Janus MoSH/WSi2N4 van der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact
Abstract
:1. Introduction
2. Results and Discussion
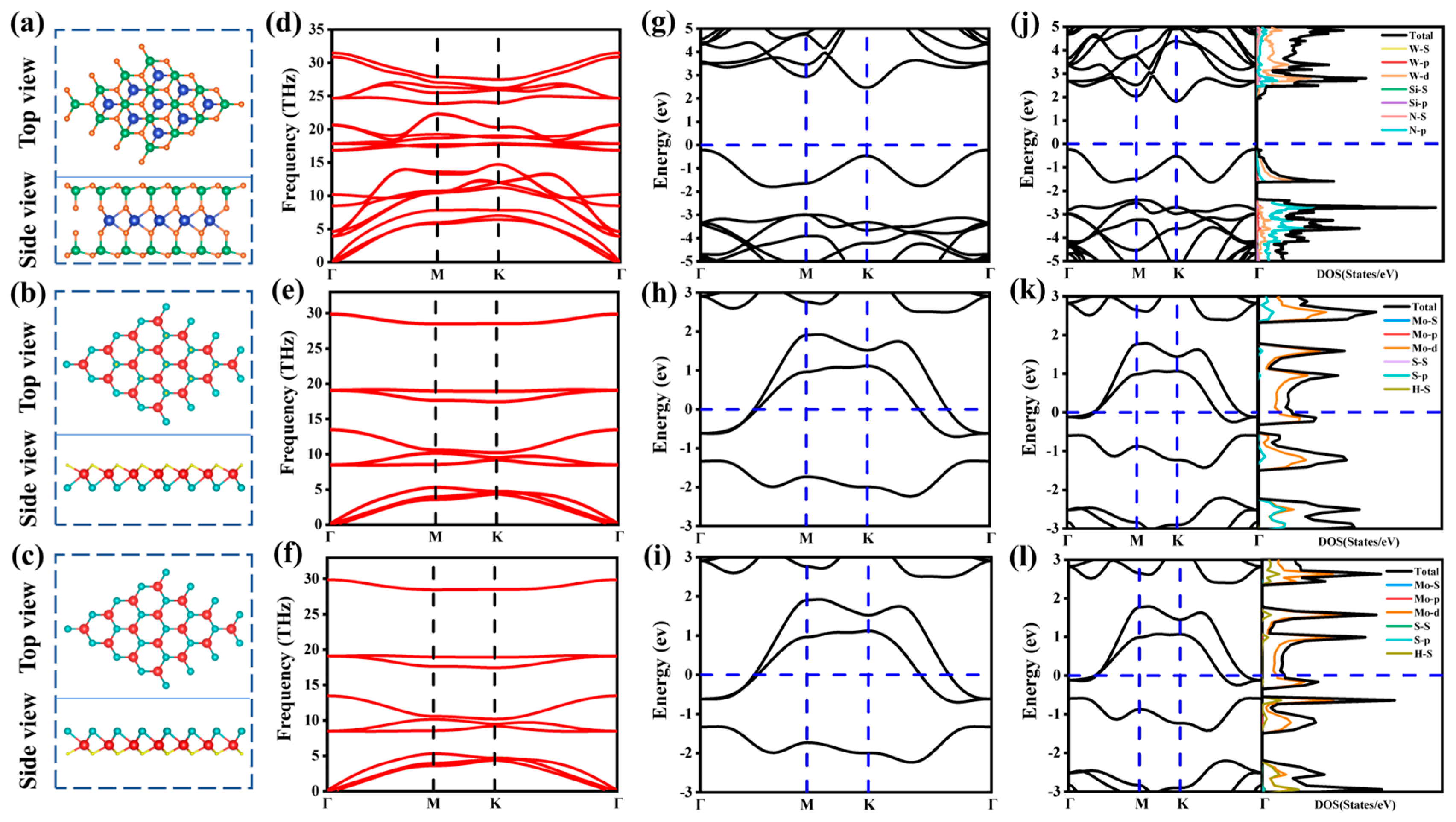
2.1. Geometric Structures and Electronic Properties
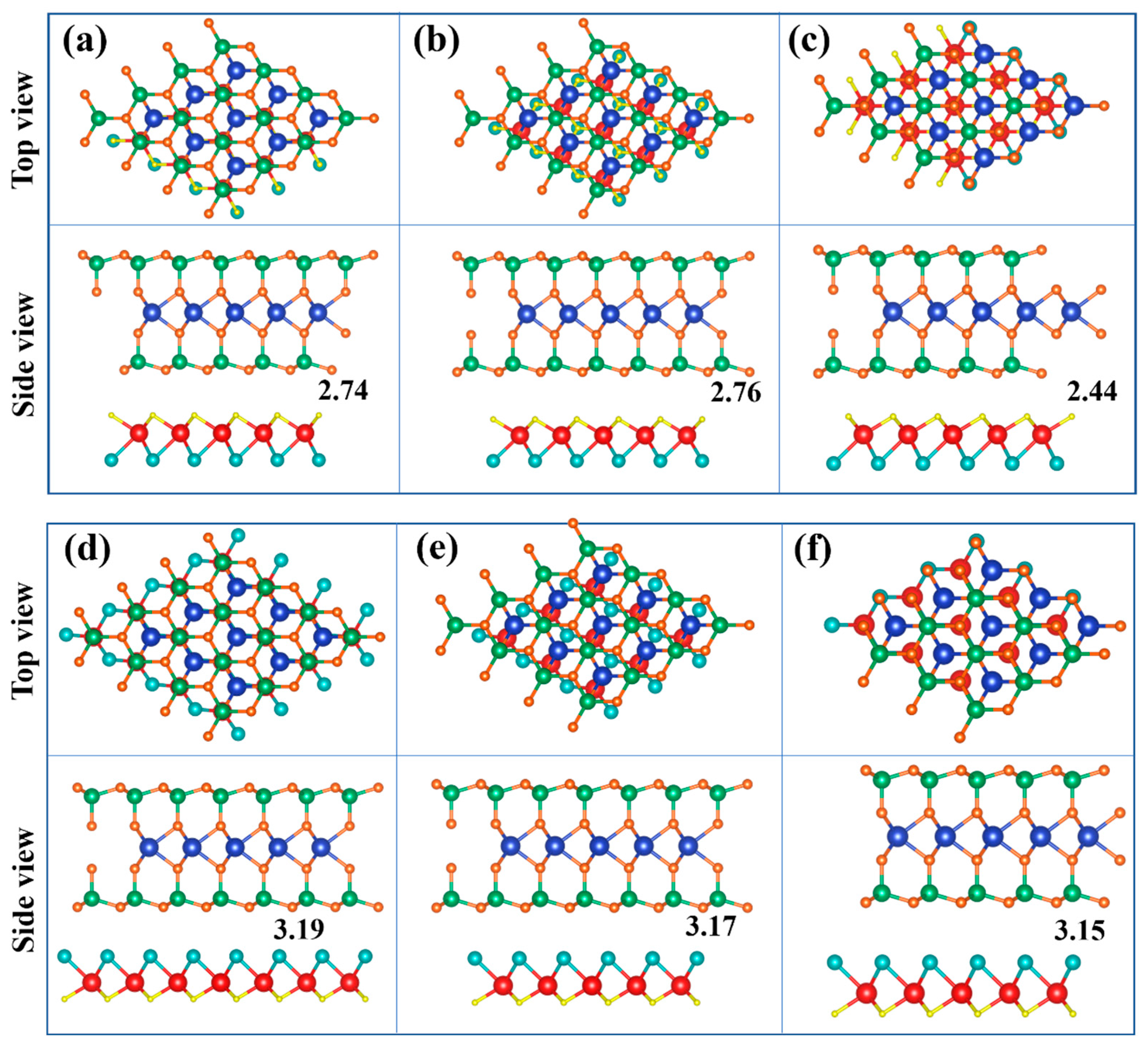
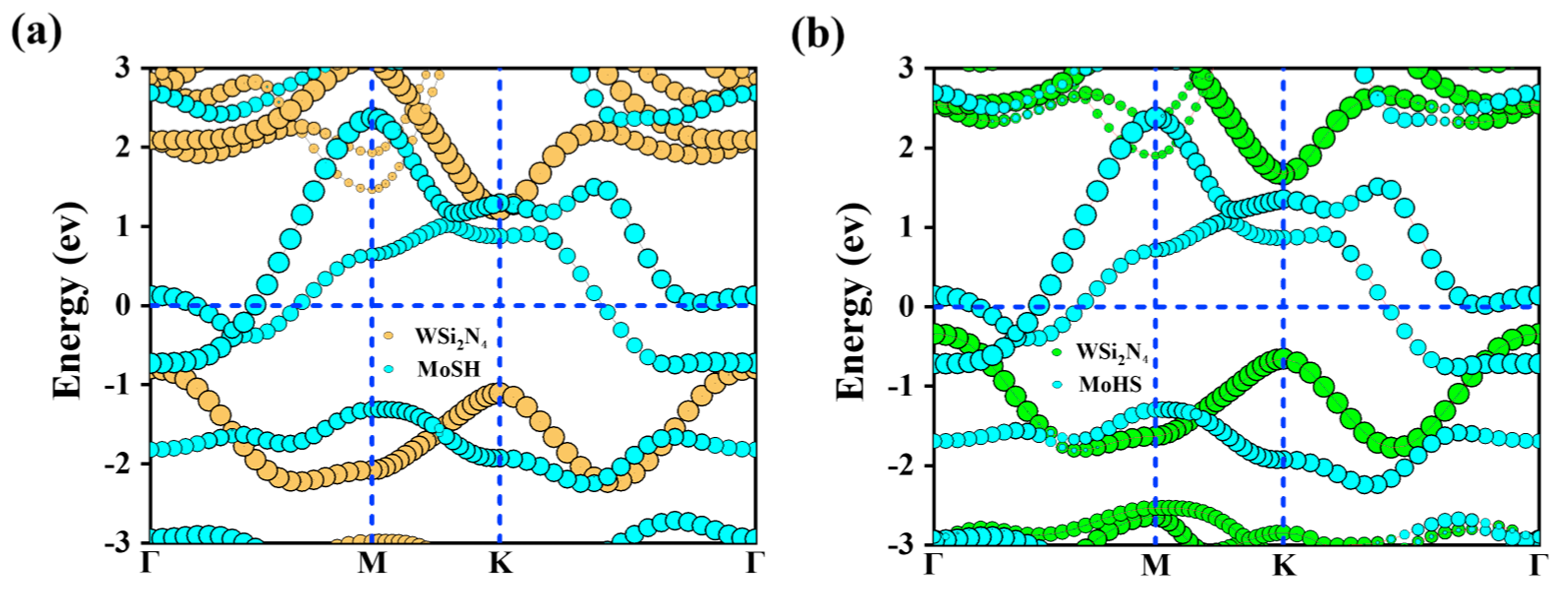
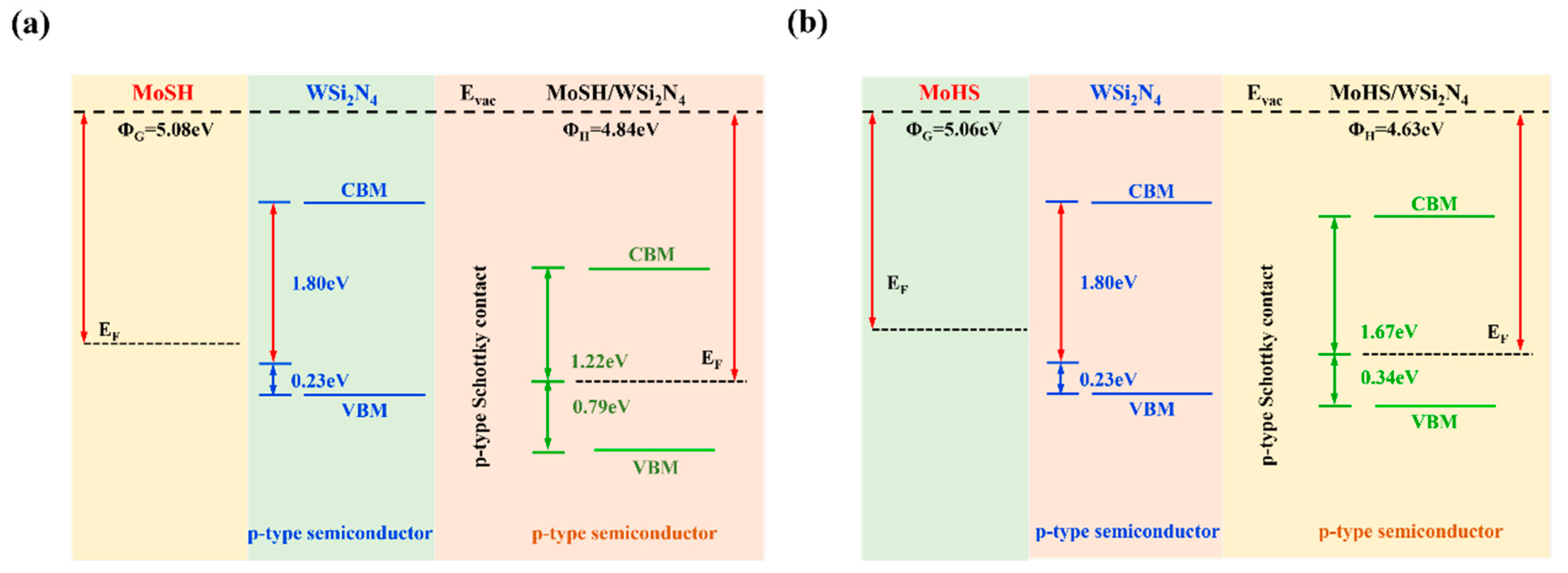
2.2. Structures and Electronic Properties of Heterostructures
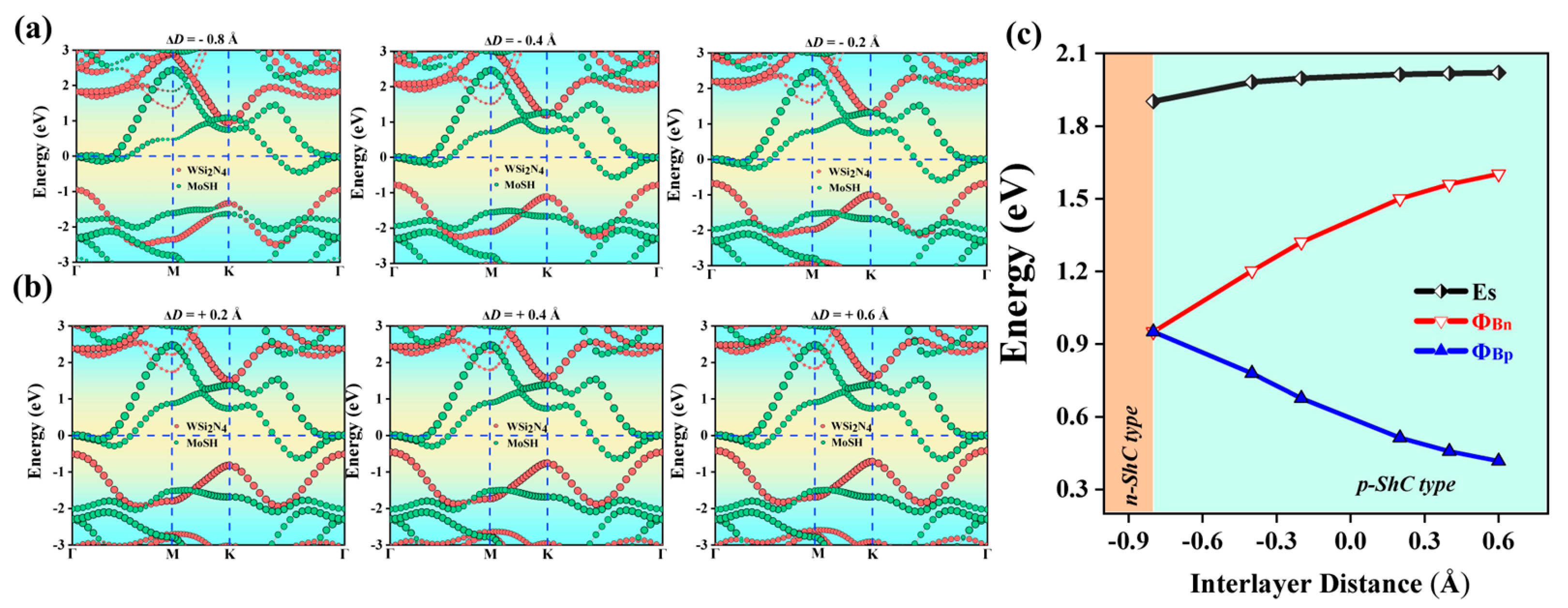
2.3. Heterostructures under Interlayer Distance
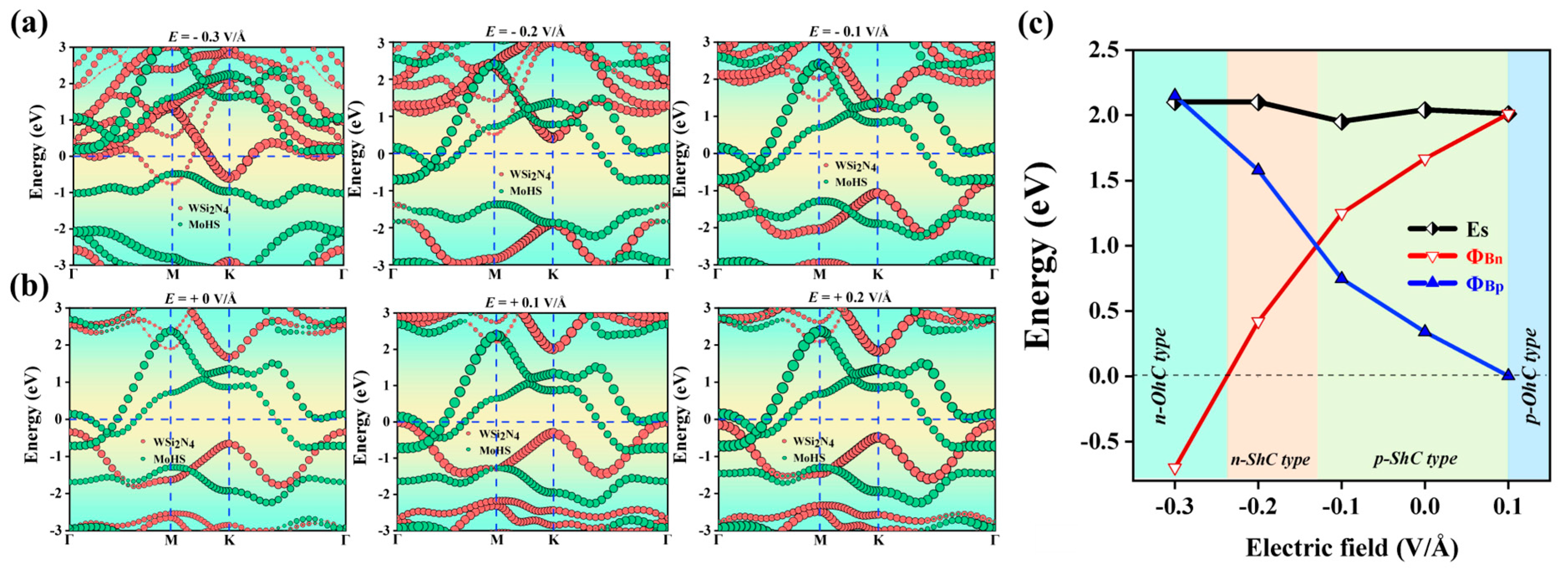
2.4. Heterostructures under Electric Field
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-Dimensional Gas of Massless Dirac Fermions in Graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Malekpour, H.; Chang, K.H.; Chen, J.C.; Lu, C.Y.; Nika, D.L.; Novoselov, K.S.; Balandin, A.A. Thermal Conductivity of Graphene Laminate. Nano Lett. 2014, 14, 5155–5161. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-Temperature Quantum Hall Effect in Graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Croitoru, M.D.; Gladilin, V.N.; Fomin, V.M.; Devreese, J.T.; Magnus, W.; Schoenmaker, W.; Sorée, B. Quantum Transport in a Nanosize Double-Gate Metal-Oxide-Semiconductor Field-Effect Transistor. J. Appl. Phys. 2004, 96, 2305–2310. [Google Scholar] [CrossRef]
- Miró, P.; Audiffred, M.; Heine, T. An Atlas of Two-Dimensional Materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Bafekry, A.; Karbasizadeh, S.; Faraji, M.; Bagheri Khatibani, A.; Sarsari, I.A.; Gogova, D.; Ghergherehchi, M. Van Der Waals Heterostructure of Graphene and Germanane: Tuning the Ohmic Contact by Electrostatic Gating and Mechanical Strain. Phys. Chem. Chem. Phys. 2021, 23, 21196–21206. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From Theory to Applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Stratakis, E. Recent Advances in 2D Metal Monochalcogenides. Adv. Sci. 2020, 7, 2001655. [Google Scholar] [CrossRef]
