C3-Alkylation of Imidazo[1,2-a]pyridines via Three-Component Aza-Friedel–Crafts Reaction Catalyzed by Y(OTf)3
Abstract
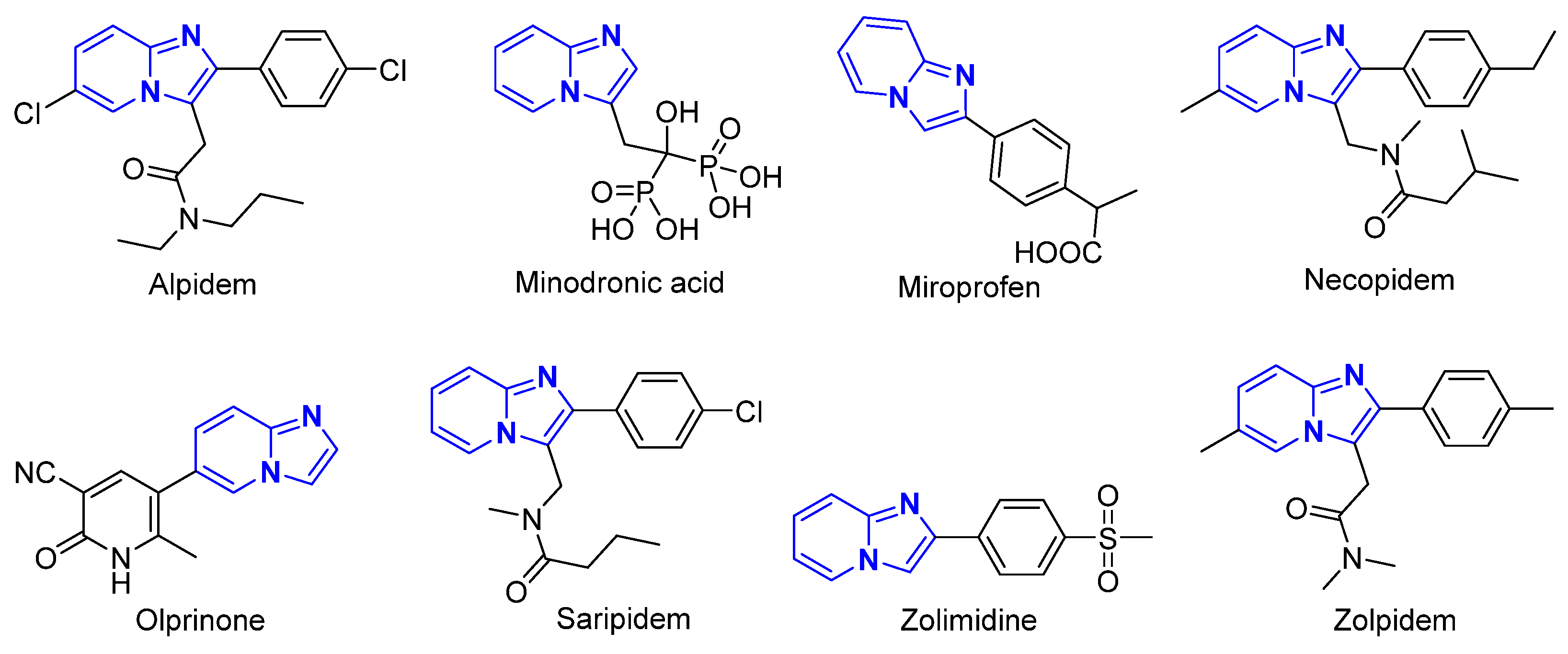
:1. Introduction
2. Results and Discussion
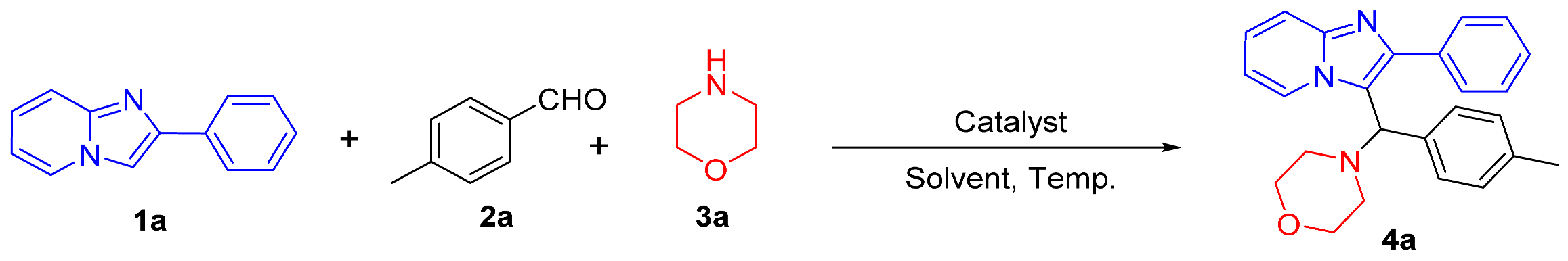
2.1. Optimization of Reaction Conditions
2.2. Scope of Benzaldehyde Substrates 2
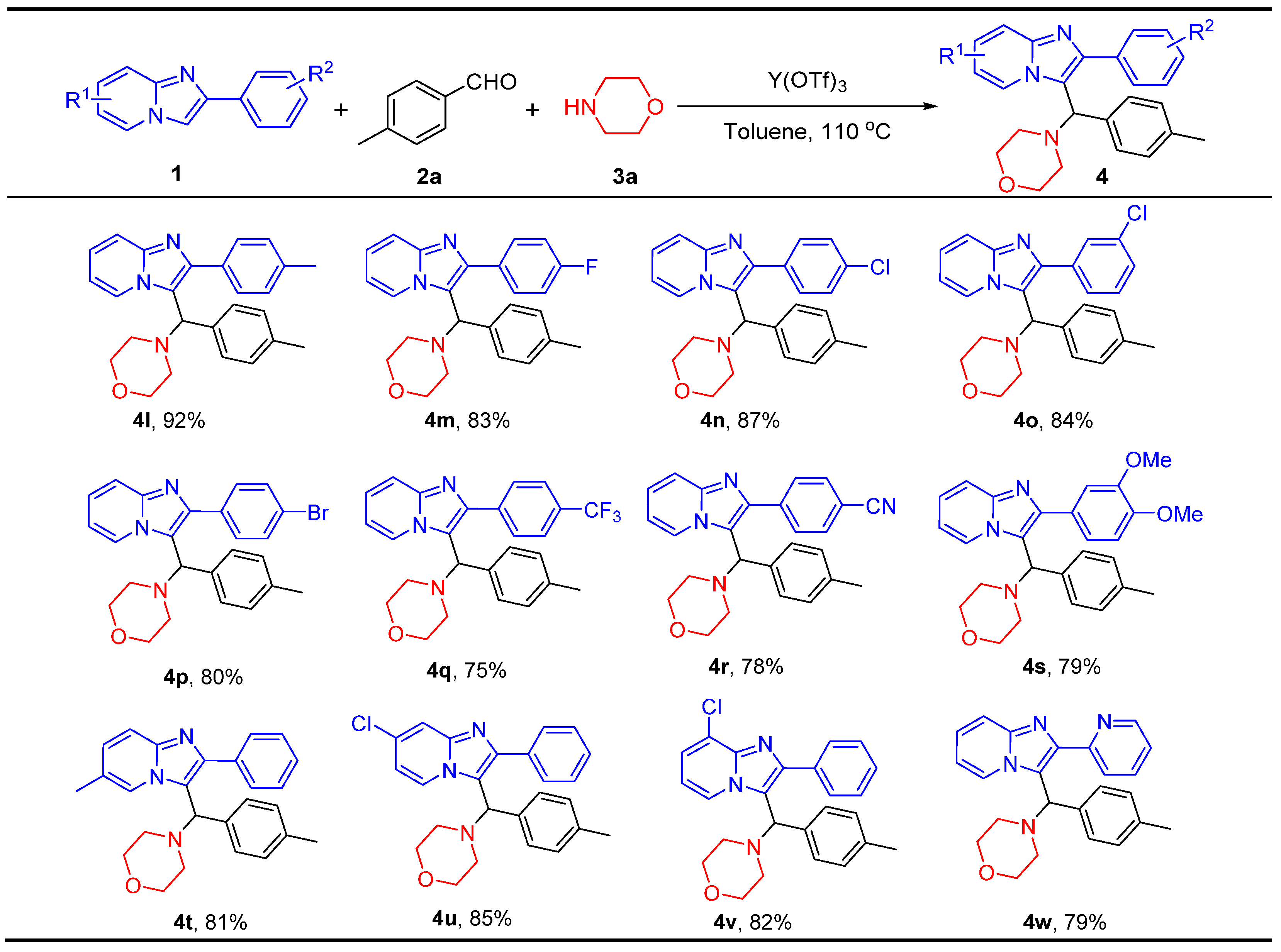
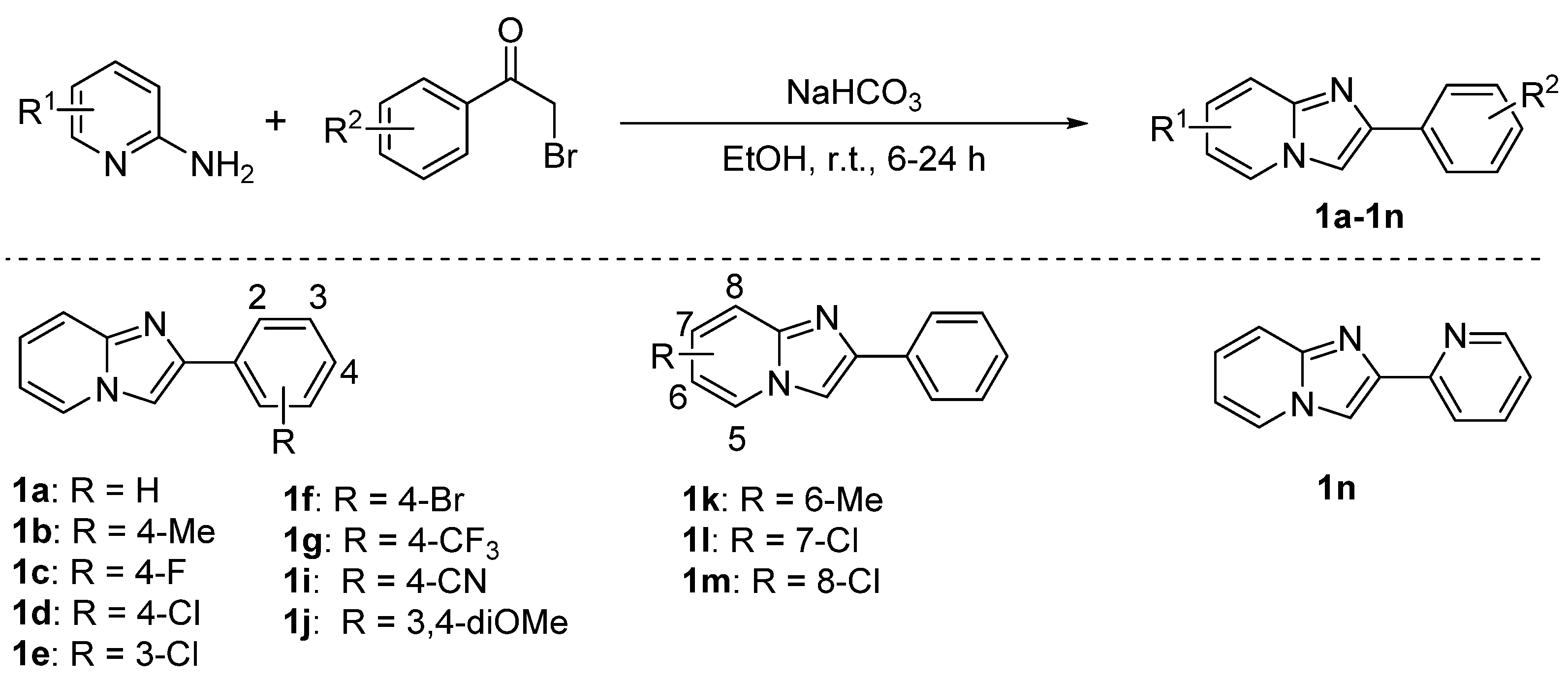
2.3. Scope of Imidazo[1,2-a]pyridine Substrates 1
2.4. Scope of Cycloamine Substrates 3
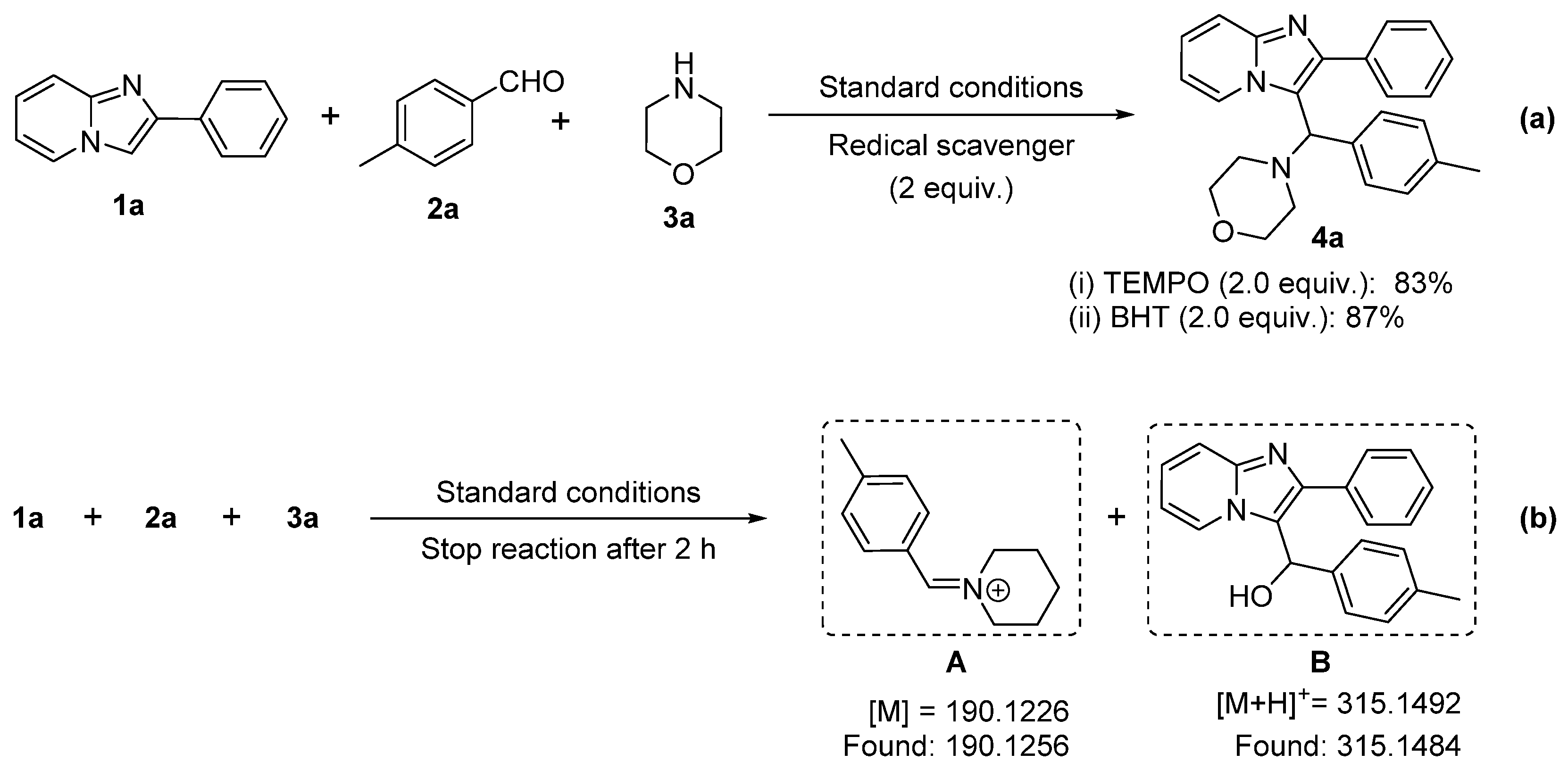
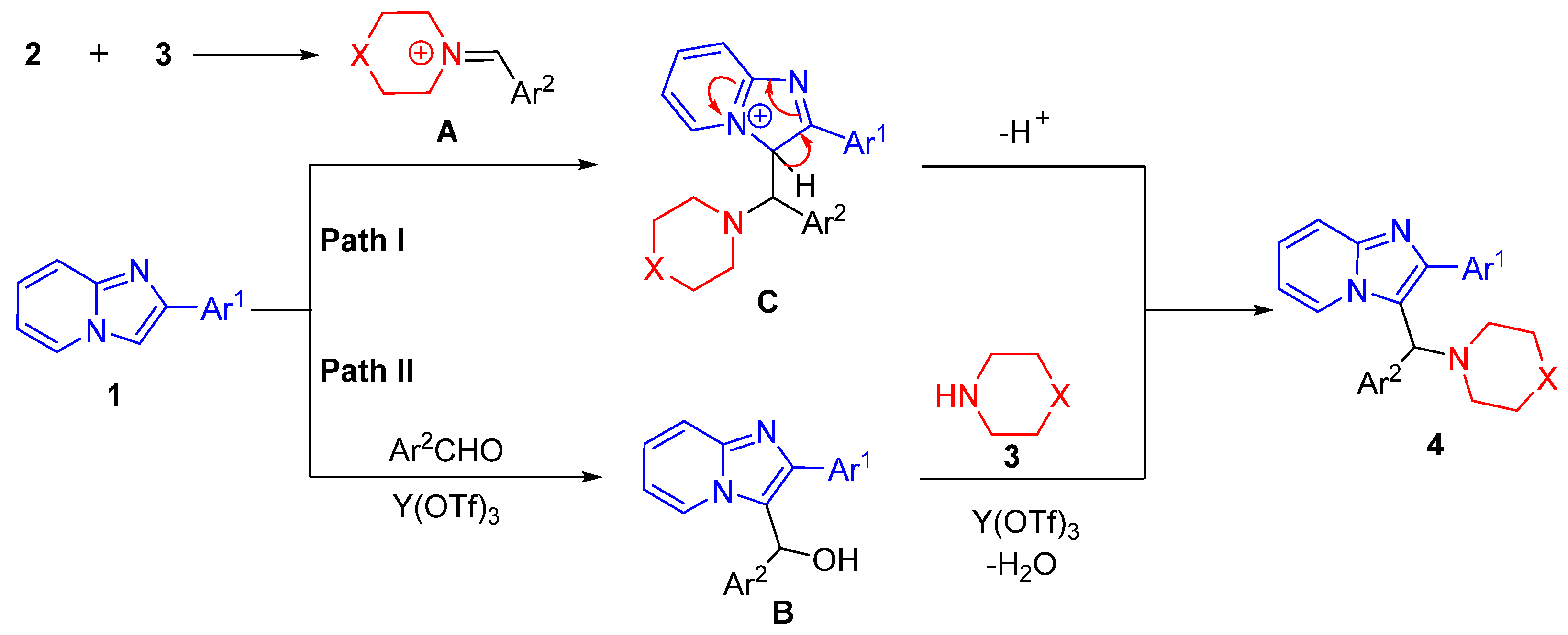
2.5. Mechanism Investigation
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for Compounds 1a–1n
3.3. Experimental Procedure for Compounds 4a–4ab
3.4. Characterization Data for All Products 4a–4ab
- 4-((2-Phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4a), white solid (69 mg, 90%); m.p. 211–213 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.24 (s, 3H), 2.32–2.35 (m, 2H), 2.64–2.67 (m, 2H), 3.70–3.79 (m, 4H), 5.10 (s, 1H), 6.78–6.81 (m, 1H), 7.04 (d, J = 8.0 Hz, 2H), 7.13–7.16 (m, 1H), 7.20 (d, J = 8.0 Hz, 2H), 7.39–7.44 (m, J = 8.0 Hz, 1H), 7.47–7.51 (m, 2H), 7.58 (d, J = 9.2 Hz, 1H), 7.71 (d, J = 7.2 Hz, 2H), 9.07 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.02, 52.67, 66.65, 67.25, 112.04, 117.54, 119.59, 124.47, 126.30, 127.46, 127.93, 128.50, 129.30, 129.52, 134.75, 135.76, 137.25, 145.04, 145.20; ESI-HRMS, m/z: Calcd for C25H26N3O [M + H]+, 384.2070, found: 384.2067.
