Bioactive Naphthoquinone and Phenazine Analogs from the Endophytic Streptomyces sp. PH9030 as α-Glucosidase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
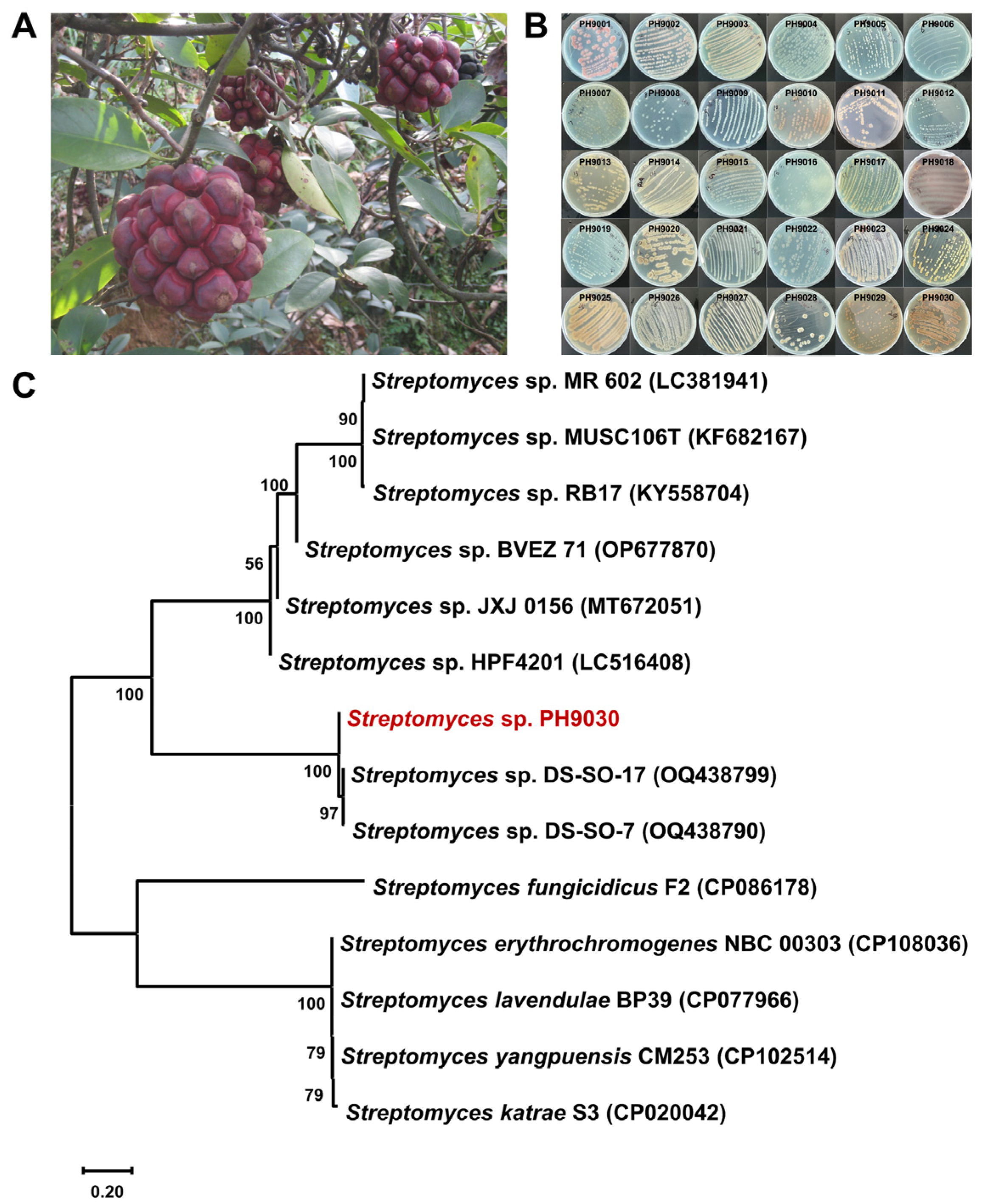
2.1. Actinomycete Isolation, Small-Scale Fermentation and Antibacterial Activity Assay
2.2. Identification and Phylogenetic Analysis of Strain PH9030
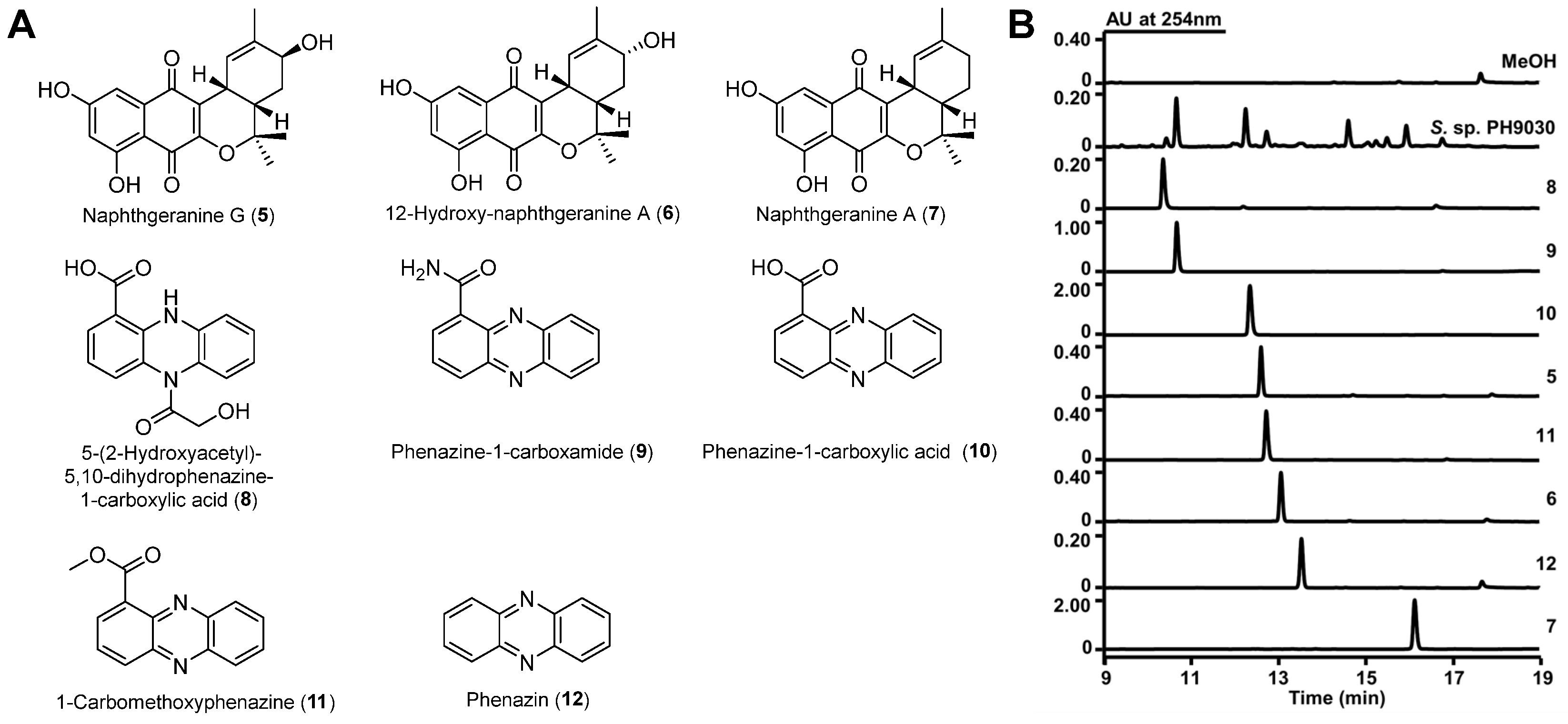
2.3. Structure Elucidation
2.4. In Vitro α-Glucosidase Inhibitory Activity
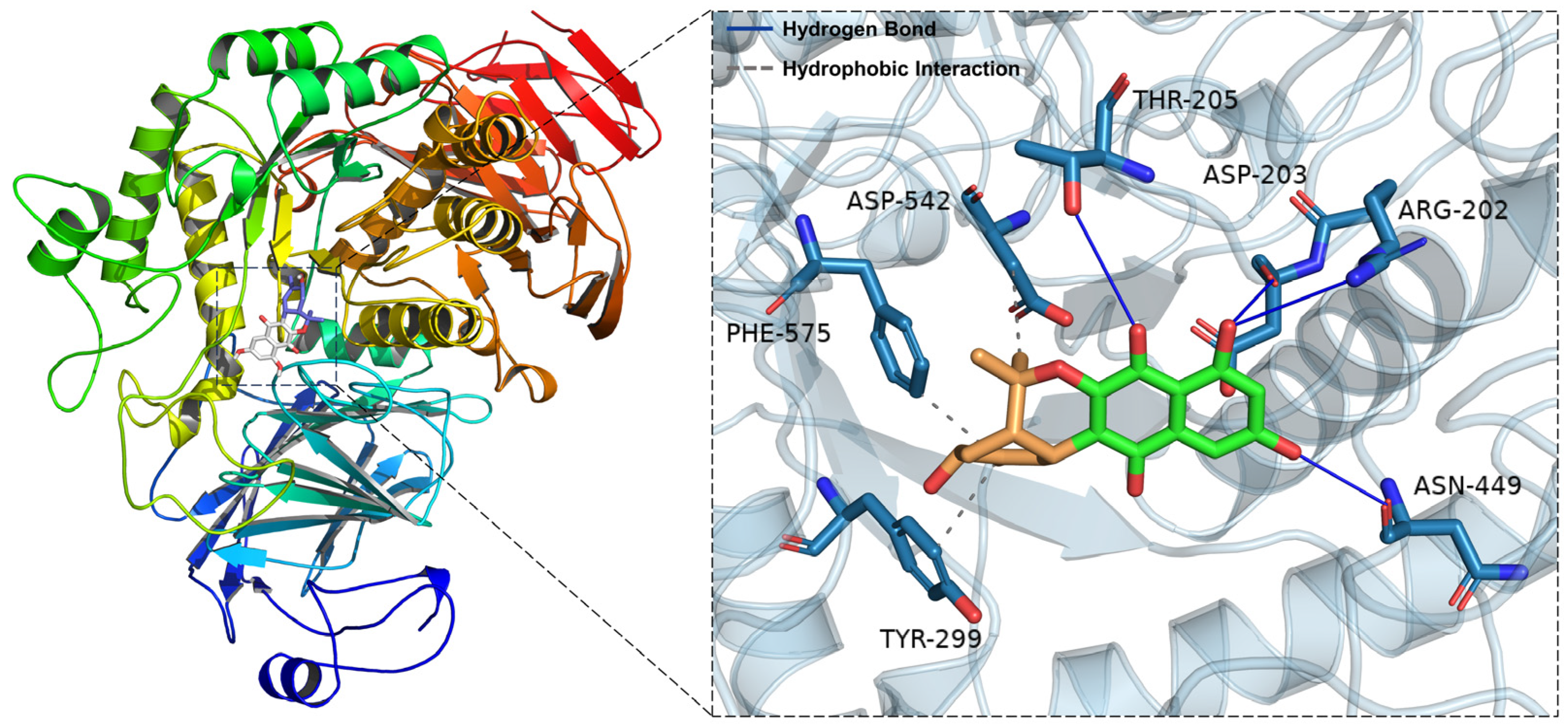
2.5. Molecular Docking Simulations of 5 with α-Glucosidase
2.6. Molecular Dynamics Simulations
2.7. Antibacterial Activities of 5–12
3. Materials and Methods
3.1. Sample Collection
3.2. Isolation of Endophytes
3.3. Genomic DNA Extraction, 16S rRNA Gene Sequencing and Phylogenetic Tree Construction
3.4. General Methods
3.5. Large-Scale Fermentation and Extraction
3.6. Isolation of Compounds 5–12
Naphthgeranine G (5)
3.7. ECD Calculation Methods
3.8. α-Glucosidase Inhibition Assay
3.9. Molecular Docking Analysis
3.10. Molecular Dynamic Simulations
3.11. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic Kidney Disease: World Wide Difference of Prevalence and Risk Factors. J. Nephropharmacol. 2016, 5, 49–56. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef]
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the Biggest Producer of New Natural Secondary Metabolites, a Current Perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Schmidt, D.D.; Frommer, W.; Junge, B.; Müller, L.; Wingender, W.; Truscheit, E.; Schäfer, D. α-Glucosidase Inhibitors. New Complex Oligosaccharides of Microbial Origin. Naturwissenschaften 1977, 64, 535–536. [Google Scholar] [CrossRef]
- Rockser, Y.; Wehmeier, U.F. The Gac-Gene Cluster for the Production of Acarbose from Streptomyces Glaucescens GLA.O–Identification, Isolation and Characterization. J. Biotechnol. 2009, 140, 114–123. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, L.L.; Wang, Y.S.; Xue, Y.P.; Zheng, Y.G.; Shen, Y.C. Actinoplanes utahensis ZJB-08196 Fed-Batch Fermentation at Elevated Osmolality for Enhancing Acarbose Production. Bioresour. Technol. 2012, 103, 337–342. [Google Scholar] [CrossRef]
