Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Proanthocyanidin Fractions
2.2. Isothermal Titration Calorimetry Analyses
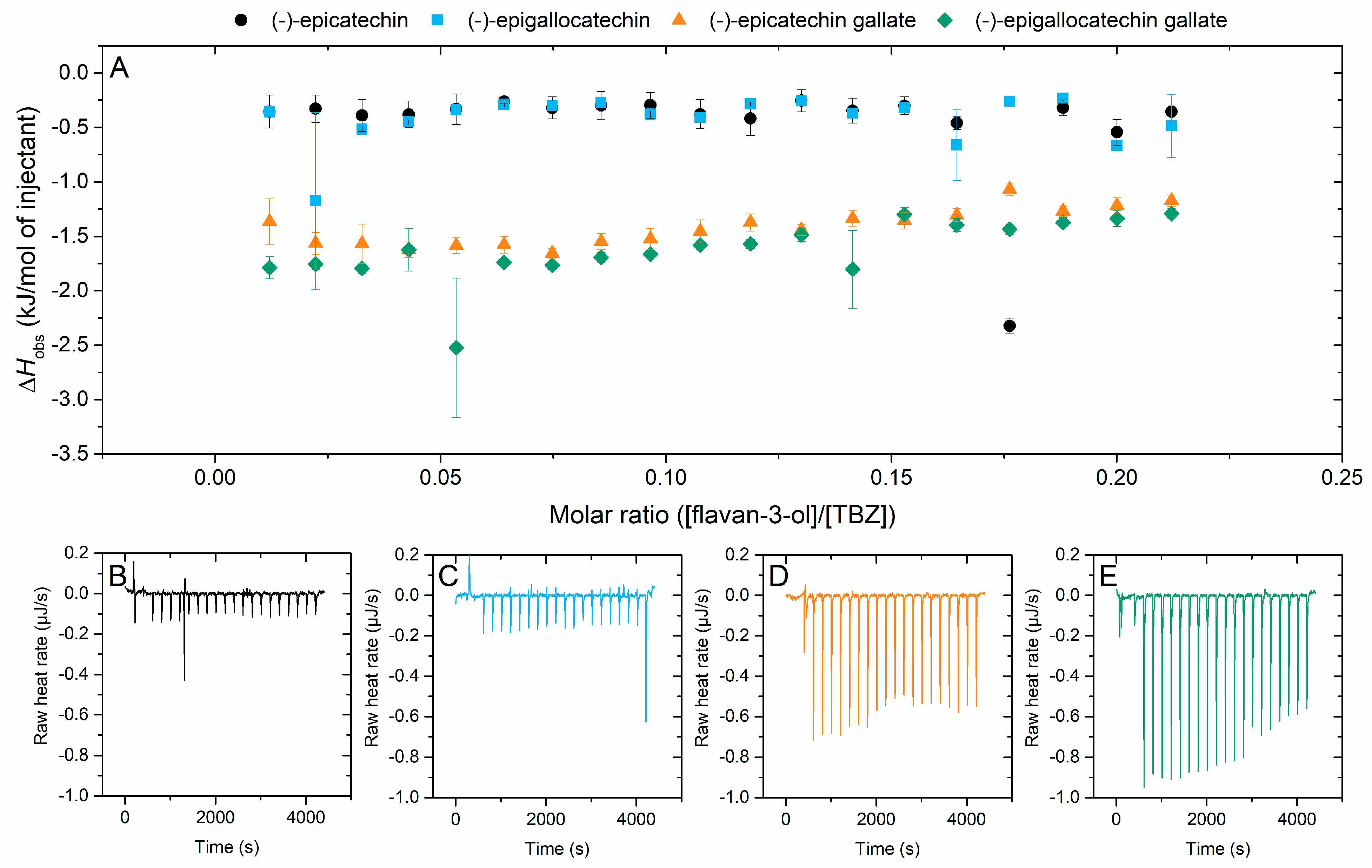
2.2.1. Interaction of Flavan-3-ols to Thiabendazole
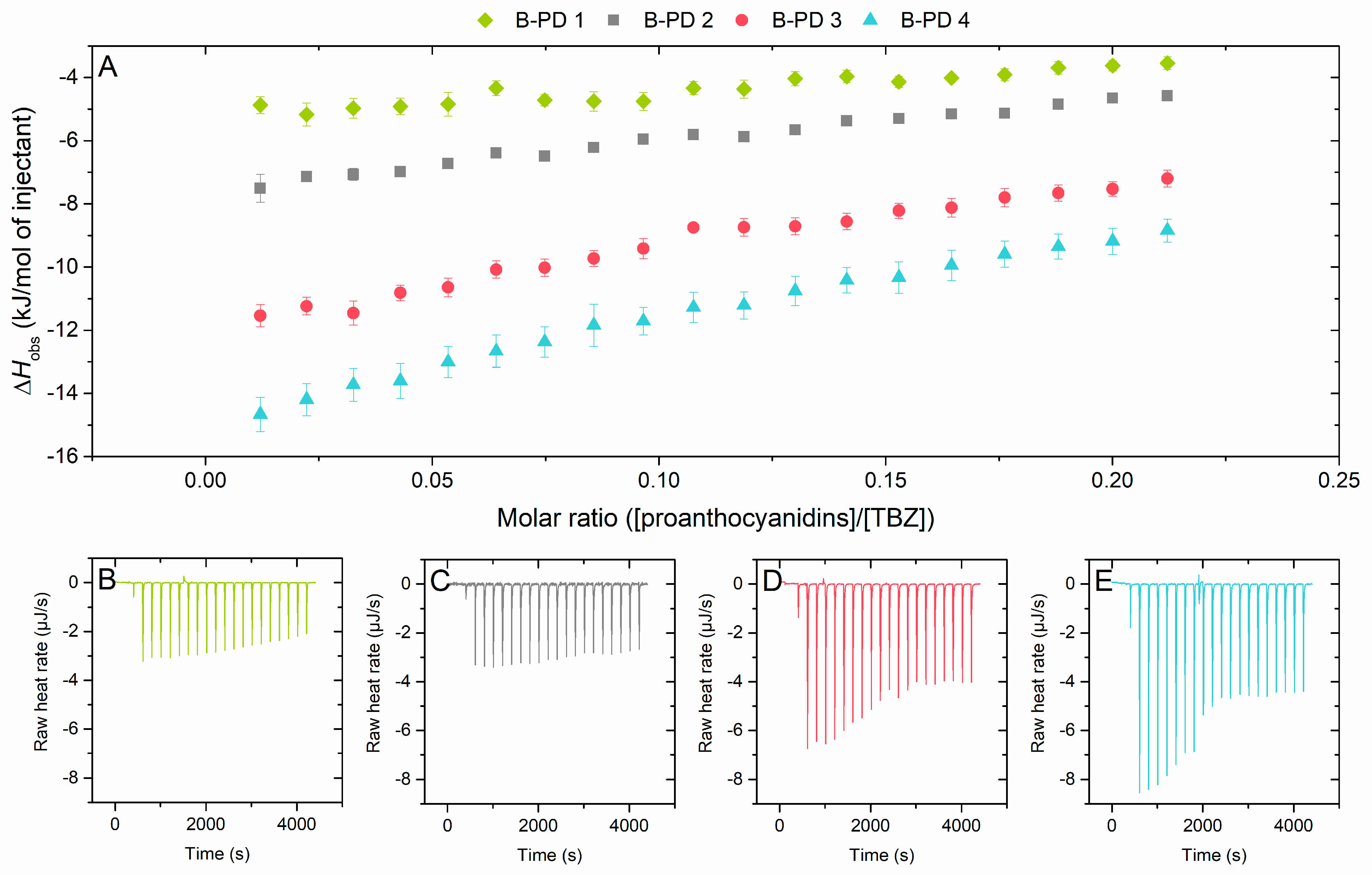
2.2.2. Effect of Mean Degree of Polymerization on the Interaction to Thiabendazole
2.2.3. Effect of the Degree of Hydroxylation on the Interaction to Thiabendazole

2.2.4. Effect of Galloylation on the Interaction to Thiabendazole

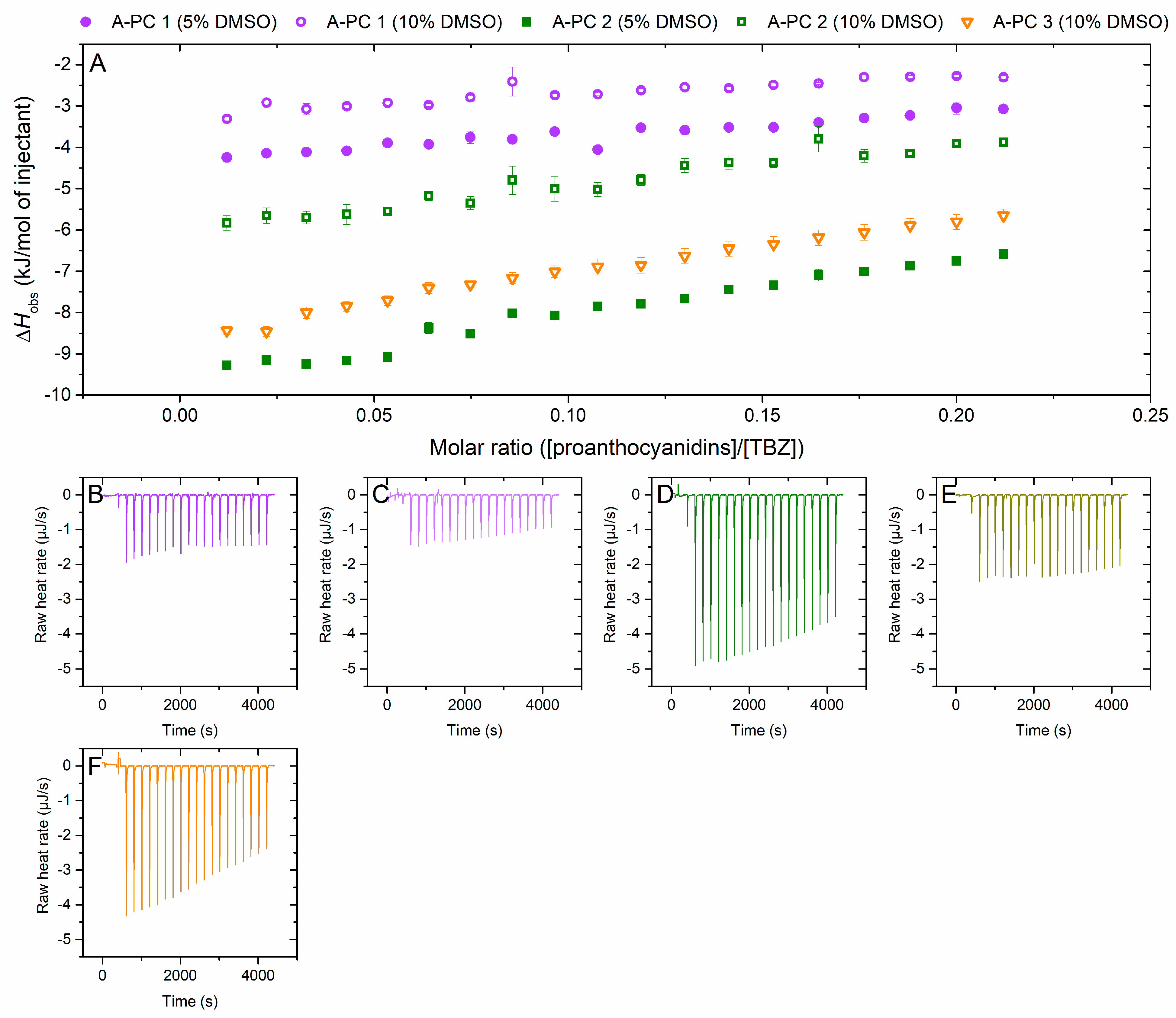
2.2.5. Interaction of A-Type Proanthocyanidins to Thiabendazole
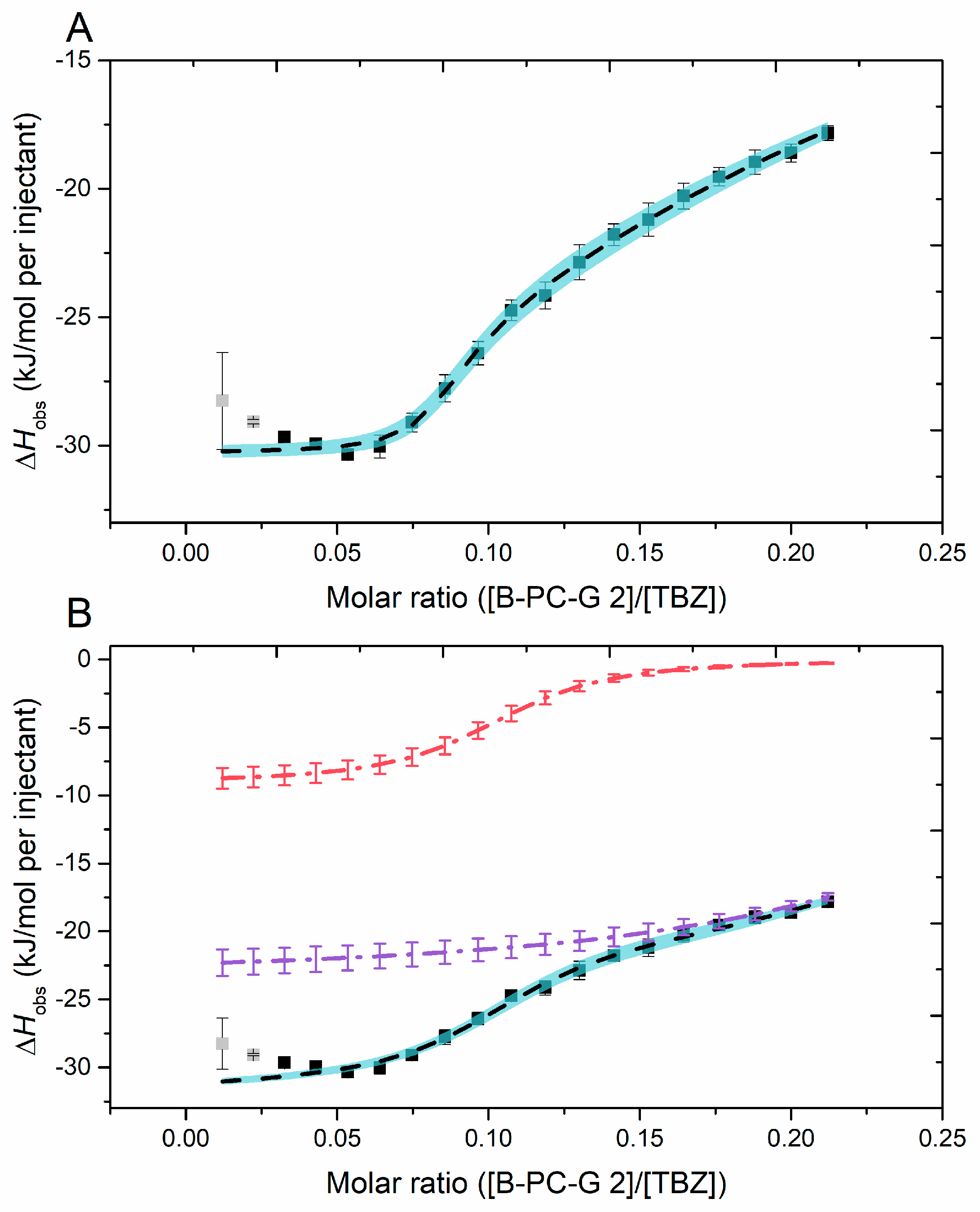
2.2.6. Fitting of the Sigmoidal Binding Isotherm
3. Materials and Methods
3.1. Reagents and Sample Collection
3.2. Plant Extraction and Fractionation
3.3. UPLC-MS/MS Analyses
3.4. Isothermal Titration Calorimetry Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esatbeyoglu, T.; Winterhalter, P. Preparation of Dimeric Procyanidins B1, B2, B5, and B7 from a Polymeric Procyanidin Fraction of Black Chokeberry (Aronia Melanocarpa). J. Agric. Food Chem. 2010, 58, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Isolation of Dimeric, Trimeric, Tetrameric and Pentameric Procyanidins from Unroasted Cocoa Beans (Theobroma Cacao L.) Using Countercurrent Chromatography. Food Chem. 2015, 179, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Oki, T.; Yoshida, T.; Nanba, F.; Yamada, K.; Toda, T. Characterisation of Proanthocyanidins from Black Soybeans: Isolation and Characterisation of Proanthocyanidin Oligomers from Black Soybean Seed Coats. Food Chem. 2013, 141, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- Köhler, N.; Wray, V.; Winterhalter, P. Preparative Isolation of Procyanidins from Grape Seed Extracts by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2008, 1177, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Leppä, M.M.; Karonen, M.; Tähtinen, P.; Engström, M.T.; Salminen, J.P. Isolation of Chemically Well-Defined Semipreparative Liquid Chromatography Fractions from Complex Mixtures of Proanthocyanidin Oligomers and Polymers. J. Chromatogr. A 2018, 1576, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E. Fifty Years of Polyphenol-Protein Complexes. In Recent Advances in Polyphenol Research. Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 3, pp. 71–97. ISBN 9781444337464. [Google Scholar]