- 4-(Phenyl(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4b), white solid (68 mg, 92%); m.p. 188–190 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.33–2.38 (m, 2H), 2.62–2.72 (m, 2H), 3.75–3.78 (m, 4H), 5.15 (s, 1H), 6.79–6.82 (m, 1H), 7.16–7.24 (m, 4H), 7.32 (d, J = 8.0 Hz, 2H), 7.41–7.45 (m, 1H), 7.49–7.52 (m, 2H), 7.59 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.0 Hz, 2H), 9.02 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.66, 66.78, 67.24, 112.10, 117.56, 119.34, 124.53, 126.16, 127.51, 127.96, 128.52, 128.59, 129.50, 134.65, 138.69, 145.07, 145.34; ESI-HRMS, m/z: Calcd for C24H24N3O [M + H]+, 370.1914, found: 370.1929.
- 4-((4-Methoxyphenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4c), white solid (69 mg, 87%); m.p. 206–208 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.31–2.36 (m, 2H), 2.63–2.66 (m, 2H), 3.73–3.77 (m, 7H), 5.08 (s, 1H), 7.76 (d, J = 8.8 Hz, 2H), 6.80–6.84 (m, 1H), 7.17–7.24 (m, 3H), 7.41–7.44 (m, 1H), 7.48–7.51 (m, 2H), 7.61 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 9.08 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.65, 55.21, 66.30, 67.25, 112.05, 113.94, 117.56, 119.72, 124.45, 126.23, 127.91, 128.47, 128.65, 129.48, 130.85, 134.65, 144.99, 145.06, 158.85; ESI-HRMS, m/z: Calcd for C25H26N3O2 [M + H]+, 400.2020, found: 400.2021.
