A High-Performance Mn/TiO2 Catalyst with a High Solid Content for Selective Catalytic Reduction of NO at Low-Temperatures
Abstract
:1. Introduction
2. Results and Discussion
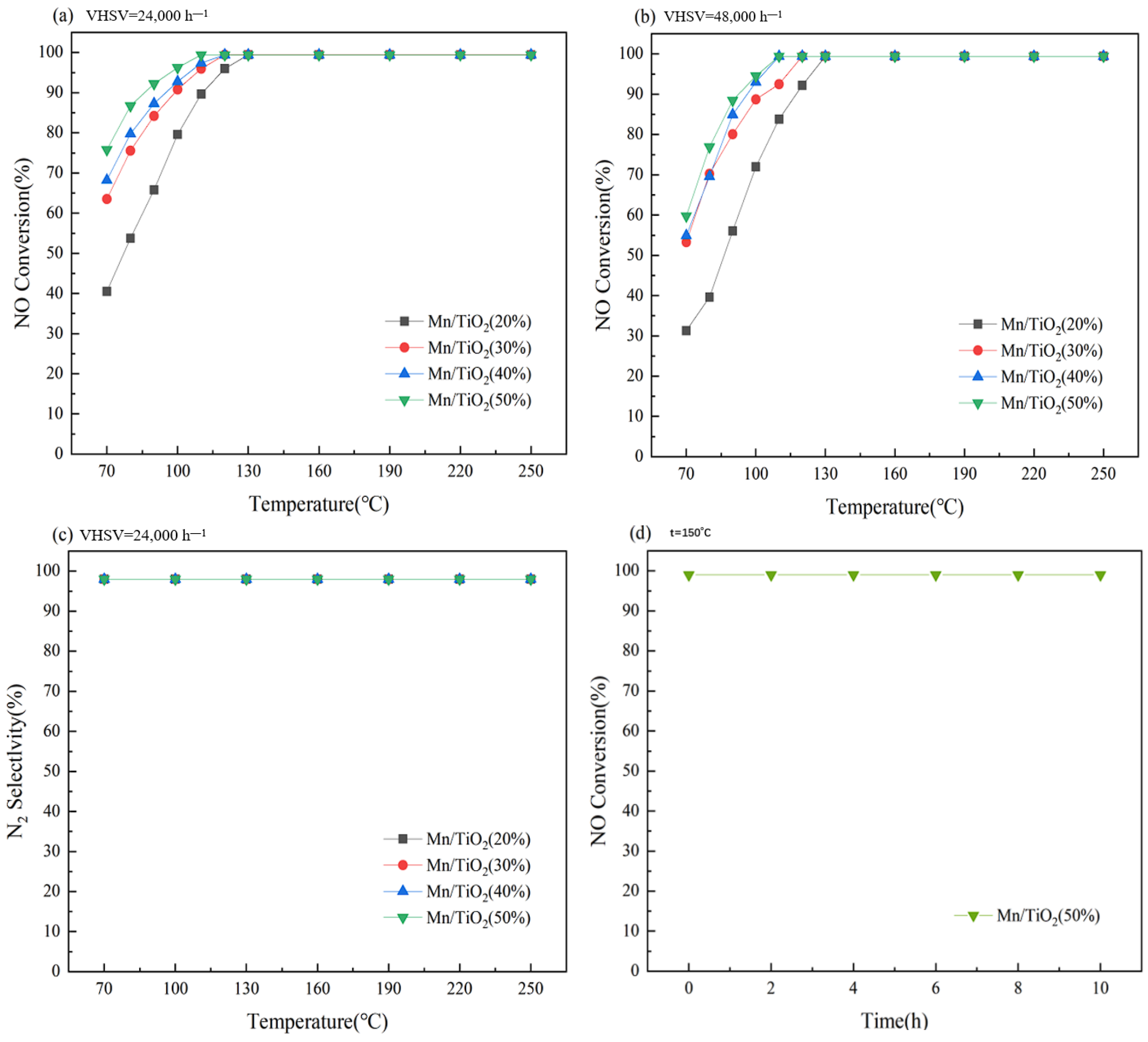
2.1. Catalytic Performance of Catalysts
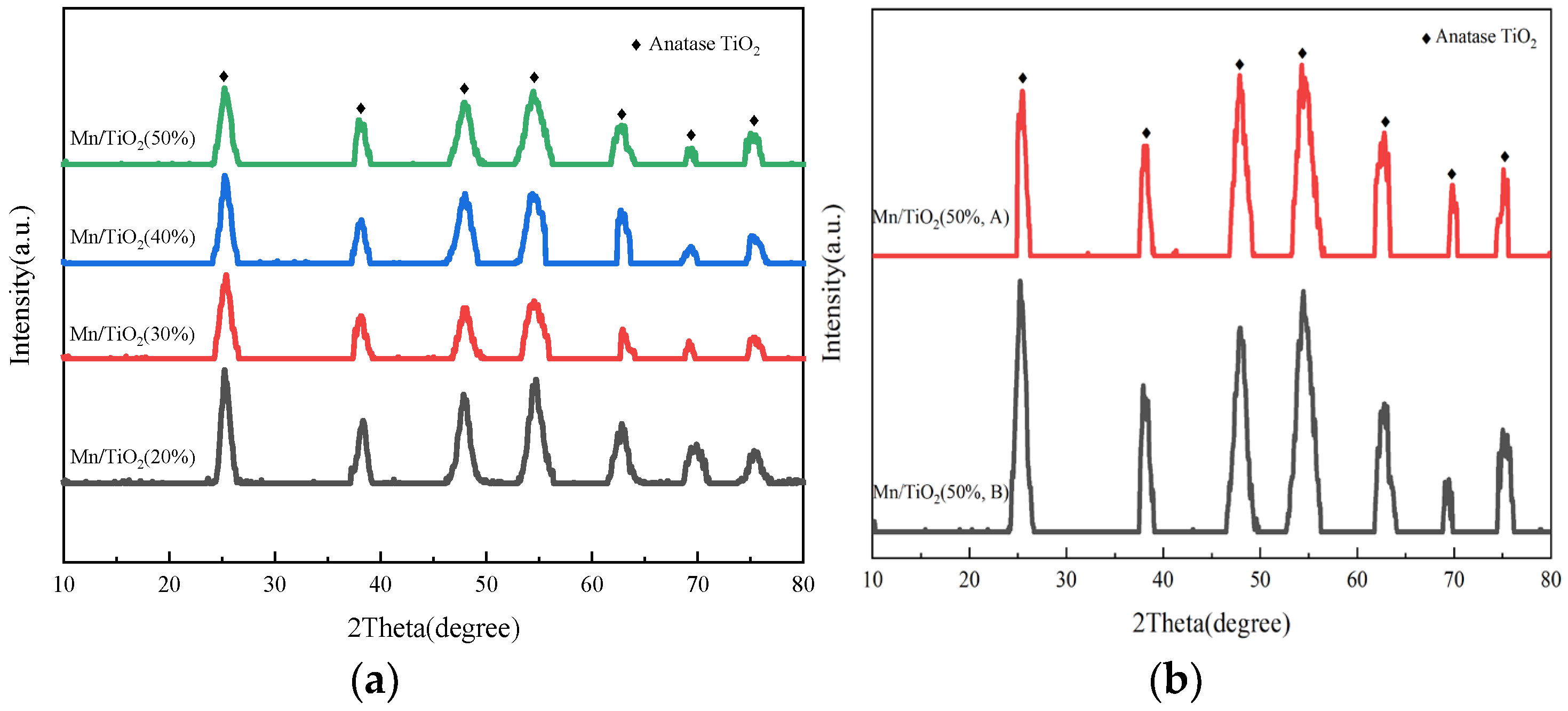
2.2. Morphology, Crystallinity, and Porous Property Analysis
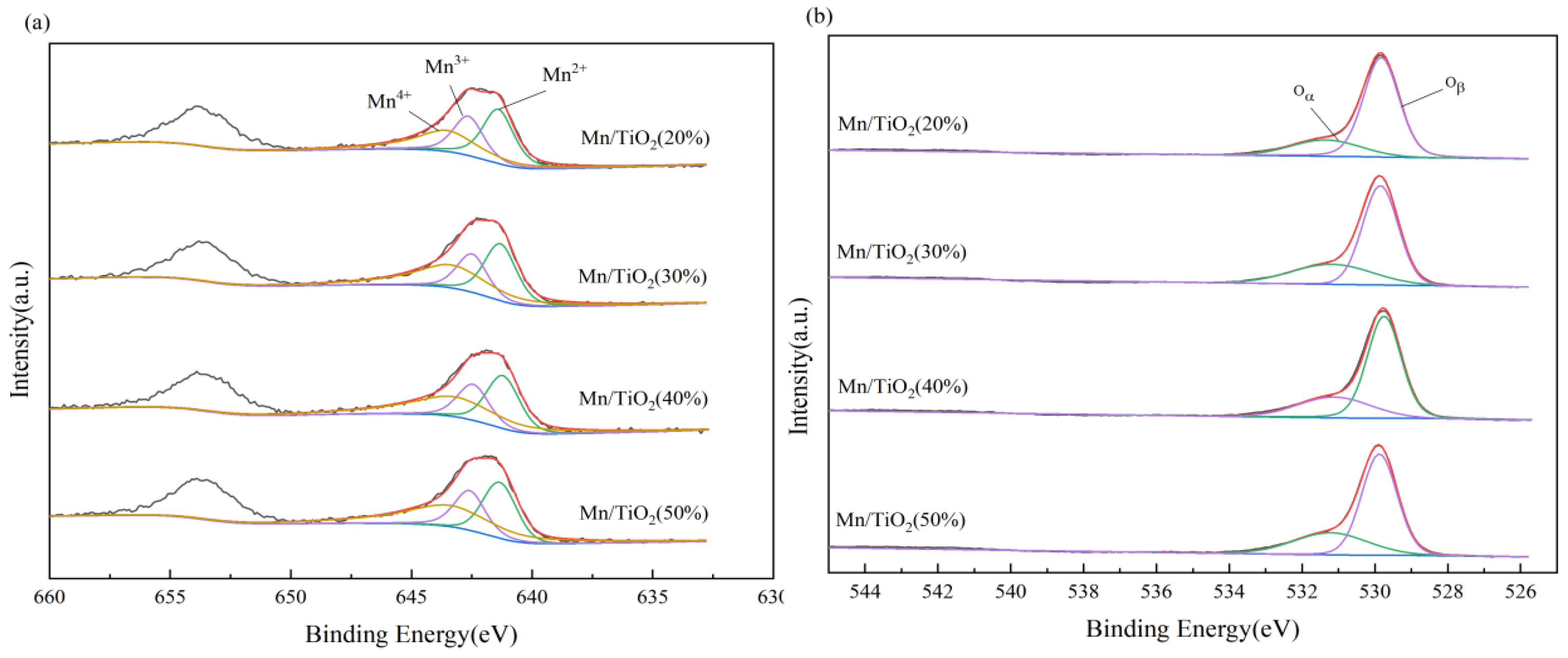
2.3. XPS Analysis
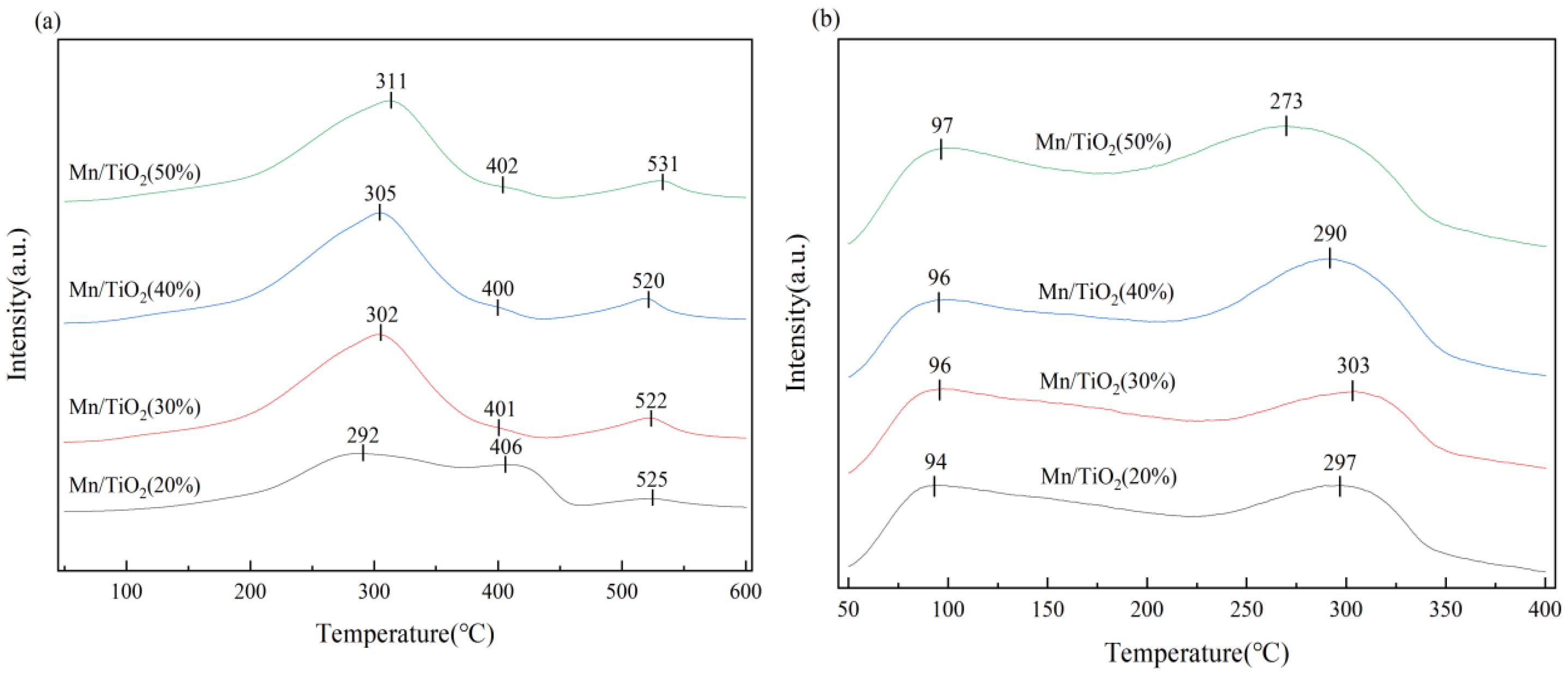
2.4. H2-TPR, NH3-TPD Analysis
2.5. In Situ DRIFTS
3. Experimental Section
3.1. Preparation of Catalysts
3.2. Characterizations
3.3. Performance Testing of Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.F.; Liao, Y.F.; Chen, Y.; Ma, X.Q. In situ DRIFTS FT-IR and DFT study on Fe-VW/Ti removal of NOx and VOCs. Environ. Sci. Pollut. Res. 2022, 29, 81571–81582. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.C.; Nguyen, V.H.; Lasek, J.; Chiang, S.W.; Li, D.X.; Jeffrey, C.S. NOx abatement from stationary emission sources by photo-assisted SCR: Lab-scale to pilot-scale studies. Appl. Catal. A 2016, 523, 294–303. [Google Scholar]
- Zhang, B.L.; Zhang, S.G.; Liu, B. Comparative study on transition element doped Mn-Zr-Ti-oxides catalysts for the low-temperature selective catalytic reduction of NO with NH3. React. Kinet. Mech. Catal. 2019, 127, 637–652. [Google Scholar] [CrossRef]
