Multifunctional Biocomposites: Synthesis, Characterization, and Prospects for Regenerative Medicine and Controlled Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Multifunctional Composite Biomaterials
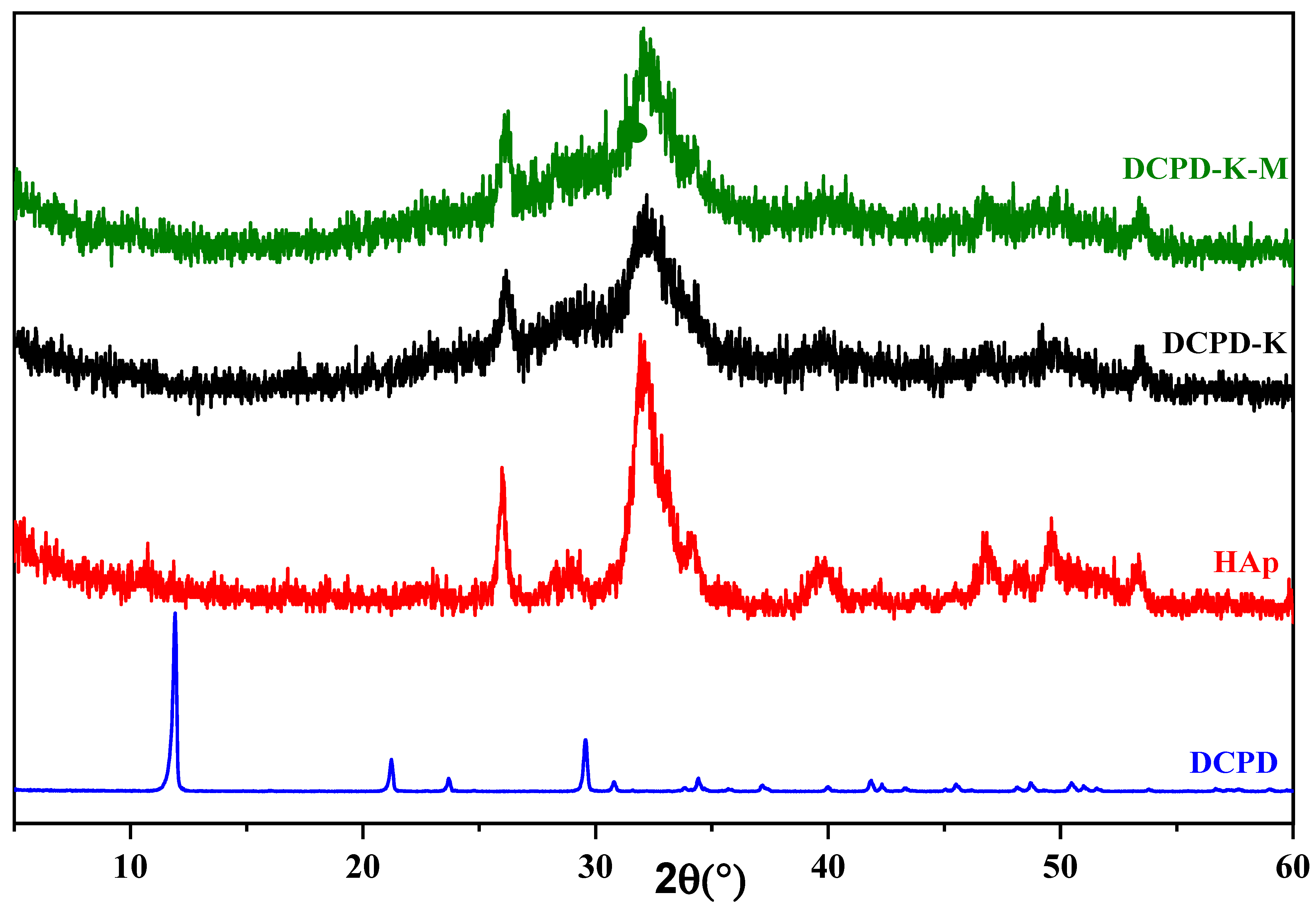
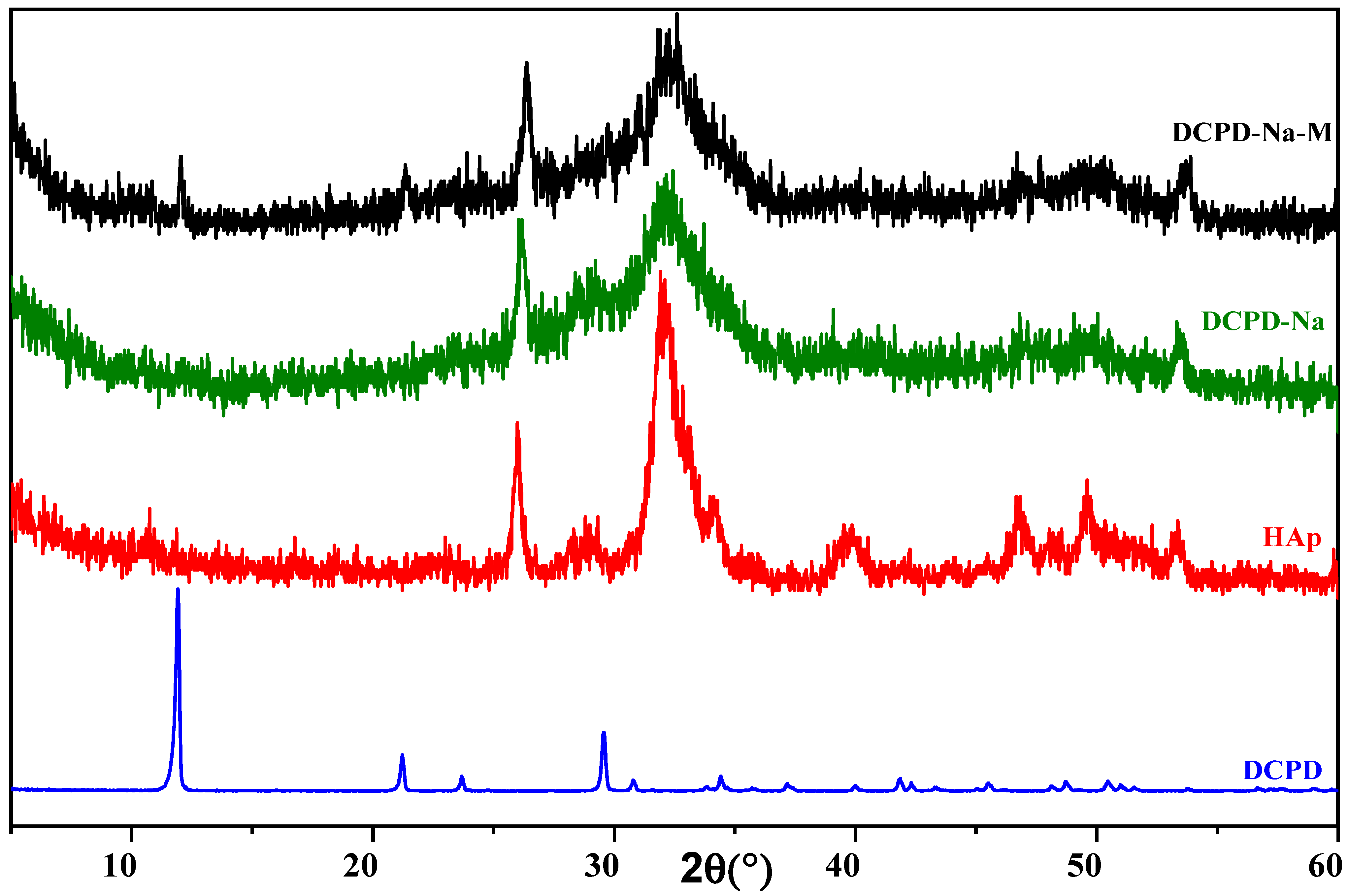
2.1.1. Characterization by X-ray Diffraction
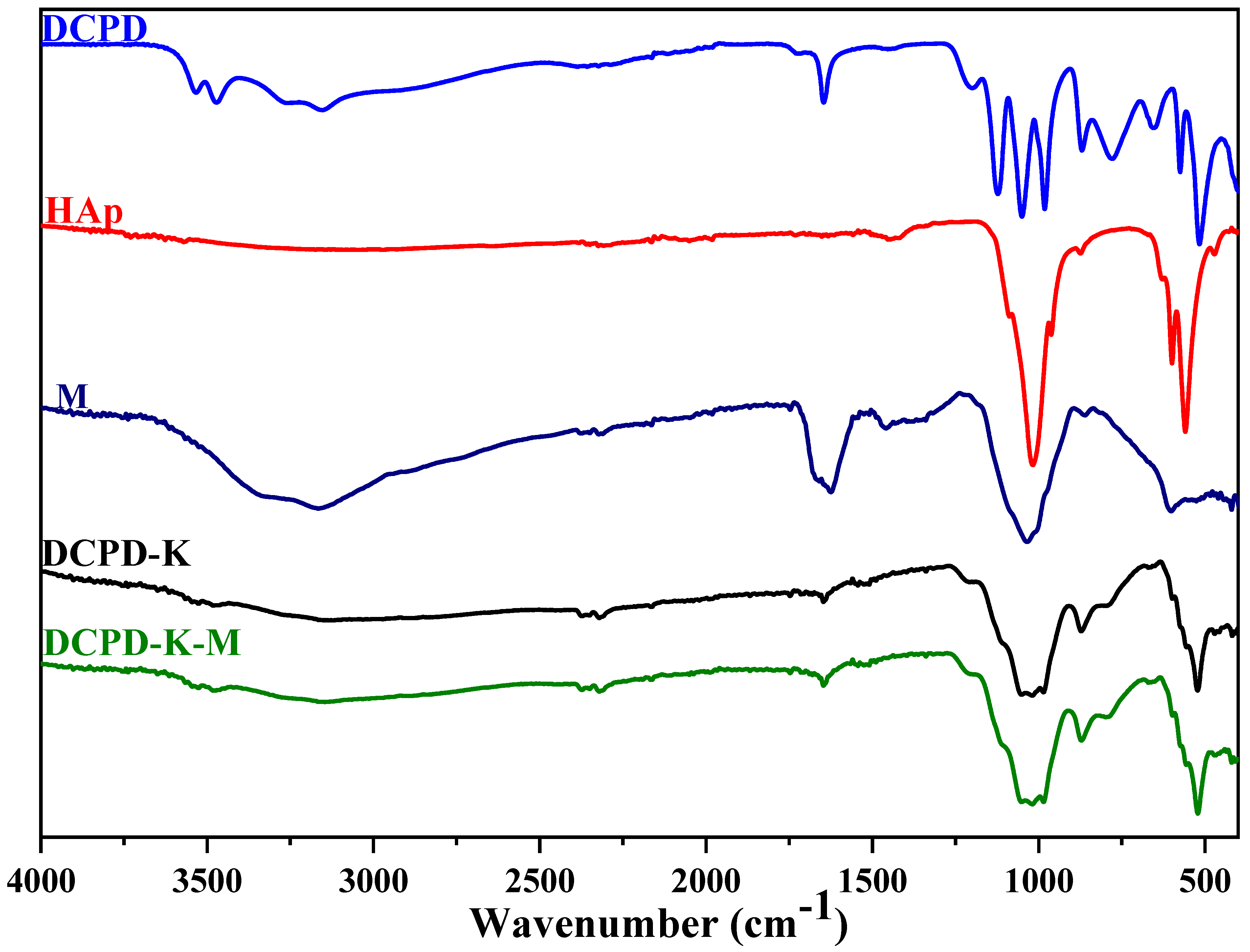
2.1.2. Characterization by Infrared Spectroscopy
2.2. Study of Biomaterial Surface Charges
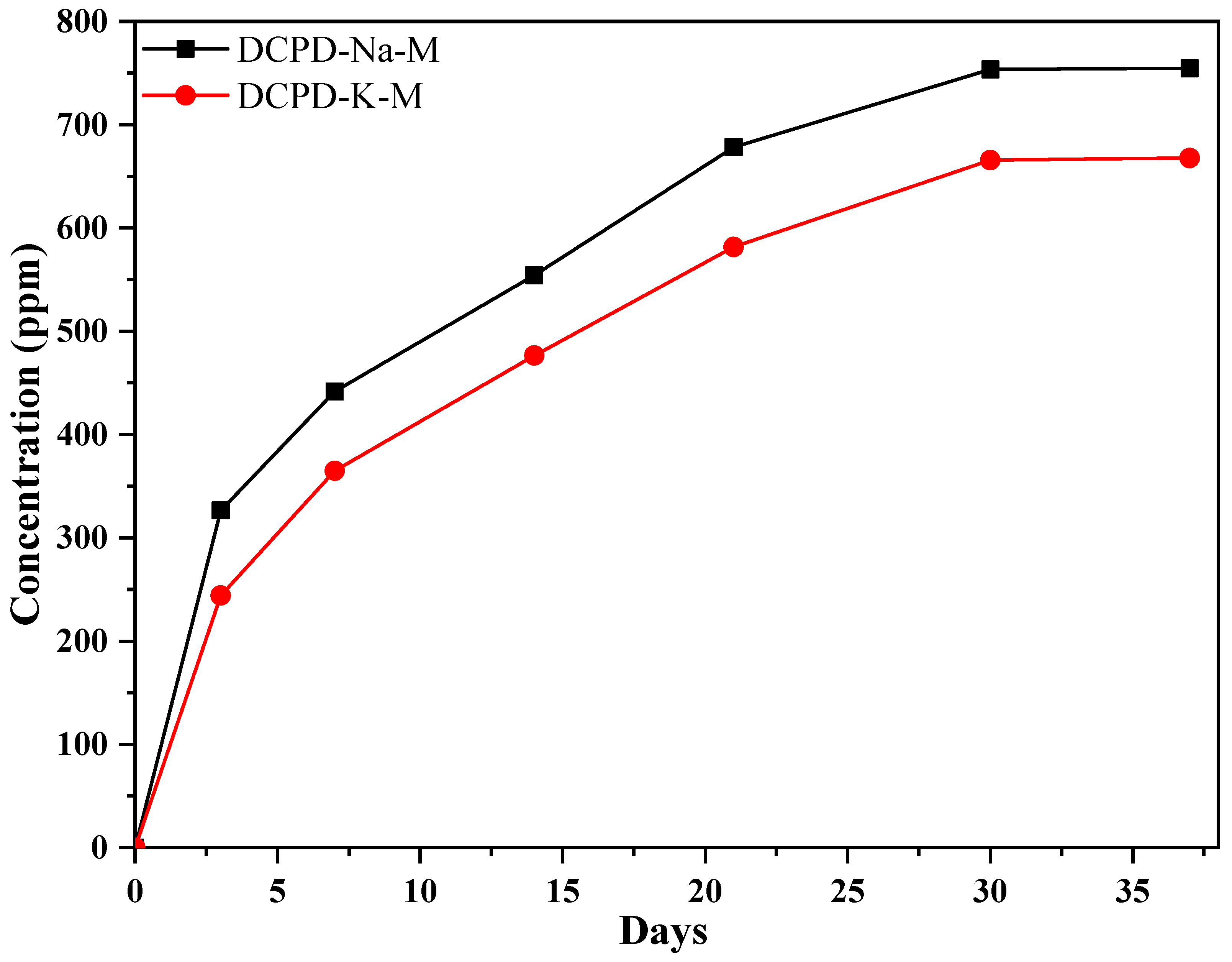
2.3. Antibiotic Release Into SBF Solution
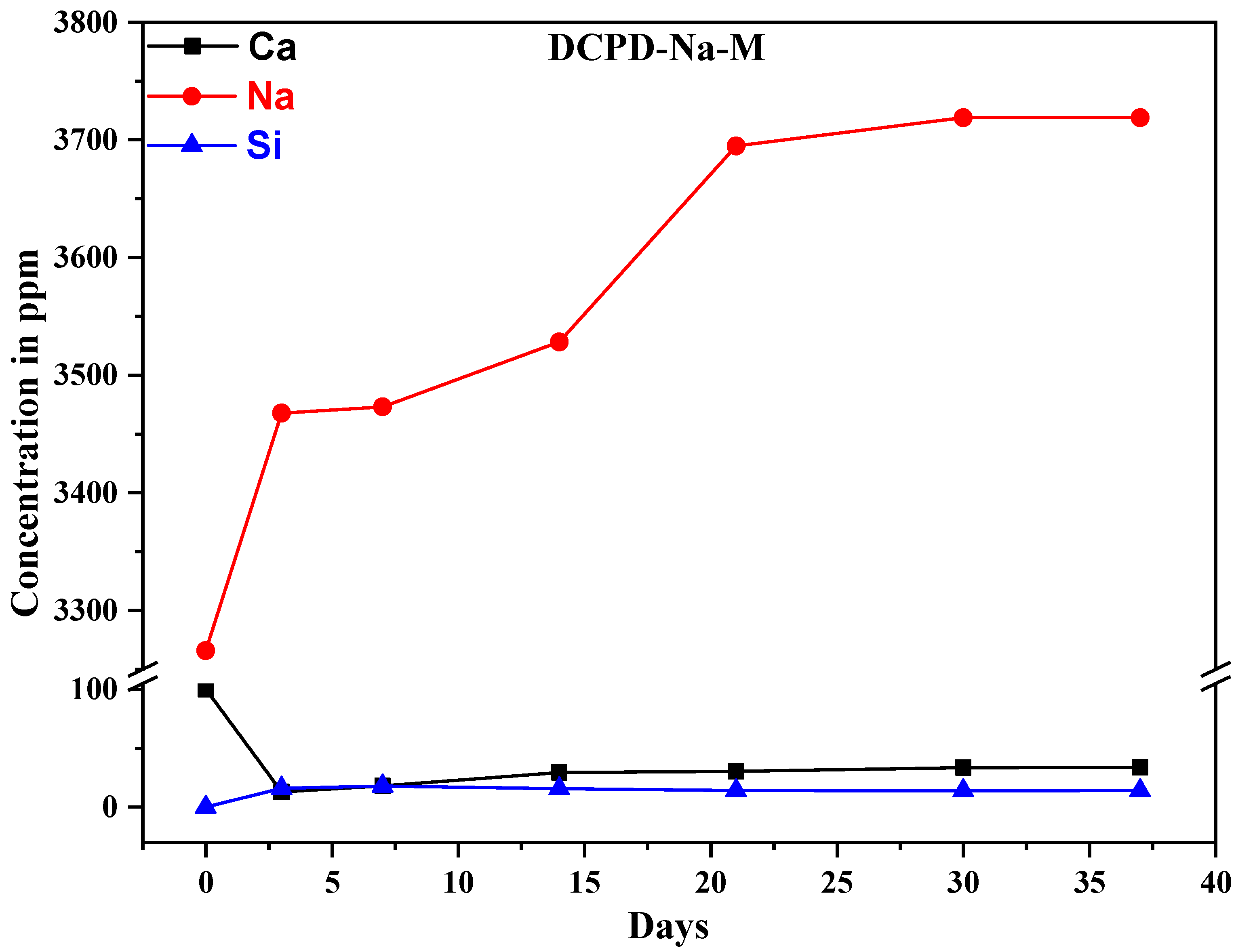
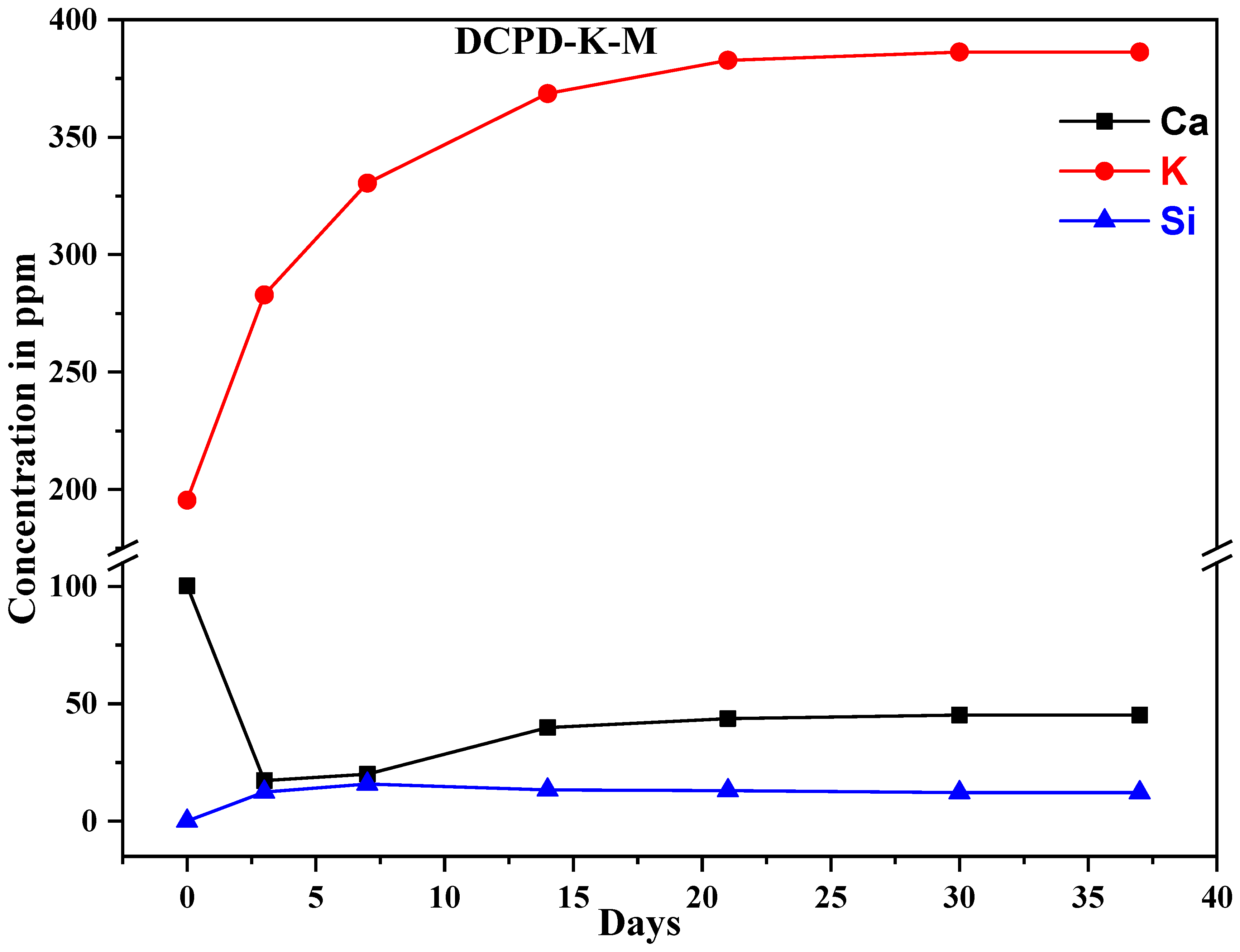
2.4. Ion Exchange with SBF Medium
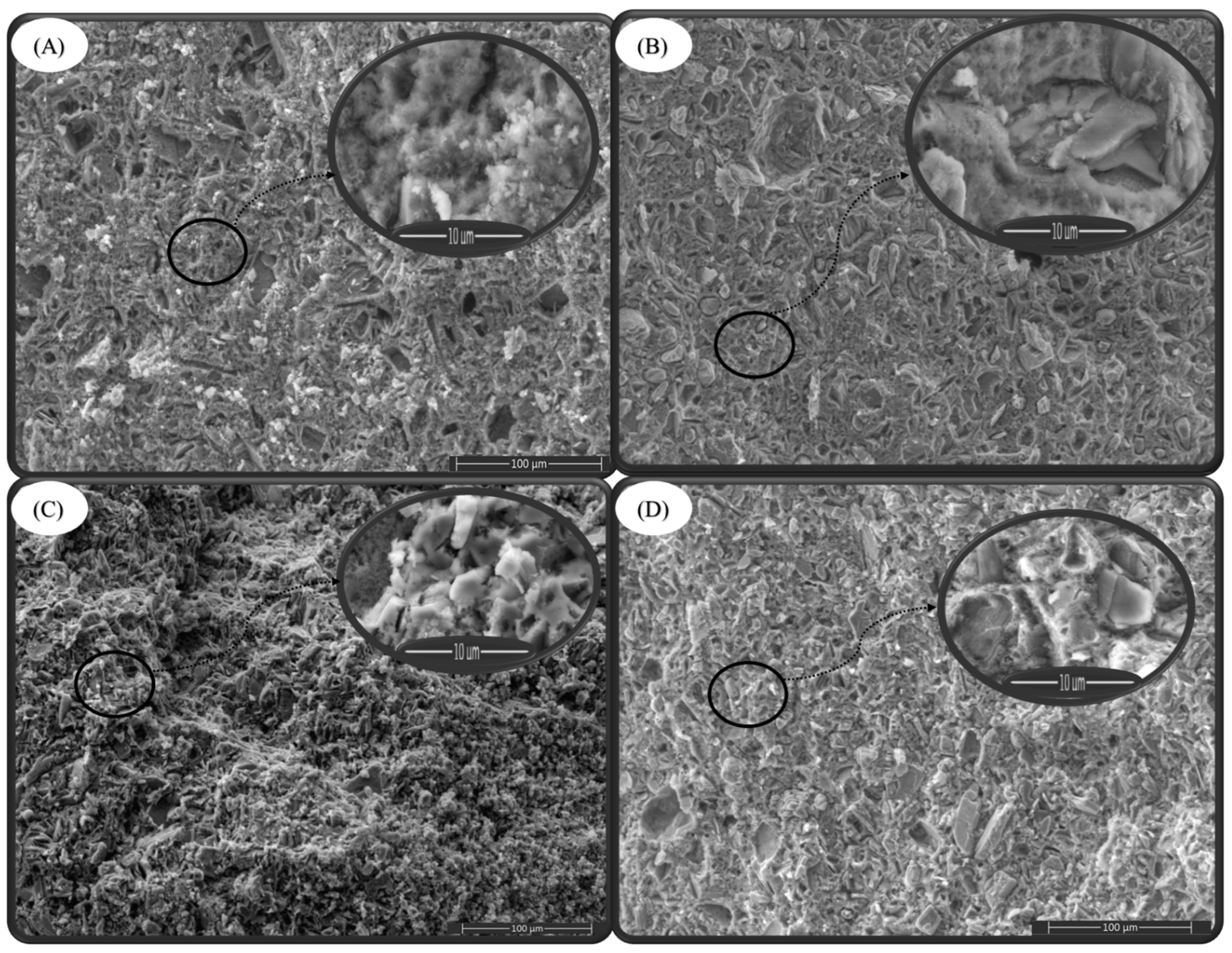
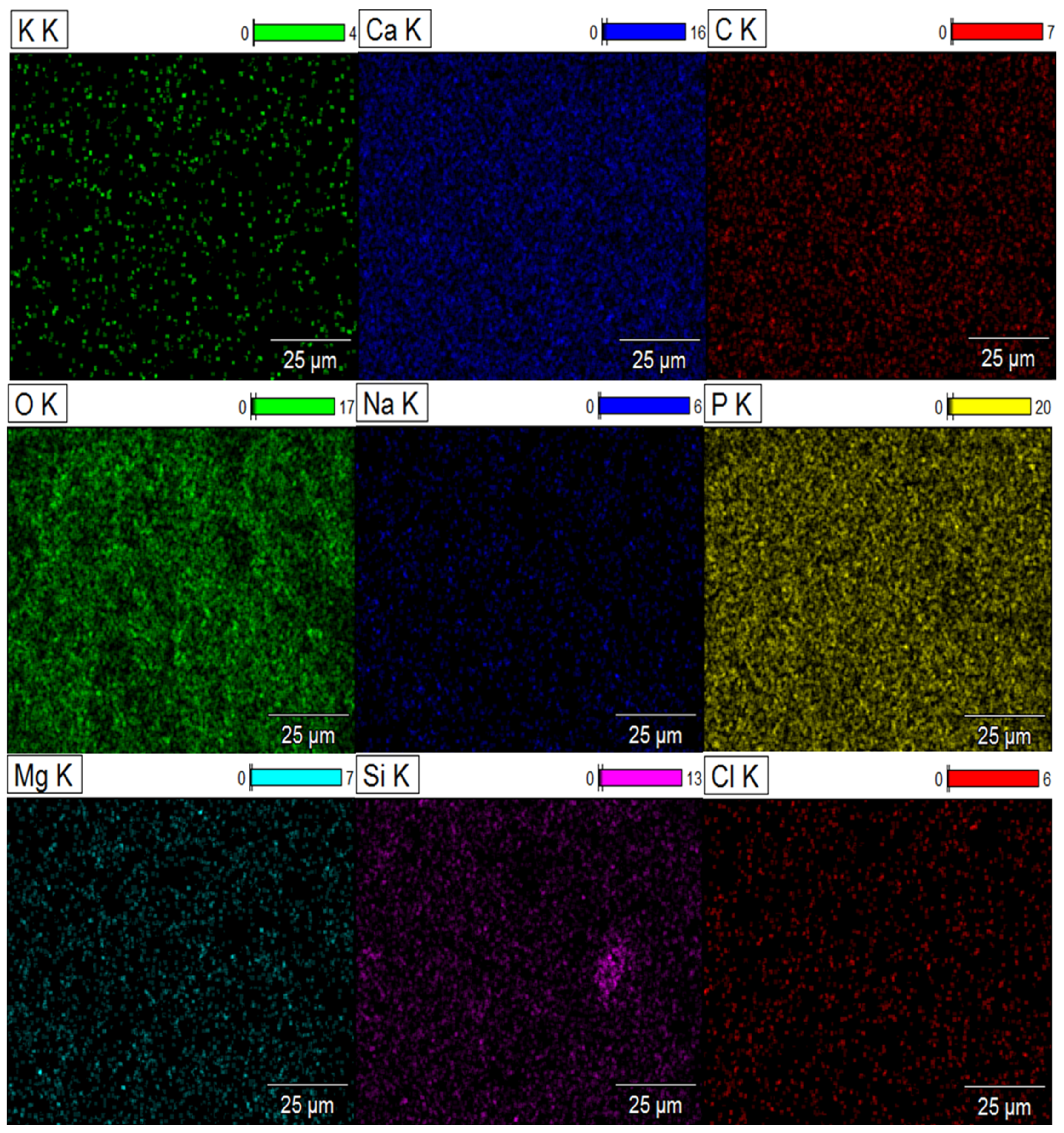
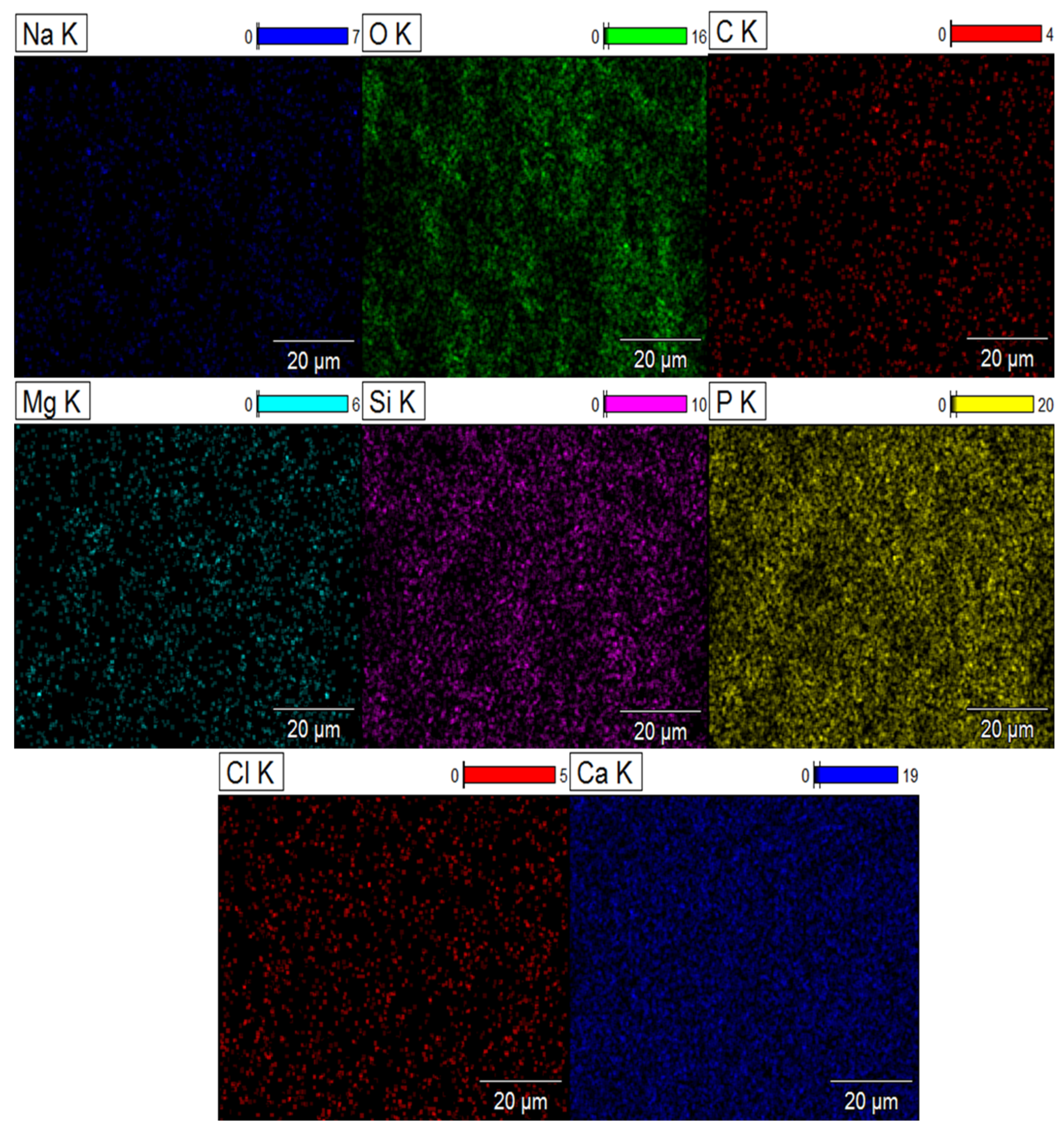
2.5. Element Distribution on the Surface of Composite Biomaterials
2.6. Toxicity Study
2.7. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.3. UV–Visible Spectrophotometry
3.4. X-ray Diffraction Analysis (XRD)
3.5. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Spectroscopy (EDX)
3.6. Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES)