- 4-((4-Fluorophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4d), white solid (67 mg, 86%); m.p. 200–202 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.34–2.39 (m, 2H), 2.66–2.70 (m, 2H), 3.76–3.79 (m, 4H), 5.14 (s, 1H), 6.80–6.84 (m, 1H), 6.89–6.94 (m, 2H), 7.18–7.22 (m, 1H), 7.25–7.29 (m, 2H), 7.42–7.46 (m, 1H), 7.49–7.53 (m, 2H), 7.61 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 8.96 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.63, 66.08, 67.21, 112.31, 115.51 (d, J = 22 Hz), 117.68, 119.12, 124.69, 125.89, 128.09, 128.61, 128.95 (d, J = 8 Hz), 129.48, 134.42 (d, J = 4 Hz), 134.49, 145.12, 145.37, 161.95 (d, J = 245 Hz); 19F NMR (376 MHz, CDCl3), δ, ppm: −114.65; ESI-HRMS, m/z: Calcd for C24H23FN3O [M + H]+, 388.1820, found: 388.1830.
- 4-((4-Chlorophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4e), white solid (73 mg, 91%); m.p. 215–217 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.34–2.39 (m, 2H), 2.66–2.70 (m, 2H), 3.73–3.82 (m, 4H), 5.14 (s, 1H), 6.79–6.83 (m, 1H), 7.17–7.26 (m, 5H), 7.42–7.45 (m, 1H), 7.49–7.53 (m, 2H), 7.60 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 8.92 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.61, 66.13, 67.17, 112.33, 117.70, 118.77, 124.69, 125.81, 128.10, 128.61, 128.67, 128.76, 129.46, 133.19, 134.48, 137.15, 145.16, 145.53; ESI-HRMS, m/z: Calcd for C24H23ClN3O [M+H]+, 404.1524, found: 404.1531.
