Biodiesel Production from Waste Cooking Oil Using Recombinant Escherichia coli Cells Immobilized into Fe3O4–Chitosan Magnetic Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Synthesis of Fe3O4 Magnetic Nanoparticle
2.3. Immobilization of E. coil Cells in Fe3O4–Chitosan Magnetic Microspheres
2.4. Characterization of Magnetic Carrier and MWCC
2.5. Lipase Activity Assay
2.6. Methanol Resistance Assays
2.7. MWCC-Catalyzed Synthesis of FAME
2.8. Components Analysis of Reaction Mixtures
2.9. Recycling of MWCC for FAME Production
2.10. Statistical Analysis
3. Results and Discussion
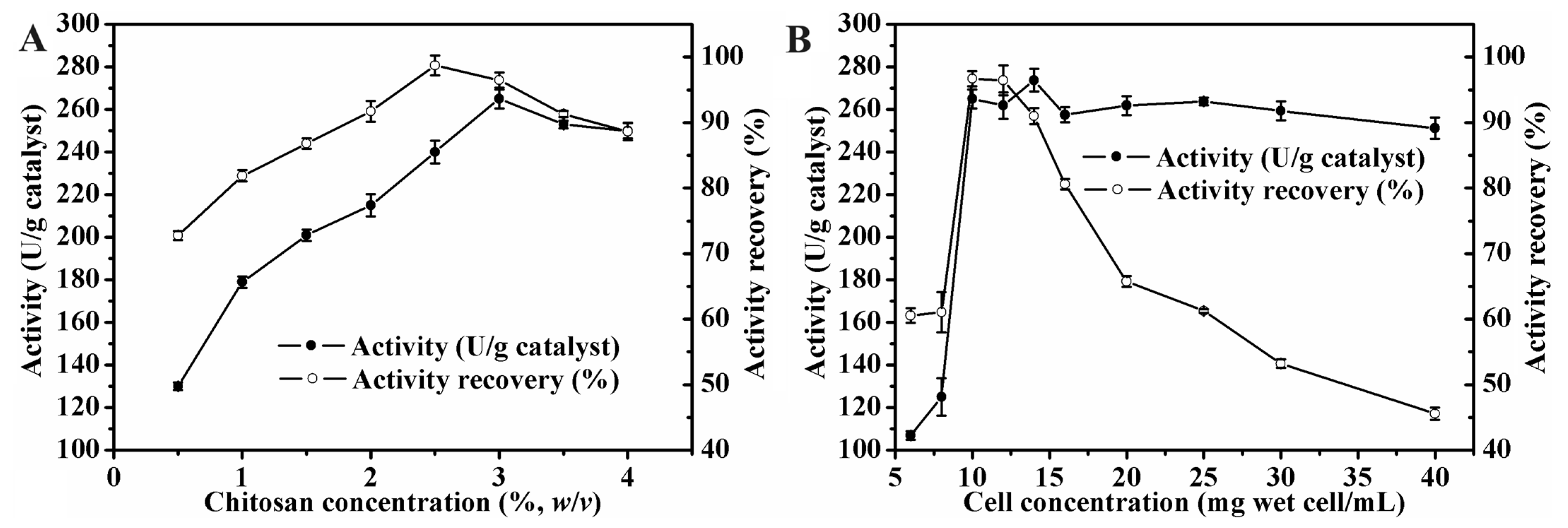
3.1. Effect of Immobilization Parameters on Lipase Activity of MWCC@MAS1
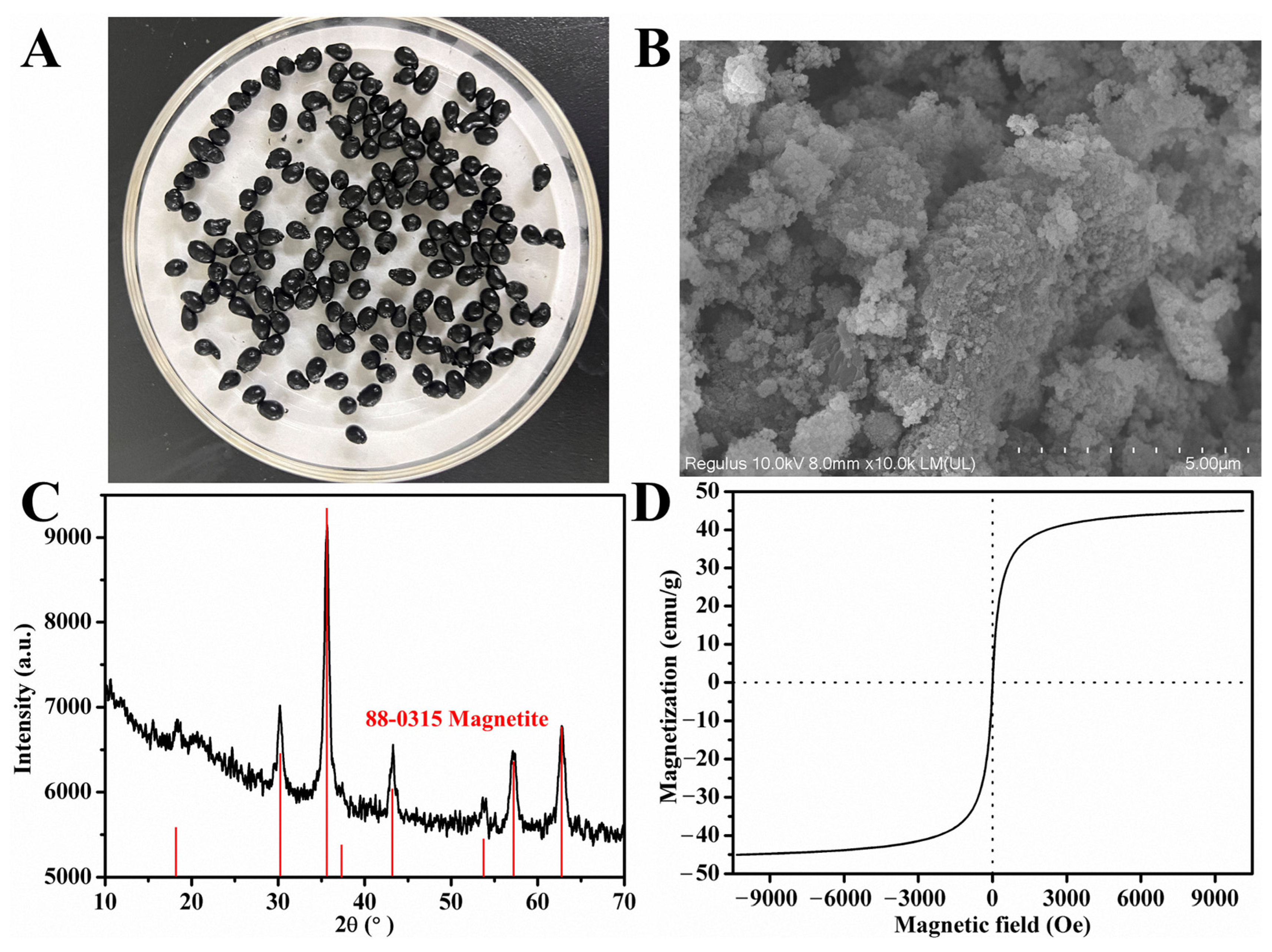
3.2. Characterization of MWCC@MAS1
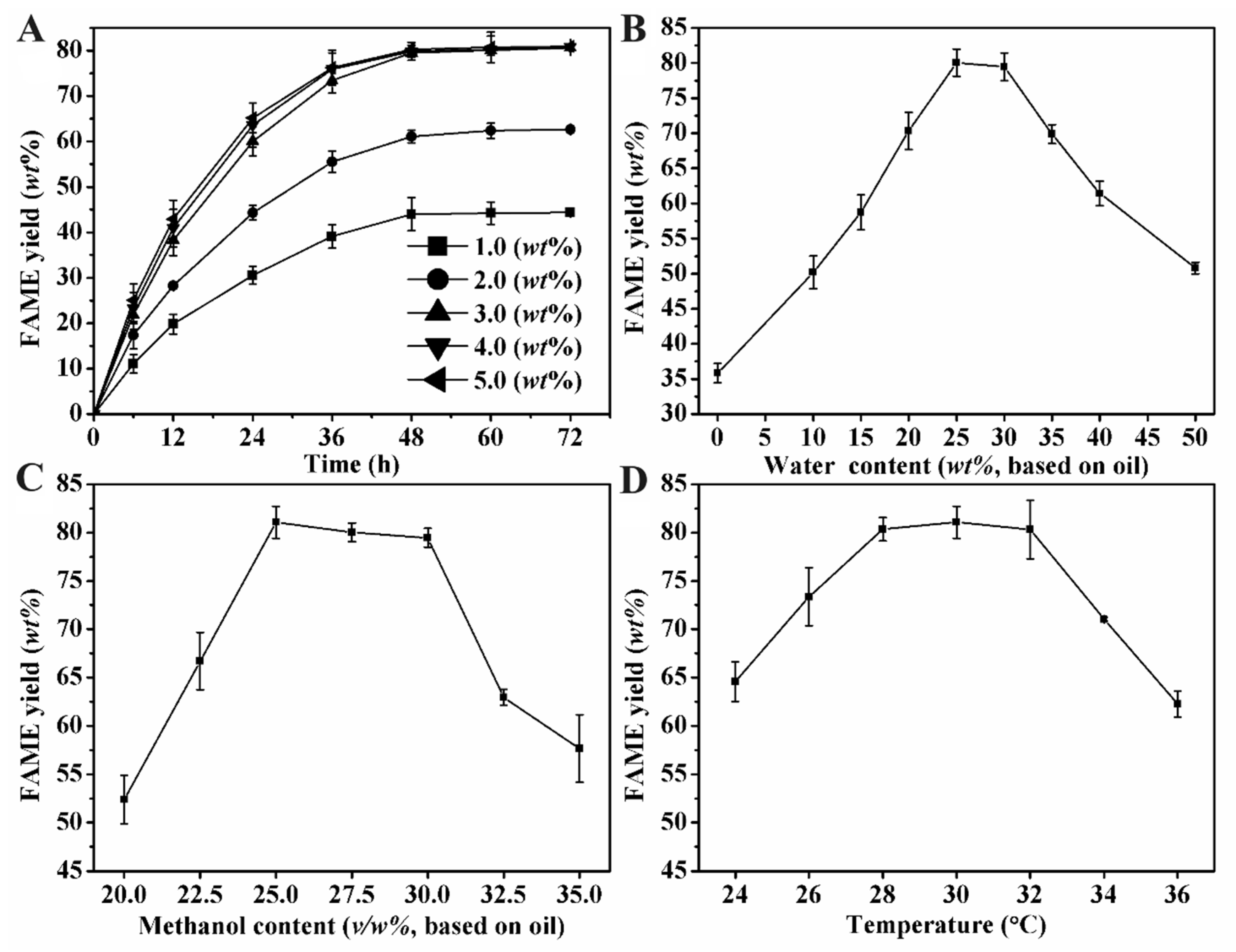
3.3. Effects of Synthesis Conditions on Biodiesel Yield
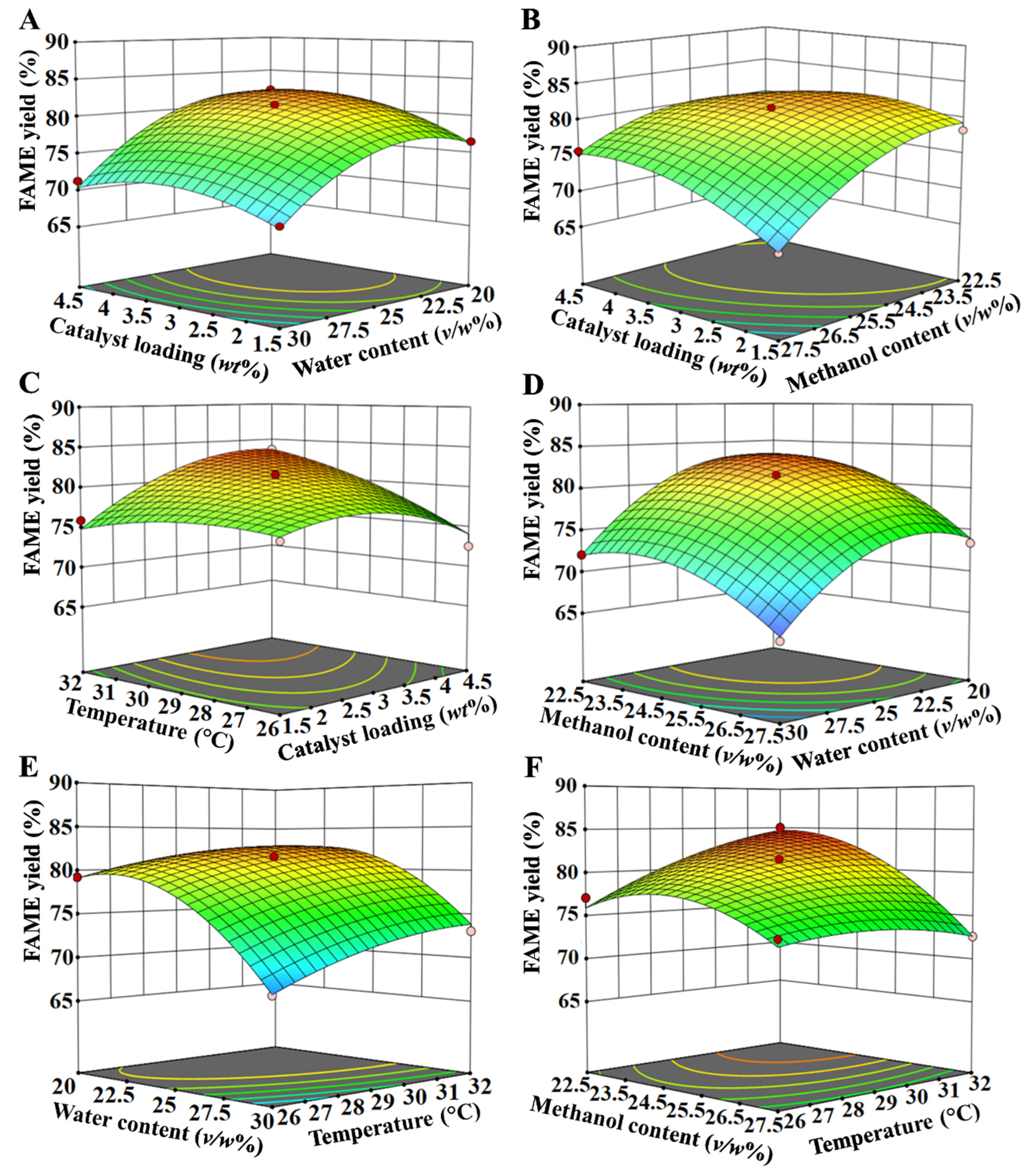
3.4. Optimization of Reaction Parameters for Biodiesel Production
− 2.65A2 − 4.34B2 − 3.21C2 − 1.25D2
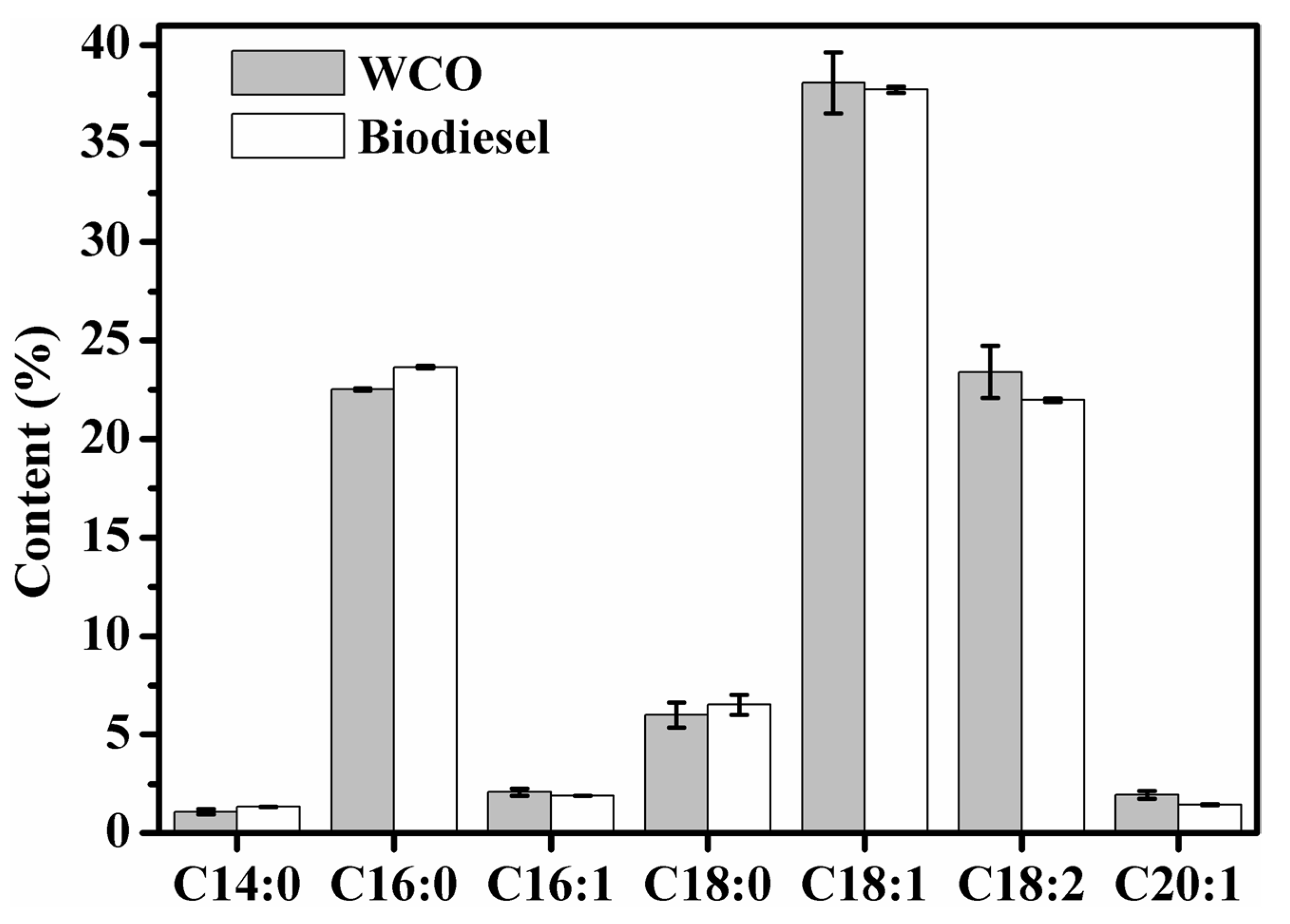
3.5. FA Composition Analysis of FAME
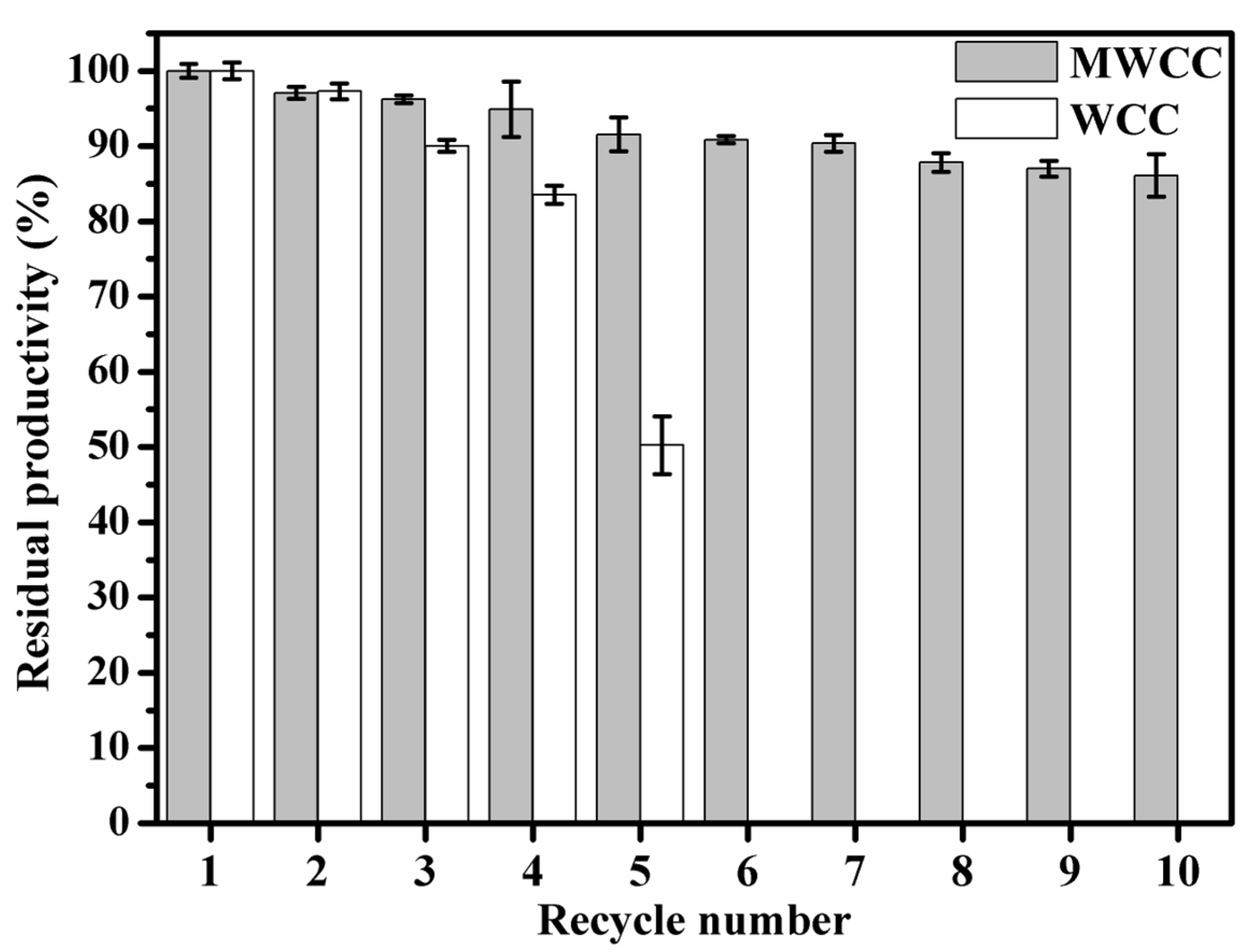
3.6. Recycling of MWCC@MAS1 in the Conversion of WCO to Biodiesel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Ndeddy Aka, R.J. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Topare, N.S.; Jogdand, R.I.; Shinde, H.P.; More, R.S.; Khan, A.; Asiri, A.M. A short review on approach for biodiesel production: Feedstock’s, properties, process parameters and environmental sustainability. Mater. Today Proc. 2022, 57, 1605–1612. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Hari, A.; Inayat, A.; Yousef, L.A.; Alarab, S.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Shanmugam, S.; Kikas, T. Recent advances on biodiesel production from waste cooking oil (WCO): A review of reactors, catalysts, and optimization techniques impacting the production. Fuel 2023, 348, 128514. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Kalogirou, S.A.; Gupta, V.K.; Park, Y.-K.; Fallahi, A.; Sulaiman, A.; Ranjbari, M.; Rahnama, H.; Aghbashlo, M.; et al. Environmental life cycle assessment of biodiesel production from waste cooking oil: A systematic review. Renew. Sustain. Energy Rev. 2022, 161, 112411. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. Production of biodiesel using high free fatty acid feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Transesterification of vegetable oil to biodiesel fuel using alkaline catalyst. Fuel 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Yeom, S.H.; Go, Y.W. Optimization of a novel two-step process comprising re-esterification and transesterification in a single reactor for biodiesel production using waste cooking oil. Biotechnol. Bioprocess Eng. 2018, 23, 432–441. [Google Scholar] [CrossRef]
- Dos Santos, L.K.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Experimental factorial design on hydroesterification of waste cooking oil by subcritical conditions for biodiesel production. Renew. Energy 2017, 114, 574–580. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. Maximizing biodiesel production from high free fatty acids feedstocks through glycerolysis treatment. Biomass Bioenergy 2021, 146, 105997. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Madavi, T.B.; Chauhan, S.; Keshri, A.; Alavilli, H.; Choi, K.-Y.; Pamidimarri, S.D.V.N. Whole-cell biocatalysis: Advancements toward the biosynthesis of fuels. Biofuels Bioprod. Biorefin. 2022, 16, 859–876. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Karishma, S. Bio-derived catalysts for production of biodiesel: A review on feedstock, oil extraction methodologies, reactors and lifecycle assessment of biodiesel. Fuel 2022, 316, 123379. [Google Scholar] [CrossRef]
