Changes in the Sensitivity of MCF-7 and MCF-7/DX Breast Cancer Cells to Cytostatic in the Presence of Metformin
Abstract
:1. Introduction
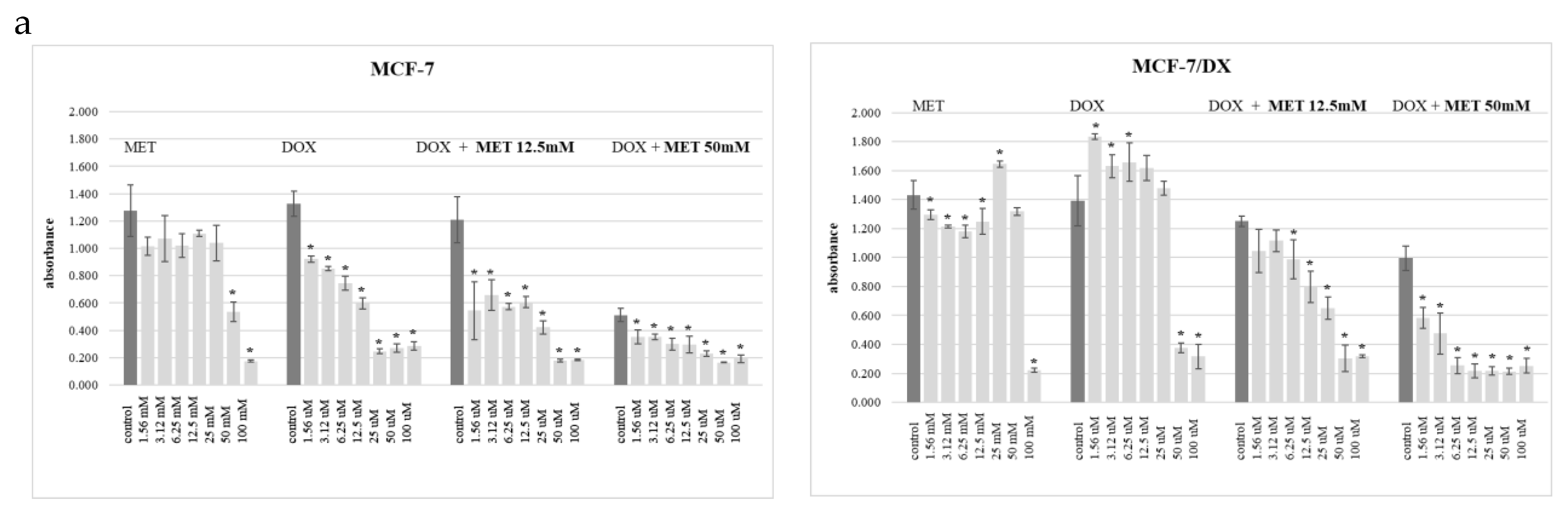
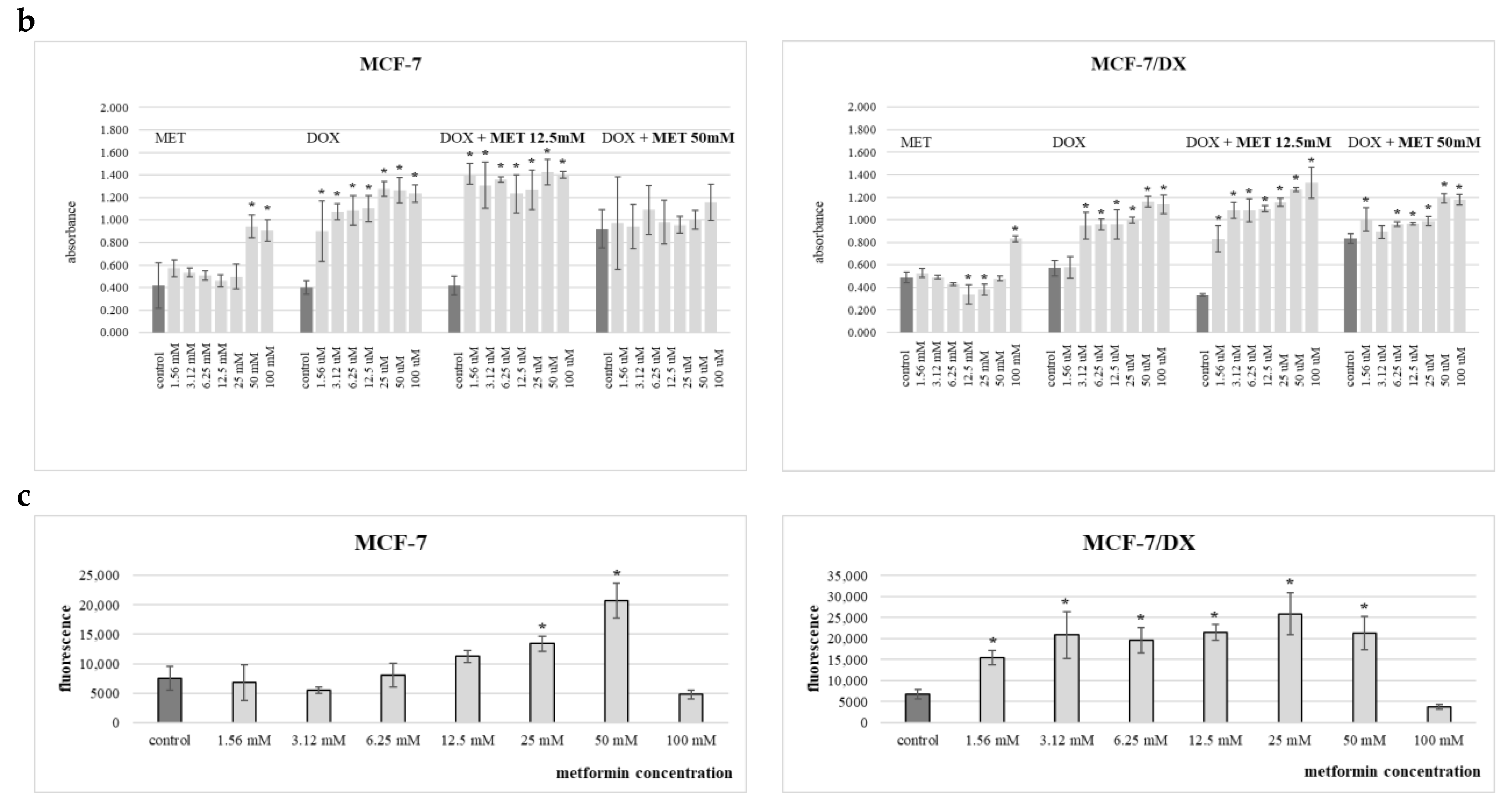
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Evaluation of the Effect of Metformin on the Sensitivity of Cells to Doxorubicin
4.3. Cell Viability
4.3.1. WST1 Test (Roche Diagnostics)
4.3.2. LDH Test (Roche Diagnostics)
4.4. MDR Test (Sigma-Aldrich)
4.5. Prediction of Potential Molecular Targets of Metformin
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Cwynar-Zając, Ł. Metformin—A new approach. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J. Mol. Endocrinol. 2019, 63, R17–R35. [Google Scholar] [CrossRef] [PubMed]
- Almaimani, R.A.; Aslam, A.; Ahmad, J.; El-Readi, M.Z.; El-Boshy, M.E.; Abdelghany, A.H.; Idris, S.; Alhadrami, M.; Althubiti, M.; Almasmoum, H.; et al. In vivo and iv vitro enhanced tumoricidal effects of metformin, active vitamin D3, and 5-fluorouracil triple therapy against colon cancer by modulating the PI3K/Akt/PTEN/mTOR network. Cancers 2022, 14, 1538. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, J.X.; Yang, S.F.; Chen, M.L.; Chen, T.H.; Hsiao, Y.H. Metformin potentiates the anticancer effect of everolimus on cervical cancer in vitro and in vivo. Cancers 2021, 13, 4612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, P.H.; Chen, P.N.; Yang, S.F.; Hsiao, Y.H. Molecular and cellular mechanisms of metformin in cervical cancer. Cancers 2021, 13, 2545. [Google Scholar] [CrossRef]
- Roshan, M.H.; Shing, Y.K.; Pace, N.P. Metformin as an adjuvant in breast cancer treatment. SAGE Open Med. 2019, 7, 2050312119865114. [Google Scholar] [CrossRef]