- 4-((4-Bromophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4f), light-yellow solid (76 mg, 85%); m.p. 193–195 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.35–2.39 (m, 2H), 2.67–2.70 (m, 2H), 3.73–3.82 (m, 4H), 5.13 (s, 1H), 6.80–6.84 (m, 1H), 7.16–7.22 (m, 3H), 7.34 (d, J = 8.0 Hz, 2H), 7.43–7.46 (m, 1H), 7.50–7.53 (m, 2H), 7.61 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 2H), 8.92 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.61, 66.19, 67.17, 112.34, 117.72, 118.66, 121.30, 124.70, 125.80, 128.10, 128.62, 129.01, 129.46, 131.71, 134.48, 137.68, 145.17, 145.56; ESI-HRMS, m/z: Calcd for C24H23BrN3O [M+H]+, 448.1019, found: 448.1028.
- 4-((2-Phenylimidazo[1,2-a]pyridin-3-yl)(4-(trifluoromethyl)phenyl)methyl)-morpholine (4g), light-yellow solid (72 mg, 82%); m.p. 196–198 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.38–2.43 (m, 2H), 2.68–2.73 (m, 2H), 3.76–3.85 (m, 4H), 5.24 (s, 1H), 6.82–6.86 (m, 1H), 7.19–7.24 (m, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.44–7.48 (m, 3H), 7.51–7.55 (m, 2H), 7.62 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 8.0 Hz, 2H), 8.92 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.63, 66.40, 67.14, 112.52, 117.79, 118.31, 123.89 (q, J = 271 Hz), 124.86, 125.24 (q, J = 3 Hz), 125.58 (q, J = 4 Hz), 127.59, 128.22, 128.71, 129.48, 129.72 (q, J = 33 Hz), 134.39, 142.63, 145.22, 145.78; 19F NMR (376 MHz, CDCl3), δ, ppm: −62.61; ESI-HRMS, m/z: Calcd for C25H23F3N3O [M + H]+, 438.1788, found: 438.1762.
- 4-(Morpholino(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)benzonitrile (4h), light-yellow solid (70 mg, 89%); m.p. 208–210 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.42–2.44 (m, 2H), 2.68–2.73 (m, 2H), 3.77–3.85 (m, 4H), 5.24 (s, 1H), 6.82–6.85 (m, 1H), 7.20–7.24 (m, 1H), 7.39 (d, J = 8.0 Hz, 2H), 7.45–7.55 (m, 5H), 7.63 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 8.81 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.58, 66.31, 67.09, 111.39, 112.77, 117.81, 118.44, 125.14, 125.37, 127.89, 128.37, 128.79, 129.44, 132.42, 134.07, 143.84, 145.29, 145.84; ESI-HRMS, m/z: Calcd for C25H23N4O [M + H]+, 395.1866, found: 395.1878.
- 4-((2,4-Dichlorophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4i), light-yellow solid (72 mg, 83%); m.p. 216–218 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.46–2.51 (m, 2H), 2.66–2.72 (m, 2H), 3.73–3.80 (m, 4H), 5.48 (s, 1H), 6.82–6.85 (m, 1H), 7.15–7.24 (m, 3H), 7.37–7.47 (m, 3H), 7.62–7.69 (m, 3H), 7.77 (d, J = 8.0 Hz, 1H), 8.87 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.07, 62.27, 67.24, 112.39, 116.73, 117.87, 124.61, 125.50, 126.81, 127.98, 128.26, 129.32, 129.60, 130.13, 133.75, 134.23, 134.83, 135.41, 145.24, 146.66; ESI-HRMS, m/z: Calcd for C24H22Cl2N3O [M + H]+, 438.1134, found: 438.1120.
- 2-Methoxy-4-(morpholino(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)phenol (4j), white solid (65 mg, 79%); m.p. 209–212 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.33–2.38 (m, 2H), 2.64–2.72 (m, 2H), 3.66 (s, 3H), 3.75–3.80 (m, 4H), 6.60 (s, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.80–6.83 (m, 1H), 6.91–6.94 (m, 1H), 7.16–7.19 (m, 1H), 7.40–7.44 (m, 1H), 7.47–7.51 (m, 2H), 7.60 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 2H), 9.06 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.69, 55.64, 66.78, 67.24, 110.17, 112.08, 114.21, 117.59, 119.68, 119.81, 124.48, 126.12, 127.96, 128.47, 129.55, 130.66, 134.74, 144.97, 145.01, 145.07, 146.60; ESI-HRMS, m/z: Calcd for C25H26N3O3 [M + H]+, 416.1969, found: 416.1960.
- 3-(Morpholino(2-phenylimidazo[1,2-a]pyridin-3-yl)methyl)-4H-chromen-4-one (4k), yellow solid (66 mg, 76%); m.p. 112–114 °C; 2.45–2.50 (m, 2H), 2.62–2.74 (m, 2H), 3.71–3.73 (m, 4H), 5.48 (s, 1H), 6.89–6.92 (m, 1H), 721–7.25 (m, 1H), 7.33–7.47 (m, 5H), 7.60–7.66 (m, 2H), 7.76 (d, J = 8.0 Hz, 2H), 8.05 (s, 1H), 8.11 (d, J = 8.0 Hz, 1H), 8.90 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 52.12, 56.87, 67.12, 112.37, 116.88, 117.78, 117.98, 120.92, 123.62, 124.44, 125.29, 125.38, 126.03, 128.03, 128.17, 129.43, 133.74, 134.59; 145.08, 145.97, 154.11, 155.92, 176.59; ESI-HRMS, m/z: Calcd for C27H24N3O3 [M + H]+, 438.1812, found: 438.1805.
- 4-(p-Tolyl(2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)methyl)morpholine (4l), white solid (73 mg, 92%); m.p. 205–207 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.15 (s, 3H), 2.22–2.27 (m, 2H), 2.35 (s, 3H), 2.55–2.58 (m, 2H), 3.62–3.70 (m, 4H), 5.02 (s, 1H), 6.67–6.71 (m, 1H), 6.93 (d, J = 8.0 Hz, 2H), 7.03–7.07 (m, 1H), 7.12 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.4 Hz, 2H), 7.47–7.53 (m, 3H), 8.96 (d, J = 6.8 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.01, 21.38, 52.66, 66.62, 67.25, 111.91, 117.44, 119.37, 124.32, 126.22, 127.47, 129.21, 129.25, 129.35, 131.79, 135.80, 137.16, 137.63, 144.97, 145.26; ESI-HRMS, m/z: Calcd for C26H28N3O [M + H]+, 398.2227, found: 398.2236.
- 4-((2-(4-Fluorophenyl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4m), white solid (67 mg, 83%); m.p. 182–184 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.26 (s, 3H), 2.30–2.34 (m, 2H), 2.61–2.64 (m, 2H), 3.71–3.78 (m, 4H), 5.01 (s, 1H), 6.80–6.83 (m, 1H), 7.04 (d, J = 7.6 Hz, 2H), 7.16–7.21 (m, 5H), 7.57 (d, J = 8.0 Hz, 1H), 7.65–7.68 (m, 2H), 9.09 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.00, 52.68, 66.83, 67.19, 112.11, 115.48 (d, J = 21 Hz), 117.51, 119.54, 124.57, 126.28, 127.42, 129.34, 130.82 (d, J = 3 Hz), 131.16 (d, J = 8 Hz), 135.67, 137.40, 144.21, 145.00, 162.66 (d, J = 245 Hz); 19F NMR (376 MHz, CDCl3), δ, ppm: −114.10; ESI-HRMS, m/z: Calcd for C25H25FN3O [M + H]+, 402.1976, found: 402.1987.
