Temperature-Sensitive Template for Preparation of ZnO/CeO2 Composite Photocatalytic Materials and Its Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD of the Ce-Doped ZnO Photocatalysts
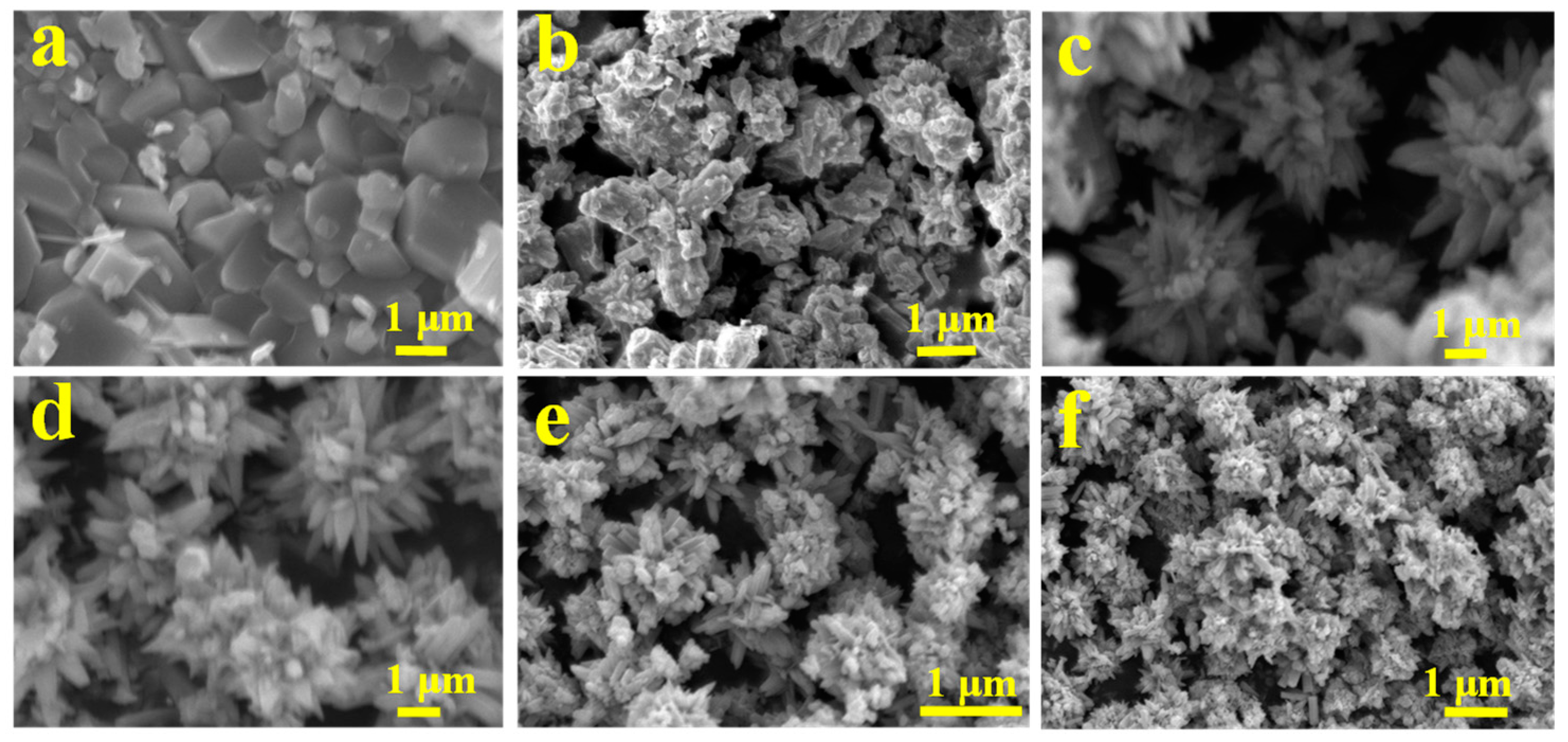
2.2. SEM of the Ce-Doped ZnO Photocatalysts
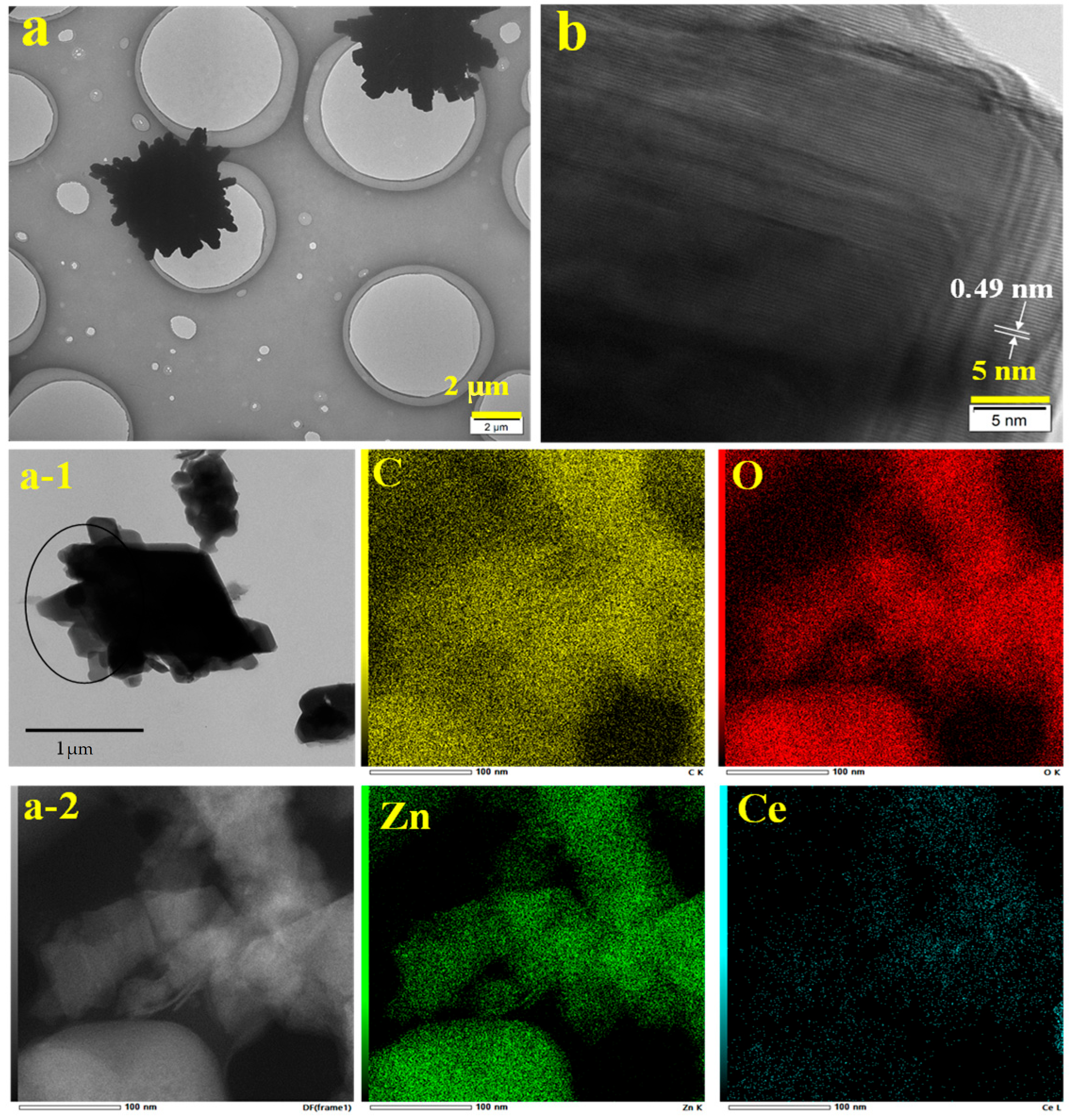
2.3. TEM and TEM Mapping of the ZnO-Ce-2‰ Photocatalyst
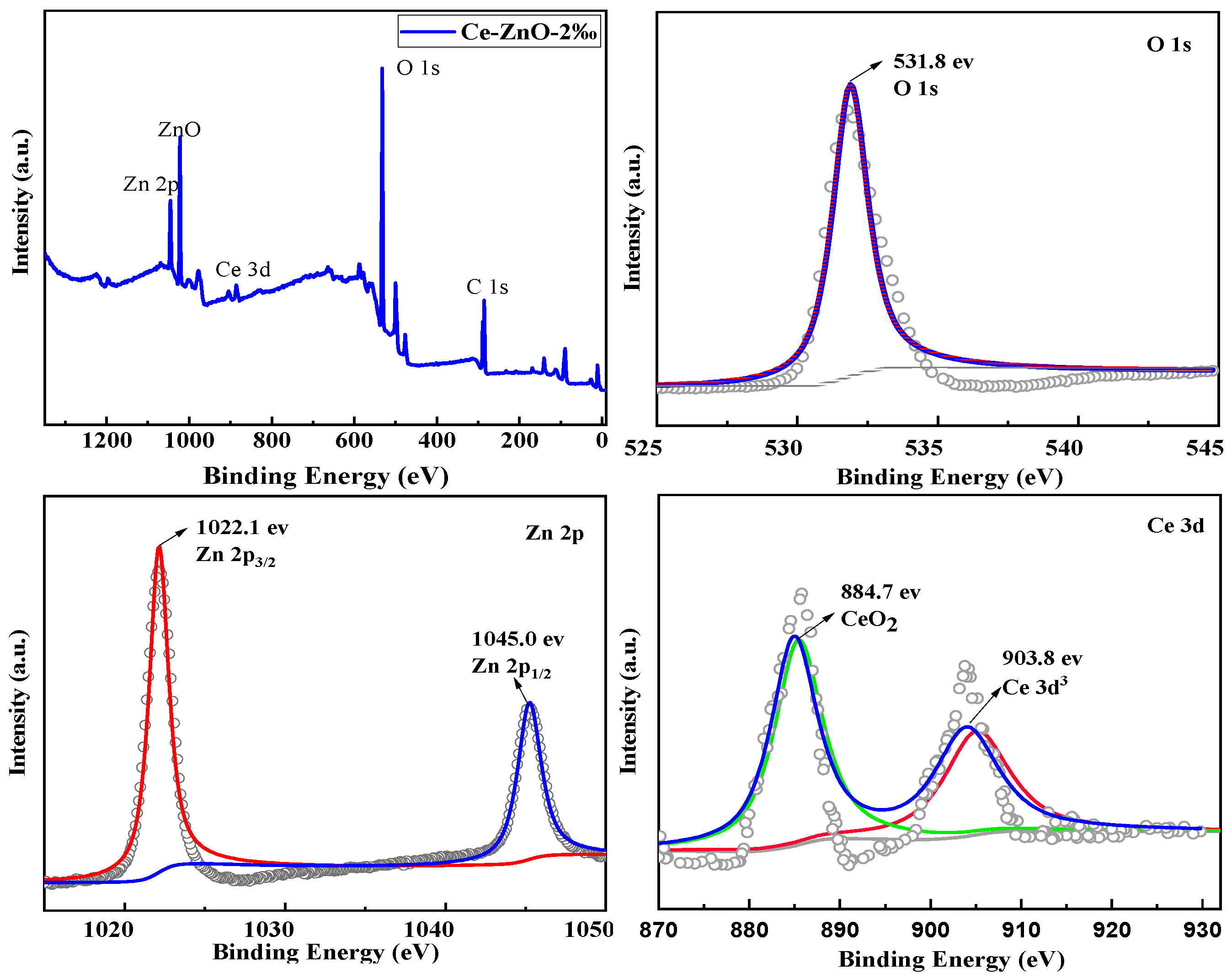
2.4. XPS of the ZnO-Ce-2‰ Photocatalyst
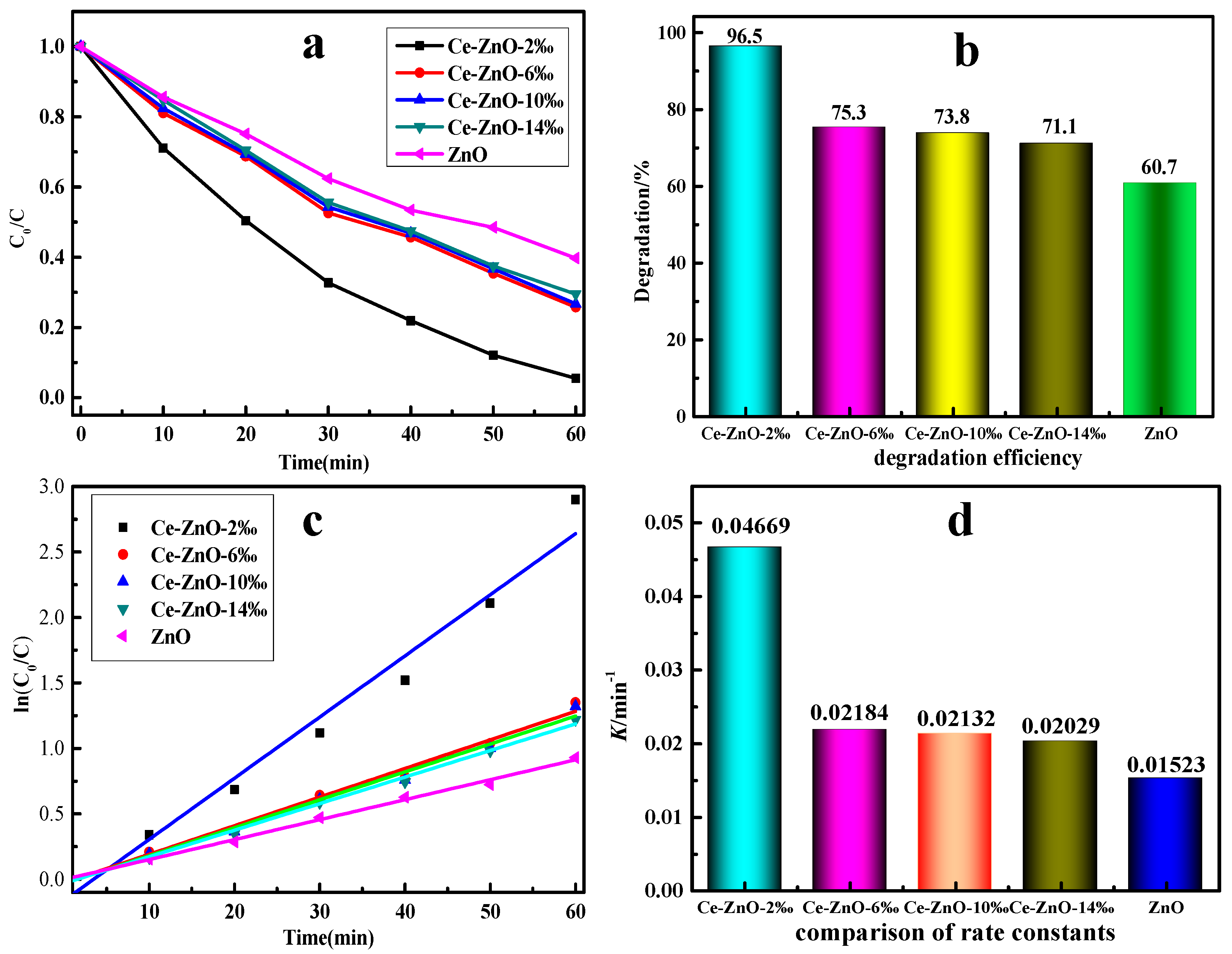
2.5. Catalytic Performance of Photocatalysts
2.6. Time-Dependent ZnO-Ce-2‰ for MO
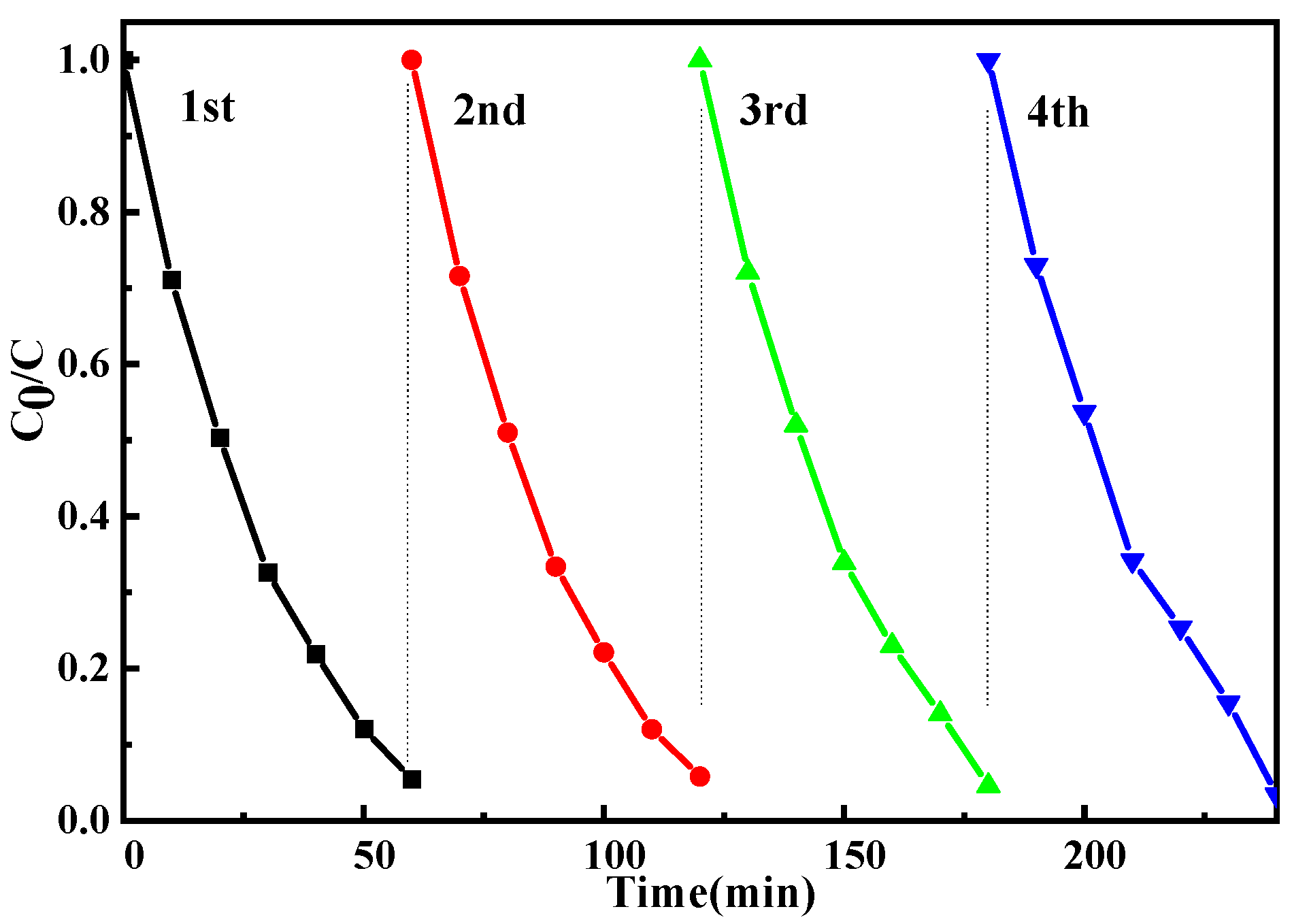
2.7. Reuse of ZnO-Ce-2‰ for MO
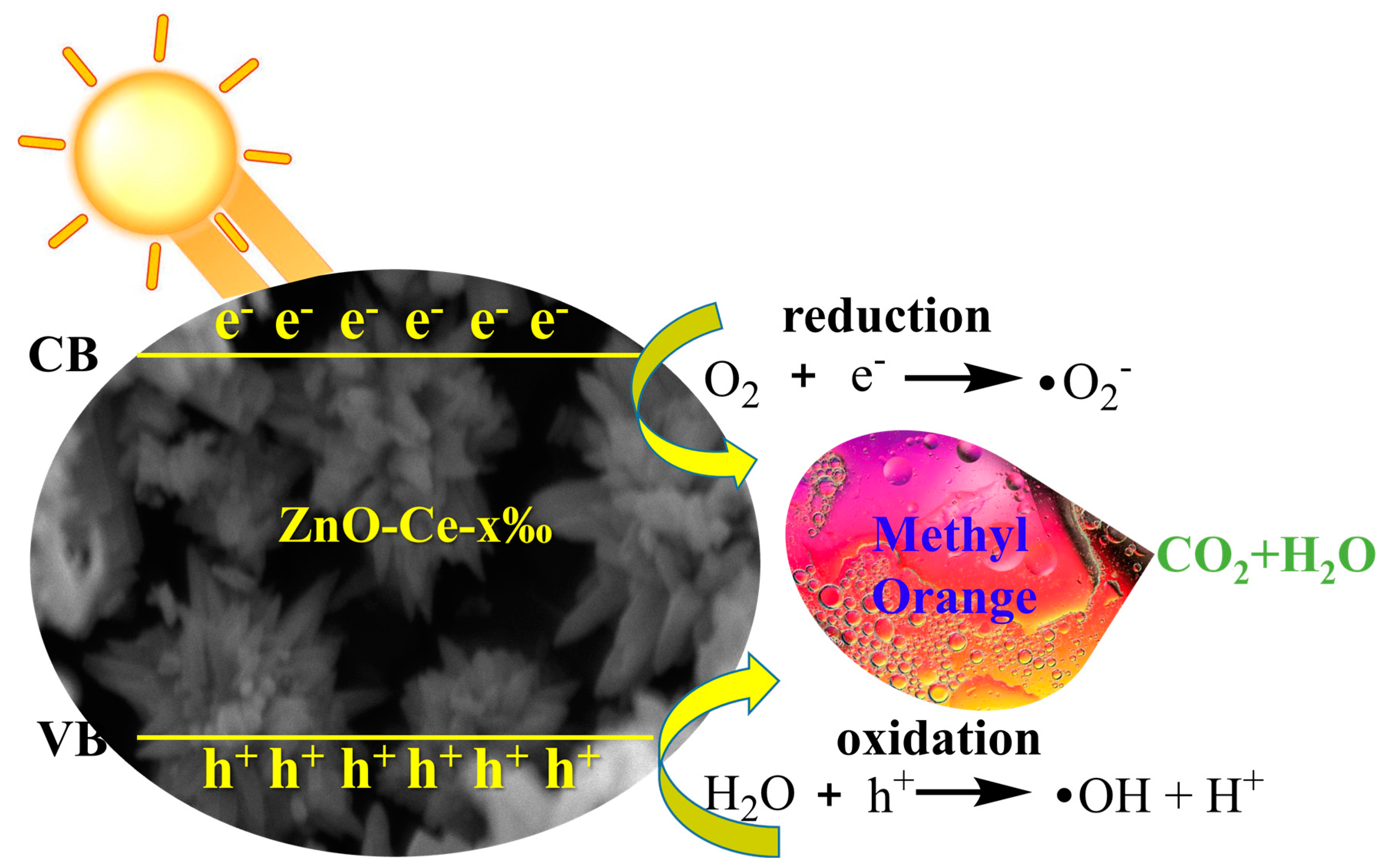
2.8. Mechanism of ZnO-Ce-2‰ for MO Degradation
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Preparation of Temperature-Sensitive Template PN64(IL)8
3.4. Preparation of Nanometer ZnO Materials Doped with Ce in Different Proportions
3.5. Photocatalytic Tests with Different Photocatalysts and Influence of Initial Concentration of MO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strack, R. Organic dyes for live imaging. Nat. Methods 2021, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; López, F.A. Organic dyes versus adsorption processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef] [PubMed]