- Ye, M.; Ye, Y.; Du, Z.; Chen, G. Cell-surface engineering of yeasts for whole-cell biocatalysts. Bioprocess Biosyst. Eng. 2021, 44, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Huang, J.; Xu, L.; Wang, C.; Cai, J. One-step production of biodiesel by wet Escherichia coli cells expressing a non-specific and methanol-resistant lipase. Process Biochem. 2023, 125, 75–83. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Xu, L.; Cai, J.; Wang, J.; Wang, Y. Structural basis for the regiospecificity of a lipase from Streptomyces sp. W007. Int. J. Mol. Sci. 2022, 23, 5822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hou, S.; Lan, D.; Wang, X.; Liu, J.; Khan, F.I.; Wang, Y. Crystal structure of a lipase from Streptomyces sp. strain W007—Implications for thermostability and regiospecificity. FEBS J. 2017, 284, 3506–3519. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Su, Y.; Li, Q.; Gu, S.; Liu, Q.; He, W.; Huang, J.; Wu, W.; Qi, F. Development of biochar-based whole-cell biocatalysts for the production of L-tryptophan and L-phenylalanine. ACS Sustain. Chem. Eng. 2023, 11, 9412–9423. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Z. Microbial biocatalysis. Catalysts 2023, 13, 629. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Yan, B.; Yi, W.; Yao, J. Biodiesel production in a magnetically fluidized bed reactor using whole-cell biocatalysts immobilized within ferroferric oxide-polyvinyl alcohol composite beads. Bioresour. Technol. 2022, 355, 127253. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Yao, J.; Qi, Y.; Yan, B. Biodiesel production from waste cooking oil in a magnetically fluidized bed reactor using whole-cell biocatalysts. Energy Convers. Manag. 2017, 138, 556–564. [Google Scholar] [CrossRef]