- 4-((2-(4-Chlorophenyl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4n), light-yellow solid (72 mg, 87%); m.p. 173–175 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.24 (s, 3H), 2.28–2.40 (m, 2H), 2.61–2.63 (m, 2H), 3.65–3.77 (m, 4H), 5.03 (s, 1H), 6.80–6.83 (m, 1H), 7.03 (d, J = 7.6 Hz, 2H), 7.14–7.20 (m, 3H), 7.46 (d, J = 6.8 Hz, 2H), 7.57 (d, J = 8.8 Hz, 1H), 7.65 (d, J = 6.8 Hz, 2H), 9.10 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.02, 52.70, 66.87, 67.18, 112.20, 117.56, 119.79, 124.71, 126.38, 127.45, 128.73, 129.39, 130.74, 133.29, 133.95, 135.61, 137.45, 143.93, 145.11; ESI-HRMS, m/z: Calcd for C25H25ClN3O [M + H]+, 418.1681, found: 418.1662.
- 4-((2-(3-Chlorophenyl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4o), light-yellow solid (70 mg, 84%); m.p. 129–131 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.19 (s, 3H), 2.23–2.28 (m, 2H), 2.53–2.56 (m, 2H), 3.63–3.71 (m, 4H), 4.96 (s, 1H), 6.74–6.78 (m, 1H), 6.98 (d, J = 8.0 Hz, 2H), 7.10–7.15 (m, 3H), 7.31–7.37 (m, 2H), 7.50–7.53 (m, 2H), 7.65 (s, 1H), 9.05 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.02, 52.68, 66.86, 67.19, 112.25, 117.65, 120.00, 124.76, 126.41, 127.50, 127.55, 128.00, 129.42, 129.55, 129.71, 134.39, 135.58, 136.57, 137.53, 143.65, 145.12; ESI-HRMS, m/z: Calcd for C25H25ClN3O [M + H]+, 418.1681, found: 418.1662.
- 4-((2-(4-Bromophenyl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4p), light-yellow solid (73 mg, 80%); m.p. 224–226 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.26 (s, 3H), 2.26–2.34 (m, 2H), 2.60–2.63 (m, 2H), 3.71–3.79 (m, 4H), 5.01 (s, 1H), 6.80–6.84 (m, 1H), 7.04 (d, J = 8.0 Hz, 2H), 7.17–7.21 (m, 3H), 7.57–7.64 (m, 5H), 9.10 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.02, 52.70, 66.86, 67.19, 112.21, 117.57, 119.79, 122.23, 124.74, 126.36, 127.45, 129.39, 131.03, 131.68, 133.69, 135.58, 137.48, 143.92, 145.12; ESI-HRMS, m/z: Calcd for C25H25BrN3O [M + H]+, 462.1176, found: 462.1188.
- 4-(p-Tolyl(2-(4-(trifluoromethyl)phenyl)imidazo[1,2-a]pyridin-3-yl)methyl)-morpholine (4q), light-yellow solid (67 mg, 75%); m.p. 161–163 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.26 (s, 3H), 2.32–2.36 (m, 2H), 2.61–2.64 (m, 2H), 3.73–3.76 (m, 4H), 5.04 (s, 1H), 6.83–6.86 (m, 1H), 7.05 (d, J = 8.0 Hz, 2H), 7.18–7.23 (m, 3H), 7.60 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.84 (d, J = 8.0 Hz, 2H), 9.15 (d, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.01, 52.71, 66.91, 67.16, 112.35, 117.71, 120.31, 124.27 (q, J = 271 Hz), 124.92, 125.42 (q, J = 3 Hz), 126.49, 127.48, 129.43, 129.67 (q, J = 24 Hz), 135.48, 137.59, 138.42, 143.55, 145.24; 19F NMR (376 MHz, CDCl3), δ, ppm: −62.39; ESI-HRMS, m/z: Calcd for C26H25F3N3O [M + H]+, 452.1944, found: 452.1965.
- 4-(3-(Morpholino(p-tolyl)methyl)imidazo[1,2-a]pyridin-2-yl)benzonitrile (4r), light-yellow solid (63 mg, 78%); m.p. 198–200 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.27 (s, 3H), 2.30–2.35 (m, 2H), 2.59–2.62 (m, 2H), 3.73–3.78 (m, 4H), 5.01 (s, 1H), 6.85–6.88 (m, 1H), 7.06 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 7.21–7.25 (m, 1H), 7.60 (d, J = 9.2 Hz, 1H), 7.78 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 8.0 Hz, 2H), 9.16 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 17.08, 48.79, 63.10, 63.17, 107.52, 108.64, 113.81, 114.98, 116.75, 121.29, 122.62, 123.51, 125.58, 125.99, 128.36, 131.34, 133.84, 135.50, 138.93, 141.41; ESI-HRMS, m/z: Calcd for C26H25N4O [M + H]+, 409.2023, found: 409.2034.
- 4-((2-(3,4-Dimethoxyphenyl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4s), light-yellow solid (70 mg, 79%); m.p. 194–196 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.25 (s, 3H), 2.31–2.32 (m, 2H), 2.62–2.66 (m, 2H), 3.71–3.78 (m, 4H), 3.95 (s, 3H), 3.97 (s, 3H), 5.10 (s, 1H), 6.79–6.83 (m, 1H), 6.99 (d, J = 8.0 Hz, 1H), 7.03 (d, J = 8.0 Hz, 2H), 7.15–7.24 (m, 5H), 7.59 (d, J = 8.0 Hz, 1H), 9.09 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.00, 52.75, 55.93, 56.03, 66.77, 67.24, 110.94, 112.00, 112.66, 117.33, 119.27, 121.81, 124.47, 126.26, 127.32, 127.51, 129.26, 135.79, 137.31, 144.82, 144.95, 148.91; ESI-HRMS, m/z: Calcd for C27H30N3O3 [M + H]+, 444.2282, found: 444.2268.
- 4-((6-Methyl-2-phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4t), light-yellow solid (64 mg, 81%); m.p. 195–197 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.17 (s, 3H), 2.22–2.25 (m, 2H), 2.28 (s, 3H), 2.54–2.61 (m, 2H), 3.65–3.71 (m, 4H), 4.98 (s, 1H), 6.92–6.97 (m, 3H), 7.12 (d, J = 8.0 Hz, 2H), 7.30–7.34 (m, 1H), 7.38–7.42 (m, 3H), 7.61 (d, J = 8.0 Hz, 2H), 7.86 (s, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 18.69, 21.02, 52.64, 66.60, 67.30, 116.77, 119.25, 121.55, 123.78, 127.52, 127.60, 127.79, 128.42, 129.24, 129.48, 134.83, 135.85, 137.16, 144.09, 144.96; ESI-HRMS, m/z: Calcd for C26H28N3O [M + H]+, 398.2227, found: 398.2236.
- 4-((7-Chloro-2-phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4u), white solid (71 mg, 85%); m.p. 197–199 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.26 (s, 3H), 2.31–2.35 (m, 2H), 2.63–2.71 (m, 2H), 3.73–3.80 (m, 4H), 5.06 (s, 1H), 6.80–6.82 (m, 1H), 7.04 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.0 Hz, 2H), 7.42–7.45 (m, 1H), 7.48–7.52 (m, 2H), 7.58–7.67 (m, 3H), 9.06 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 20.96, 52.61, 66.52, 67.14, 113.84, 116.07, 120.01, 126.61, 127.33, 128.27, 128.57, 129.18, 129.36, 129.41, 129.57, 133.84, 135.28, 137.56, 144.75; ESI-HRMS, m/z: Calcd for C25H25ClN3O [M + H]+, 418.1681, found: 418.1662.
- 4-((8-Chloro-2-phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4v), white solid (68 mg, 82%); m.p. 189–191 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.25 (s, 3H), 2.30–2.35 (m, 2H), 2.64–2.67 (m, 2H), 3.70–3.79 (m, 4H), 5.06 (s, 1H), 6.73–6.77 (m, 1H), 7.03 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 1H), 7.41–7.51 (m, 3H), 7.69 (d, J = 8.0 Hz, 2H), 9.05 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.01, 52.65, 66.67, 67.18, 111.62, 121.44, 123.16, 123.42, 125.04, 127.38, 128.16, 128.48, 129.35, 129.78, 134.21, 135.36, 137.44, 142.38, 145.91; ESI-HRMS, m/z: Calcd for C25H25ClN3O [M + H]+, 418.1681, found: 418.1662.