- Singh, A.K.; Hennig, R.G. Computational Prediction of Two-Dimensional Group-IV Mono-Chalcogenides. Appl. Phys. Lett. 2014, 105, 042103. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Ciesielski, A.; Samorì, P. 2D Materials Beyond Graphene for High-Performance Energy Storage Applications. Adv. Energy Mater. 2016, 6, 1600671. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Alzakia, F.I.; Tan, S.C. Liquid-Exfoliated 2D Materials for Optoelectronic Applications. Adv. Sci. 2021, 8, 2003864. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Transition Metal Dichalcogenides. Nat. Publ. Gr. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and Optoelectronics of 2D Semiconductor Transition Metal Dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Allain, A.; Kang, J.; Banerjee, K.; Kis, A. Electrical Contacts to Two-Dimensional Semiconductors. Nat. Mater. 2015, 14, 1195–1205. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. Two-Dimensional Semiconductors for Transistors. Nat. Rev. Mater. 2016, 1, 16052. [Google Scholar] [CrossRef]
- Mitta, S.B.; Choi, M.S.; Nipane, A.; Ali, F.; Kim, C.; Teherani, J.T.; Hone, J.; Yoo, W.J. Electrical Characterization of 2D Materials-Based Field-Effect Transistors. 2D Mater. 2021, 8, 012002. [Google Scholar] [CrossRef]
- Yang, X.; Sa, B.; Lin, P.; Xu, C.; Zhu, Q.; Zhan, H.; Sun, Z. Tunable Contacts in Graphene/InSe van Der Waals Heterostructures. J. Phys. Chem. C 2020, 124, 23699–23706. [Google Scholar] [CrossRef]
