Study on the Pyrolysis and Fire Extinguishing Performance of High-Temperature-Resistant Ultrafine Dry Powder Fire Extinguishing Agents for Aviation Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
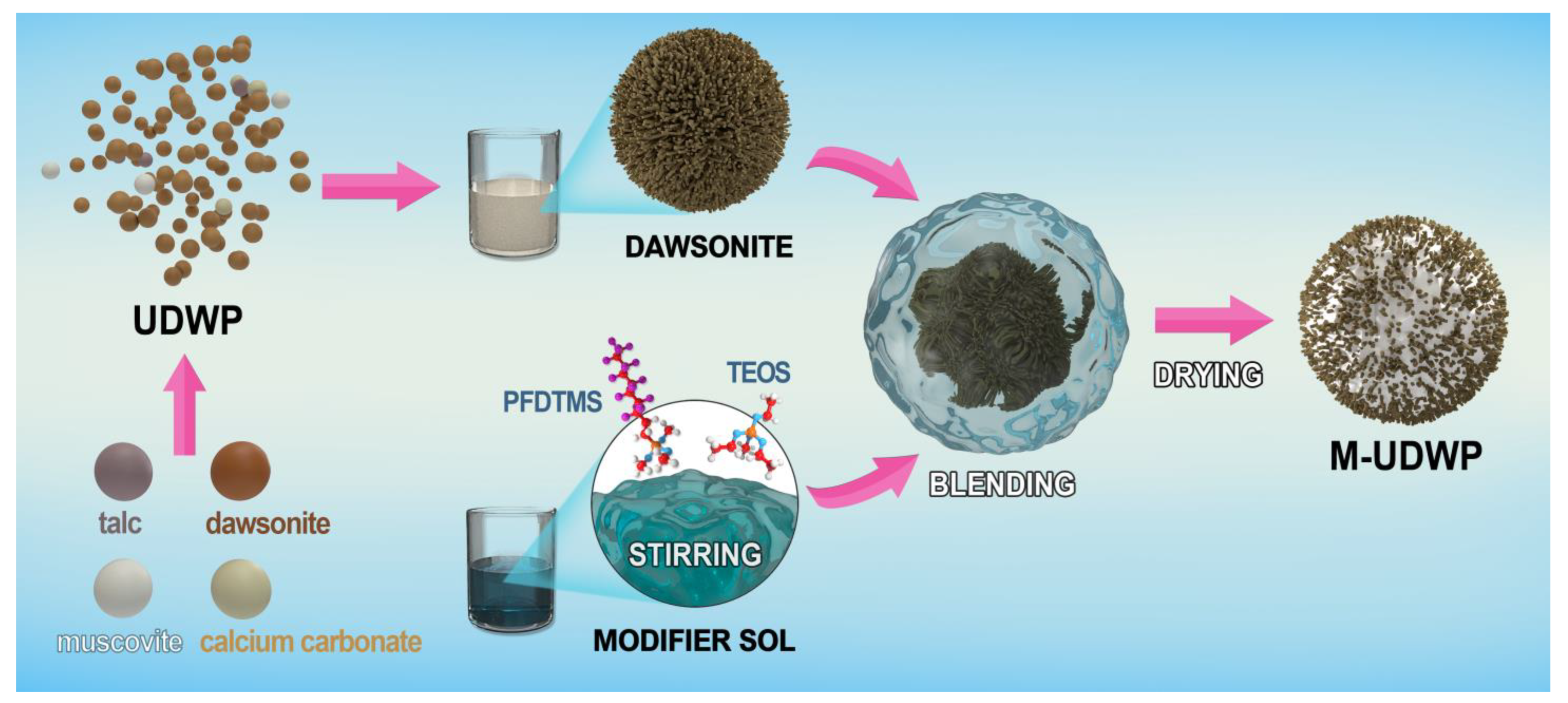
2.2. Preparation of M-UDWP
2.3. Thermal Analysis Experiment
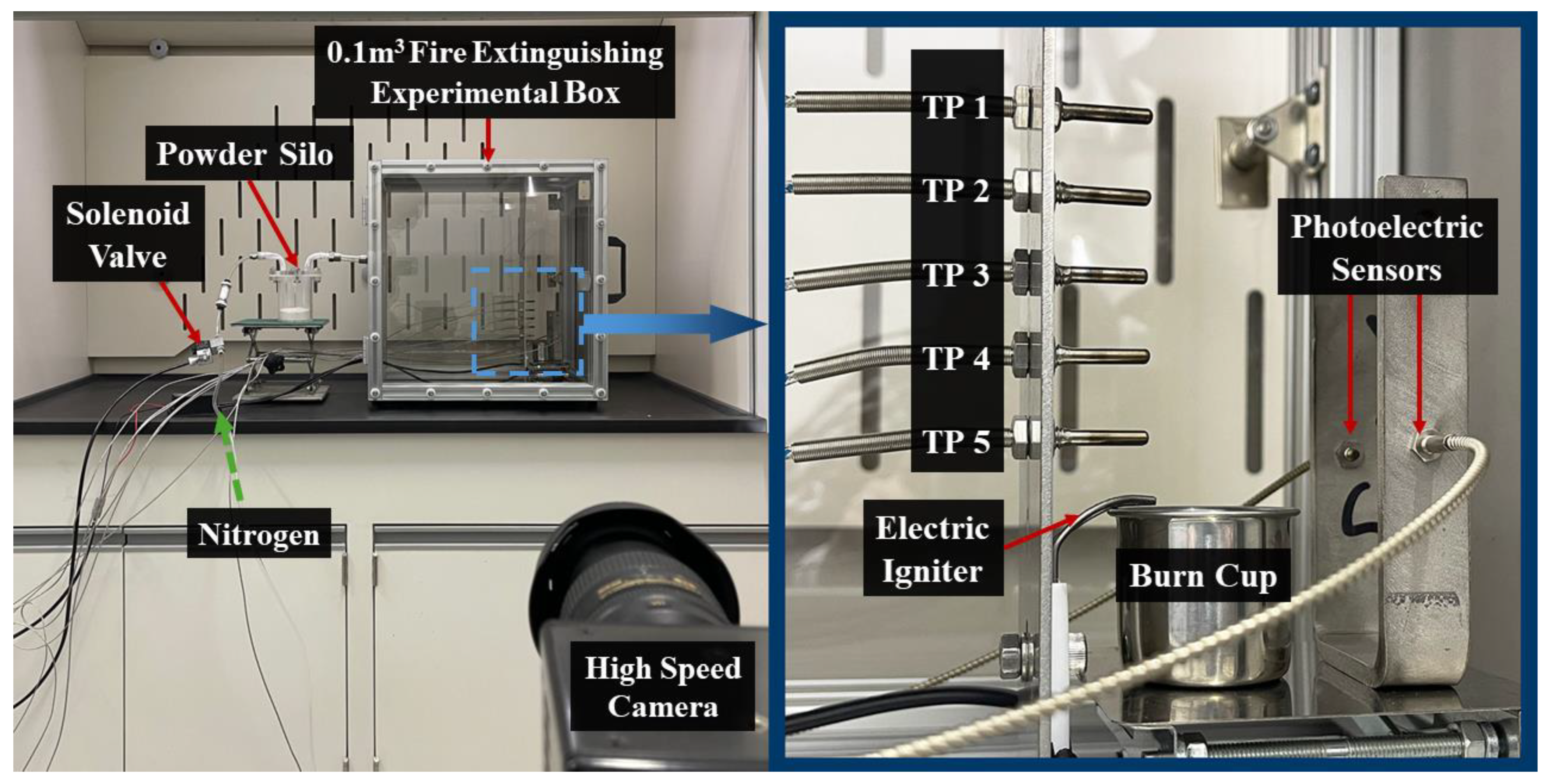
2.4. Fire Extinguishing Tests
2.5. Characterizations
3. Results and Discussion
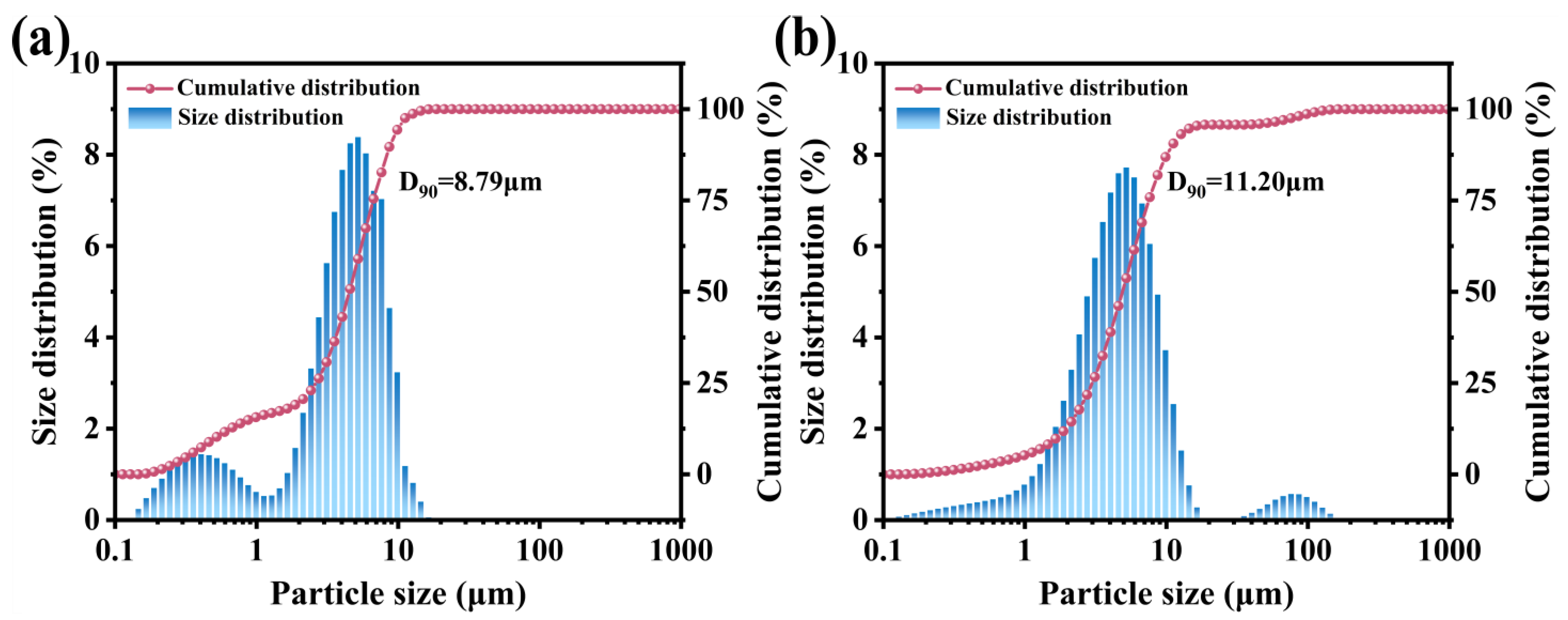
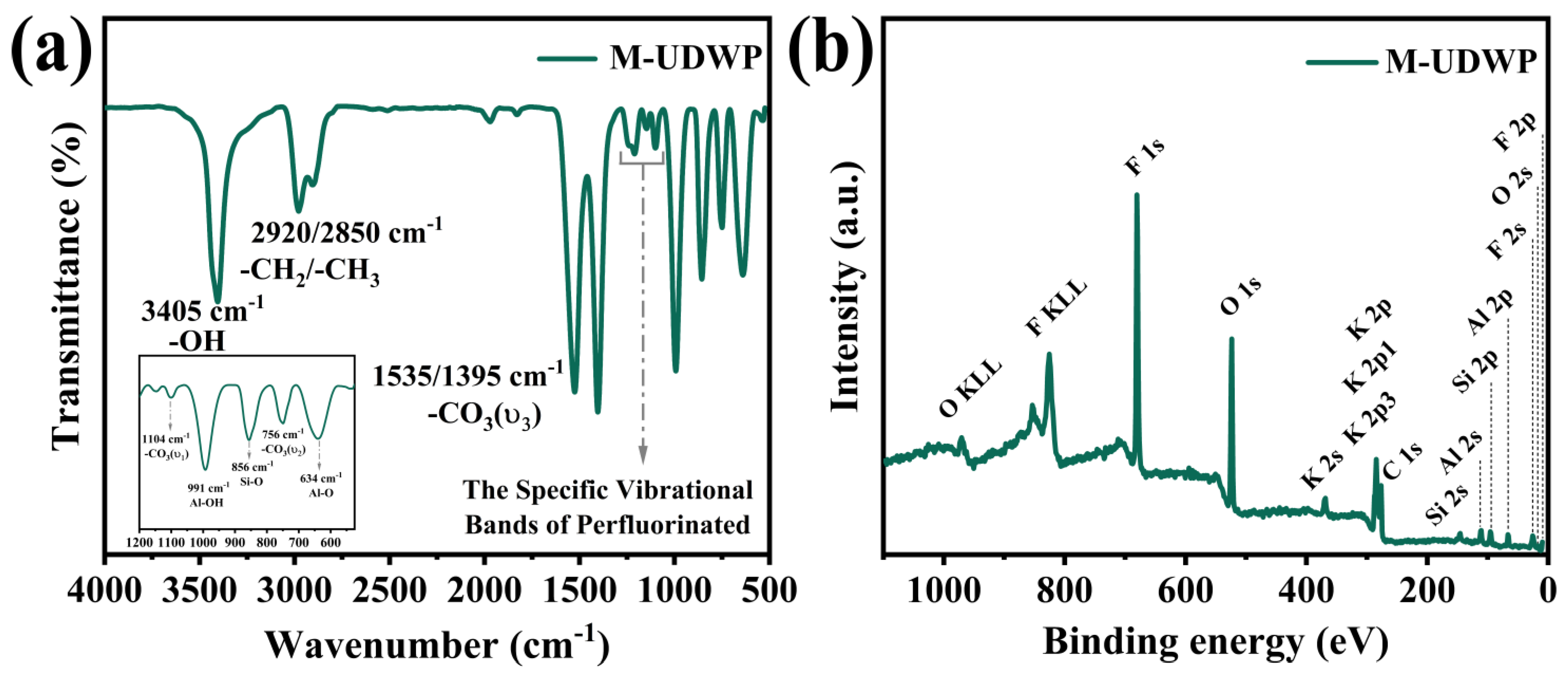
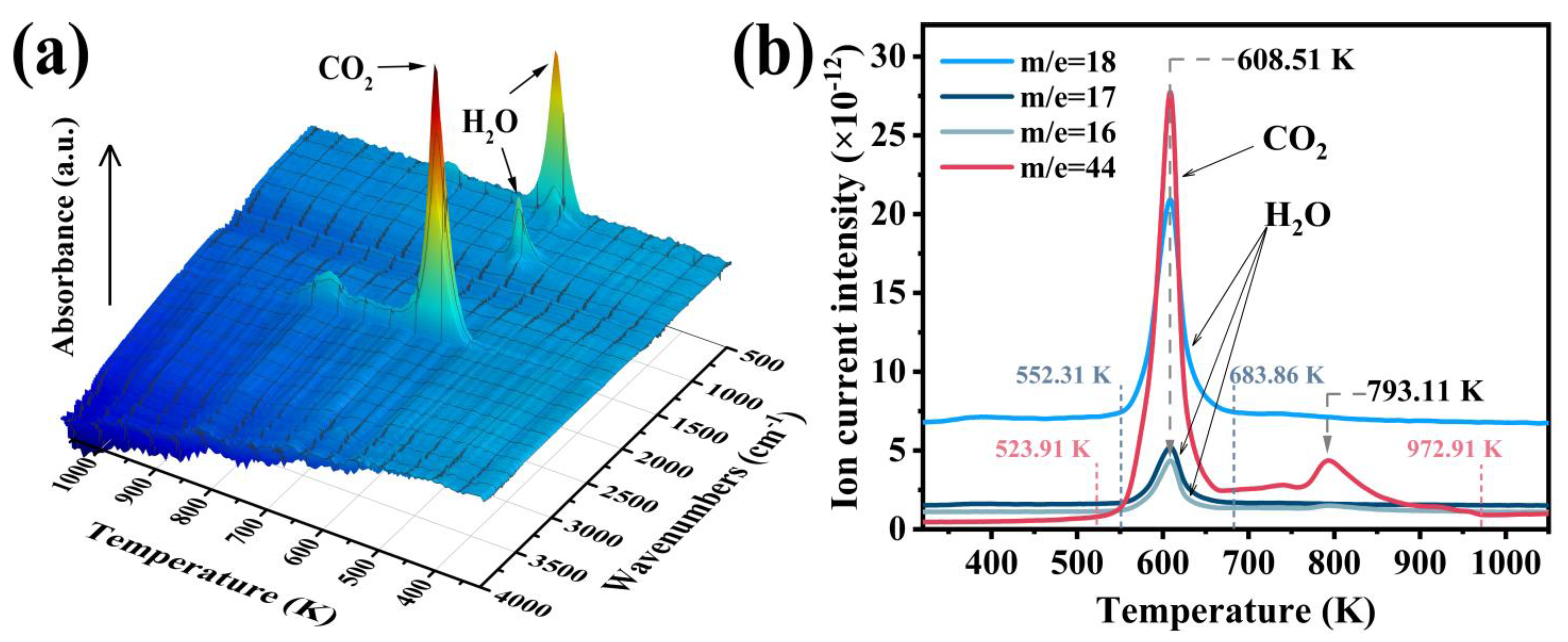
3.1. Characterization of the M-UDWP Sample
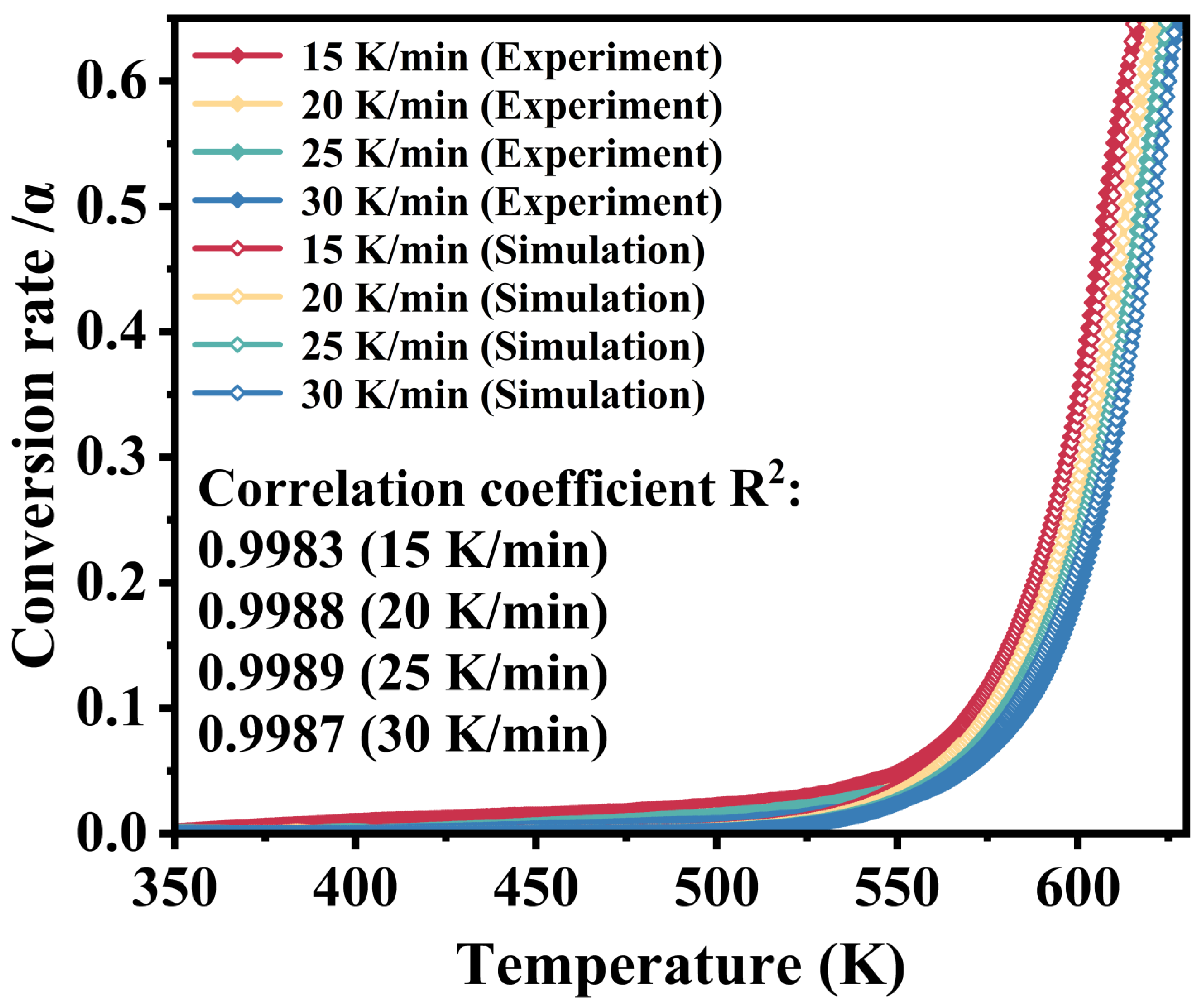
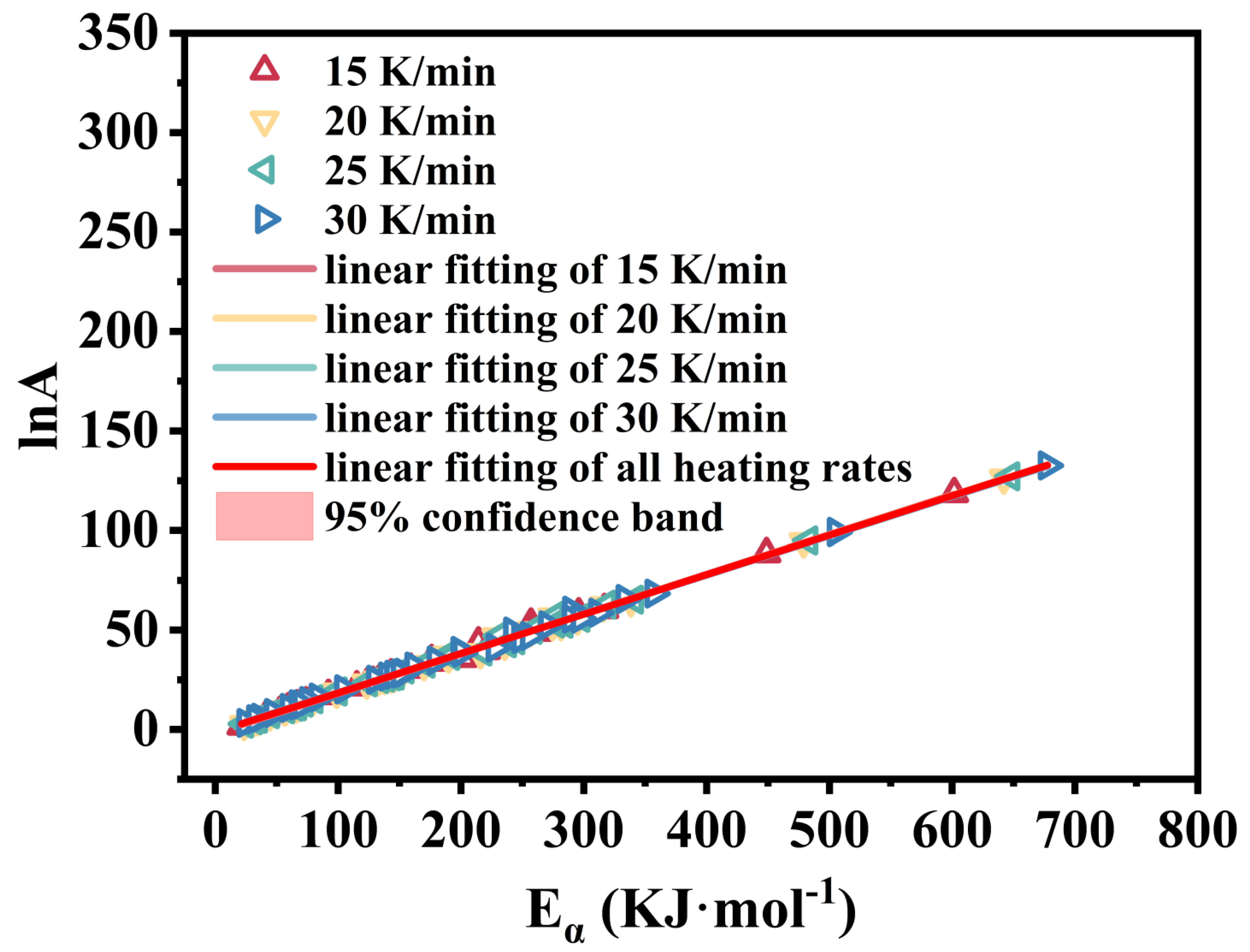
3.2. Kinetic Analysis
3.3. Thermal Pyrolysis Kinetic
3.4. Fire Extinguishing Performance of M-UDWP
4. Conclusions
- (1)