3.7. Zeta Potential
3.8. Preparation of Antibiotic-Loaded Composite Biomaterials
3.8.1. Synthesis of Dicalcium Phosphate by the Double Decomposition Method
(NH4)2HPO4 + Ca(NO3)2.4H2O
 CaHPO4.2H2O + 2NH4NO3
CaHPO4.2H2O + 2NH4NO3
3.8.2. Synthesis of Sodium Silicate and Potassium Silicate Solutions
3.8.3. Synthesis of Antibiotic-Loaded Composite Biomaterials
3.8.4. Study of the Release of Active Ingredients from Engineered Composite Biomaterials
3.9. Test In Vitro
3.9.1. Antibacterial Activity
3.9.2. Toxicity Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dornelas, J.; Dornelas, G.; Rossi, A.; Piattelli, A.; Di Pietro, N.; Romasco, T.; Mourão, C.F.; Alves, G.G. The Incorporation of Zinc into Hydroxyapatite and Its Influence on the Cellular Response to Biomaterials: A Systematic Review. J. Funct. Biomater. 2024, 15, 178. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Khalifa, H.O.; Ki, M.-R.; Pack, S.P. Nanoengineered Silica-Based Biomaterials for Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 6125. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Azzaoui, K.; Jodeh, S.; Mejdoubi, E.; Hammouti, B.; Taleb, M.; Ennabety, G.; Berisha, A.; Aaddouz, M.; Youssouf, M.; Shityakov, S. Synthesis of hydroxyapatite/polyethylene glycol 6000 composites by novel dissolution/precipitation method: Optimization of the adsorption process using a factorial design: DFT and molecular dynamic. BMC Chem. 2023, 17, 150. [Google Scholar] [CrossRef]
- Magnaterra, G. Additive Manufacturing of Hydroxyapatite Scaffolds for Bone Repair. Ph.D. Thesis, Politecnico di Torino, Turin, Italy, 2020. [Google Scholar]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar]
- DileepKumar, V.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef]
- El Hammari, L.; Latifi, S.; Saoiabi, S.; Azzaoui, A.; Hammouti, B.; Chetouani, A.; Sabbahi, R. Toxic heavy metals removal from river water using a porous phospho-calcic hydroxyapatite. Moroc. J. Chem. 2022, 10, 62–72. [Google Scholar]
- Apelt, D.; Theiss, F.; El-Warrak, A.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Şahin, E. Synthesis and Characterization of Calcium Phosphate Cement Based Macroporous Scaffolds; Izmir Institute of Technology: Urla, Turkey, 2012. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics and its clinical applications. In Clinical Applications of Biomaterials; Kaur, G., Ed.; Springer: Cham, Switzerland, 2017; pp. 123–226. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Atila, D.; Dalgic, A.D.; Krzemińska, A.; Pietrasik, J.; Gendaszewska-Darmach, E.; Bociaga, D.; Lipinska, M.; Laoutid, F.; Passion, J.; Kumaravel, V. Injectable Liposome-Loaded Hydrogel Formulations with Controlled Release of Curcumin and α-Tocopherol for Dental Tissue Engineering. Adv. Healthc. Mater. 2024, 2400966. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug delivery systems in regenerative medicine: An updated review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone diseases: Current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Jin, S.; Fu, L.; Zhang, H.; Huang, X.; Long, H.; Ming, W.; Zhao, J. An update on the advances in the field of nanostructured drug delivery systems for a variety of orthopedic applications. Drug Deliv. 2023, 30, 2241667. [Google Scholar] [CrossRef]
- Aaddouz, M.; Azzaoui, K.; Akartasse, N.; Mejdoubi, E.; Hammouti, B.; Taleb, M.; Sabbahi, R.; Alshahateet, S. Removal of methylene blue from aqueous solution by adsorption onto hydroxyapatite nanoparticles. J. Mol. Struct. 2023, 1288, 135807. [Google Scholar] [CrossRef]