- Tohfegar, E.; Habibi, A. Immobilization of Candida catenulata cells by surface-loading of an amino-functionalized Fe3O4 nanoparticles and its application as the sustainable whole-cell biocatalyst for enzymatic biodiesel production. Energy Convers. Manag. 2023, 293, 117503. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, S.; Tao, Y.; Wang, P.; Ma, X.; Chen, L. Enahanced biosorption of Cu(II) by magnetic chitosan microspheres immobilized Aspergillus sydowii (MCMAs) from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123813. [Google Scholar] [CrossRef]
- Negi, H.; Verma, P.; Singh, R.K. A comprehensive review on the applications of functionalized chitosan in petroleum industry. Carbohydr. Polym. 2021, 266, 118125. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, J.; Qi, Y.; Yao, J.; Yan, B. Biodiesel production using magnetic whole-cell biocatalysts by immobilization of Pseudomonas mendocina on Fe3O4-chitosan microspheres. Biochem. Eng. J. 2016, 113, 86–92. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mai, Q.-Y.; Qin, X.-L.; Yang, B.; Wang, Z.-L.; Chen, H.-T. Establishment of an evaluation model for human milk fat substitutes. J. Agric. Food Chem. 2010, 58, 642–649. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, G.; Lin, Q.; Gu, Y.; Wang, X.; Feng, Z.; Sengupta, A. Application of 3D magnetic nanocomposites: MXene-supported Fe3O4@CS nanospheres for highly efficient adsorption and separation of dyes. Sci. Total Environ. 2022, 822, 153544. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Zhu, Y.; Jiang, W.; Shi, F.; Hu, J.; Jiang, S.; Jian, S. Epichlorohydrin and triethylenetetramine functionalized electrosprayed Fe3O4/Chitosan magnetic microspheres for removal and separation of Congo red. Chem. Eng. J. 2023, 476, 146907. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Bai, X.; Xu, J.; Zhang, J.; Zhang, G.; Huang, C.; Liu, W.; Huang, C.; Xiong, X. Microfluidic preparation of magnetic chitosan microsphere and its adsorption towards Congo red. J. Polym. Res. 2023, 30, 1–10. [Google Scholar] [CrossRef]