- The water contact angle (WCA) and oil contact angle (OCA) of the modified M-UDWP are 135.3° and 128.3°, respectively, with hydrophobicity and oleophobicity ratios of 90.34% and 86.59%.
- (2)
- The first-stage thermal decomposition of M-UDWP can be considered a single-step reaction with thresholds at α = 0.65 and α = 0.85. The activation energy (E) for the first stage is 239.17 KJ·mol−1. The adopted chemical reaction model is three-dimensional diffusion (No.32 g(α) = [(1 − α)1/3 − 1]2).
- (3)
- For common combustibles in two types of aircraft engine nacelles, the extinguishing times for #RP-3 and #RP-5 flames using M-UDWP are 243 ms and 224 ms, respectively, with MEC values of 25.9 g/m3 and 23.4 g/m3. The maximum cooling rates are similar. It is evident that #RP-3 fires in aircraft engine nacelles are more challenging to suppress.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravishankara, A. Findings from the 2006 ozone scientific assessment for the Montreal Protocol. In Twenty Years of Ozone Decline; Zerefos, C., Contopoulos, G., Skalkeas, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 387–391. [Google Scholar]
- Konstantinova, A.; Yudaev, P.; Orlov, A.; Loban, O.; Lukashov, N.; Chistyakov, E. Aryloxyphosphazene-Modified and Graphite-Filled Epoxy Compositions with Reduced Flammability and Electrically Conductive Properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Konstantinova, A.; Yudaev, P.; Shapagin, A.; Panfilova, D.; Palamarchuk, A.; Chistyakov, E. Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)phenoxy-phenoxycyclotriphosphazenes with Increased Adhesion to Metals. Science 2024, 6, 30. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Guo, X.; Li, S.; Zhang, H.; Pan, X.; Hua, M. Experimental and theoretical studies on the effect of Al(OH)3 on the fire-extinguishing performance of superfine ABC dry powder. Powder Technol. 2021, 393, 280–290. [Google Scholar] [CrossRef]
- Pan, J.; Mu, J.; Wu, Z.; Zhang, X. Effect of nitrogen–phosphorus fire retardant blended with Mg(OH)2/Al(OH)3 and nano-SiO2 on fire-retardant behavior and hygroscopicity of poplar. Fire Mater. 2014, 38, 817–826. [Google Scholar] [CrossRef]
- European Aviation Safety Agency. Certification Specifications and Acceptable Means of Compliance for Large Aeroplanes CS-25; European Aviation Safety Agency Cologne: Koln, Germany, 2019.