- Yang, S.J.; Qi, F.H.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J.H.; Xiong, H.C. MnOx supported on Fe-Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B-Environ. 2016, 181, 570–580. [Google Scholar] [CrossRef]
- Xu, S.Y.; Chen, J.W.; Li, Z.G.; Liu, Z.M. Highly ordered mesoporous MnOx catalysis for the NH3-SCR of NOx at low temperatures. Appl. Catal. A-Gen. 2023, 649, 118966. [Google Scholar] [CrossRef]
- Gao, Q.; Han, S.; Ye, Q.; Cheng, S.Y.; Kang, T.F.; Dai, H.X. Effects of Lanthanide Doping on the Catalytic Activity and Hydrothermal Stability of Cu-SAPO-18 for the Catalytic Removal of NOx (NH3-SCR) from Diesel Engines. Catalysts 2020, 10, 336. [Google Scholar] [CrossRef]
- Jung, Y.J.; Cha, J.S.; Kim, B.S. Characteristics of deactivation and thermal regeneration of Nb-doped V2O5-WO3/TiO2 catalyst for NH3-SCR reaction. Environ. Res. 2023, 227, 115744. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Serrano, L.A.; Monte, M.; Iglesias, J.A.; Pavon, C.P.; Portela, R.; Avila, P. MnOx-support interactions in catalytic bodies for selective reduction of NO with NH3. Appl. Catal. B-Environ. 2019, 256, 117821. [Google Scholar] [CrossRef]