- Hansen, T.V.A.; Fryganas, C.; Acevedo, N.; Caraballo, L.; Thamsborg, S.M.; Mueller-Harvey, I.; Williams, A.R. Proanthocyanidins Inhibit Ascaris Suum Glutathione-S-Transferase Activity and Increase Susceptibility of Larvae to Levamisole in Vitro. Parasitol. Int. 2016, 65, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Butter, N.L.; Dawson, J.M.; Wakelin, D.; Buttery, P.J. Effect of Dietary Condensed Tannins on Gastrointestinal Nematodes. J. Agric. Sci. 2001, 137, 461–469. [Google Scholar] [CrossRef]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic Inhibition of Haemonchus Contortus Exsheathment by Flavonoid Monomers and Condensed Tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Desrues, O.; Fryganas, C.; Ropiak, H.M.; Mueller-Harvey, I.; Enemark, H.L.; Thamsborg, S.M. Impact of Chemical Structure of Flavanol Monomers and Condensed Tannins on in Vitro Anthelmintic Activity against Bovine Nematodes. Parasitology 2016, 143, 444–454. [Google Scholar] [CrossRef]
- Novobilský, A.; Stringano, E.; Hayot Carbonero, C.; Smith, L.M.J.J.; Enemark, H.L.; Mueller-Harvey, I.; Thamsborg, S.M. In Vitro Effects of Extracts and Purified Tannins of Sainfoin (Onobrychis viciifolia) against Two Cattle Nematodes. Vet. Parasitol. 2013, 196, 532–537. [Google Scholar] [CrossRef]
- Quijada, J.; Fryganas, C.; Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I.; Hoste, H. Anthelmintic Activities against Haemonchus Contortus or Trichostrongylus Colubriformis from Small Ruminants Are Influenced by Structural Features of Condensed Tannins. J. Agric. Food Chem. 2015, 63, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Ropiak, H.M.; Fryganas, C.; Desrues, O.; Mueller-Harvey, I.; Thamsborg, S.M. Assessment of the Anthelmintic Activity of Medicinal Plant Extracts and Purified Condensed Tannins against Free-Living and Parasitic Stages of Oesophagostomum Dentatum. Parasit. Vectors 2014, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Azuhnwi, B.N.; Hertzberg, H.; Arrigo, Y.; Gutzwiller, A.; Hess, H.D.; Mueller-Harvey, I.; Torgerson, P.R.; Kreuzer, M.; Dohme-Meier, F. Investigation of Sainfoin (Onobrychis viciifolia) Cultivar Differences on Nitrogen Balance and Fecal Egg Count in Artificially Infected Lambs. J. Anim. Sci. 2013, 91, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for Suppression of Internal Parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar]