- Patrascu, M.T.; Busuioc, A.D.; Busuioc, C.; Cotarta, A.; Cojocaru, A.; Visan, T.; Vaireanu, D.I. Experimental Study on the Corrosion of Carbon Steel and Aluminum Alloy in Firefighting Protein Foam Concentrates. Materials 2021, 14, 7259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ismail, M.H.S.; Hee, C. Hot Aerosol Fire Extinguishing Agents and The Associated Technologies: A Review. Braz. J. Chem. Eng. 2015, 32, 707–724. [Google Scholar] [CrossRef]
- Ma, W.; Fu, Y.; Zhao, J.; Lu, S.; Zhang, H. Ultra-fine powder extinguishing agent concentration measurement based on extinction method. Opt. Eng. 2021, 60, 94110. [Google Scholar] [CrossRef]
- Hamins, A. Flame extinction by sodium bicarbonate powder in a cup burner. Symp. (Int.) Combust. 1998, 27, 2857–2864. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, F.; Fu, Y.; Lu, S.; Zhang, H. Insights into the particle diameter and base chosen for dry powder fire extinguishing agents. Fire Mater. 2022, 47, 774–783. [Google Scholar] [CrossRef]
- Yan, C.; Pan, X.; Hua, M.; Li, S.; Guo, X.; Zhang, C. Study on the fire extinguishing efficiency and mechanism of composite superfine dry powder containing ferrocene. Fire Saf. J. 2022, 130, 103606. [Google Scholar] [CrossRef]
- McClure, J.D.; Springer, R.J. Advanced Fire Extinguishant for Aircraft; JTCG Report AS-74-T-001; General Dynamics: Fort Worth, TX, USA, 1974. [Google Scholar]
- McClure, J.D.; Springer, R.J. Environmental and Operating Requirements for Fire Extinguishing Systems on Advanced Aircraft; JTCG Report AS-74-T-002; General Dynamics: Fort Worth, TX, USA, 1974. [Google Scholar]
- Takaya, Y.; Wu, M.; Kato, Y. Unique Environmental Conditions Required for Dawsonite Formation: Implications from Dawsonite Synthesis Experiments under Alkaline Conditions. ACS Earth Space Chem. 2019, 3, 285–294. [Google Scholar] [CrossRef]
- Stoica, G.; Abelló, S.; Pérez-Ramírez, J. Na-dawsonite derived aluminates for DMC production by transesterification of ethylene carbonate. Appl. Catal. A Gen. 2009, 365, 252–260. [Google Scholar] [CrossRef]
- Tang, J.; Liu, G.-H.; Qi, T.-G.; Zhou, Q.-S.; Peng, Z.-H.; Li, X.-B. Fibrous activated alumina prepared through phase transformation using dawsonite as a template. J. Central South Univ. 2022, 29, 1147–1160. [Google Scholar] [CrossRef]
- Ndez-Carrasco, L.F.; Rius, J. Synthesis and crystal structure determination of hydrated potassium dawsonite from powder diffraction data. Eur. J. Miner. 2006, 18, 99–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Liu, J.; Li, Q.; Pan, R.; Zhou, X. Alkaline potassium aluminum carbonate: A novel high-efficiency dry powder extinguishing agent with high heat-resistant. J. Anal. Appl. Pyrolysis 2023, 173, 106038. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, Q.; Pan, R.; Zhou, X. A novel approach for enhancing fire suppression efficiency of dry powder extinguishant: From the synergistic effect of dawsonite. Powder Technol. 2024, 431, 119052. [Google Scholar] [CrossRef]