- Boyles, C.; Sobeck, S.J.S. Photostability of organic red food dyes. Food Chem. 2020, 315, 126249. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, A.; Anselmi, M.; Calogero, F.; Potenti, S.; Bassan, E.; Ceroni, P.; Cozzi, P.G. Metallaphotoredox catalysis with organic dyes. Org. Biomol. Chem. 2021, 19, 3527–3550. [Google Scholar] [CrossRef]
- Gsänger, M.; Bialas, D.; Huang, L.; Stolte, M.; Würthner, F. Organic semiconductors based on dyes and color pigments. Adv. Mater. 2016, 28, 3615–3645. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Scown, T.M.; Moger, J.; Cumberland, S.A.; Baalousha, M.; Linge, K.; van Aerle, R.; Jarvis, K.; Lead, J.R.; Tyler, C.R. Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Environ. Sci. Technol. 2010, 44, 1144–1151. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, S.; Mittal, A.; Sharma, S.; Kumari, K.; Kumar, N. Hydrothermally synthesized nano-carrots ZnO with CeO2 heterojunctions and their photocatalytic activity towards different organic pollutants. J. Mater. Sci. Mater. Electron. 2020, 31, 5227–5240. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, G.; Hu, W.; Xiong, D.; Zhou, B.; Chang, S.; Huang, W.Q. Construction of g-C3N4/CeO2/ZnO ternary photocatalysts with enhanced photocatalytic performance. J. Phys. Chem. Solids 2017, 106, 1–9. [Google Scholar] [CrossRef]