- Waghorn, G.C.; McNabb, W.C. Consequences of Plant Phenolic Compounds for Productivity and Health of Ruminants. Proc. Nutr. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Solaiman, S.; Gurung, N.; Behrends, J.; Eun, J.S.; Taha, E.; Rose, J. Effects of Pine Bark Supplementation on Performance, Rumen Fermentation, and Carcass Characteristics of Kiko Crossbred Male Goats. J. Anim. Sci. 2012, 90, 3556–3567. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.; Alonso-diaz, M.Á.; Brunet, S.; Sandoval-Castro, C.; Adote, S.H. Identification and Validation of Bioactive Plants for the Control of Gastrointestinal Nematodes in Small Ruminants. Trop. Biomed. 2008, 25, 56–72. [Google Scholar] [PubMed]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and Indirect Effects of Bioactive Tannin-Rich Tropical and Temperate Legumes against Nematode Infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef]
- Malisch, C.S.; Lüscher, A.; Baert, N.; Engström, M.T.; Studer, B.; Fryganas, C.; Suter, D.; Mueller-Harvey, I.; Salminen, J.P. Large Variability of Proanthocyanidin Content and Composition in Sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2015, 63, 10234–10242. [Google Scholar] [CrossRef]
- Sottie, E.T.; Acharya, S.N.; McAllister, T.; Thomas, J.; Wang, Y.; Iwaasa, A. Alfalfa Pasture Bloat Can Be Eliminated by Intermixing with Newly-Developed Sainfoin Population. Agron. J. 2014, 106, 1470–1478. [Google Scholar] [CrossRef]
- Shalaby, H.A. Anthelmintics Resistance; How to Overcome It? Iran. J. Parasitol. 2013, 8, 18. [Google Scholar] [PubMed]
- Rose Vineer, H.; Morgan, E.R.; Hertzberg, H.; Bartley, D.J.; Bosco, A.; Charlier, J.; Chartier, C.; Claerebout, E.; De Waal, T.; Hendrickx, G.; et al. Increasing Importance of Anthelmintic Resistance in European Livestock: Creation and Meta-Analysis of an Open Database. Parasite 2020, 27, 69. [Google Scholar] [CrossRef]
- Ahuir-Baraja, A.E.; Cibot, F.; Llobat, L.; Garijo, M.M. Anthelmintic Resistance: Is a Solution Possible? Exp. Parasitol. 2021, 230, 108169. [Google Scholar] [CrossRef] [PubMed]
- Lanusse, C.; Lifschitz, A.; Alvarez, L. Basic and Clinical Pharmacology Contribution to Extend Anthelmintic Molecules Lifespan. Vet. Parasitol. 2015, 212, 35–46. [Google Scholar] [CrossRef]