- Kameda, Y.; Asano, N.; Yoshikawa, M.; Takeuchi, M.; Yamaguchi, T.; Matsui, K.; Horii, S.; Fukase, H. Valiolamine, a New α-Glucosidase Inhibiting Aminocyclitol Produced by Streptomyces hygroscopicus. J. Antibiot. 1984, 37, 1301–1307. [Google Scholar] [CrossRef]
- Ghani, U. Re-Exploring Promising α-Glucosidase Inhibitors for Potential Development into Oral Anti-Diabetic Drugs: Finding Needle in the Haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- Mackenzie, T.A.; Reyes, F.; Martínez, M.; González-Menéndez, V.; Sánchez, I.; Genilloud, O.; Tormo, J.R.; Ramos, M.C. Naphthoquinone Derivatives from Angustimassarina populi CF-097565 Display Anti-Tumour Activity in 3D Cultures of Breast Cancer Cells. Molecules 2024, 29, 425. [Google Scholar] [CrossRef]
- Efeoglu, C.; Selcuk, O.; Demir, B.; Sahin, E.; Sari, H.; Türkeş, C.; Demir, Y.; Nural, Y.; Beydemir, Ş. New Naphthoquinone Thiazole Hybrids as Carbonic Anhydrase and Cholinesterase Inhibitors: Synthesis, Crystal Structure, Molecular Docking, and Acid Dissociation Constant. J. Mol. Struct. 2024, 1301, 137365. [Google Scholar] [CrossRef]
- Matsuda, Y.; Abe, I. Biosynthesis of Fungal Meroterpenoids. Nat. Prod. Rep. 2015, 33, 26–53. [Google Scholar] [CrossRef]
- Kashi, M.E.; Ghorbani, M.; Badibostan, H.; Seidel, V.; Hosseini, S.H.; Asili, J.; Shakeri, A.; Sahebkar, A. Antimicrobial and Cytotoxic Naphthoquinones from Microbial Origin: An Updated Review. Mini Rev. Med. Chem. 2024, 24, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.A.M.; McKinnie, S.M.K.; Moore, B.S.; George, J.H. Meroterpenoid Natural Products from Streptomyces Bacteria–the Evolution of Chemoenzymatic Syntheses. Nat. Prod. Rep. 2020, 37, 1334–1366. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, K.; Funayama, S.; Anraku, Y.; Ishibashi, M.; Takahashi, Y.; Omura, S. Novel Antibiotics, Furaquinocins A and B. Taxonomy, Fermentation, Isolation and Physico-Chemical and Biological Characteristics. J. Antibiot. 1990, 43, 247–252. [Google Scholar] [CrossRef]