- Ning, W.; Yang, Y.; Zhang, D.; Pan, R. Surface modification of sodium bicarbonate ultrafine powder extinguishing agent by environmental friendly fluorinated acrylate copolymers. Polym. Degrad. Stab. 2021, 187, 109558. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Zhou, X.; Pan, R. Fluorosilane-Modified Antireflash Sodium Bicarbonate Fire Extinguishing Agent with Advanced Hydrophobic and Oleophobic Properties. ACS Appl. Eng. Mater. 2023, 1, 341–349. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, S.; Wang, J.; Wang, Y.; Pan, R. Construction of NaHCO3 fire extinguishing agent modified by fluoroalkyl silane containing short fluorocarbon Chain: Improve its anti-reignition performance. Therm. Sci. Eng. Prog. 2023, 41, 101814. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, H.; Wang, Y.; Pan, R.; Zhou, X.; Zhang, Y.; Hou, H.; Wang, Y.; Pan, R.; Zhou, X. Preparation of NaHCO3 fire extinguishing agent with perfluoropolyether groups to inhibit rapid re-ignition of Class B fires. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133881. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, Z.; Shahid, M.U.; Xing, H.; Cheng, X.; Fu, Y.; Lu, S. Superhydrophobic and oleophobic ultra-fine dry chemical agent with higher chemical activity and longer fire-protection. J. Hazard. Mater. 2019, 380, 120625. [Google Scholar] [CrossRef] [PubMed]
- Ning, W. Technical Research on Improving the Fluidity of Ultrafine Dry Powder Fire Extinguishing Agent. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2021. [Google Scholar]
- Wang, J.S.; Wang, G.H.; Liu, Y.; Jiao, Y.H.; Liu, D. Thermal Stability, Combustion Behavior, and Toxic Gases in Fire Effluents of an Intumescent Flame-Retarded Polypropylene System. Ind. Eng. Chem. Res. 2014, 53, 6978–6984. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Wang, J.; Lian, W.; Li, P.; Zhang, Z.; Yang, J.; Hao, X.; Huang, W.; Guan, G. Simulation of pyrolysis in low rank coal particle by using DAEM kinetics model: Reaction behavior and heat transfer. Fuel 2017, 207, 126–135. [Google Scholar] [CrossRef]
- Miura, K. A New and Simple Method to Estimate f(E) and k0(E) in the Distributed Activation Energy Model from Three Sets of Experimental Data. Energy Fuels 1995, 9, 302–307. [Google Scholar] [CrossRef]
- Málek, J.; Criado, J.M.; Šesták, J.; Militký, J. The boundary conditions for kinetic models. Thermochim. Acta 1989, 153, 429–432. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, Y.; Li, Y.; Liao, S.; Long, Q.; Liang, J. LaPO4: Ce, Tb, Yb phosphor–synthesis and kinetics study for thermal process of precursor by Vyazovkin, OFW, KAS, Starink, and Mastplosts methods. J. Therm. Anal. Calorim. 2015, 120, 1635–1643. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Qing, Y.; Hu, C.; Yang, C.; An, K.; Tang, F.; Tan, J.; Liu, C. Rough Structure of Electrodeposition as a Template for an Ultrarobust Self-Cleaning Surface. ACS Appl. Mater. Interfaces 2017, 9, 16571–16580. [Google Scholar] [CrossRef]