- Azzaoui, K.; Aaddouz, M.; Akartasse, N.; Mejdoubi, E.; Jodeh, S.; Hammouti, B.; Taleb, M.; ES-Sehli, S.; Berisha, A.; Rhazi, L. Synthesis of β-Tricalcium Phosphate/PEG 6000 Composite by Novel Dissolution/Precipitation Method: Optimization of the Adsorption Process Using a Factorial Design—DFT and Molecular Dynamic. Arab. J. Sci. Eng. 2023, 49, 711–732. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Siliqi, D.; Giannini, C.; Iafisco, M. The effect of chemical structure of carboxylate molecules on hydroxyapatite nanoparticles. A structural and morphological study. Bioact. Mater. 2021, 6, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Kingston, J.L.; Heywood, B.R.; Mann, S. Influence of monosaccharides and related molecules on the morphology of hydroxyapatite. J. Cryst. Growth 1993, 133, 1–12. [Google Scholar] [CrossRef]
- Aaddouz, M.; Azzaoui, K.; Sabbahi, R.; Youssoufi, M.H.; Yahyaoui, M.I.; Asehraou, A.; El Miz, M.; Hammouti, B.; Shityakov, S.; Siaj, M. Cheminformatics-based design and synthesis of hydroxyapatite/collagen