- Pathirana, C.; Jensen, P.R.; Fenical, W. Marinone and Debromomarinone: Antibiotic Sesquiterpenoid Naphthoquinones of a New Structure Class from a Marine Bacterium. Tetrahedron Lett. 1992, 33, 7663–7666. [Google Scholar] [CrossRef]
- Guttenberger, N.; Blankenfeldt, W.; Breinbauer, R. Recent Developments in the Isolation, Biological Function, Biosynthesis, and Synthesis of Phenazine Natural Products. Bioorg. Med. Chem. 2017, 25, 6149–6166. [Google Scholar] [CrossRef]
- Blankenfeldt, W.; Parsons, J.F. The Structural Biology of Phenazine Biosynthesis. Curr. Opin. Struct. Biol. 2014, 29, 26–33. [Google Scholar] [CrossRef]
- Kankanamge, S.; Khalil, Z.G.; Capon, R.J. Tepuazines A–E: Phenazine Glycosides from a Venezuelan Quartz-Rich (Tepui) Cave Soil-Derived Streptomyces virginiae CMB-CA091. J. Nat. Prod. 2024, 87, 1084–1091. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine Natural Products: Biosynthesis, Synthetic Analogues, and Biological Activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- Noakes, F.F.; Smitten, K.L.; Maple, L.E.C.; Bernardino de la Serna, J.; Robertson, C.C.; Pritchard, D.; Fairbanks, S.D.; Weinstein, J.A.; Smythe, C.G.W.; Thomas, J.A. Phenazine Cations as Anticancer Theranostics. J. Am. Chem. Soc. 2024, 146, 12836–12849. [Google Scholar] [CrossRef]
- Liu, K.; Li, Z.; Liang, X.; Xu, Y.; Cao, Y.; Wang, R.; Li, P.; Li, L. Biosynthesis and Genetic Engineering of Phenazine-1-Carboxylic Acid in Pseudomonas chlororaphis Lzh-T5. Front. Microbiol. 2023, 14, 1186052. [Google Scholar] [CrossRef]
- Mahdally, N.H.; ElShiekh, R.A.; Thissera, B.; Eltaher, A.; Osama, A.; Mokhtar, M.; Elhosseiny, N.M.; Kashef, M.T.; Magdeldin, S.; El Halawany, A.M.; et al. Dihydrophenazine: A Multifunctional New Weapon That Kills Multidrug-Resistant Acinetobacter Baumannii and Restores Carbapenem and Oxidative Stress Susceptibilities. J. Appl. Microbiol. 2024, 135, lxae100. [Google Scholar] [CrossRef]
