Chemical Structure and Thermal Properties versus Accelerated Aging of Bio-Based Poly(ether-urethanes) with Modified Hard Segments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Analysis of Chemical Structure and Degree of Phase Separation in Bio-TPUs
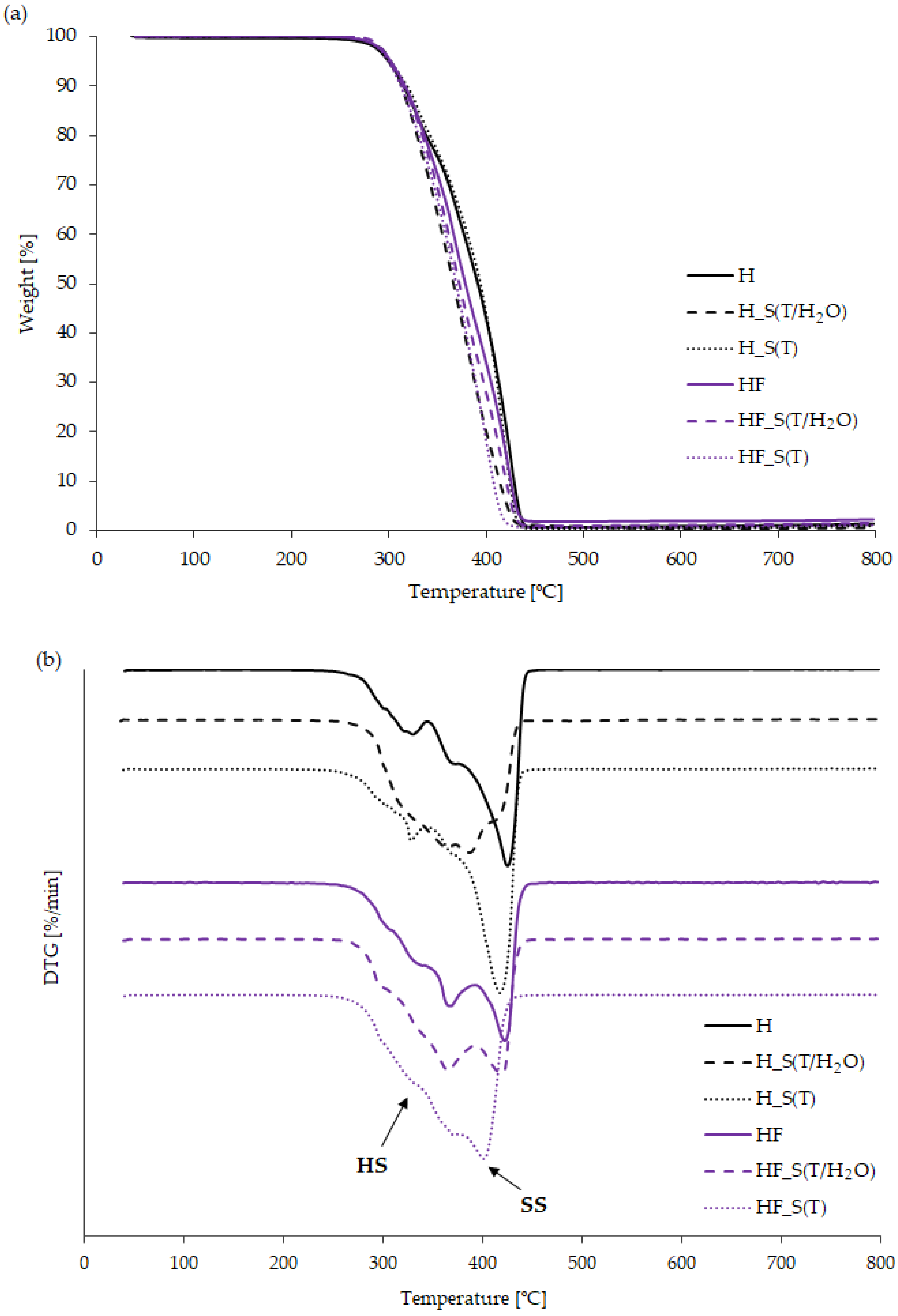
2.2. Thermal Properties of Bio-TPUs–Influence—Influence of Aging Factors on the Thermal Stability, Phase Transitionthermal Stability, Phase Transition, and Thermomechanical Behaviorthermo-Mechanical Behavior
3. Materials and Methods
3.1. Materials
3.2. TPU Preparation and Accelerated Aging
3.3. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic aging processes: Environmental relevance and analytical implications. TrAC-Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Starkova, O.; Gagani, A.I.; Karl, C.W.; Rocha, I.B.C.M.; Burlakovs, J.; Krauklis, A.E. Modelling of environmental ageing of polymers and polymer composites—Durability prediction methods. Polymers 2022, 14, 907. [Google Scholar] [CrossRef]
- Mouren, A.; Avérous, L. Aromatic thermoplastic polyurethanes synthesized from different potential sustainable resources. Eur. Polym. J. 2023, 197, 112338. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Głowińska, E.; Parcheta-Szwindowska, P.; Rohde, K.; Datta, J. Green TPUs from prepolymer mixtures designed by controlling the chemical structure of flexible segments. Int. J. Mol. Sci. 2021, 22, 7438. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Głowińska, E.; Datta, J. Structure and properties comparison of poly(ether-urethane)s based on nonpetrochemical and petrochemical polyols obtained by solvent free two-step method. Eur. Polym. J. 2021, 157, 110673. [Google Scholar] [CrossRef]
- Baysal, G.; Aydın, H.; Hoşgören, H.; Uzan, S.; Karaer, H. Improvement of Synthesis and Dielectric Properties of Polyurethane/Mt-QASs+ (Novel Synthesis). J. Polym. Environ. 2016, 24, 139–147. [Google Scholar] [CrossRef]
- Gallu, R.; Méchin, F.; Dalmas, F.; Gérard, J.F.; Perrin, R.; Loup, F. On the use of solubility parameters to investigate phase separation-morphology-mechanical behavior relationships of TPU. Polymer 2020, 207, 122882. [Google Scholar] [CrossRef]