- Shen, T.; Ren, J.-C.; Liu, X.; Li, S.; Liu, W. Van Der Waals Stacking Induced Transition from Schottky to Ohmic Contacts: 2D Metals on Multilayer InSe. J. Am. Chem. Soc. 2019, 141, 3110–3115. [Google Scholar] [CrossRef]
- Mohanta, M.K.; De Sarkar, A. 2D HfN2/Graphene Interface Based Schottky Device: Unmatched Controllability in Electrical Contacts and Carrier Concentration via Electrostatic Gating and out-of-Plane Strain. Appl. Surf. Sci. 2021, 540, 148389. [Google Scholar] [CrossRef]
- Mohanta, M.K.; De Sarkar, A. Giant Tunability in Electrical Contacts and Doping via Inconsiderable Normal Electric Field Strength or Gating for a High-Performance in Ultrathin Field Effect Transistors Based on 2D BX/Graphene (X = P, As) van Der Waals Heterobilayer. Appl. Surf. Sci. 2020, 526, 146749. [Google Scholar] [CrossRef]
- Li, R.; Cheng, Y.; Huang, W. Recent Progress of Janus 2D Transition Metal Chalcogenides: From Theory to Experiments. Small 2018, 14, 1802091. [Google Scholar] [CrossRef]
- Dong, L.; Lou, J.; Shenoy, V.B. Large In-Plane and Vertical Piezoelectricity in Janus Transition Metal Dichalchogenides. ACS Nano 2017, 11, 8242–8248. [Google Scholar] [CrossRef]
- Zhuang, G.; Yan, J.; Wen, Y.; Zhuang, Z.; Yu, Y. Two-Dimensional Transition Metal Oxides and Chalcogenides for Advanced Photocatalysis: Progress, Challenges, and Opportunities. Sol. RRL 2021, 5, 2000403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Lv, T.; Chu, P.K.; Huo, K. Two-Dimensional Transition Metal Chalcogenides for Alkali Metal Ions Storage. ChemSusChem 2020, 13, 1114–1154. [Google Scholar] [CrossRef]
- Kanade, C.K.; Seok, H.; Kanade, V.K.; Aydin, K.; Kim, H.U.; Mitta, S.B.; Yoo, W.J.; Kim, T. Low-Temperature and Large-Scale Production of a Transition Metal Sulfide Vertical Heterostructure and Its Application for Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 8710–8717. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus Monolayer Transition-Metal Dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef]