- Xun, W.; Gong, B.; Liu, X.; Yang, X.; Zhou, X.; Jin, L. Antifungal Mechanism of Phenazine-1-Carboxylic Acid against Pestalotiopsis kenyana. Int. J. Mol. Sci. 2023, 24, 11274. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.Y.; Cong, M.J.; Pang, X.Y.; Chen, B.; Liu, Y.H.; Liao, L.; Wang, J.F. Cytotoxic Phenazine and Antiallergic Phenoxazine Alkaloids from an Arctic Nocardiopsis dassonvillei SCSIO 502F. Nat. Prod. Bioprospect. 2023, 13, 41. [Google Scholar] [CrossRef]
- Borrero, N.V.; Bai, F.; Perez, C.; Duong, B.Q.; Rocca, J.R.; Jin, S.; Huigens Iii, R.W. Phenazine Antibiotic Inspired Discovery of Potent Bromophenazine Antibacterial Agents against Staphylococcus aureus and Staphylococcus epidermidis. Org. Biomol. Chem. 2014, 12, 881–886. [Google Scholar] [CrossRef]
- Kennedy, R.K.; Naik, P.R.; Veena, V.; Lakshmi, B.S.; Lakshmi, P.; Krishna, R.; Sakthivel, N. 5-Methyl Phenazine-1-Carboxylic Acid: A Novel Bioactive Metabolite by a Rhizosphere Soil Bacterium That Exhibits Potent Antimicrobial and Anticancer Activities. Chem. Biol. Interact. 2015, 231, 71–82. [Google Scholar] [CrossRef]
- Yang, Y.P.; Hussain, N.; Zhang, L.; Jia, Y.Z.; Jian, Y.Q.; Li, B.; Iqbal Choudhary, M.; Rahman, A.U.; Wang, W. Kadsura coccinea: A Rich Source of Structurally Diverse and Biologically Important Compounds. Chin. Herb. Med. 2020, 12, 214–223. [Google Scholar] [CrossRef]
- Dong, J.J.; Ma, J.Y.; Yang, W.Y.; Cai, W.; Wu, W.H. Characterization of the Volatile Profile and Its Estrogenic Activity in Kadsura coccinea Fruit. J. Ethnopharmacol. 2023, 309, 116341. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Xiang, J.; Su, M.; Yan, X.; Yang, D.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Huanglongmycin A-C, Cytotoxic Polyketides Biosynthesized by a Putative Type II Polyketide Synthase from Streptomyces sp. CB09001. Front. Chem. 2018, 6, 254. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Qin, X.; Liu, J.; Wen, Z.; Huang, Y.; Xiang, J.; Xiang, Y.; Ju, J.; Duan, Y.; et al. Syn-2, 3-Diols and Anti-Inflammatory Indole Derivatives from Streptomyces sp. CB09001. Nat. Prod. Res. 2021, 35, 144–151. [Google Scholar] [CrossRef]