- Farahi, A.; Abedini, M.R.; Javdani, H.; Arzi, L.; Chamani, E.; Farhoudi, R.; Talebloo, N.; Hoshyar, R. Crocin and metformin suppress metastatic breast cancer progression via VEGF and MMP9 downregulations: In vitro and in vivo studies. Mol. Cell. Biochem. 2021, 476, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, D.; Zhou, H.; Sima, X.; Wu, Z.; Sun, Y.; Wang, L.; Ruan, Y.; Wu, Q.; Wu, F.; et al. Metformin: A promising drug for human cancers (review). Oncol. Lett. 2022, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular targeting therapy of cancer: Drug resistance, apoptosis and survival signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2021, 12, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updates 2021, 59, 100796. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Mir, S.A.; Hamid, L.; Bader, G.N.; Shoaib, A.; Rahamathulla, M.; Alshahrani, M.Y.; Alam, P.; Shakeel, F. Role of nanotechnology in overcoming the multidrug resistance in cancer therapy: A review. Molecules 2022, 27, 6608. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Imani, S.; Jomhori, M.; Ling, K.H. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs the current chemotherapy. Am. J. Cancer Res. 2021, 11, 5155–5183. [Google Scholar]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R.; Chakraborty, P.P. Diabetes monotherapies versus metformin-based combination therapy for the treatment of type 2 diabetes. Int. J. Gen. Med. 2021, 14, 3833–3848. [Google Scholar] [CrossRef]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980225. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Detaille, D.; Guigas, B. Role of mitochondria in the mechanism(s) of action of metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Kölz, C.; Schaeffeler, E.; Schwab, M.; Nies, A.T. Genetic and epigenetic regulation of organic cation transporters. Handb. Exp. Pharmacol. 2021, 266, 81–100. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Lam, T.G.; Jeong, S.; Ahn, S.G. Metformin derivative HL156A reverses multidrug resistance by inhibiting HOXC6/ERK1/2 signaling in multidrug-resistant human cancer cells. Pharmaceuticals 2020, 13, 218. [Google Scholar] [CrossRef]