- Aloulou, H.; Aloulou, W.; Daramola, M.O.; Ben Amar, R. Silane-grafted sand membrane for the treatment of oily wastewater via air gap membrane distillation: Study of the efficiency in comparison with microfiltration and ultrafiltration ceramic membranes. Mater. Chem. Phys. 2021, 261, 124186. [Google Scholar] [CrossRef]
- Picard, C. Elaboration de Membranes Céramiques pour la Diffusion sans Bulle d’Ozone dans le Traitement des Eaux Polluées. Ph.D. Thesis, University of Montpellier, Montpellier, France, 2001; p. 191. [Google Scholar]
- Li, R.; Shan, Y. Contact Angle and Local Wetting at Contact Line. Langmuir 2012, 28, 15624–15628. [Google Scholar] [CrossRef] [PubMed]
- Chesna, J.W.; Wiedmaier, B.F.; Wang, J.; Samara, A.; Leach, R.K.; Her, T.-H.; Smith, S.T. Aerial wetting contact angle measurement using confocal microscopy. Meas. Sci. Technol. 2016, 27, 125202. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M.; Pilati, F.; Taurino, R.; Tonelli, C.; Toselli, M. Hydrophobic and oleophobic coatings based on perfluoropolyether/silica hybrids by the sol-gel method. Adv. Polym. Technol. 2007, 26, 182–190. [Google Scholar] [CrossRef]
- Krasnyansky, M. Studies of fundamental physical–chemical mechanisms and processes of flame extinguishing by powder aerosols. Fire Mater. 2007, 32, 27–47. [Google Scholar] [CrossRef]
- Chang, L.; Cao, F.; Cai, J.; Liu, W.; Zhang, J.; Cao, C. Formation and transformation of Mg(OH)2 in anodic coating using FTIR mapping. Electrochem. Commun. 2009, 11, 2245–2248. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Zhang, G.; Ren, M.; Xie, W.; Sun, S. Torrefaction-assisted oxy-fuel co-combustion of textile dyeing sludge and bamboo residues toward enhancing emission-to-ash desulfurization in full waste circularity. Fuel 2022, 318, 123603. [Google Scholar] [CrossRef]
- Zhao, N.; Yao, E.; Ma, H.; Zeng, J.; Yu, Z.; An, T.; Zhao, F.; Yu, X. Studies on thermokinetic and reactive mechanism of graphdiyne-based NC composite via multi isoconversional methods and model reconstruction. Cellulose 2022, 29, 4365–4379. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Liu, J.; Chang, K.; Evrendilek, F.; Liang, G. Flue gas-to-ash desulfurization of combustion of textile dyeing sludge: Its dependency on temperature, lignocellulosic residue, and CaO. Chem. Eng. J. 2020, 417, 127906. [Google Scholar] [CrossRef]
- Gaidukova, O.; Misyura, S.; Donskoy, I.; Morozov, V.; Volkov, R. Pool Fire Suppression Using CO2 Hydrate. Energies 2022, 15, 9585. [Google Scholar] [CrossRef]

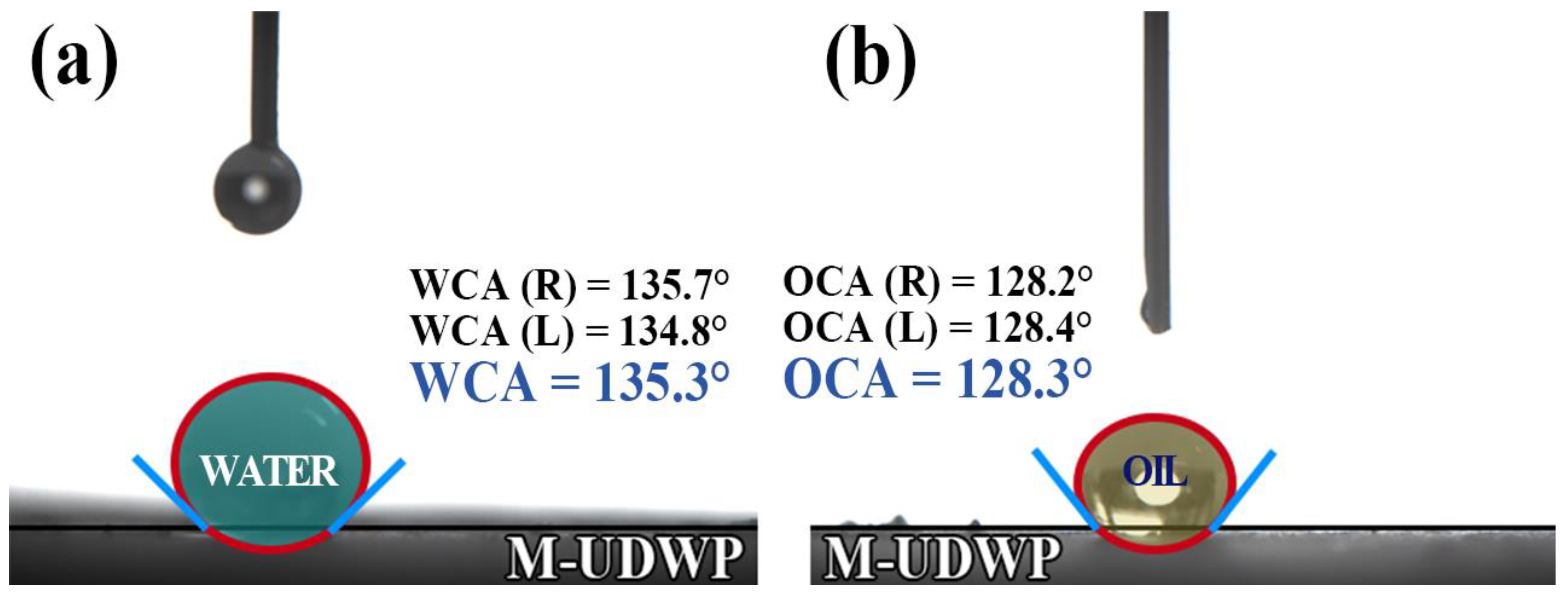
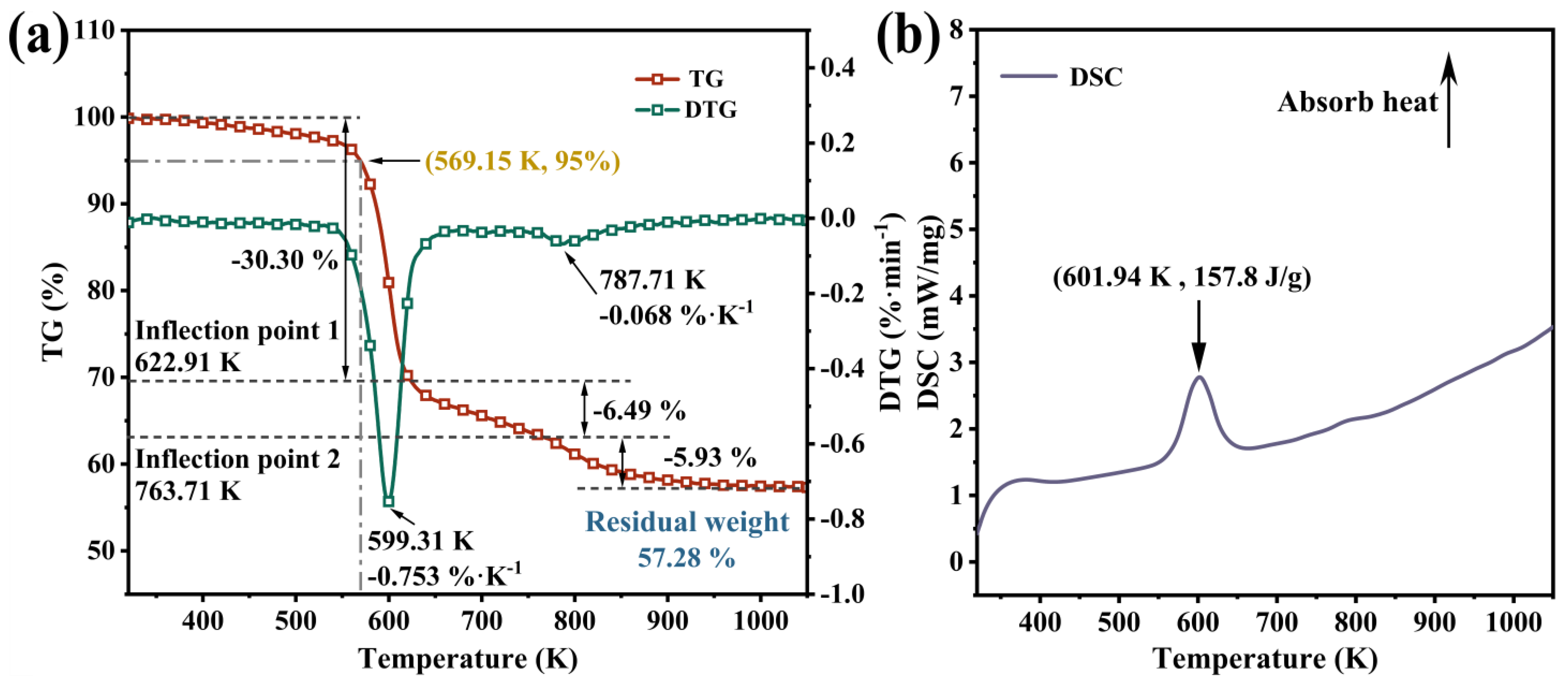
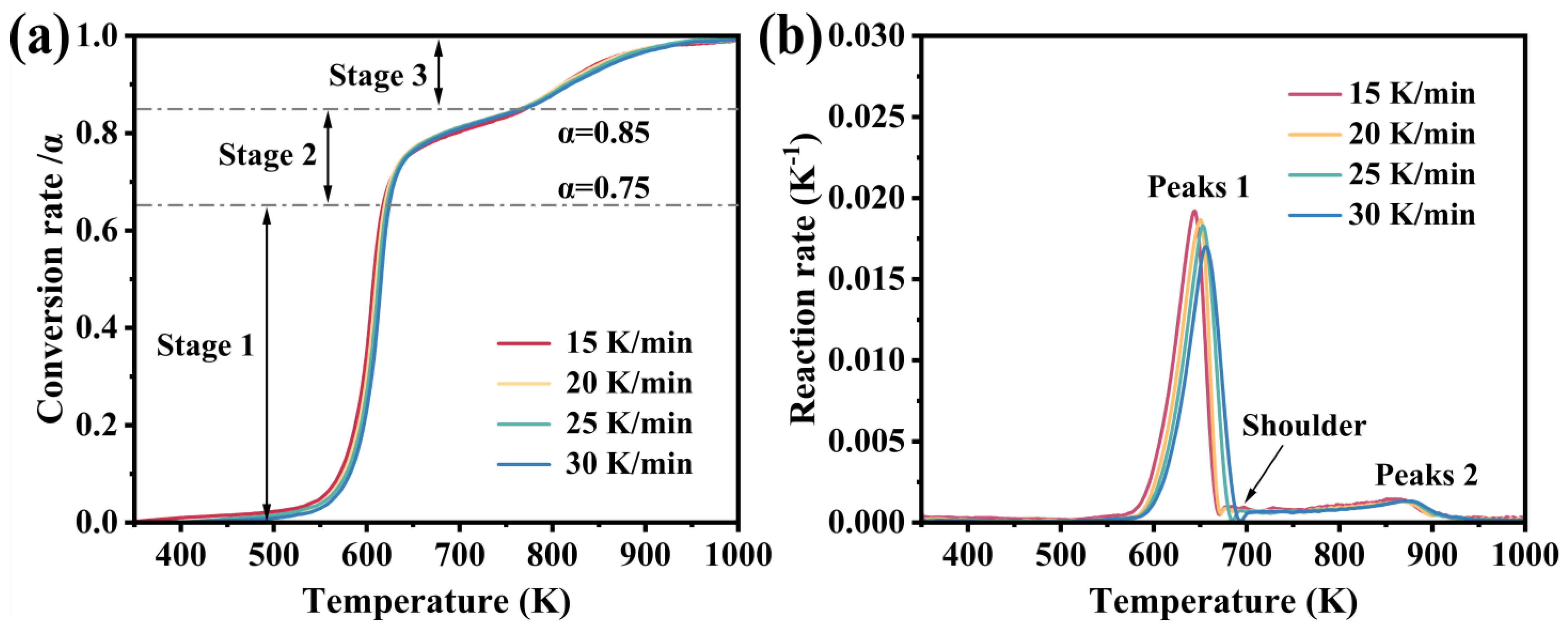
