 22, 10094. [Google Scholar] [CrossRef]
- Banerjee, D.; Hu, Z.; Li, J. Luminescent metal–organic frameworks as explosive sensors. Dalton Trans. 2014, 43, 10668. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Chem. Rev. 2014, 273, 76. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. Engineering metal–organic frameworks for aqueous phase 2, 4, 6-trinitrophenol (TNP) sensing. CrystEngComm 2016, 18, 2994. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly selective detection of nitro explosives by a luminescent metal-organic framework. Chem. Int. Ed. 2013, 52, 2881. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A fluorescent metal–organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915. [Google Scholar] [CrossRef]
- Joarder, B.; Desai, A.V.; Samanta, P.; Mukherjee, S.; Ghosh, S.K. Selective and sensitive aqueous-phase detection of 2, 4, 6-trinitrophenol (TNP) by an amine-functionalized metal–organic framework. Chem. Eur. J. 2015, 21, 965. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Inamdar, A.I.; Manna, B.; Ghosh, S.K. Selective detection of 2, 4, 6-trinitrophenol (TNP) by a π-stacked organic crystalline solid in water. Cryst. Growth Design 2015, 15, 3493. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Manna, B.; Inamdar, A.I.; Ghosh, S.K. Exploitation of guest accessible aliphatic amine functionality of a metal–organic framework for selective detection of 2, 4, 6-trinitrophenol (TNP) in water. Cryst. Growth Des. 2015, 15, 4627. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Samanta, P.; Ghosh, S.K. Aqueous phase selective detection of 2, 4, 6-trinitrophenol using a fluorescent metal–organic framework with a pendant recognition site. Dalton Trans. 2015, 44, 15175. [Google Scholar] [CrossRef]
- Karmakar, A.; Kumar, A.; Chaudhari, A.K.; Samanta, P.; Desai, A.V.; Krishna, R.; Ghosh, S.K. Bimodal Functionality in a Porous Covalent Triazine Framework by Rational Integration of an Electron-Rich and-Deficient Pore Surface. Chem.-Eur. J. 2016, 22, 4931. [Google Scholar] [CrossRef]
- Surya, S.G.; Nagarkar, S.S.; Ghosh, S.K.; Sonar, P.; Rao, V.R. OFET based explosive sensors using diketopyrrolopyrrole and metal organic framework composite active channel material. Sens. Actuators. B. 2016, 223, 114. [Google Scholar] [CrossRef]
- Liu, X.G.; Wang, H.; Chen, B.; Zou, Y.; Gu, Z.G.; Zhao, Z.; Shen, L. A luminescent metal–organic framework constructed using a tetraphenylethene-based ligand for sensing volatile organic compounds. Chem. Commun. 2015, 51, 1677. [Google Scholar] [CrossRef]
- Wang, G.Y.; Song, C.; Kong, D.M.; Ruan, W.J.; Chang, Z.; Li, Y. Two luminescent metal–organic frameworks for the sensing of nitroaromatic explosives and DNA strands. J. Mater. Chem. A 2014, 2, 2213. [Google Scholar] [CrossRef]
- Tian, H.R.; Gao, C.Y.; Yang, Y.; Ai, J.; Liu, C.; Xua, Z.G.; Sun, Z.M. A microporous Cd-MOF based on a hexavalent silicon-centred connector and luminescence sensing of small molecules. New J. Chem. 2017, 41, 1137. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.G.; Dang, S.; Sun, Z.M. Self-Assembly of Tunable Heterometallic Ln–Ru Coordination Polymers with Near-Infrared Luminescence and Magnetocaloric Effect. Chem.-Eur. J. 2017, 23, 2852. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, A.; Yousufuddin, M.; Sarma, M.; Biswas, S. 3D luminescent amide-functionalized cadmium tetrazolate framework for selective detection of 2, 4, 6-trinitrophenol. Cryst. Growth Des. 2016, 16, 842. [Google Scholar] [CrossRef]
- Huang, R.W.; Wei, Y.S.; Dong, X.Y.; Wu, X.H.; Du, C.X.; Zang, S.Q.; Mak, T.C.W. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal–organic framework. Nat. Chem. 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, L.; Li, X.; Qin, C.; Zhao, L.; Shao, K.Z.; Su, Z.M. Metal–organic frameworks constructed from tib and carboxylate acid ligands: Selective sensing of nitro explosives and magnetic properties. Dalton Trans. 2017, 46, 7567. [Google Scholar] [CrossRef] [PubMed]
- Gole, B.; Bar, A.K.; Mukherjee, P.S. Modification of extended open frameworks with fluorescent tags for sensing explosives: Competition between size selectivity and electron deficiency. Chem.-Eur. J. 2014, 20, 2276–2291. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Wang, J.X.; Huang, P.P.; Liu, J.; Zheng, N.; Shi, J.; Xu, H.T.; Yue, S.Y.; Lu, J.F. A new pyrazine carboxyl derivative and its two d10 metal coordination polymers: Syntheses, characterization, DFT and property. J. Mol. Struct. 2023, 1290, 135935. [Google Scholar] [CrossRef]
- Elantabli, F.M.; Mohamed, R.G.; El-Medani, S.M.; Haukka, M.; Ramadan, R.M.; Afifi, M.A. Structural investigations of new tridentate-phenylacetohydrazide Schiff base metal chelates: X-ray diffraction, Hirshfeld surface analyses, DFT, antibacterial and molecular docking studies. J. Mol Struct. 2024, 1299, 137230. [Google Scholar] [CrossRef]
- Najafi, Z.; Marandi, F.; Bahrami, A.; Fuhrmann, D.; Janghouri, M. Four new Zn (II) complexes based on 2-thienoyltrifluoroacetone and N-donor auxiliary bridging and chelating ligands: Synthesis, spectroscopic and structural studies, thermal behavior and Hirshfeld surface analysis. Polyhedron 2023, 242, 116486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Song, J.; Zhou, Y.-L.; Chen, H.-H.; Duan, B.-F.; Jin, L.-X.; Ren, C.-Q.; Lu, J.-F. A Series of Lanthanide Coordination Polymers as Luminescent Sensors for Selective Detection of Inorganic Ions and Nitrobenzene. Molecules 2024, 29, 3438. https://doi.org/10.3390/molecules29143438
Wu M, Song J, Zhou Y-L, Chen H-H, Duan B-F, Jin L-X, Ren C-Q, Lu J-F. A Series of Lanthanide Coordination Polymers as Luminescent Sensors for Selective Detection of Inorganic Ions and Nitrobenzene. Molecules. 2024; 29(14):3438. https://doi.org/10.3390/molecules29143438
Chicago/Turabian StyleWu, Miao, Juan Song, Yun-Long Zhou, Hui-Hui Chen, Bo-Feng Duan, Ling-Xia Jin, Chuan-Qing Ren, and Jiu-Fu Lu. 2024. "A Series of Lanthanide Coordination Polymers as Luminescent Sensors for Selective Detection of Inorganic Ions and Nitrobenzene" Molecules 29, no. 14: 3438. https://doi.org/10.3390/molecules29143438
APA StyleWu, M., Song, J., Zhou, Y.-L., Chen, H.-H., Duan, B.-F., Jin, L.-X., Ren, C.-Q., & Lu, J.-F. (2024). A Series of Lanthanide Coordination Polymers as Luminescent Sensors for Selective Detection of Inorganic Ions and Nitrobenzene. Molecules, 29(14), 3438. https://doi.org/10.3390/molecules29143438