- Pu, H.; Jiang, T.; Peng, D.; Xia, J.; Gao, J.; Wang, Y.; Yan, X.; Huang, X.; Duan, Y.; Huang, Y. Degradation of Mirubactin to Multiple Siderophores with Varying Fe(III) Chelation Properties. Org. Biomol. Chem. 2022, 20, 5066–5070. [Google Scholar] [CrossRef]
- Lu, C.; Yang, C.H.; Xu, Z. Three Naphthoquinones from Streptomyces sp. XZYN-4. Rec. Nat. Prod. 2016, 10, 430–440. [Google Scholar]
- Wessels, P.; Göhrt, A.; Zeeck, A.; Drautz, H.; Zähner, H. Metabolic Products of Microorganisms, 260. Naphthgeranines, New Naphthoquinone Antibiotics from Streptomyces sp. J. Antibiot. 1991, 44, 1013–1018. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, F.X.; Liu, C.; Wang, L.; Qi, Y.; Cao, M.; Guo, X.; Li, J.; Huang, X.; Yang, J.; et al. Isolation and Biosynthesis of Phenazine-Polyketide Hybrids from Streptomyces sp. KIB-H483. J. Nat. Prod. 2022, 85, 1324–1331. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an Antarctic Sponge-Associated Bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef]
- Geiger, A.; Keller-Schierlein, W.; Brandl, M.; Zähner, H. Metabolites of Microorganisms. 247. Phenazines from Streptomyces antibioticus, Strain Tü 2706. J. Antibiot. 1988, 41, 1542–1551. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Q.; Li, J. Research Progress of Phenazine-1-carboxylic Acid and Its Analogue. Chin. J. Org. Chem. 2019, 39, 2744. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, R.; Dong, L.; Chi, J.; Huang, F.; Dong, L.; Zhang, M.; Jia, X. α-Glucosidase Inhibitors from Brown Rice Bound Phenolics Extracts (BRBPE): Identification and Mechanism. Food Chem. 2022, 372, 131306. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.; Chater, K.; Butter, M.; Hopwood, D.; Bittner, M.; Buttner, M. Practical Streptomyces Genetics: A Laboratory Manual; John Innes Centre Foundation: Norwich, UK, 2000. [Google Scholar]