- Alvarez, L.; Lifschitz, A.; Entrocasso, C.; Manazza, J.; Mottier, L.; Borda, B.; Virkel, G.; Lanusse, C. Evaluation of the Interaction between Ivermectin and Albendazole Following Their Combined Use in Lambs. J. Vet. Pharmacol. Ther. 2008, 31, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Klein, D.R.; Whitney, T.R.; Scott, C.B.; Muir, J.P.; Lambert, B.D.; Craig, T.M. Effect of Using Redberry Juniper (Juniperus Pinchotii) to Reduce Haemonchus Contortus in Vitro Motility and Increase Ivermectin Efficacy. Vet. Parasitol. 2013, 197, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Whitney, T.R.; Wildeus, S.; Zajac, A.M. The Use of Redberry Juniper (Juniperus Pinchotii) to Reduce Haemonchus Contortus Fecal Egg Counts and Increase Ivermectin Efficacy. Vet. Parasitol. 2013, 197, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, E.; Simon, M.; Quijada, J.; Schelcher, F.; Sutra, J.F.; Lespine, A.; Hoste, H. Efficacy of Sainfoin (Onobrychis viciifolia) Pellets against Multi Resistant Haemonchus Contortus and Interaction with Oral Ivermectin: Implications for on-Farm Control. Vet. Parasitol. 2016, 227, 122–129. [Google Scholar] [CrossRef]
- Malsa, J.; Courtot, É.; Boisseau, M.; Dumont, B.; Gombault, P.; Kuzmina, T.A.; Basiaga, M.; Lluch, J.; Annonay, G.; Dhorne-Pollet, S.; et al. Effect of Sainfoin (Onobrychis viciifolia) on Cyathostomin Eggs Excretion, Larval Development, Larval Community Structure and Efficacy of Ivermectin Treatment in Horses. Parasitology 2022, 149, 1439. [Google Scholar] [CrossRef]
- Sillanpää, M.; Engström, M.T.; Tähtinen, P.; Green, R.J.; Käpylä, J.; Näreaho, A.; Karonen, M. Tannins Can Have Direct Interactions with Anthelmintics: Investigations by Isothermal Titration Calorimetry. Molecules 2023, 28, 5261. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Bin Imran, I.; Engström, M.T.; Salminen, J.P. Characterization of Natural and Alkaline-Oxidized Proanthocyanidins in Plant Extracts by Ultrahigh-Resolution UHPLC-MS/MS. Molecules 2021, 26, 1873. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Suominen, E.; Savila, S.; Sillanpää, M.; Damlin, P.; Karonen, M. Affinity of Tannins to Cellulose: A Chromatographic Tool for Revealing Structure-Activity Patterns. Molecules 2023, 28, 5370. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zheng, Y.; Li, X.; Li, S.; Xie, B.; Sun, Z. Characterization and Preparation of Oligomeric Procyanidins from Litchi Chinensis Pericarp. Fitoterapia 2016, 112, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of Procyanidins in Pine Bark with Reversed-Phase and Normal-Phase High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Lin, L.Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMS n profiling Method to Identify and Quantify Oligomeric Proanthocyanidins in Plant Products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, J.; Chen, L.; Hu, C.; Chen, P.; Xie, B.; Sun, Z. Identification of A-Series Oligomeric Procyanidins from Pericarp of Litchi Chinensis by FT-ICR-MS and LC-MS. Food Chem. 2012, 135, 31–38. [Google Scholar] [CrossRef]