- Głowińska, E.; Smorawska, J.; Niesiobędzka, J.; Datta, J. Structure versus hydrolytic and thermal stability of bio-based thermoplastic polyurethane elastomers composed of hard and soft building blocks with high content of green carbon. J. Therm. Anal. Calorim. 2024, 149, 2147–2160. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Linda Fernando, T. Studies on ageing performance of some novel polyurethanes. J. Chem. Pharm. Res. 2011, 3, 848–862. [Google Scholar]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Boubakri, A.; Haddar, N.; Elleuch, K.; Bienvenu, Y. Influence of thermal aging on tensile and creep behavior of thermoplastic polyurethane. Comptes Rendus-Mec. 2011, 339, 666–673. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Rosu, D.; Oprea, S. Effect of thermal aging on the physico-chemical and optical properties of poly(Ester urethane) elastomers designed for passive damping (pads) of the railway. Polymers 2021, 13, 192. [Google Scholar] [CrossRef]
- Boubakri, A.; Elleuch, K.; Guermazi, N.; Ayedi, H.F. Investigations on hygrothermal aging of thermoplastic polyurethane material. Mater. Des. 2009, 30, 3958–3965. [Google Scholar] [CrossRef]
- Hong, S.; Park, N.Y.; Ju, S.; Lee, A.; Shin, Y.; Kim, J.S.; Um, M.K.; Yi, J.W.; Chae, H.G.; Park, T. Molecular degradation mechanism of segmented polyurethane and life prediction through accelerated aging test. Polym. Test. 2023, 124, 108086. [Google Scholar] [CrossRef]
- Jana, R.N.; Bhunia, H. Accelerated hygrothermal and UV aging of thermoplastic polyurethanes. High Perform. Polym. 2010, 22, 3–15. [Google Scholar] [CrossRef]
- Bistričić, L.; Baranović, G.; Leskovac, M.; Bajsić, E.G. Hydrogen bonding and mechanical properties of thin films of polyether-based polyurethane-silica nanocomposites. Eur. Polym. J. 2010, 46, 1975–1987. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Datta, J. Novel bio-based thermoplastic poly(ether-urethane)s. Correlations between the structure, processing and properties. Polymer 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Błażek, K.; Parcheta, P.; Datta, J. Green thermoplastic poly(ether-urethane)s–synthesis, chemical structure and selected properties investigation. Polimery/Polymers 2020, 65, 672–680. [Google Scholar] [CrossRef]