- Li, H.R.; Schill, L.; Gao, Q.; Mossin, S.; Riisager, A. The effect of dopants (Fe, Al) on the low-temperature activity and SO2 tolerance in solvothermally synthesized MnOx NH3-SCR catalysts. Fuel 2024, 358, 130111. [Google Scholar] [CrossRef]
- Zhai, G.P.; Han, Z.T.; Wu, X.T.; Du, H.; Gao, Y.; Yang, S.L.; Song, L.G.; Dong, J.M.; Pan, X.X. Pr-modified MnOx catalysts for selective reduction of NO with NH3 at low temperature. J. Taiwan Inst. Chem. Eng. 2021, 125, 132–140. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Huang, T.J.; Xiao, R.; Xu, H.T.; Shen, K.; Zhou, C.C. A comparative study on the Mn/TiO2-M (M = Sn, Zr or Al) Ox catalysts for NH3-SCR reaction at low temperature. Environ. Technol. 2018, 39, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, K.H.; Hong, S.C. MnOx/CeO2-TiO2 mixed oxide catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chem. Eng. J. 2012, 195, 323–331. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Nam, I.S.; Choung, J.W.; Kil, J.K.; Kim, H.J.; Cha, M.S.; Yeo, G.K. High deNOx performance of Mn/TiO2 catalyst by NH3. Catal. Today 2010, 151, 244–250. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B-Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhao, X.T.; Zheng, P.W.; Jiang, H.; Chen, T.; Zhang, Y.R.; Cao, H.L.; Lin, H.; Zhan, R.G. Pd-M-TiO2 (M = Mn, Cu, Ce and Fe) as passive NOx adsorber (PNA) at low temperature. J. Cent. South Univ. 2022, 29, 2253–2265. [Google Scholar] [CrossRef]