- Turnbull, W.B.; Daranas, A.H. On the Value of c: Can Low Affinity Systems Be Studied by Isothermal Titration Calorimetry? J. Am. Chem. Soc. 2003, 125, 14859–14866. [Google Scholar] [CrossRef] [PubMed]
- Tellinghuisen, J. Statistical Error in Isothermal Titration Calorimetry. Methods Enzymol. 2004, 383, 245–282. [Google Scholar] [CrossRef]
- Tellinghuisen, J. Stupid Statistics! Methods Cell Biol. 2008, 84, 737–780. [Google Scholar]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of Tea Tannins and Condensed Tannins with Proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Effects of Green Tea Catechins on Membrane Fluidity. Pharmacology 1999, 59, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zeller, W.E.; Reinhardt, L.A.; Robe, J.T.; Sullivan, M.L.; Panke-Buisse, K. Comparison of Protein Precipitation Ability of Structurally Diverse Procyanidin-Rich Condensed Tannins in Two Buffer Systems. J. Agric. Food Chem. 2020, 68, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Leppä, M.M.; Laitila, J.E.; Salminen, J.P. Distribution of Protein Precipitation Capacity within Variable Proanthocyanidin Fingerprints. Molecules 2020, 25, 5002. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, R.L.; Faulkner, P.; Downey, M.O.; Darby, S.J.; Falconer, R.J. The Complexity of Condensed Tannin Binding to Bovine Serum Albumin—An Isothermal Titration Calorimetry Study. Food Chem. 2016, 190, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Kilmister, R.L.; Kelm, M.A.; Downey, M.O. Impact of Condensed Tannin Size as Individual and Mixed Polymers on Bovine Serum Albumin Precipitation. Food Chem. 2014, 160, 16–21. [Google Scholar] [CrossRef]
- Cala, O.; Pinaud, N.; Simon, C.; Fouquet, E.; Laguerre, M.; Dufourc, E.J.; Pianet, I. NMR and Molecular Modeling of Wine Tannins Binding to Saliva Proteins: Revisiting Astringency from Molecular and Colloidal Prospects. Faseb J. 2010, 24, 4281–4290. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4 → 8) Catechin (Procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Simon, C.; Barathieu, K.; Laguerre, M.; Schmitter, J.M.; Fouquet, E.; Pianet, I.; Dufourc, E.J. Three-Dimensional Structure and Dynamics of Wine Tannin-Saliva Protein Complexes. A Multitechnique Approach. Biochemistry 2003, 42, 10385–10395. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure–Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Virtanen, V.; Green, R.J.; Karonen, M. Interactions between Hydrolysable Tannins and Lipid Vesicles from Escherichia Coli with Isothermal Titration Calorimetry. Molecules 2022, 27, 3204. [Google Scholar] [CrossRef] [PubMed]
- Poncet-Legrand, C.; Gautier, C.; Cheynier, V.; Imberty, A. Interactions between Flavan-3-Ols and Poly(L-Proline) Studied by Isothermal Titration Calorimetry: Effect of the Tannin Structure. J. Agric. Food Chem. 2007, 55, 9235–9240. [Google Scholar] [CrossRef]
- Soares, S.; García-Estévez, I.; Ferrer-Galego, R.; Brás, N.F.; Brandão, E.; Silva, M.; Teixeira, N.; Fonseca, F.; Sousa, S.F.; Ferreira-da-Silva, F.; et al. Study of Human Salivary Proline-Rich Proteins Interaction with Food Tannins. Food Chem. 2018, 243, 175–185. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Falconer, R.J.; Kennedy, J.A. Thermodynamics of Grape and Wine Tannin Interaction with Polyproline: Implications for Red Wine Astringency. J. Agric. Food Chem. 2010, 58, 12510–12518. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of Structural Features of Condensed Tannins That Affect Protein Aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef]
- Frazier, R.A.; Papadopoulou, A.; Mueller-Harvey, I.; Kissoon, D.; Green, R.J. Probing Protein-Tannin Interactions by Isothermal Titration Microcalorimetry. J. Agric. Food Chem. 2003, 51, 5189–5195. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Binding of an Oligomeric Ellagitannin Series to Bovine Serum Albumin (BSA): Analysis by Isothermal Titration Calorimetry (ITC). J. Agric. Food Chem 2015, 63, 9. [Google Scholar] [CrossRef]
- Sun, Q.; He, J.; Yang, H.; Li, S.; Zhao, L.; Li, H. Analysis of Binding Properties and Interaction of Thiabendazole and Its Metabolite with Human Serum Albumin via Multiple Spectroscopic Methods. Food Chem. 2017, 233, 190–196. [Google Scholar] [CrossRef]