- Rainey, F.A.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E. The Genus Nocardiopsis Represents a Phylogenetically Coherent Taxon and a Distinct Actinomycete Lineage: Proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.T.; Deng, X.Y.; Chen, J.; Liang, Q.M.; Zhang, K.; Li, D.L.; Wu, P.P.; Zheng, X.; Zhou, R.P.; Jiang, Z.Y.; et al. Synthesis and Biological Evaluation of Coumarin Derivatives as α-Glucosidase Inhibitors. Eur. J. Med. Chem. 2020, 189, 112013. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Stephens, P.J.; Harada, N. ECD Cotton Effect Approximated by the Gaussian Curve and Other Methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. WIREs Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Sagui, C.; Darden, T.A. Molecular Dynamics Simulations of Biomolecules: Long-Range Electrostatic Effects. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 155–179. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A Fast SHAKE Algorithm to Solve Distance Constraint Equations for Small Molecules in Molecular Dynamics Simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Larini, L.; Mannella, R.; Leporini, D. Langevin Stabilization of Molecular-Dynamics Simulations of Polymers by Means of Quasisymplectic Algorithms. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Fong, P.; Mao, S.; Wang, Q. The Application of the MM/GBSA Method in the Binding Pose Prediction of FGFR Inhibitors. Phys. Chem. Chem. Phys. 2020, 22, 9656–9663. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Rastelli, G.; Rio, A.D.; Degliesposti, G.; Sgobba, M. Fast and Accurate Predictions of Binding Free Energies Using MM-PBSA and MM-GBSA. J. Comput. Chem. 2010, 31, 797–810. [Google Scholar] [CrossRef]
- Nguyen, H.; Roe, D.R.; Simmerling, C. Improved Generalized Born Solvent Model Parameters for Protein Simulations. J. Chem. Theory Comput. 2013, 9, 2020–2034. [Google Scholar] [CrossRef]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate Atomic Surfaces from Linear Combinations of Pairwise Overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]