- Yan, D.J.; Zhao, J.X.; Li, J.; Abbas, G.; Chen, Z.H.; Guo, T. Improvement of Sb-Modified Mn-Ce/TiO2 Catalyst for SO2 and H2O Resistance at Low-Temperature SCR. Catal. Lett. 2022, 153, 2838–2852. [Google Scholar] [CrossRef]
- Xie, H.D.; He, P.W.; Chen, C.; Yang, C.; Chai, S.; Wang, N.; Ge, C.M. Enhanced Water and Sulfur Resistance by Sm3+ Modification of Ce-Mn/TiO2 for NH3-SCR. Catal. Lett. 2023, 153, 850–862. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wu, P.; Li, G.B.; Zhuang, K.; Shen, W.; Huang, T.J. Improved activity of Ho-modified Mn/Ti catalysts for the selective catalytic reduction of NO with NH. Environ. Sci. Pollut. Res. 2020, 27, 26954–26964. [Google Scholar] [CrossRef]
- Qi, Y.F.; Shan, X.W.; Wang, M.T.; Hu, D.D.; Song, Y.B.; Ge, P.L.; Wu, J. Study on Low-Temperature SCR Denitration Mechanisms of Manganese-Based Catalysts with Different Carriers. Water Air Soil Pollut. 2020, 231, 1–16. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jiang, B.Q.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ. 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Lu, X.L.; Song, C.Y.; Jia, S.H.; Tong, Z.S.; Tang, X.L.; Teng, Y.X. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2-graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Chen, L.Q.; Yuan, F.L.; Li, Z.B.; Niu, X.Y.; Zhu, Y.J. Synergistic effect between the redox property and acidity on enhancing the low temperature NH3-SCR activity for NOx removal over the Co0.2CexMn0.8-xTi10 (x = 0–0.40) oxides catalysts. Chem. Eng. J. 2018, 354, 393–406. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Sreekanth, P.M.; Pena, D.A.; Jenkins, R.G. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2: A comparison for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6436–6443. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.W.; Liu, C.; Gao, C.; Fan, Z.Y.; Niu, C.M. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Zhao, K.; Meng, J.P.; Lu, J.Y.; He, Y.; Huang, H.Z.; Tang, Z.C.; Zhen, X.P. Sol-gel one-pot synthesis of efficient and environmentally friendly iron-based catalysts for NH3-SCR. Appl. Surf. Sci. 2018, 445, 454–461. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Liu, Y.; Wu, Z.B. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Niu, C.H.; Wang, B.R.; Xing, Y.; Su, W.; He, C.; Xiao, L.; Xu, Y.; Zhao, S.; Cheng, Y.H.; Shi, J.W. Thulium modified MnOx/TiO2 catalyst for the low-temperature selective catalytic reduction of NO with ammonia. J. Clean. Prod. 2021, 290, 125858. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhang, T.S.; Su, Z.H.; Long, H.M.; Kong, M.; Yao, L. Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NO. Chem. Eng. J. 2020, 379, 122398. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Y.P.; Zhuang, K.; Shen, K.; Wang, S.; Huang, T.J. Promoting effect and mechanism of neodymium on low-temperature selective catalytic reduction with NH3 over Mn/TiO2 catalysts. J. Rare Earths 2020, 38, 1215–1223. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhu, J.Z.; Li, J.H.; Ma, L.L.; Woo, S.I. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.C.; Meng, T.T.; Xu, B.L.; Gao, F.; Ding, Y.H.; Yu, L.; Fan, Y.N. Advanced MnOx/TiO2 catalyst with preferentially exposed anatase {001} facet for low-temperature SCR of NO. ACS Catal. 2016, 6, 5807–5815. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B-Environ. 2015, 165, 628–635. [Google Scholar] [CrossRef]
- Yao, X.J.; Ma, K.L.; Zou, W.X.; He, S.G.; An, J.B.; Yang, F.M.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]

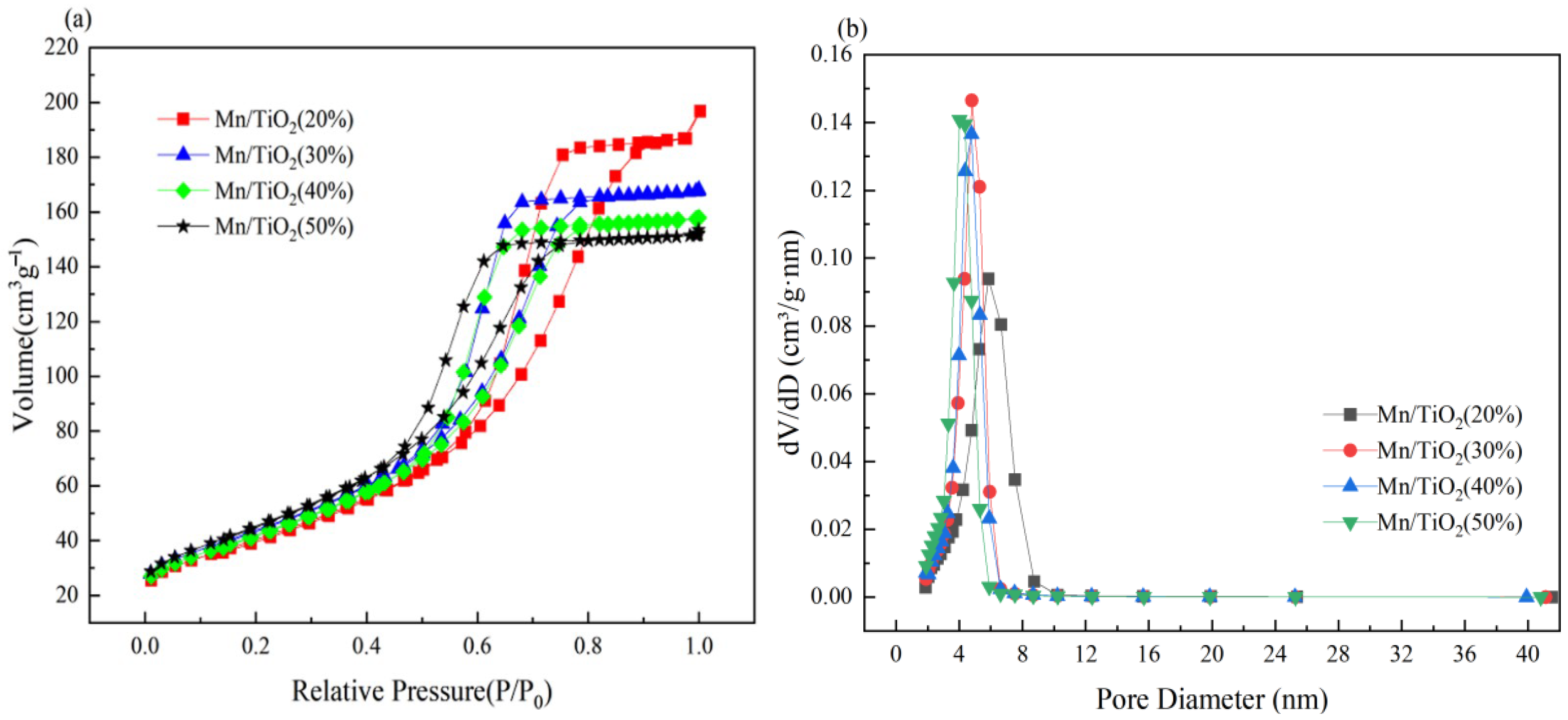



| Catalyst Sample | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Mn/TiO2 (20%) | 146.44 | 0.2908 | 6.15 |
| Mn/TiO2 (30%) | 158.76 | 0.2613 | 5.03 |