- Stepniak, A.; Erdenebayar, B.; Biernacka, M.; Buczkowski, A.; Zavodnik, L.; Zavodnik, I.; Palecz, B. Calorimetric Studies of α-Cyclodextrin Inclusion Complexes with Carbendazim and Thiabendazole. Phys. Chem. Liq. 2020, 59, 495–502. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, R.F.; Khalifa, I.; Li, C.M. Understanding toward the Biophysical Interaction of Polymeric Proanthocyanidins (Persimmon Condensed Tannins) with Biomembranes: Relevance for Biological Effects. J. Agric. Food Chem. 2019, 67, 11044–11052. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Pälijärvi, M.; Salminen, J.P. Rapid Fingerprint Analysis of Plant Extracts for Ellagitannins, Gallic Acid, and Quinic Acid Derivatives and Quercetin-, Kaempferol- and Myricetin-Based Flavonol Glycosides by UPLC-QqQ-MS/MS. J. Agric. Food Chem. 2015, 63, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Stringano, E.; Brown, R.H.; Mueller-Harvey, I. In Situ Analysis and Structural Elucidation of Sainfoin (Onobrychis viciifolia) Tannins for High-Throughput Germplasm Screening. J. Agric. Food Chem. 2011, 59, 495–503. [Google Scholar] [CrossRef] [PubMed]
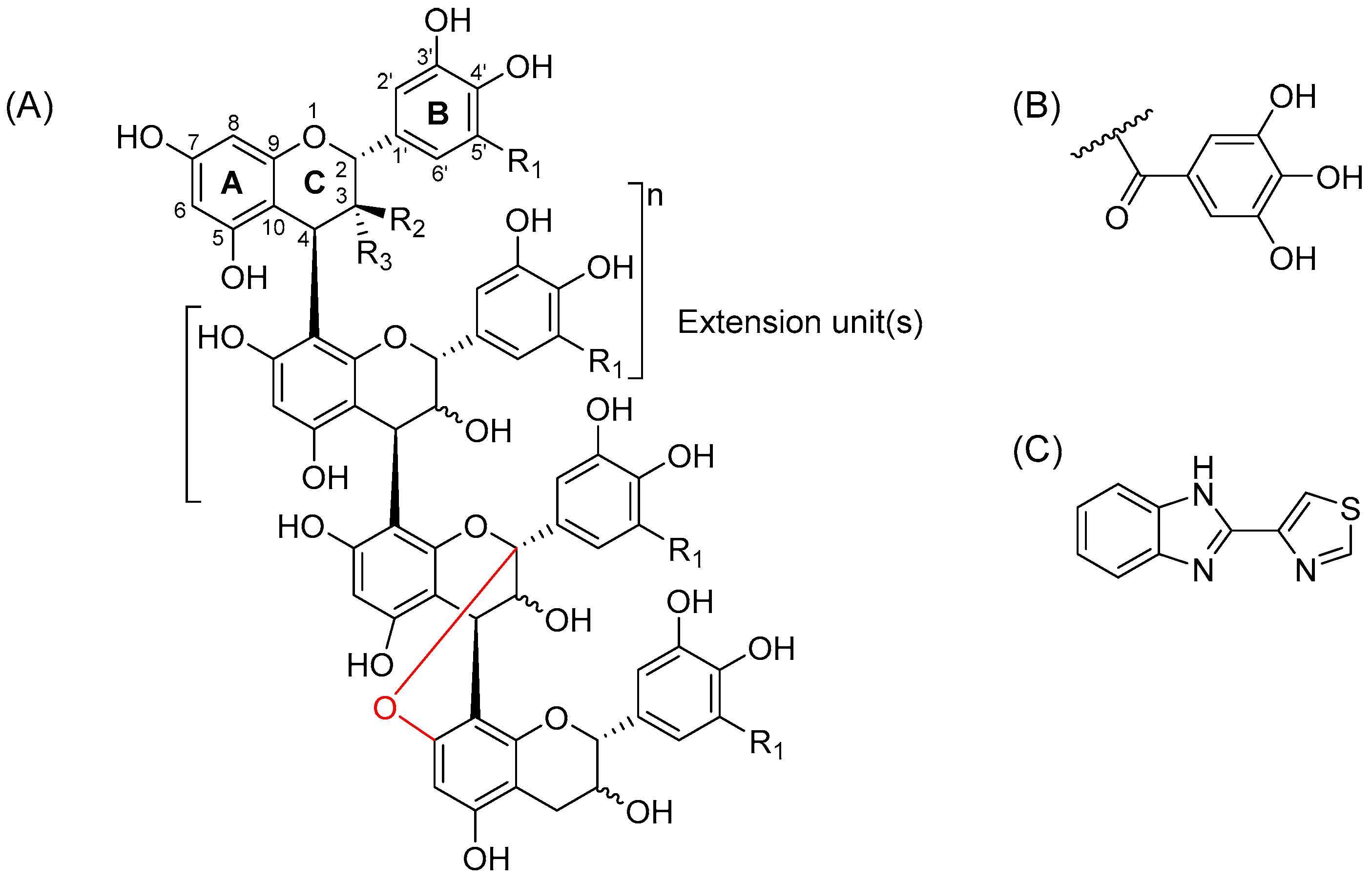
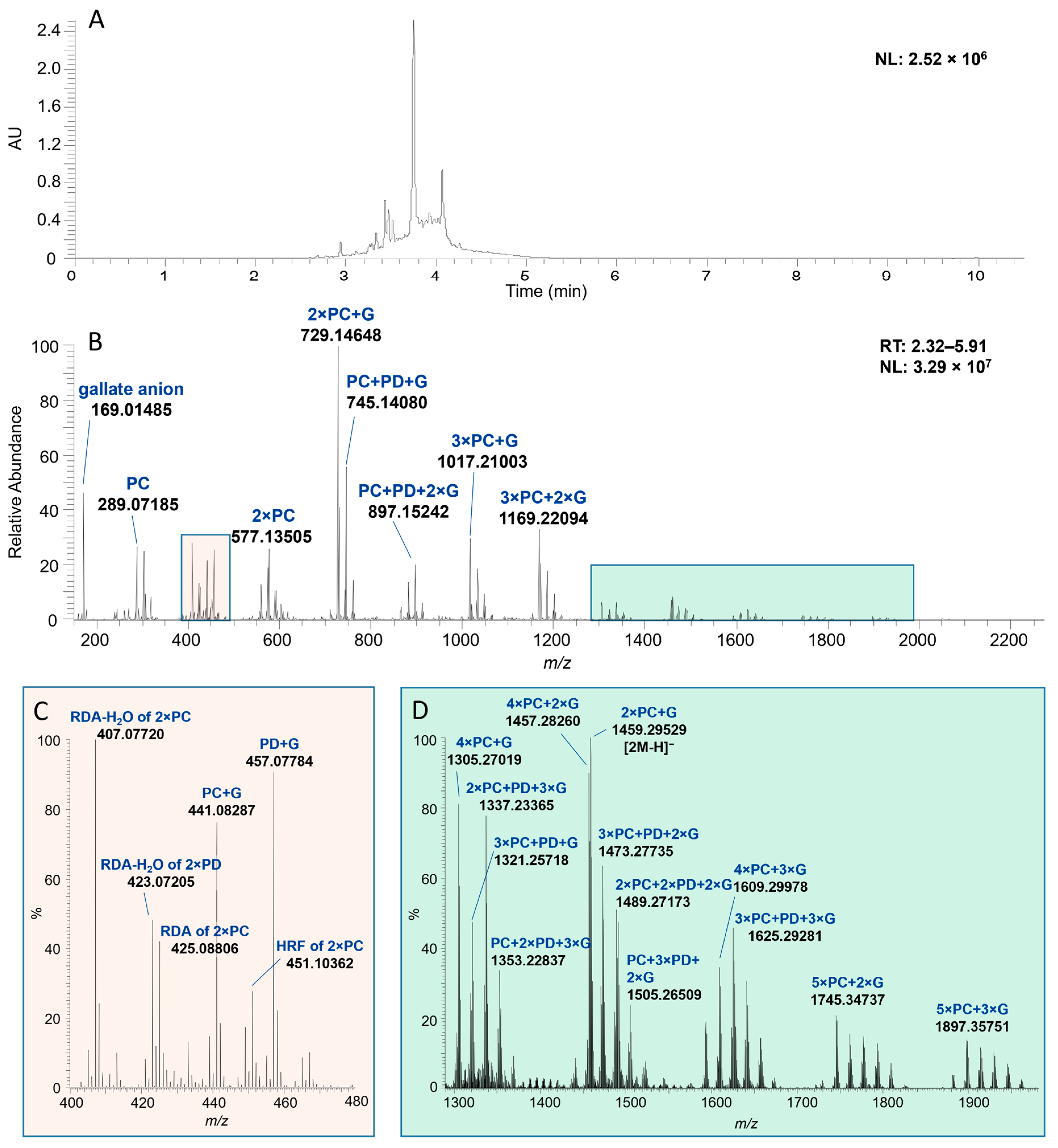

| Scientific Name | Common Name | Code | mDP | PD-% | #Galloyls | #A-Type Bonds a | mDa b |
|---|---|---|---|---|---|---|---|
| Pellaea rotundifolia | Button fern | A-PD 1 | 11 | 95% | 0.0 | 1.0 | 3336.6 |
| A-PD 2 c | 11 | 93% | 0.0 | 0.8 | 3332.6 | ||
| A-PD 3 | 11 | 70% | 0.0 | 0.5 | 3323.0 | ||
| Ixora coccinea | Jungle geranium | A-PC 1 | 6 | 1% | 0.0 | 2.0 | 1727.2 |
| A-PC 2 c | 10 | 1% | 0.0 | 3.0 | 2993.1 | ||
| A-PC 3 | 13 | 1% | 0.0 | 3.0 | 3742.7 | ||
| Ribes alpinum | Alpine currant | B-PD 1 | 12 | 98% | 0.0 | 0.0 | 3540.8 |
| B-PD 2 | 12 | 96% | 0.0 | 0.0 | 3700.7 | ||
| B-PD 3 | 16 | 95% | 0.0 | 0.0 | 4900.4 | ||
| B-PD 4 | 18 | 94% | 0.0 | 0.0 | 5322.0 | ||
| Cephalotaxus harringtonia drupacea | Japanese plum yew | B-PC 1 c | 6 | 23% | 0.0 | 0.0 | 1810.5 |
| B-PC 2 | 7 | 5% | 0.0 | 0.0 | 1879.5 | ||
| Coccoloba uvifera | Sea grape | B-PC-G 1 c | 5 | 28% | 2.1 | 0.0 | 1663.1 |
| B-PC-G 2 | 10 | 12% | 3.6 | 0.0 | 3593.1 | ||
| Podocarpus macrophyllus | Yew plum pine | B-PC/PD | 11 | 69% | 0.0 | 0.0 | 3174.8 |
| DP | Molecular Formula | Calculated Mass | Monomeric Composition | [M-H]− | [2M-H]− |
|---|---|---|---|---|---|
| - | C7H6O5 | 170.02153 | gallate anion | 169.01485 | |
| 1 | C15H14O6 | 290.07904 | PC | 289.07185 | |
| 2 | C30H26O12 | 578.14243 | 2 × PC | 577.13505 | |
| 2 | C37H30O16 | 730.15339 | 2 × PC + G | 729.14648 | |
| 2 | C37H30O16 | 730.15339 | 2 × PC + G | 1459.29529 | |
| 2 | C37H30O17 | 746.14831 | PC + PD + G | 745.14080 | |
| 2 | C37H30O18 | 762.14322 | 2 × PD + G | 761.13542 | |
| 2 | C44H34O20 | 882.16435 | 2 × PC + 2 × G | 881.15817 | |