- Velusamy, P.; Lakshmi, G. Enhanced photocatalytic performance of (ZnO/CeO2)-β-CD system for the effective decolorization of Rhodamine B under UV light irradiation. Appl. Water Sci. 2017, 7, 4025–4036. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Ghica, D.; Vlaicu, I.D.; Stefan, M.; Maraloiu, V.A.; Joita, A.C.; Ghica, C. Tailoring the dopant distribution in ZnO: Mn nanocrystals. Sci. Rep. 2019, 9, 6894. [Google Scholar] [CrossRef] [PubMed]
- Yahmadi, B.; Kamoun, O.; Alhalaili, B.; Alleg, S.; Vidu, R.; Turki, N.K. Physical investigations of (Co, Mn) Co-doped ZnO nanocrystalline films. Nanomaterials 2020, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, R.; Soheyli, E.; Kaboutari, P.; Daneshfar, A.; Soheyli, S. Antireflective and nanocolumnar-shaped Mn:ZnO films grown by chemical bath deposition. Mater. Sci. Eng. B 2022, 278, 115634. [Google Scholar] [CrossRef]
- Saadi, H.; Rhouma, F.; Benzarti, Z.; Bougrioua, Z.; Guermazi, S.; Khirouni, K. Electrical conductivity improvement of Fe doped ZnO nanopowders. Mater. Res. Bull. 2020, 129, 110884. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Matysik, J.; Matyjasik, W.; Banach, M. Fabrication of ZnO/Ag and ZnO/Fe nanoparticles with immobilised peroxidase as a biocatalyst for photodegradation of ibuprofen. Sustain. Mater. Technol. 