- Shafiei-Irannejad, V.; Samadi, N.; Salehi, R.; Yousefi, B.; Rahimi, M.; Akbarzadeh, A.; Zarghami, N. Reversion of multidrug resistance by co-encapsulation of doxorubicin and metformin in poly(lactide-co-glycolide)-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles. Pharm. Res. 2018, 35, 119. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, N.; Jiang, S.; Bai, Y.; Guan, C.; Zhang, W.; Fan, S.; Huang, Y.; Lin, H.; Ying, Y. The chemosensitizing role of metformin in anti-cancer therapy. Anticancer Agents Med. Chem. 2021, 21, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, J.; Ma, L.; Zhao, Y.; Xu, S.; Shi, J.; Qian, K.; Gu, M.; Tan, H.; Xu, L.; et al. Reversing multi-drug resistance by polymeric metformin to enhance antitumor efficacy of chemotherapy. Int. J. Pharm. 2022, 624, 121931. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Zhou, Y.; Qiu, S.; Wu, Y.; Xie, M.; Zhu, G.; Liang, S.; Li, H.; Zhou, D.; et al. Metformin reverses the drug resistance of cisplatin in irradiated CNE-1 human nasopharyngeal carcinoma cells through PECAM-1 mediated MRPs down-regulation. Int. J. Med. Sci. 2020, 17, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, S.; Zong, Q.; Yin, Z. Co-delivery of metformin and paclitaxel via folate-modified pH-sensitive micelles for enhanced anti-tumor efficacy. AAPS Pharm. Sci. Tech. 2018, 19, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Lobanova, L.; Dawicki, W.; Groot, G.; Gordon, J.R.; Bowen, M.; Harkness, T.; Arnason, T. Metformin inhibits the development, and promotes the resensitization, of treatment-resistant breast cancer. PLoS ONE 2017, 12, e0187191. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.; Sterner, G.N.; Londahl, M.; Wiklander, C.; Cato, A.; Vinge, E.; Andersson, A. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: Clinical recommendations. Diabetes Care 2010, 33, 1291–1293. [Google Scholar] [CrossRef]
- (FDA) Food and Drug Administration; US Department of Health and Human Services. Metformin Hydrochloride Tablets: Fda.gov Accessed 30 May 2017. Available online: www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf (accessed on 3 December 2022).