- Tian, K.; Li, Z. High-yielding, one-pot, and green production of biodiesel from waste grease using wet cells of a recombinant Escherichia coli strain as catalyst. Biochem. Eng. J. 2016, 115, 30–37. [Google Scholar] [CrossRef]
- Yan, J.; Li, A.; Xu, Y.; Ngo, T.P.N.; Phua, S.; Li, Z. Efficient production of biodiesel from waste grease: One-pot esterification and transesterification with tandem lipases. Bioresour. Technol. 2012, 123, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sudalai, S.; Vairaprakash, P.; Devanesan, M.G.; Arumugam, A. Sustainable biodiesel production from Madhuca indica oil using a functionalized industrial waste as a catalyst: Ready to scale-up approach. Ind. Crop. Prod. 2023, 193, 116233. [Google Scholar] [CrossRef]
- Karkal, S.S.; Rathod, D.R.; Jamadar, A.S.; Mamatha, S.S.; Kudre, T.G. Production optimization, scale-up, and characterization of biodiesel from marine fishmeal plant oil using Portunus sanguinolentus crab shell derived heterogeneous catalyst. Biocatal. Agric. Biotechnol. 2023, 47, 102571. [Google Scholar] [CrossRef]
- Abdulla, R.; Derman, E.; K. Mathialagan, T.; Yaser, A.Z.; Abu Samah, M.A.; Gansau, J.A.; Syed Najmuddin, S.U.F. Biodiesel production from waste palm cooking oil using immobilized Candida rugosa lipase. Sustainability 2022, 14, 13632. [Google Scholar] [CrossRef]
- Karunanithi, G.; Varadappan, A.M.S. Enzymatic transesterification of Phoenix dactylifera L. oil using lipase immobilized on activated carbon and optimization using response surface methodology. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Dharmalingam, B.; Balamurugan, S.; Wetwatana, U.; Tongnan, V.; Sekhar, C.; Paramasivam, B.; Cheenkachorn, K.; Tawai, A.; Sriariyanun, M. Comparison of neural network and response surface methodology techniques on optimization of biodiesel production from mixed waste cooking oil using heterogeneous biocatalyst. Fuel 2023, 340, 127503. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; dos Santos, H.C.L.; da Silva, M.A.R.; da Cas Viegas, A.; da Rocha Filho, G.N.; da Conceição, L.R.V. Biodiesel production from waste cooking oil using an innovative magnetic solid acid catalyst based on Ni–Fe ferrite: RSM-BBD optimization approach. J. Ind. Eng. Chem. 2024, 135, 270–285. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Liu, M.; Ma, L.; Zhang, W. Co-Immobilization of lipases with different specificities for efficient and recyclable biodiesel production from waste oils: Optimization using response surface methodology. Int. J. Mol. Sci. 2023, 24, 4726. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.M.R.; Lacson, C.F.Z.; Choi, A.E.S.; Chung, T.-W.; Retumban, J.D.; Abarca, R.R.M.; Grisdanurak, N.; de Luna, M.D.G. Biodiesel production from soybean oil via LiOH-pumice catalytic transesterification and BBD-RSM optimization. Energy Rep. 2024, 11, 4032–4043. [Google Scholar] [CrossRef]
- Buasri, A.; Sirikoom, P.; Pattane, S.; Buachum, O.; Loryuenyong, V. Process optimization of biodiesel from used cooking oil in a microwave reactor: A case of machine learning and Box–Behnken design. ChemEngineering 2023, 7, 65. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Qin, X.; Zhao, Z.; Yang, B.; Wang, Y. Immobilized MAS1 lipase-catalyzed synthesis of n-3 PUFA-rich triacylglycerols in deep eutectic solvents. J. Oleo Sci. 2021, 70, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Rashidi Nodeh, H.; Oryani, B.; Rezania, S. Lipase enzyme immobilized over magnetic titanium graphene oxide as catalyst for biodiesel synthesis from waste cooking oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Q.; Zhang, R.; Liu, M.; Zhang, W. Efficient biodiesel production from waste cooking oil by fast co-immobilization of lipases from Aspergillus oryzae and Rhizomucor miehei in magnetic chitosan microcapsules. Process Biochem. 