2023, 38, e00763. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Zhang, Y.; Zhang, R.; Ge, H.; Bi, J.; Tang, M. Strong metal–support interactions between Ni and ZnO particles and their effect on the methanation performance of Ni/ZnO. Catal. Sci. Technol. 2017, 7, 4413–4421. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 2018, 448, 481. [Google Scholar] [CrossRef]
- Huang, P.-S.; Qin, F.; Lee, J.-K. Role of the interface between Ag and ZnO in the electric conductivity of Ag nanoparticle-embedded ZnO. ACS Appl. Mater. Interfaces 2019, 12, 4715–4721. [Google Scholar] [CrossRef]
- Nie, M.; Sun, H.; Cai, H.; Xue, Z.; Yang, C.; Li, Q.; Qin, L.; Wu, M. Study on electrocatalytic property of ZnO and Ag/ZnO. Mater. Lett. 2020, 271, 127785. [Google Scholar] [CrossRef]
- Rabell, G.O.; Cruz, M.A.; Juárez-Ramírez, I. Hydrogen production of ZnO and ZnO/Ag films by photocatalysis and photoelectrocatalysis. Mater. Sci. Semicond. Process. 2021, 134, 105985. [Google Scholar] [CrossRef]
- Moslehnejad, N.; Jahangiri, M.; Vafaee, F.; Salavati-Niasari, M. Synthesis and characterization of ZnO-Ce nanophotocatalyst and their application for the removal of dye (reactive red 198) by degradation process: Kinetics, thermodynamics and experimental design. Int. J. Hydrogen Energy 2022, 47, 23980. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Hashim, A.M.; Esmaeilzadeh, J. Role of CeO2 and calcination temperature on the structural and optical properties of (ZnO)1-x/(CeO2)x nanocomposites in the UV-visible region. Ceram. Int. 2024, 50, 4167–4177. [Google Scholar] [CrossRef]
- Sasirekha, C.; Arumugam, S.; Muralidharan, G. Green synthesis of ZnO/carbon (ZnO/C) as an electrode material for symmetric supercapacitor devices. Appl. Surf. Sci. 2018, 449, 521–527. [Google Scholar] [CrossRef]
- Chao, J.; Chen, Y.; Xing, S.; Zhang, D.; Shen, W. Facile fabrication of ZnO/C nanoporous fibers and ZnO hollow spheres for high performance gas sensor. Sens. Actuators B Chem. 2019, 298, 126927. [Google Scholar] [CrossRef]
- Kumar, P.; Malik, H.K.; Ghosh, A.; Thangavel, R.; Asokan, K. An insight to origin of ferromagnetism in ZnO and N implanted ZnO thin films: Experimental and DFT approach. J. Alloys Compd. 2018, 768, 323–328. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Y.; Ji, X.; Zheng, W.; Lin, Z.; Lu, X.; Huang, F. Experimental evidence on stability of N substitution for O in ZnO lattice. J. Phys. Chem. Lett. 2020, 11, 8901–8907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, M.; Chabungbam, A.S.; Kim, D.-E.; Park, H.-H. Suppressed oxygen vacancy in pristine/N doped ZnO and improved ZnO homogenous p-n junction performance by H2O2 oxidant. Appl. Surf. Sci. 2022, 579, 152170. [Google Scholar] [CrossRef]