- Lu, A.Y.; Zhu, H.; Xiao, J.; Chuu, C.P.; Han, Y.; Chiu, M.H.; Cheng, C.C.; Yang, C.W.; Wei, K.H.; Yang, Y.; et al. Janus Monolayers of Transition Metal Dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Zhu, Z.Y.; Tahir, M.; Schwingenschlögl, U. Spin-Orbit–Induced Spin Splittings in Polar Transition Metal Dichalcogenide Monolayers. EPL (Europhys. Lett.) 2013, 102, 57001. [Google Scholar] [CrossRef]
- Wan, X.; Chen, E.; Yao, J.; Gao, M.; Miao, X.; Wang, S.; Gu, Y.; Xiao, S.; Zhan, R.; Chen, K.; et al. Synthesis and Characterization of Metallic Janus MoSH Monolayer. ACS Nano 2021, 15, 20319–20331. [Google Scholar] [CrossRef]
- Hong, Y.-L.; Liu, Z.; Wang, L.; Zhou, T.; Ma, W.; Xu, C.; Feng, S.; Chen, L.; Chen, M.-L.; Sun, D.-M.; et al. Chemical Vapor Deposition of Layered Two-Dimensional MoSi2N4 Materials. Science 2020, 369, 670–674. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Bagheri Khatibani, A.; Abdolahzadeh Ziabari, A.; Ghergherehchi, M.; Nedaei, S.; Shayesteh, S.F.; Gogova, D. Tunable Electronic and Magnetic Properties of MoSi2N4 Monolayer via Vacancy Defects, Atomic Adsorption and Atomic Doping. Appl. Surf. Sci. 2021, 559, 149862. [Google Scholar] [CrossRef]
- Jian, C.; Ma, X.; Zhang, J.; Yong, X. Strained MoSi2N4 Monolayers with Excellent Solar Energy Absorption and Carrier Transport Properties. J. Phys. Chem. C 2021, 125, 15185–15193. [Google Scholar] [CrossRef]
- Guo, X.-S.; Guo, S.-D. Tuning Transport Coefficients of Monolayer MoSi2N4 with Biaxial Strain*. Chinese Phys. B 2021, 30, 067102. [Google Scholar] [CrossRef]