- Allan, D.; Daly, J.H.; Liggat, J.J. Oxidative and non-oxidative degradation of a TDI-based polyurethane foam: Volatile product and condensed phase characterisation by FTIR and solid state 13 C NMR spectroscopy. Polym. Degrad. Stab. 2019, 161, 57–73. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P. Thermoplastic polyurethanes derived from petrochemical or renewable resources: A comprehensive review. Polym. Eng. Sci. 2018, 58, E14–E35. [Google Scholar] [CrossRef]
- Saiani, A.; Novak, A.; Rodier, L.; Eeckhaut, G.; Leenslag, J.W.; Higgins, J.S. Origin of Multiple Melting Endotherms in a High Hard Block Content Polyurethane. 1. Thermodynamic Investigation. Macromolecules 2007, 40, 7252–7262. [Google Scholar] [CrossRef]
- Chang, B.P.; Mohanty, A.K.; Misra, M. Studies on durability of sustainable biobased composites: A review. RSC Adv. 2020, 10, 17955–17999. [Google Scholar] [CrossRef]
- Smorawska, J.; Wloch, M.; Głowińska, E. Structure-property relationship, and multiple processing studies of novel bio-based thermoplastic polyurethane elastomers. Materials 2022, 16, 6246. [Google Scholar] [CrossRef] [PubMed]
- Głowińska, E.; Kasprzyk, P.; Datta, J. The green approach to the synthesis of bio-based thermoplastic polyurethane elastomers with partially bio-based hard blocks. Materials 2021, 14, 2334. [Google Scholar] [CrossRef] [PubMed]
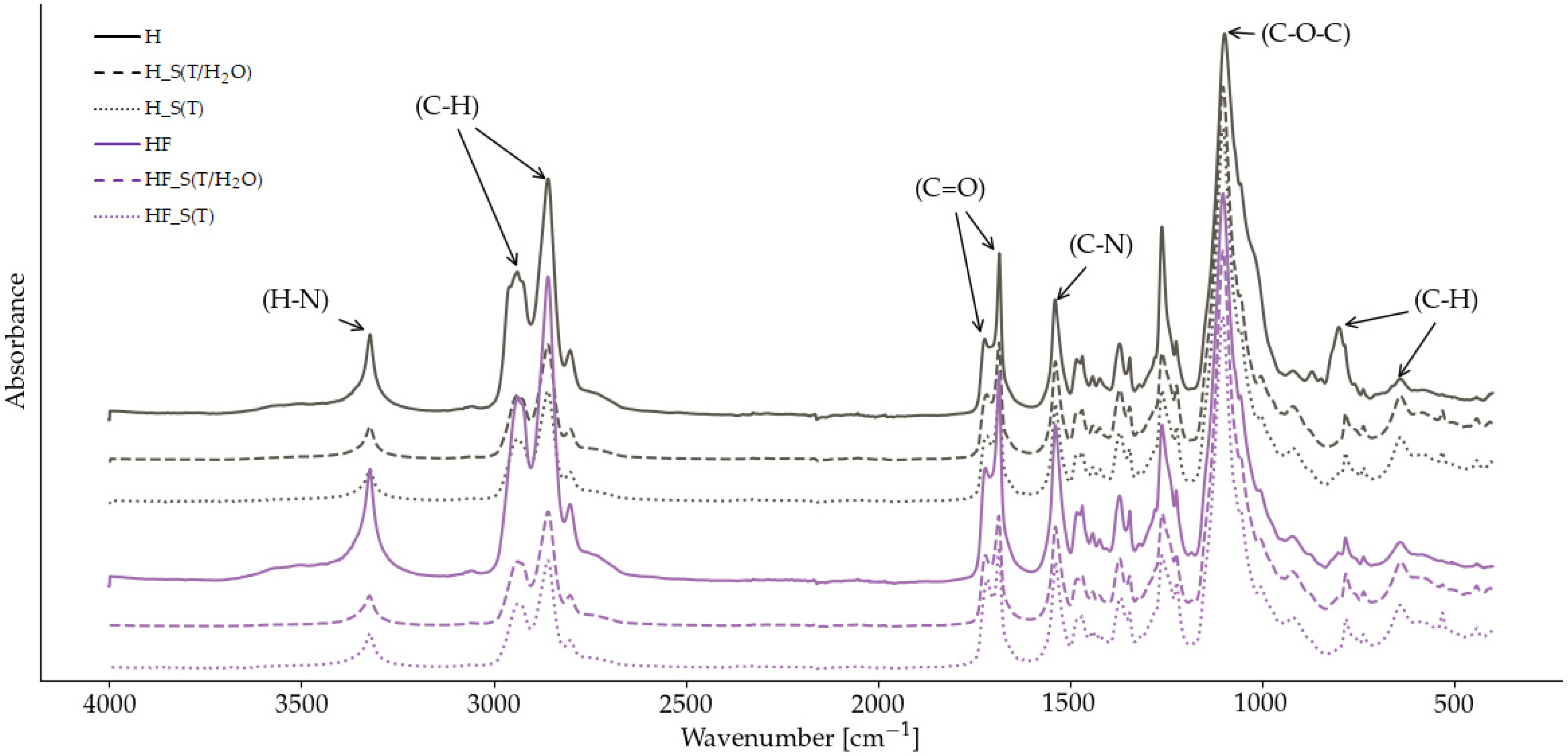



| Sample | R | DPS |
|---|---|---|
| H | 0.83 | 0.45 |
| H_S(T/H2O) | 1.50 | 0.60 |
| H_S(T) | 1.49 | 0.60 |
| HF | 0.74 | 0.42 |
| HF_S(T/H2O) | 1.31 | 0.57 |
| HF_S(T) | 1.52 | 0.60 |
| Sample | Temperature [°C] | Residue at 800 °C [%] | ||||
|---|---|---|---|---|---|---|
| T5% | T10% | T50% | TDTG1 | TDTG2 | ||
| H | 300 | 316 | 392 | 329 | 424 | 1.40 |
| H_S(T/H2O) | 303 | 314 | 367 | 364 | 387 | 0.52 |
| H_S(T) | 301 | 319 | 394 | 329 | 418 | 0.95 |
| HF | 302 | 316 | 378 | 368 | 423 | 2.14 |
| HF_S(T/H2O) | 300 | 314 | 372 | 367 | 421 | 1.65 |