| Mn/TiO2 (40%) | 152.77 | 0.2458 | 4.89 |
| Mn/TiO2 (50%) | 165.34 | 0.2365 | 4.38 |
| Catalyst Sample | Mn4+/Mnn+ (%) | Oα/(Oα + Oβ) (%) | H2-TPR Peak Area (%) | NH3-TPD Peak Area (%) |
|---|---|---|---|---|
| Mn/TiO2 (20%) | 35.24 | 22.77 | 83.21 | 76.28 |
| Mn/TiO2 (30%) | 42.07 | 31.06 | 95.87 | 78.57 |
| Mn/TiO2 (40%) | 44.55 | 29.87 | 97.44 | 84.28 |
| Mn/TiO2 (50%) | 45.40 | 31.41 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, Z.; Xu, B.; Hu, J.; Pan, D.; Fan, G.; Zhang, L.; Zhou, Z. A High-Performance Mn/TiO2 Catalyst with a High Solid Content for Selective Catalytic Reduction of NO at Low-Temperatures. Molecules 2024, 29, 3467. https://doi.org/10.3390/molecules29153467
Yang L, Wang Z, Xu B, Hu J, Pan D, Fan G, Zhang L, Zhou Z. A High-Performance Mn/TiO2 Catalyst with a High Solid Content for Selective Catalytic Reduction of NO at Low-Temperatures. Molecules. 2024; 29(15):3467. https://doi.org/10.3390/molecules29153467
Chicago/Turabian StyleYang, Lei, Zhen Wang, Bing Xu, Jie Hu, Dehua Pan, Guozhi Fan, Lei Zhang, and Ziyang Zhou. 2024. "A High-Performance Mn/TiO2 Catalyst with a High Solid Content for Selective Catalytic Reduction of NO at Low-Temperatures" Molecules 29, no. 15: 3467. https://doi.org/10.3390/molecules29153467