- Bafekry, A.; Faraji, M.; Hoat, D.M.; Shahrokhi6, M.; Fadlallah, M.M.; Shojaei, F.; Feghhi, S.A.H.; Ghergherehchi, M.; Gogova, D. MoSi2N4 Single-Layer: A Novel Two-Dimensional Material with Outstanding Mechanical, Thermal, Electronic, Optical, and Photocatalytic Properties. J. Phys. D Appl. Phys. 2021, 54, 155303. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.-L.; Wang, X.; Liang, J.-S.; Xu, Z.; Wang, P.; Liao, Y.-T.; Peng, Y.; Miao, L. Theoretical Prediction of Two-Dimensional WSi2N4 Materials for Photocatalytic Water Splitting. J. Appl. Phys. 2022, 132, 203102. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, X.-Y.; Wang, Y.-X.; Liang, Y.-C.; Xie, Q. Modulation of Schottky Barrier in XSi2N4/Graphene (X = Mo and W) Heterojunctions by Biaxial Strain. Chinese Phys. B 2022, 31, 087101. [Google Scholar] [CrossRef]
- Wang, J.; Bai, L.; Zhao, X.; Chen, C.; Niu, L. Controllable Contact Types of Janus MoSH and WSi2N4 van Der Waals Heterostructures via Biaxial Strain and External Electric Field. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 149, 115668. [Google Scholar] [CrossRef]
- Nguyen, C.V. Electric Gating and Interlayer Coupling Controllable Electronic Structure and Schottky Contact of Graphene/BiI3 van Der Waals Heterostructure. Phys. Rev. B 2021, 103, 115429. [Google Scholar] [CrossRef]
- Mohanta, M.K.; Arora, A.; De Sarkar, A. Effective modulation of ohmic contact and carrier concentration in a graphene-MgX (X=S, Se) van der Waals heterojunction with tunable band-gap opening via strain and electric field. Phys. Rev. B 2021, 104, 165421. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, L.; Liang, S.