- 4-((2-(Pyridin-2-yl)imidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4w), white solid (60 mg, 79%); m.p. 201–203 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.25 (s, 3H), 2.28–2.33 (m, 2H), 2.71–2.74 (m, 2H), 3.74–3.79 (m, 4H), 6.66 (s, 1H), 6.76–6.79 (m, 1H), 7.04 (d, J = 8.0 Hz, 2H), 7.13–7.17 (m, 1H), 7.23–7.24 (m, 1H), 7.55–7.60 (m, 3H), 7.76–7.80 (m, 1H), 8.25 (d, J = 8.0 Hz, 1H), 8.72 (d, J = 8.0 Hz, 1H), 9.14 (d, J = 6.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.04, 52.52, 64.47, 67.29, 112.05, 117.59, 122.13, 122.55, 122.62, 124.68, 126.92, 127.70, 129.06, 136.37, 136.48, 136.73, 141.76, 144.68, 148.61, 154.61; ESI-HRMS, m/z: Calcd for C24H25N4O [M + H]+, 385.2033, found: 385.2059.
- 2-Methyl-4-((2-phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)morpholine (4x), white solid (38 mg, 48%); m.p. 182–184 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 1.12 (d, J = 8.0 Hz, 3H), 1.84–1.87 (m, 1H), 2.26 (s, 3H), 2.40–2.44 (m, 2H), 2.87 (d, J = 8.8 Hz, 1H), 3.66–3.79 (m, 2H), 3.82–3.86 (m, 1H), 5.08 (s, 1H), 6.78–6.81 (m, 1H), 7.03 (d, J = 8.0 Hz, 2H), 7.15–7.20 (m, 3H), 7.41–7.44 (m, 1H), 7.48–7.51 (m, 2H), 7.58 (d, J = 8.8 Hz, 1H), 7.70 (d, J = 8.0 Hz, 2H), 9.03 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 19.18, 21.02, 51.64, 59.22, 66.39, 67.12, 71.94, 112.02, 117.50, 119.71, 124.47, 126.23, 127.39, 127.91, 128.48, 129.27, 129.52, 134.66, 135.70, 137.19, 144.98, 145.04; ESI-HRMS, m/z: Calcd for C26H28N3O [M + H]+, 398.2227, found: 398.2236.
- 4-((2-phenylimidazo[1,2-a]pyridin-3-yl)(p-tolyl)methyl)thiomorpholine (4y), yellow solid (42 mg, 62%); m.p. 190–192 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 2.23 (s, 3H), 2.63–2.74 (m, 6H), 2.89–2.94 (m, 2H), 5.20 (s, 1H), 6.75–6.79 (m, 1H), 7.01 (d, J = 8.0 Hz, 2H), 7.10–7.16 (m, 3H), 7.41–7.43 (m, 1H), 7.46–7.50 (m, 2H), 7.54 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 8.0 Hz, 2H), 8.87 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.00, 28.47, 53.92, 66.28, 112.02, 117.47, 119.51, 124.57, 126.20, 127.22, 127.95, 128.54, 129.27, 129.51, 134.62, 135.84, 137.12, 145.08, 145.25; ESI-HRMS, m/z: Calcd for C25H26N3S [M + H]+, 400.1842, found: 400.1857.
- 2-Phenyl-3-((4-phenylpiperazin-1-yl)(p-tolyl)methyl)imidazo[1,2-a]pyridine (4z), yellow solid (43 mg, 47%); m.p. 95–97 °C; 2.26 (s, 3H), 2.51–2.55 (m, 2H), 2.82–2.87 (m, 2H), 3.19–3.28 (m, 4H), 5.17 (s, 1H), 6.79–6.80 (m, 1H), 6.85–6.88 (m, 1H), 6.92 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 8.0 Hz, 2H), 7.15–7.19 (m, 1H), 7.21–7.24 (m, 2H), 7.25–7.28 (m, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.48–7.51 (m, 2H), 7.60 (d, J = 9.2 Hz, 1H), 7.73 (d, J = 8.0 Hz, 2H), 9.06 (d, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.04, 49.53, 52.09, 66.19, 112.00, 116.02, 117.51, 119.89, 119.94, 124.51, 126.42, 127.37, 127.93, 128.52, 128.77, 129.17, 129.28, 129.57, 134.74, 136.05, 137.19, 145.06, 151.15; ESI-HRMS, m/z: Calcd for C31H31N4 [M + H]+, 459.2543, found: 459.2557.
- 3-((4-Methylpiperidin-1-yl)(p-tolyl)methyl)-2-phenylimidazo[1,2-a]pyridine (4aa), light-yellow solid (40 mg, 52%); m.p. 156–158 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 0.94 (d, J = 8.0 Hz, 3H), 1.23–1.27 (m, 3H), 1.63 (d, J = 9.2 Hz, 1H), 1.88–1.94 (m, 1H), 2.15–2.21 (m, 1H), 2.23 (s, 3H), 2.68 (d, J = 9.2 Hz, 1H), 2.98 (d, J = 9.2 Hz, 1H), 5.08 (s, 1H), 6.74–6.78 (m, 1H), 6.99 (d, J = 8.0 Hz, 2H), 7.11–7.14 (m, 1H), 7.17 (d, J = 8.0 Hz, 2H), 7.38–7.42 (m, 1H), 7.47–7.50 (m, 2H), 7.56 (d, J = 9.2 Hz, 1H), 7.72 (d, J = 8.0 Hz, 2H), 9.06 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 19.93, 20.84, 33.55, 51.37, 52.04, 65.41, 110.67, 116.20, 119.72, 123.23, 125.66, 126.13, 126.65, 127.34, 128.00, 128.51, 133.91, 135.68, 136.02, 143.44, 143.82; ESI-HRMS, m/z: Calcd for C27H30N3 [M + H]+, 396.2434, found: 396.2445.