| Position | Naphthgeranine G (5) | |
|---|---|---|
| δC, Type | δH (J in Hz) | |
| 1 | 178.5, C | |
| 2 | 154.3, C | |
| 3 | 123.0, C | |
| 4 | 184.2, C | |
| 4a | 134.0, C | |
| 5 | 105.9, CH | 6.64 (s) |
| 6 | 164.9, C | |
| 7 | 104.3, CH | 6.10 (d, 4.8) |
| 8 | 164.8, C | |
| 8a | 107.6, C | |
| 9 | 30.8, CH | 3.42 (m) |
| 10 | 120.1, CH | 6.03 (s) |
| 11 | 136.5, C | |
| 12 | 65.3, CH | 3.75 (m) |
| 13 | 29.4, CH2 | 1.83 (dd, 2.7, 13.6); 1.23 (m) |
| 14 | 33.9, CH | 2.04 (ddd, 2.9, 6.2, 13.2) |
| 15 | 79.8, C | |
| 16 | 21.2, CH3 | 1.69 (s) |
| 17 | 25.5, CH3 | 1.42 (s) |
| 18 | 24.7, CH3 | 1.26 (s) |
| Compounds | IC50 (µM) a | Compounds | IC50 (µM) a |
|---|---|---|---|
| 5 | 66.4 ± 6.7 | 10 | >800 |
| 6 | 115.6 ± 4.4 | 11 | NA b |
| 7 | 185.9 ± 0.2 | 12 | NA b |
| 8 | NA b | Acarbose | 671.5 ± 0.2 |
| 9 | 105.4 ± 10.5 |
| Compound | −log (FBE) | Targeting Residues |
|---|---|---|
| Naphthgeranine G (5) | −7.2 | Phe-575, Asp-542, Thr-205 |
| Asp-203, Arg-202, Asn-449, Tyr-299 | ||
| Acarbose | −6.7 | Trp-406, Tyr-299, Tyr-605 |
| Thr-205, Arg-526, Asp-443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Zhong, Y.; Huang, P.; Li, A.; Jiang, T.; Jiang, L.; Yang, H.; Wang, Z.; Wu, G.; Huang, X.; et al. Bioactive Naphthoquinone and Phenazine Analogs from the Endophytic Streptomyces sp. PH9030 as α-Glucosidase Inhibitors. Molecules 2024, 29, 3450. https://doi.org/10.3390/molecules29153450
Ma Q, Zhong Y, Huang P, Li A, Jiang T, Jiang L, Yang H, Wang Z, Wu G, Huang X, et al. Bioactive Naphthoquinone and Phenazine Analogs from the Endophytic Streptomyces sp. PH9030 as α-Glucosidase Inhibitors. Molecules. 2024; 29(15):3450. https://doi.org/10.3390/molecules29153450
Chicago/Turabian StyleMa, Qingxian, Yani Zhong, Pingzhi Huang, Aijie Li, Ting Jiang, Lin Jiang, Hao Yang, Zhong Wang, Guangling Wu, Xueshuang Huang, and et al. 2024. "Bioactive Naphthoquinone and Phenazine Analogs from the Endophytic Streptomyces sp. PH9030 as α-Glucosidase Inhibitors" Molecules 29, no. 15: 3450. https://doi.org/10.3390/molecules29153450