2023, 125, 171–180. [Google Scholar] [CrossRef]
- Liu, M.; Ma, L.; Zhang, Z.; Zhang, X.; Zhang, W. Development of a sustainable co-immobilized lipases on Co2+-chelated magnetic metal-organic framework for efficient biodiesel production from waste oil. Mol. Catal. 2024, 563, 114241. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef]
| WCC@MAS1 | MWCC@MAS1 | |
|---|---|---|
| 51.01 ± 1.23 [15] | 72.82 ± 0.58 | |
| 6.80 ± 0.01 [15] | 9.42 ± 0.32 |
| Variables | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Catalyst loading (wt%) | A | 30 | 60 | 90 |
| Water content (v/w%) | B | 400 | 500 | 600 |
| Methanol content (v/w%) | C | 450 | 500 | 550 |
| Temperature (°C) | D | 26 | 29 | 32 |
| Run | Coded Value of Reaction Variables | FAME Yield (%) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | Predicted | Experimental | |
| 1 | −1 | −1 | 0 | 0 | 76.32 | 76.53 |
| 2 | 1 | −1 | 0 | 0 | 81.26 | 82.07 |
| 3 | −1 | 1 | 0 | 0 | 68.68 | 69 |
| 4 | 1 | 1 | 0 | 0 | 70.34 | 71.28 |
| 5 | 0 | 0 | −1 | −1 | 75.93 | 77.1 |
| 6 | 0 | 0 | 1 | −1 | 73.39 | 74.19 |
| 7 | 0 | 0 | −1 | 1 | 84.71 | 85.06 |
| 8 | 0 | 0 | 1 | 1 | 72.65 | 72.63 |
| 9 | −1 | 0 | 0 | −1 | 76.35 | 75.89 |
| 10 | 1 | 0 | 0 | −1 | 74.11 | 72.56 |
| 11 | −1 | 0 | 0 | 1 | 74.83 | 75.89 |
| 12 | 1 | 0 | 0 | 1 | 83.67 | 83.64 |
| 13 | 0 | −1 | −1 | 0 | 82.74 | 82.43 |
| 14 | 0 | 1 | 0 | 0 | 72.15 | 72.06 |
| 15 | 0 | −1 | 1 | 0 | 73.97 | 73.41 |
| 16 | 0 | 1 | 1 | 0 | 65.89 | 65.45 |
| 17 | −1 | 0 | −1 | 0 | 79.34 | 78.31 |
| 18 | 1 | 0 | −1 | 0 | 78.52 | 77.95 |
| 19 | −1 | 0 | 1 | 0 | 67.92 | 67.8 |