- 2-Phenyl-3-((4-phenylpiperidin-1-yl)(p-tolyl)methyl)imidazo[1,2-a]pyridine (4ab), light-yellow solid (41 mg, 45%); m.p. 202–204 °C; 1H NMR (400 MHz, CDCl3), δ, ppm: 1.77–1.90 (m, 4H), 2.05–2.09 (m, 1H), 2.24 (s, 3H), 2.31–2.37 (m, 1H), 2.56–2.64 (m, 1H), 2.86 (d, J = 9.2 Hz, 1H), 2.16 (d, J = 9.2 Hz, 1H), 5.16 (s, 1H), 6.77–6.81 (m, 1H), 7.02 (d, J = 8.0 Hz, 2H), 7.13–7.26 (m, 7H), 7.29–7.33 (m, 2H), 7.40–7.44 (m, 1H), 7.49–7.53 (m, 2H), 7.58 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 9.08 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3), δ, ppm: 21.04, 33.66, 34.24, 42.74, 52.79, 53.50, 66.43, 111.88, 117.39, 120.54, 124.39, 126.39, 126.60, 126.84, 127.25, 127.80, 128.46, 128.47, 129.17, 129.61, 134.97, 136.84, 136.91, 144.72, 144.97, 146.21; ESI-HRMS, m/z: Calcd for C32H32N3 [M + H]+, 458.2591, found: 458.2598.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devi, N.; Singh, D.; K. Rawal, R.; Bariwal, J.; Singh, V. Medicinal attributes of imidazo[1,2-a]pyridine derivatives: An update. Curr. Top. Med. Chem. 2016, 16, 2963–2994. [Google Scholar] [CrossRef]
- Quattrini, L.; Gelardi, E.L.M.; Coviello, V.; Sartini, S.; Ferraris, D.M.; Mori, M.; Nakano, I.; Garavaglia, S.; La Motta, C. Imidazo[1,2-a]pyridine derivatives as aldehyde dehydrogenase inhibitors: Novel chemotypes to target glioblastoma stem cells. J. Med. Chem. 2020, 63, 4603–4616. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.L.; Jia, Y.H.; Li, Q.; Feng, Z.W.; Zhang, S.D.; Zheng, W.; Zhou, Y.L.; Li, L.L.; Liu, X.C.; et al. Imidazo[1,2-a]pyridine derivatives as novel dual-target inhibitors of ABCB1 and ABCG2 for reversing multidrug resistance. J. Med. Chem. 2023, 66, 2804–2831. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hong, D.; Wang, B.; Zheng, X.; Miao, K.; Wang, L.; Yun, H.; Gao, L.; Zhao, S.; Shen, H.C. Discovery of imidazopyridine derivatives as highly potent respiratory syncytial virus fusion inhibitors. ACS Med. Chem. Lett. 2015, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Demizu, R.; Ohkado, R. Tandem Flavin-iodine-catalyzed aerobic oxidative sulfenylation of imidazo[1,2-a]pyridines with thiols. J. Org. Chem. 2018, 83, 12291–12296. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Kumar, S.; Aratikatla, E.K.; Ghorpade, S.R.; Singh, V. Recent developments of imidazo[1,2-a]pyridine analogues as antituberculosis agents. RSC Med. Chem. 2023, 14, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Khoramjouy, M.; Movahed, M.A.; Amidi, S.; Faizi, M.; Zarghi, A. Design, synthesis, in vitro and in vivo evaluation of new imidazo[1,2-a]pyridine derivatives as cyclooxygenase-2 inhibitors. Anti Cancer Agents Med. Chem. 2024, 24, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Valla, L.; Pitrat, D.; Mulatier, J.-C.; Bahers, T.; Jeanneau, E.; Ali, L.M.A.; Nguyen, C.; Gary-Bobo, M.; Andraud, C.; Bretonniere, Y. Imidazo[1,2-a]pyridine and Imidazo[1,5-a]pyridine: Electron Donor Groups in the Design of D–π–A Dyes. J. Org. Chem. 2024, 89, 8407–8419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; An, Y.N.; Li, J.W.; Yang, S.R.; Wu, W.Q.; Jiang, H.F. Palladium-catalyzed C-S bond activation and functionalization of 3-sulfenylindoles and related electron-rich heteroarenes. Org. Chem. Front. 2017, 4, 1590–1594. [Google Scholar] [CrossRef]
- Yu, Y.; Su, Z.Q.; Cao, H. Strategies for synthesis of imidazo[1,2-a]pyridine derivatives: Carbene transformations or C-H functionalizations. Chem. Rec. 2019, 19, 2105–2118. [Google Scholar] [CrossRef]
- Yan, J.; Zhong, S.J.; Chen, X.; Luo, Y.H.; Cao, H.; Liu, X.; Zhao, L.M. Controlled and site-selective C-H/N-H alkenylation, dialkenylation, and dehydrogenative β-alkenylation of various N-heterocycles. J. Org. Chem. 2024, 89, 4840–4850. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, Q.; Fan, X.; Kayaat, F.; Lv, R.; Li, J.; Wang, Y. The discovery of novel imidazo[1,2-a]pyridine derivatives as covalent anticancer agents. Org. Biomol. Chem. 2024, 22, 5374–5384. [Google Scholar] [CrossRef]
- Tzara, A.; Xanthopoulos, D.; Kourounakis, A.P. Morpholine as a scaffold in medicinal chemistry: An update on synthetic strategies. ChemMedChem 2020, 15, 392–403. [Google Scholar] [CrossRef]
- Lenci, E.; Calugi, L.; Trabocchi, A. Occurrence of morpholine in central nervous system drug discovery. ACS Chem. Neurosci. 2021, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srinivasa, R.V.; Kapur, S. Emphasizing morpholine and its derivatives (Maid): Typical candidate of pharmaceutical importance. Int. J. Chem. Sci. 2016, 14, 1777–1788. [Google Scholar]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2019, 40, 709–752. [Google Scholar] [CrossRef]
- Tang, S.A.; Fults, A.; Boyd, S.R.; Gattu, N.; Tran, K.A.; Fan, J.Y.; Mackenzie, K.R.; Palzkill, T.; Young, D.W.; Chamakuri, S. Expanding complex morpholines using systematic chemical diversity. Org. Lett. 2024, 26, 3493–3497. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Fan, Y.S.B.; Qiang, C.C.; Liu, P.; Sun, P.P. Electrochemical C3-methylthiolation of imidazopyridines with dimethyl sulfoxide. Green Chem. 2024, 26, 3517–3521. [Google Scholar] [CrossRef]
- Jin, S.Z.; Xie, B.; Lin, S.; Min, C.; Deng, R.H.; Yan, Z.H. Metal-free site-specific hydroxyalkylation of imidazo[1,2-a]pyridines with alcohols through radical reaction. Org. Lett. 2019, 21, 3436–3440. [Google Scholar] [CrossRef]
- Wu, Y.R.; Li, L.; Wen, K.M.; Deng, J.; Chen, J.W.; Shi, J.; Wu, T.; Pang, J.X.; Tang, X.D. Copper-catalyzed C3 functionalization of imidazo[1,2-a]pyridines with 3-indoleacetic acids. J. Org. Chem. 2021, 86, 12394–12402. [Google Scholar] [CrossRef]
- Ji, J.J.; Zhu, Z.Q.; Xiao, L.J.; Guo, D.; Zhu, X.; Tang, J.; Wu, J.; Xie, Z.B.; Le, Z.G. Photocatalyst-free decarboxylative aminoalkylation of imidazo[1,2-a]pyridines with N-aryl glycines enabled by visible light. Org. Chem. Front. 2019, 6, 3693–3697. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, Z.L.; Zhang, L.; Li, L.S.; Lei, A.W. Electrochemical oxidative C3 acyloxylation of imidazo[1,2-a]pyridines with hydrogen evolution. Org. Lett. 2021, 23, 5932–5936. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Wang, T.M.; Li, X.C.; Fang, L.J.; Zhai, H.B.; Cheng, B. Switchable electrooxidative N-methyl amines: Access to C3-aminomethylated and C3-arylmethylated imidazo[1,2-a] pyridines. Green Chem. 2022, 24, 9482–9488. [Google Scholar] [CrossRef]