- Marinello, P.C.; Panis, C.; Silva, T.N.X.; Binato, R.; Abdelhay, E.; Rodrigues, J.A.; Mencalha, A.L.; Lopes, N.M.D.; Luiz, R.C.; Cecchini, R.; et al. Metformin prevention of doxorubicin resistance in MCF-7 and MDA-MB-231 involves oxidative stress generation and modulation of cell adaptation genes. Sci. Rep. 2019, 9, 5864. [Google Scholar] [CrossRef]
- Swiss Institute of Bioinformatics. Available online: http://swisstargetprediction.ch (accessed on 3 December 2022).
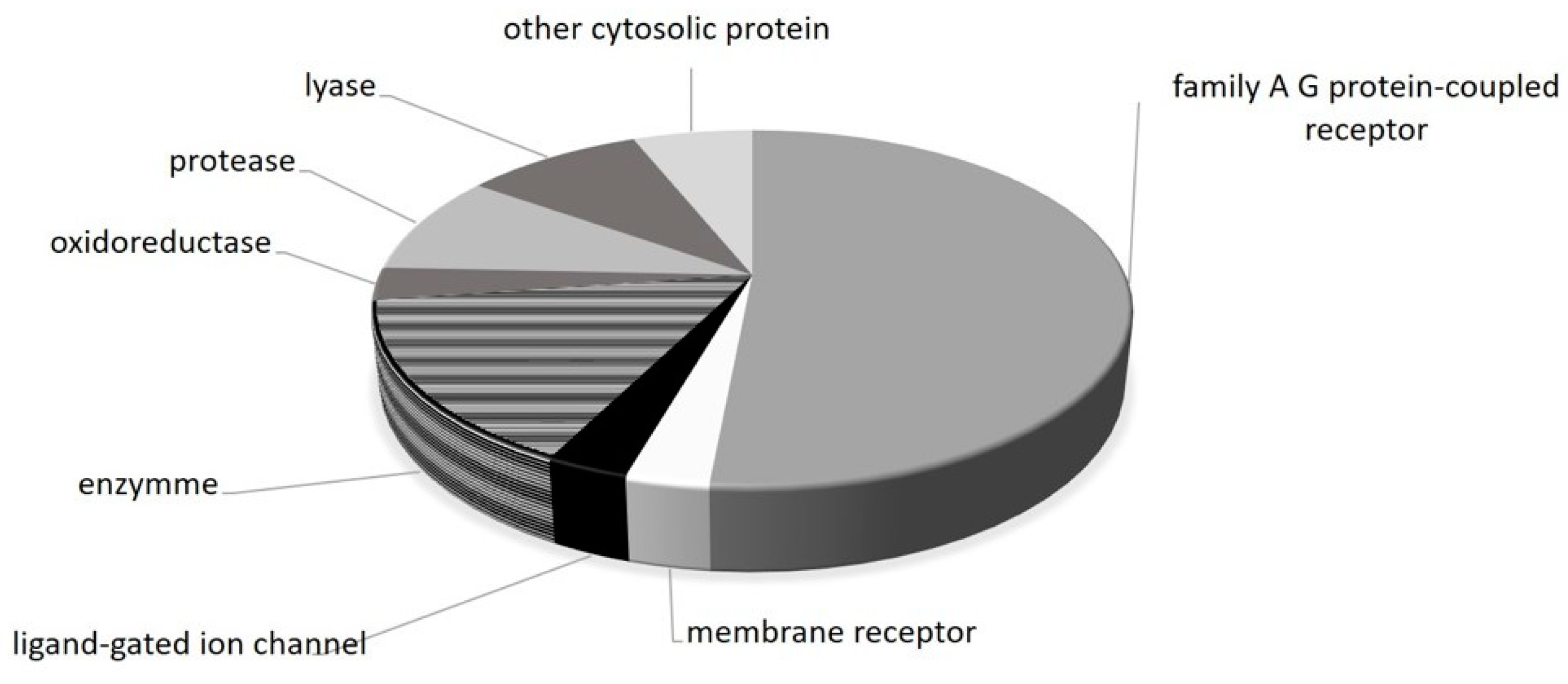
| Query Molecule | 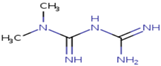 | |
|---|---|---|
| Target | Target Class | Common Name |
| Adrenergic receptor alpha-2 | Family A G protein-coupled receptor | ADRA2C |
| Alpha-1a adrenergic receptor | Family A G protein-coupled receptor | ADRA1A |
| Alpha-1b adrenergic receptor | Family A G protein-coupled receptor | ADRA1B |
| Alpha-1d adrenergic receptor | Family A G protein-coupled receptor | ADRA1D |
| Alpha-2a adrenergic receptor | Family A G protein-coupled receptor | ADRA2A |
| Alpha-2b adrenergic receptor | Family A G protein-coupled receptor | ADRA2B |
| Amine oxidase, copper containing | Enzyme | AOC3 |
| Carbonic anhydrase I | Lyase | CA1 |
| Carbonic anhydrase II | Lyase | CA2 |
| Carbonic anhydrase IX | Lyase | CA9 |
| Dihydrofolate reductase | Oxidoreductase | DHFR |
| Histamine H3 receptor | Family A G protein-coupled receptor | HRH3 |
| Histamine H4 receptor | Family A G protein-coupled receptor | HRH4 |
| Indoleamine 2,3-dioxygenase | Enzyme | IDO1 |
| Integrin alpha-V/beta-3 | Membrane receptor | ITGAV ITGB3 |
| Nischarin | Other cytosolic protein | NISCH |
| Nitric oxide synthase, inducible | Enzyme | NOS2 |
| Nitric oxide synthase, brain | Enzyme | NOS1 |
| Nitric oxide synthase, endothelial | Enzyme | NOS3 |