nanocomposites for biomedical applications. Polymers 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of fenofibrate/randomly methylated β-cyclodextrin-loaded Eudragit® RL 100 nanoparticles for ocular delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.; Luppi, B. Chitosan based micro-and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Đošić, M.S.; Mitrić, M.; Mišković-Stanković, V.B. The porosity and roughness of electrodeposited calcium phosphate coatings in simulated body fluid. J. Serbian Chem. Soc. 2015, 80, 237–251. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Celiktas, O.Y.; Kocabas, E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Pundir, C.S.; Rawal, R. Determination of sulfite with emphasis on biosensing methods: A review. Anal. Bioanal. Chem. 2013, 405, 3049–3062. [Google Scholar] [CrossRef]


| Charged Particle | |||
|---|---|---|---|
| Composite Biomaterial | Average Zeta Potential ζ [mV] | Distribution Peak [mV] | Electron Mobility [µm × cm/Vs] |
| DCPD | −26.6 | −24.0 | −2.076 |
| DCPD-Na | −23.9 | −0.0202 | −1.8658 |
| DCPD-Na-M | −19.7 | −0.0158 | −1.5318 |
| DCPD-K | −24.6 | −19.5 | −1.9183 |
| DCPD-K-M | −16.5 | −0.0125 | −1.2847 |
| DCPD-Na | DCPD-K | DCPD-Na-M | DCPD-K-M | Cycloheximide | DMSO | |
|---|---|---|---|---|---|---|
| Escherichia coli | ND * | ND | 10.5 ± 0.1 | 12.1 ± 0.3 | 31 ± 0.1 | ND |
| Pseudomonas aeruginosa | ND | ND | 9.5 ± 0.1 | 8.1 ± 0.1 | 21 ± 0.2 | ND |
| Staphylococcus aureus | ND | ND | 10.1 ± 0.2 | 8.5 ± 0.1 | 30.7 ± 0.3 | ND |
| Listeria monocytogenes | ND | ND | 10.1 ± 0.3 | 8.5 ± 0.2 | 30.3 ± 0.2 | ND |
| Composite | SiO2/M2O | Antibiotic 1% |
|---|---|---|
| DCPD-Na | SiO2/Na2O | 0% |
| DCPD-K | SiO2/K2O | 0% |
| DCPD-Na-M * | SiO2/Na2O | 1% |
| DCPD-K-M * | SiO2/K2O | 1% |
| Ions (mM) | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− |
|---|---|---|---|---|---|---|---|---|
| SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 |
| Plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaddouz, M.; El Yousfi, R.; Sabbahi, R.; Azzaoui, K.; Yahyaoui, M.I.; Asehraou, A.; Hammouti, B.; Laoutid, F.; Alanazi, M.M.; Mejdoubi, E. Multifunctional Biocomposites: Synthesis, Characterization, and Prospects for Regenerative Medicine and Controlled Drug Delivery. Molecules 2024, 29, 3483. https://doi.org/10.3390/molecules29153483