| 2 | C44H34O21 | 898.15927 | PC + PD + 2 × G | 897.15242 | |
| 2 | C44H34O22 | 914.15418 | 2 × PD + 2 × G | 913.14639 | |
| 3 | C52H42O22 | 1018.21678 | 3 × PC + G | 1017.21003 | |
| 3 | C52H42O23 | 1034.21170 | 2 × PC + PD + G | 1033.20370 | |
| 3 | C52H42O24 | 1050.20661 | PC + 2 × PD + G | 1049.19849 | |
| 3 | C59H46O26 | 1170.22774 | 3 × PC + 2 × G | 1169.22094 | |
| 3 | C59H46O27 | 1186.22266 | 2 × PC + PD + 2 × G | 1185.21531 | |
| 3 | C59H46O28 | 1202.21757 | PC + 2 × PD + 2 × G | 1201.20948 | |
| 4 | C67H54O28 | 1306.28017 | 4 × PC + G | 1305.27019 | |
| 4 | C74H58O32 | 1458.29113 | 4 × PC + 2 × G | 1457.28260 | |
| 4 | C74H58O33 | 1474.28605 | 3 × PC + PD + 2 × G | 1473.27735 | |
| 5 | C82H66O34 | 1594.34356 | 5 × PC + G | 1593.33421 | |
| 4 | C81H62O36 | 1610.30209 | 4 × PC + 3 × G | 1609.29978 | |
| 5 | C89H70O38 | 1746.35452 | 5 × PC + 2 × G | 1745.34737 | |
| 5 | C89H70O39 | 1762.34944 | 4 × PC + PD + 2 × G | 1761.33710 | |
| 5 | C96H74O42 | 1898.36548 | 5 × PC + 3 × G | 1897.35751 |
| Model | Ka1 (M−1) a | Ka2 (M−1) a | n1 b | n2 b | ΔH1 (kJ/mol) c | ΔH2 (kJ/mol) c |
|---|---|---|---|---|---|---|
| Multiple Sites | (5.99 ± 2.08) × 105 | (4.66 ± 0.84) × 102 | 0.08 ± 0.05 | 0.16 ± 0.02 | −30 ± 0 | −165 ± 24 |
| Two Independent Sites | (9.99 ± 0.83) × 104 | (3.17 ± 0.55) × 104 | 0.10 ± 0.00 | 0.30 ± 0.01 | −9 ± 1 | −23 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillanpää, M.; Engström, M.T.; Tähtinen, P.; Green, R.J.; Käpylä, J.; Näreaho, A.; Karonen, M. Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry. Molecules 2024, 29, 3492. https://doi.org/10.3390/molecules29153492
Sillanpää M, Engström MT, Tähtinen P, Green RJ, Käpylä J, Näreaho A, Karonen M. Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry. Molecules. 2024; 29(15):3492. https://doi.org/10.3390/molecules29153492
Chicago/Turabian StyleSillanpää, Mimosa, Marica T. Engström, Petri Tähtinen, Rebecca J. Green, Jarmo Käpylä, Anu Näreaho, and Maarit Karonen. 2024. "Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry" Molecules 29, no. 15: 3492. https://doi.org/10.3390/molecules29153492
APA StyleSillanpää, M., Engström, M. T., Tähtinen, P., Green, R. J., Käpylä, J., Näreaho, A., & Karonen, M. (2024). Exploring the Interactions between Plant Proanthocyanidins and Thiabendazole: Insights from Isothermal Titration Calorimetry. Molecules, 29(15), 3492. https://doi.org/10.3390/molecules29153492