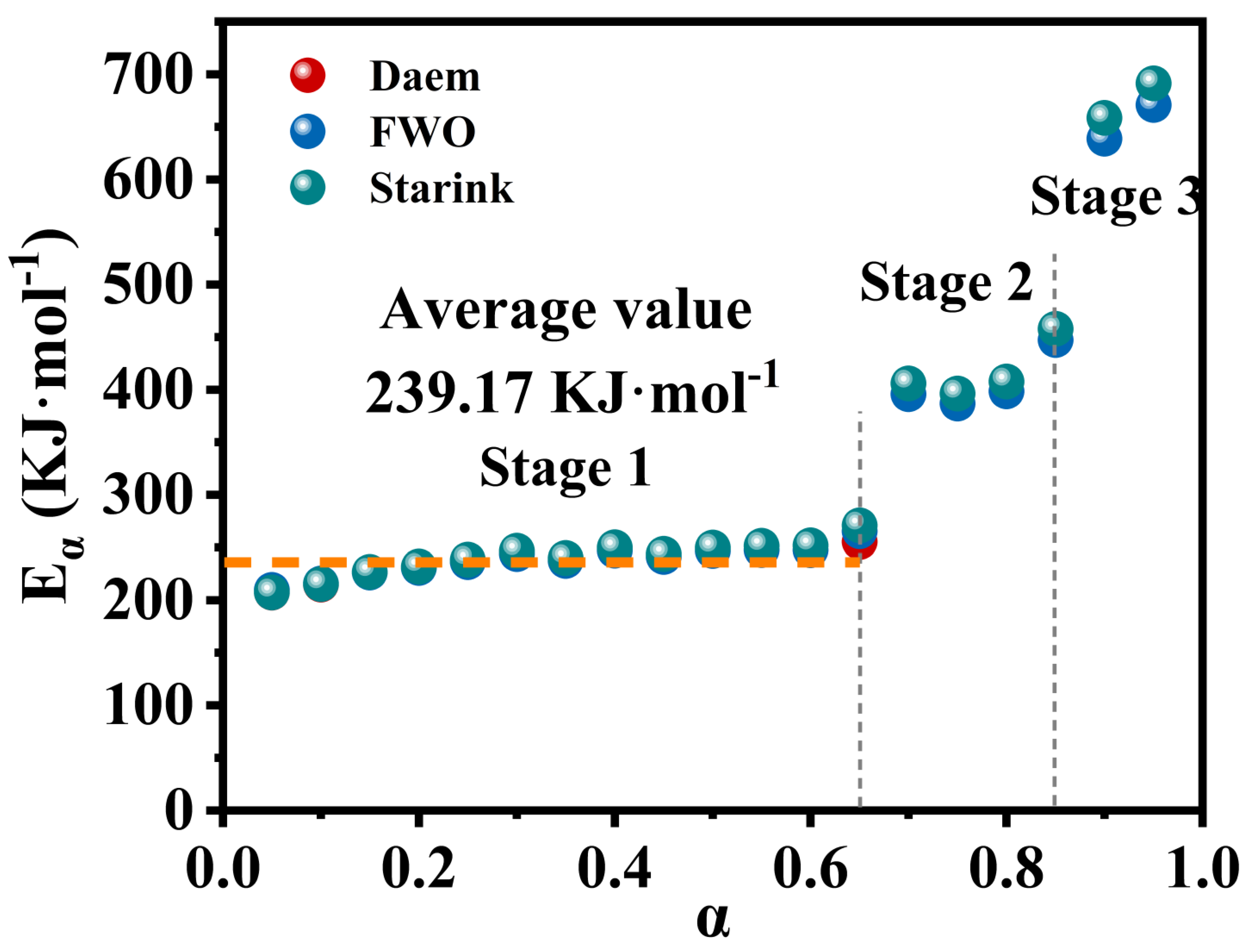



| M0 | M1 | M1/M0 | |
|---|---|---|---|
| water | 10 | 9.034 | 90.34% |
| Aviation Kerosene | 10 | 8.659 | 86.59% |
| Heating Rate (K·min−1) | Temperature (K) /Reaction Rate (K−1) | Temperature (K) /Average Reaction Rate (K−1) | Total Average Reaction Rate (K−1) | ||||
|---|---|---|---|---|---|---|---|
| Peak 1 | Shoulder | Peak 2 | Stage 1 | Stage 2 | Stage 3 | ||
| 15 | 644.0 /0.0192 | 678.4 /0.0097 | 865.4 /0.0014 | 350–651 /0.0024 | 651–787 /0.0016 | 787–1000 /0.0007 | 0.0019 |
| 20 | 650.5 /0.0187 | 677.2 /0.0087 | 866.5 /0.0013 | 350–657 /0.0022 | 657–782 /0.0017 | 782–1000 /0.0007 | 0.0018 |
| 25 | 652.7 /0.0183 | 694.0 /0.0073 | 869.4 /0.0013 | 350–661 /0.0021 | 661–776 /0.0018 | 776–1000 /0.0007 | 0.0017 |
| 30 | 656.2 /0.0170 | 704.2 /0.0062 | 871.7 /0.0013 | 350–666 /0.0019 | 666–792 /0.0019 | 792–1000 /0.0007 | 0.0017 |
| Heating Rate (K/min) | Kinetic Parameter | No. 32 g(α) = [(1 − α)1/3 − 1]2 |
|---|---|---|
| 15 K/min | Eα (kJ·mol−1) | 210.6493 |
| lnA (s−1) | 37.5603 | |
| R2 | 0.9983 | |
| 20 K/min | Eα (kJ·mol−1) | 222.7939 |
| lnA (s−1) | 40.0954 | |
| R2 | 0.9988 | |
| 25 K/min | Eα (kJ·mol−1) | 225.9828 |
| lnA (s−1) | 40.7834 | |
| R2 | 0.9989 | |
| 30 K/min | Eα (kJ·mol−1) | 232.6409 |
| lnA (s−1) | 42.0824 | |
| R2 | 0.9987 | |
| Average value | Eα (kJ·mol−1) | 223.0200 |
| lnA (s−1) | 40.1300 | |
| R2 | 0.9987 |
| Heating Rate (K/min) | a (1/min) | 95% CI of a | B (kJ/mol) | 95% CI of b | kiso | Tiso (K) | R2 |
|---|---|---|---|---|---|---|---|
| 15 | −1.8654 | (−2.6183, −1.1126) | 0.1548 | (0.1960, 0.2032) | 0.1478 | 602.7 | 0.9975 |
| 20 | −1.5118 | (−2.2660, −0.7576) | 0.1986 | (0.1952, 0.2019) | 0.2205 | 605.7 | 0.9978 |
| 25 | −1.2863 | (−2.0406, −0.5321) | 0.1978 | (0.1945, 0.2011) | 0.2641 | 608.1 | 0.9978 |
| 30 | −1.0645 | (−1.8199, −0.3092) | 0.1968 | (0.1937, 0.2000) | 0.3449 | 611.0 | 0.9980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, Y.; Liu, Y.; Wang, J.; Zhou, X.; Pan, R. Study on the Pyrolysis and Fire Extinguishing Performance of High-Temperature-Resistant Ultrafine Dry Powder Fire Extinguishing Agents for Aviation Applications. Molecules 2024, 29, 3500. https://doi.org/10.3390/molecules29153500
Wang Z, Zhang Y, Liu Y, Wang J, Zhou X, Pan R. Study on the Pyrolysis and Fire Extinguishing Performance of High-Temperature-Resistant Ultrafine Dry Powder Fire Extinguishing Agents for Aviation Applications. Molecules. 2024; 29(15):3500. https://doi.org/10.3390/molecules29153500
Chicago/Turabian StyleWang, Zhixuan, Yi Zhang, Yurong Liu, Jun Wang, Xia Zhou, and Renming Pan. 2024. "Study on the Pyrolysis and Fire Extinguishing Performance of High-Temperature-Resistant Ultrafine Dry Powder Fire Extinguishing Agents for Aviation Applications" Molecules 29, no. 15: 3500. https://doi.org/10.3390/molecules29153500