Aaddouz M, El Yousfi R, Sabbahi R, Azzaoui K, Yahyaoui MI, Asehraou A, Hammouti B, Laoutid F, Alanazi MM, Mejdoubi E. Multifunctional Biocomposites: Synthesis, Characterization, and Prospects for Regenerative Medicine and Controlled Drug Delivery. Molecules. 2024; 29(15):3483. https://doi.org/10.3390/molecules29153483
Chicago/Turabian StyleAaddouz, Mohamed, Ridouan El Yousfi, Rachid Sabbahi, Khalil Azzaoui, Meryem Idrissi Yahyaoui, Abdeslam Asehraou, Belkheir Hammouti, Fouad Laoutid, Mohammed M. Alanazi, and Elmiloud Mejdoubi. 2024. "Multifunctional Biocomposites: Synthesis, Characterization, and Prospects for Regenerative Medicine and Controlled Drug Delivery" Molecules 29, no. 15: 3483. https://doi.org/10.3390/molecules29153483
APA StyleAaddouz, M., El Yousfi, R., Sabbahi, R., Azzaoui, K., Yahyaoui, M. I., Asehraou, A., Hammouti, B., Laoutid, F., Alanazi, M. M., & Mejdoubi, E. (2024). Multifunctional Biocomposites: Synthesis, Characterization, and Prospects for Regenerative Medicine and Controlled Drug Delivery. Molecules, 29(15), 3483. https://doi.org/10.3390/molecules29153483