- Gernet, A.; Sevrain, N.; Volle, J.N.; Ayad, T.; Pirat, J.L.; Virieux, D. Diversity-oriented synthesis toward aryl- and phosphoryl-functionalized imidazo[1,2-a]pyridines. J. Org. Chem. 2020, 85, 14730–14743. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Samanta, S.; Jana, S.; Hajra, A. (Diacetoxy)iodobenzene-mediated oxidative C-H amination of imidazopyridines at ambient temperature. J. Org. Chem. 2017, 82, 4504–4510. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Samanta, S.; Singsardar, M.; Hajra, A. Aminomethylation of imidazoheterocycles with morpholine. Org. Lett. 2017, 19, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, F.; Kilic, H. Metal- and additive-free C3-functionalization of imidazo[1,2-a]pyridines with para-quinone methides. Asian J. Org. Chem. 2021, 10, 1814–1821. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, G.; Dheer, D.; Jyoti; Kushwaha, M.; Ahmed, Q.N.; Shankar, R. BCl3-mediated C-N, C-S, and C-O bond formation of imidazo[1,2-a]pyridine benzylic ethers. ACS Omega 2019, 4, 4530–4539. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, X.H.; Chen, Z.X.; Wu, L.H.; Yang, K. Metal-free synthesis of trifluoromethyl carbinol-containing imidazo[1,2-a]pyridines via dehydrative coupling of imidazo[1,2-a]pyridines with trifluoroacetaldehyde. Synthesis 2024, 56, 1756–1764. [Google Scholar]
- Meena, N.; Bhawani, N.; Sonam; Rangan, K.; Kumar, A. Ball-Milling-Enabled Zn(OTf)2-catalyzed Friedel-Crafts hydroxyalkylation of imidazo[1,2-a]pyridines and indoles. J. Org. Chem. 2023, 88, 3022–3034. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Guo, X.; Wu, L.; Chen, Z.; Yang, K. 1,1,1,3,3,3-Hexafluoro-2-propanol-promoted Friedel-Crafts reaction: Metal-free synthesis of C3-difluoromethyl carbinol-containing imidazo[1,2-a]pyridines at room temperature. Molecules 2023, 28, 7522. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Luo, S.-H.; Chen, S.-H.; Cao, X.-Y.; Zhou, Y.-J.; Lin, J.-Y.; Huo, Y.-P.; Wang, Z.-Y. Simple inorganic base promoted C-N and C-C formation: Synthesis of benzo[4,5]imidazo[1,2-a]pyridines as functional AIEgens used for detecting picric acid. Org. Biomol. Chem. 2021, 19, 8133–8139. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Z.-X.; Zhou, Y.-J.; Chen, Q.; Yu, S.-W.; Luo, S.-H.; Wang, Z.-Y. Simple inorganic base promoted polycyclic construction using mucohalic acid as a C3 synthon: Synthesis and AIE probe application of benzo[4,5]imidazo[1,2-a]pyridines. Org. Chem. Front. 2022, 9, 1127–1136. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Liu, Z.; Chen, Z.-X.; Fu, X.; Yang, K. Metal-free synthesis of difluoro/trifluoromethyl carbinol-containing chromones via tandem cyclization of o-hydroxyaryl enaminone. Org. Biomol. Chem. 2023, 21, 9236–9241. [Google Scholar] [CrossRef] [PubMed]
- Pericherla, K.; Khungar, B.; Kumar, A. One-pot, three-component synthesis of 1-amidomethyl-imidazo[1,2-a]pyridines catalyzed by ytterbium triflate. Tetrahedron Lett. 2012, 53, 1253–1257. [Google Scholar] [CrossRef]
- Xue, Y.T.; Shi, L.L.; Wang, X.; Yu, X.N.; Zhu, X.J.; Hao, X.Q.; Song, M.P. Regioselective N-F and α C(sp3)-H Arylation of Aliphatic N-Fluorosulfonamides with Imidazopyridines. Org. Lett. 2021, 23, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-X.; Zhang, J.; Ma, F.-L.; Li, M.; Yu, J.-Y.; Luo, W.; Li, X.-Q. Synthesis and biological activities of dithiocarbamates containing 2(5H)-furanone-piperazine. Eur. J. Med. Chem. 2018, 155, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-X.; Yu, J.-Y.; Liu, X.-X.; Li, X.-Q.; Yang, J.-H.; Zhang, M.-W.; Yang, P.-W.; Zhang, S.-S.; He, Y. Synthesis and biological evaluation of novel artemisone-piperazine-tetronamide hybrids. RSC Adv. 2021, 11, 18333–18341. [Google Scholar] [CrossRef] [PubMed]
- Karunanidhi, S.; Chandrasekaran, B.; Karpoormath, R.; Patel, H.M.; Kayamba, F.; Merugu, S.R.; Kumar, V.; Dhawan, S.; Kushwaha, B.; Mahlalela, M.C. Novel thiomorpholine tethered isatin hydrazones as potential inhibitors of resistant Mycobacterium tuberculosis. Bioorg. Chem. 2021, 115, 105133. [Google Scholar] [CrossRef]
- CCDC2325330 (for 4a) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 14 January 2024).
- Vachhani, D.D.; Sharma, A.; Van der Eycken, E. Copper-catalyzed direct secondary and tertiary C-H alkylation of azoles through a heteroarene-amine-aldehyde/ketone coupling reaction. Angew. Chem. Int. Ed. 2013, 52, 2547–2550. [Google Scholar] [CrossRef]
- Gunaganti, N.; Kharbanda, A.; Lakkaniga, N.R.; Zhang, L.T.; Cooper, R.; Li, H.Y.; Frett, B. Catalyst free, C-3 functionalization of imidazo[1,2-a]pyridines to rapidly access new chemical space for drug discovery efforts. Chem. Commun. 2018, 54, 12954–12957. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Yu, B. HFIP-promoted de novo synthesis of biologically relevant nonnatural α-arylated amino esters and dipeptide mimetics. Chem. Eur. J. 2019, 25, 16528–16532. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.H.; Masurier, N. Recent advances in aza Friedel-Crafts reaction: Strategies for achiral and stereoselective synthesis. Org. Chem. Front. 2023, 10, 1847–1866. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Z.; Chai, L.; Liu, Z.-Q. A Free-radical-promoted site-specific cross-dehydrogenative-coupling of N-heterocycles with fluorinated alcohols. Org. Lett. 2016, 18, 4662–4665. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Wen, K.M.; Wu, Y.R.; Shi, J.; Yao, X.G.; Tang, X.D. Transition metal catalyst-free C3 sulfonylmethylation of imidazo[1,2-a]pyridines with glyoxylic acid and sodium sulfinates in water. J. Org. Chem. 2022, 87, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, D.; Znidar, D.; Glotz, G.; Stadler, A.; Dallinger, D.; Kappe, C.O. Design and performance validation of a conductively heated sealed-vessel reactor for organic Synthesis. J. Org. Chem. 2016, 81, 11788–11801. [Google Scholar] [CrossRef]
- Ghosh, P.; Ganguly, B.; Kar, B.; Dwivedi, S.; Das, S. Green procedure for highly efficient, rapid synthesis of imidazo[1,2-a]pyridine and its late stage functionalization. Synth. Commun. 2018, 48, 1076. [Google Scholar] [CrossRef]



 | ||||
| Entry | Catalyst | Solvent | Temp. (°C) | Yield of 4a (%) [b] |
| 1 | - | Toluene | 110 | trace |
| 2 | TFA (20%) | Toluene | 110 | trace |
| 3 | TsOH (20%) | Toluene | 110 | trace |
| 4 | HFIP (20%) | Toluene | 110 | trace |
| 5 | Sc(OTf)3 (20%) | Toluene | 110 | 75 |
| 6 | Y(OTf)3 (20%) | Toluene | 110 | 90 |
| 7 | Y(OTf)3 (10%) | Toluene | 110 | 72 |
| 8 | Y(OTf)3 (25%) | Toluene | 110 | 90 |
| 9 | Y(OTf)3 (20%) | DMF | 110 | 42 |
| 10 | Y(OTf)3 (20%) | 1,4-Dioxane | 110 | 35 |
| 11 | Y(OTf)3 (20%) | Acetonitrile | 110 | 18 |
| 12 | Y(OTf)3 (20%) | Toluene | 100 | 62 |
| 13 | Y(OTf)3 (20%) | Toluene | 120 | 90 |
| 14 [c] | Y(OTf)3 (20%) | Toluene | 110 | 66 |
| 15 [d] | Y(OTf)3 (20%) | Toluene | 110 | 82 |
| 16 [e] | Y(OTf)3 (20%) | Toluene | 110 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Chen, C.-B.; Liu, Z.-W.; Li, Z.-L.; Zeng, Y.; Wang, Z.-Y. C3-Alkylation of Imidazo[1,2-a]pyridines via Three-Component Aza-Friedel–Crafts Reaction Catalyzed by Y(OTf)3. Molecules 2024, 29, 3463. https://doi.org/10.3390/molecules29153463
Yang K, Chen C-B, Liu Z-W, Li Z-L, Zeng Y, Wang Z-Y. C3-Alkylation of Imidazo[1,2-a]pyridines via Three-Component Aza-Friedel–Crafts Reaction Catalyzed by Y(OTf)3. Molecules. 2024; 29(15):3463. https://doi.org/10.3390/molecules29153463
Chicago/Turabian StyleYang, Kai, Cai-Bo Chen, Zhao-Wen Liu, Zhen-Lin Li, Yu Zeng, and Zhao-Yang Wang. 2024. "C3-Alkylation of Imidazo[1,2-a]pyridines via Three-Component Aza-Friedel–Crafts Reaction Catalyzed by Y(OTf)3" Molecules 29, no. 15: 3463. https://doi.org/10.3390/molecules29153463