-J.; Wu, W.; Wang, G.; Lee, C.H.; Ong, W.L.; Yang, H.Y.; Ang, L.K.; Yang, S.A.; et al. Efficient Ohmic Contacts and Built-in Atomic Sublayer Protection in MoSi2N4 and WSi2N4 Monolayers. npj 2D Mater. Appl. 2021, 5, 71. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Qian, G.; Liang, Q.; Luo, X.; Xie, Q. Monolayers of Germanene/Janus Ga2 SeTe van Der Waals Heterostructures by First-Principles Calculations for High-Performance Optoelectronic Devices. ACS Appl. Nano Mater. 2023, 6, 3453–3462. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Ang, Y.S.; Nguyen, S.T.; Hoang, N.V.; Hung, N.M.; Nguyen, C.V. Tunable Type-II Band Alignment and Electronic Structure of C3 N4/MoSi2N4 Heterostructure: Interlayer Coupling and Electric Field. Phys. Rev. B 2022, 105, 045303. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Nguyen, C.Q.; Nguyen, S.-T.; Ang, Y.S.; Hieu, N.V. Two-Dimensional Metal/Semiconductor Contact in a Janus MoSH/MoSi2N4 van Der Waals Heterostructure. J. Phys. Chem. Lett. 2022, 13, 2576–2582. [Google Scholar] [CrossRef]
- Born, M.; Huang, K.; Lax, M. Dynamical Theory of Crystal Lattices. Am. J. Phys. 1955, 23, 474. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B—Condens. Matter Mater. Phys. 2014, 90, 4–7. [Google Scholar] [CrossRef]
- Aldakov, D. Two-Dimensional van Der Waals Heterojunctions for Functional Materials and Devices. J. Mater. Chem 2020, 1, 3777. [Google Scholar] [CrossRef]
- He, C.; Cheng, M.; Li, T.; Zhang, W. Tunable Ohmic, p-Type Quasi-Ohmic, and n-Type Schottky Contacts of Monolayer SnSe with Metals. ACS Appl. Nano Mater. 2019, 2, 2767–2775. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, H.; Zhang, Y.; Wang, D.; Yang, L.; Fan, L.; Chen, Y.; Qu, X.; Liu, Y. Tunable Electrical Contact Properties in Two-Dimensional van Der Waals V2C/MoSi2N4 Heterostructures. J. Phys. Condens. Matter 2023, 35, 445501. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, H.; Zhang, Y.; Wang, D.; Fan, L.; Chen, Y.; Qu, X.; Yang, L.; Liu, Y. Tunable Contact Types and Interfacial Electronic Properties in TaS2/MoS2 and TaS2/WSe2 Heterostructures. Molecules 2023, 28, 5607. [Google Scholar] [CrossRef]
- Bardeen, J. Surface States and Rectification at a Metal Semi-Conductor Contact. Phys. Rev. 1947, 71, 717–727. [Google Scholar] [CrossRef]
- Zhang, W.X.; Yin, Y.; He, C. Spontaneous Enhanced Visible-Light-Driven Photocatalytic Water Splitting on Novel Type-II GaSe/CN and Ga2SSe/CN VdW Heterostructures. J. Phys. Chem. Lett. 2021, 12, 5064–5075. [Google Scholar] [CrossRef]
- He, C.; Liang, Y.; Zhang, W.X. Constructing a Novel Metal-Free g-C3N4/g-CN VdW Heterostructure with Enhanced Visible-Light-Driven Photocatalytic Activity for Water Splitting. Appl. Surf. Sci. 2021, 553, 149550. [Google Scholar] [CrossRef]
- Barber, H.D. Effective Mass and Intrinsic Concentration in Silicon. Solid State Electron. 1967, 10, 1039–1051. [Google Scholar] [CrossRef]
- Yankowitz, M.; Watanabe, K.; Taniguchi, T.; San-Jose, P.; LeRoy, B.J. Pressure-Induced Commensurate Stacking of Graphene on Boron Nitride. Nat. Commun. 2016, 7, 13168. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Fan, W.; Kang, J.; Park, J.; Koldemir, U.; Suh, J.; Narang, D.S.; Liu, K.; Ji, J.; Li, J.; et al. Tuning Interlayer Coupling in Large-Area Heterostructures with CVD-Grown MoS2 and WS2 Monolayers. Nano Lett. 2014, 14, 3185–3190. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for Density Functional Theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Vargas-Hernández, R.A. Bayesian Optimization for Calibrating and Selecting Hybrid-Density Functional Models. J. Phys. Chem. A 2020, 124, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Paier, J.; Hirschl, R.; Marsman, M.; Kresse, G. The Perdew–Burke–Ernzerhof Exchange-Correlation Functional Applied to the G2-1 Test Set Using a Plane-Wave Basis Set. J. Chem. Phys. 2005, 122, 234102. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First Principles Phonon Calculations in Materials Science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]



| a (Å) | D (Å) | Eg (eV) | mex/m0 | µe (cm2/Vs) | mhy/m0 | µh (cm2/Vs) | Contact Types | |
|---|---|---|---|---|---|---|---|---|
| 1T-WSi2N4 | 2.91 | - | 2.03 | 0.36 | 1.35 | - | ||
| MoSH/WSi2N4 | 2.91 | 2.74 | 2.01 | 1.21 | 145 | 1.05 | 96 | p-ShC |
| MoHS/WSi2N4 | 2.91 | 3.15 | 2.02 | 1.84 | 167 | 1.17 | 150 | p-ShC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, X.; Zhang, H.; He, S.; Liu, Y.; Zhao, W.; Liu, H.; Qu, X. Janus MoSH/WSi2N4 van der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact. Molecules 2024, 29, 3554. https://doi.org/10.3390/molecules29153554
Wang Y, Zhu X, Zhang H, He S, Liu Y, Zhao W, Liu H, Qu X. Janus MoSH/WSi2N4 van der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact. Molecules. 2024; 29(15):3554. https://doi.org/10.3390/molecules29153554
Chicago/Turabian StyleWang, Yongdan, Xiangjiu Zhu, Hengshuo Zhang, Shitong He, Ying Liu, Wenshi Zhao, Huilian Liu, and Xin Qu. 2024. "Janus MoSH/WSi2N4 van der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact" Molecules 29, no. 15: 3554. https://doi.org/10.3390/molecules29153554
APA StyleWang, Y., Zhu, X., Zhang, H., He, S., Liu, Y., Zhao, W., Liu, H., & Qu, X. (2024). Janus MoSH/WSi2N4 van der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact. Molecules, 29(15), 3554. https://doi.org/10.3390/molecules29153554