| HF_S(T) | 299 | 312 | 369 | 370 | 401 | 1.17 |
| Sample | TGss [°C] | TmSS [°C] | ΔHmSS [J/g] | TcSS [°C] | ΔHmSS [J/g] | TgHS [°C] | TmHS [°C] | ΔHmHS [J/g] | TcHS [°C] | ΔHcHS [J/g] |
|---|---|---|---|---|---|---|---|---|---|---|
| H | −67 | 5.4 | 26.0 | −27.7 | −67.3 | 34.0 | 147 | 17.5 | 76.9 | −15.0 |
| H_S(T/H2O) | −68 | 5.7 | 27.2 | −25.8 | −67.7 | 44.3 | 147 | 17.7 | 84.0 | −18.2 |
| H_S(T) | −68 | 6.5 | 25.8 | −24.8 | −67.7 | 34.0 | 147 | 14.7 | 80.2 | −14.2 |
| HF | −68 | 4.2 | 7.8 | - | - | 63.2 | 143 | 14.1 | 25.9 | −3.7 |
| HF_S(T/H2O) | −68 | 4.2 | 12.8 | −37.1 | −3.7 | 42.7 | 143 | 15.0 | 63.7 | −8.2 |
| HF_S(T) | −67 | 4.4 | 12.3 | - | - | 48,7 | 144 | 16.9 | 25.8 | −10.1 |
| Sample | E’ [MPa] | E’ at 25 °C [MPa] | E” [MPa] | Tan δ [-] | TgSS [°C] |
|---|---|---|---|---|---|
| H | 3857 | 139 | 441 | 0.284 | −52.5 |
| H_S(T/H2O) | 2837 | 130 | 305 | 0.236 | −52.4 |
| H_S(T) | 3780 | 137 | 411 | 0.268 | −54.2 |
| HF | 3589 | 104 | 458 | 0.384 | −53.3 |
| HF_S(T/H2O) | 2853 | 112 | 349 | 0.365 | −52.8 |
| HF_S(T) | 3485 | 108 | 441 | 0.369 | −53.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewska, J.; Smorawska, J.; Głowińska, E. Chemical Structure and Thermal Properties versus Accelerated Aging of Bio-Based Poly(ether-urethanes) with Modified Hard Segments. Molecules 2024, 29, 3585. https://doi.org/10.3390/molecules29153585
Godlewska J, Smorawska J, Głowińska E. Chemical Structure and Thermal Properties versus Accelerated Aging of Bio-Based Poly(ether-urethanes) with Modified Hard Segments. Molecules. 2024; 29(15):3585. https://doi.org/10.3390/molecules29153585
Chicago/Turabian StyleGodlewska, Julia, Joanna Smorawska, and Ewa Głowińska. 2024. "Chemical Structure and Thermal Properties versus Accelerated Aging of Bio-Based Poly(ether-urethanes) with Modified Hard Segments" Molecules 29, no. 15: 3585. https://doi.org/10.3390/molecules29153585
APA StyleGodlewska, J., Smorawska, J., & Głowińska, E. (2024). Chemical Structure and Thermal Properties versus Accelerated Aging of Bio-Based Poly(ether-urethanes) with Modified Hard Segments. Molecules, 29(15), 3585. https://doi.org/10.3390/molecules29153585