| S-100 protein beta chain | Other cytosolic protein | S100B |
| Serotonin 1a (5-HT1a) receptor | Family A G protein-coupled receptor | HTR1A |
| Serotonin 1b (5-HT1b) receptor | Family A G protein-coupled receptor | HTR1B |
| Serotonin 1d (5-HT1d) receptor | Family A G protein-coupled receptor | HTR1D |
| Serotonin 2a (5-HT2a) receptor | Family A G protein-coupled receptor | HTR2A |
| Serotonin 2b (5-HT2b) receptor | Family A G protein-coupled receptor | HTR2B |
| Serotonin 2c (5-HT2c) receptor | Family A G protein-coupled receptor | HTR2C |
| Serotonin 3a (5-HT3a) receptor | Ligand-gated ion channel | HTR3A |
| Serotonin 5a (5-HT5a) receptor | Family A G protein-coupled receptor | HTR5A |
| Serotonin 6 (5-HT6) receptor | Family A G protein-coupled receptor | HTR6 |
| Serotonin 7 (5-HT7) receptor | Family A G protein-coupled receptor | HTR7 |
| Thrombin | Protease | F2 |
| Thrombin and coagulation factor X | Protease | F10 |
| Urokinase-type plasminogen activator | Protease | PLAU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płonka-Czerw, J.; Żyrek, L.; Latocha, M. Changes in the Sensitivity of MCF-7 and MCF-7/DX Breast Cancer Cells to Cytostatic in the Presence of Metformin. Molecules 2024, 29, 3531. https://doi.org/10.3390/molecules29153531
Płonka-Czerw J, Żyrek L, Latocha M. Changes in the Sensitivity of MCF-7 and MCF-7/DX Breast Cancer Cells to Cytostatic in the Presence of Metformin. Molecules. 2024; 29(15):3531. https://doi.org/10.3390/molecules29153531
Chicago/Turabian StylePłonka-Czerw, Justyna, Luiza Żyrek, and Małgorzata Latocha. 2024. "Changes in the Sensitivity of MCF-7 and MCF-7/DX Breast Cancer Cells to Cytostatic in the Presence of Metformin" Molecules 29, no. 15: 3531. https://doi.org/10.3390/molecules29153531