| 20 | 1 | 0 | 1 | 0 | 75.34 | 75.68 |
| 21 | 0 | −1 | 0 | −1 | 79.11 | 79.23 |
| 22 | 0 | 1 | 0 | −1 | 67.95 | 67.87 |
| 23 | 0 | −1 | 0 | 1 | 81.25 | 80.65 |
| 24 | 0 | 1 | 0 | 1 | 73.85 | 73.05 |
| 25 | 0 | 0 | 0 | 0 | 81.13 | 81.01 |
| 26 | 0 | 0 | 0 | 0 | 81.13 | 81.58 |
| 27 | 0 | 0 | 0 | 0 | 81.13 | 80.8 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 703.07 | 14 | 50.22 | 55.13 | < 0.0001 | significant |
| Residual | 10.93 | 12 | 0.9109 | - | - | - |
| Lack of Fit | 10.61 | 10 | 1.06 | 6.51 | 0.1404 | not significant |
| Pure Error | 0.3258 | 2 | 0.1629 | - | - | - |
| Cor Total | 714 | 26 | - | - | - | - |
| R2 = 0.9848; RAdj2 = 0.9668 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Han, M.; Zhou, L.; Wang, C.; Lin, J.; Du, X.; Cai, J. Biodiesel Production from Waste Cooking Oil Using Recombinant Escherichia coli Cells Immobilized into Fe3O4–Chitosan Magnetic Microspheres. Molecules 2024, 29, 3469. https://doi.org/10.3390/molecules29153469
Zhao Z, Han M, Zhou L, Wang C, Lin J, Du X, Cai J. Biodiesel Production from Waste Cooking Oil Using Recombinant Escherichia coli Cells Immobilized into Fe3O4–Chitosan Magnetic Microspheres. Molecules. 2024; 29(15):3469. https://doi.org/10.3390/molecules29153469
Chicago/Turabian StyleZhao, Zexin, Meiling Han, Ling Zhou, Changgao Wang, Jianguo Lin, Xin Du, and Jun Cai. 2024. "Biodiesel Production from Waste Cooking Oil Using Recombinant Escherichia coli Cells Immobilized into Fe3O4–Chitosan Magnetic Microspheres" Molecules 29, no. 15: 3469. https://doi.org/10.3390/molecules29153469
APA StyleZhao, Z., Han, M., Zhou, L., Wang, C., Lin, J., Du, X., & Cai, J. (2024). Biodiesel Production from Waste Cooking Oil Using Recombinant Escherichia coli Cells Immobilized into Fe3O4–Chitosan Magnetic Microspheres. Molecules, 29(15), 3469. https://doi.org/10.3390/molecules29153469