- Muñoz-Chilito, J.F.; Rodríguez-Páez, J.E. ZnO-CeO2 nanocomposites: Synthesis, characterization and evaluation of their action on polluting gases emitted by motorcycles. J. Environ. Chem. Eng. 2021, 9, 104890. [Google Scholar] [CrossRef]
- Alabyadh, T.; Albadri, R.; Es-Haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 nanocomposites: Metal-organic framework-mediated synthesis, characterization, and estimation of cellular toxicity toward liver cancer cells. J. Funct. Biomater. 2022, 13, 139. [Google Scholar] [CrossRef]
- Pujar, M.S.; Hunagund, S.M.; Khanapure, S.; Vootla, S.K.; Sidarai, A.H. Multifunctional ZnO/CeO2 nanocomposites for Photoinduced dye degradation and Antibacterial activities. J. Sol-Gel Sci. Technol. 2022, 101, 356–369. [Google Scholar] [CrossRef]
- Peng, W.; Cai, L.; Lu, Y.; Zhang, Y. Preparation of Mn-Co-MCM-41 molecular sieve with thermosensitive template and its degradation performance for rhodamine B. Catalysts 2023, 13, 991. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, R.; Gao, M.; Hao, P.; Yin, D. Bio-inspired single-chain polymeric nanoparticles containing a chiral salen TiIV complex for highly enantioselective sulfoxidation in water. Green Chem. 2017, 19, 1182. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Alex, Z.C.; Meher, S.R. Experimental and ab initio studies of the structural and optoelectronic properties of hexagonal wurtzite ZnO1-xSx alloys. Mater. Chem. Phys. 2024, 312, 128674. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Soundarrajan, P.; Ravi, G. Synthesis of ZnO nanosheets morphology by Ce doping for photocatalytic activity. J. Electron. Mater. 2019, 48, 684–695. [Google Scholar] [CrossRef]
- Pathak, T.K.; Coetsee-Hugo, E.; Swart, H.; Swart, C.; Kroon, R. Preparation and characterization of Ce doped ZnO nanomaterial for photocatalytic and biological applications. Mater. Sci. Eng. B 2020, 261, 114780. [Google Scholar] [CrossRef]
- Ribić, V.; Rečnik, A.; Komelj, M.; Kokalj, A.; Branković, Z.; Zlatović, M.; Branković, G. New inversion boundary structure in Sb-doped ZnO predicted by DFT calculations and confirmed by experimental HRTEM. Acta Mater. 2020, 199, 633–648. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, M.; Krishnan, V.; Mohapatra, S. Facile synthesis of Ce doped ZnO nanowires for efficient photocatalytic removal of organic pollutants from water. Mater. Today Commun. 2023, 34, 105361. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. CeO2-ZnO hexagonal nanodisks: Efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J. Alloys Compd. 2015, 620, 67–73. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Dimitropoulos, M.; Govatsi, A.; Sygellou, L.; Tsakiroglou, C.D.; Yannopoulos, S.N. Influence of the surface-to-bulk defects ratio of ZnO and TiO2 on their UV-mediated photocatalytic activity. Appl. Catal. B Environ. 2017, 205, 292–301. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L. Temperature dependence of Raman scattering in ZnO. Phys. Rev. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Tayyebi, A.; Outokesh, M.; Tayebi, M.; Shafikhani, A.; Şengör, S.S. ZnO quantum dots-graphene composites: Formation mechanism and enhanced photocatalytic activity for degradation of methyl orange dye. J. Alloys Compd. 2016, 663, 738–749. [Google Scholar] [CrossRef]
- Abbasi, S.; Hasanpour, M. The effect of pH on the photocatalytic degradation of methyl orange using decorated ZnO nanoparticles with SnO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 1307–1314. [Google Scholar] [CrossRef]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of pH value on the bandgap energy and particles size for biosynthesis of ZnO nanoparticles: Efficiency for photocatalytic adsorption of methyl orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Hannachi, E.; Slimani, Y.; Nawaz, M.; Sivakumar, R.; Trabelsi, Z.; Vignesh, R.; Akhtar, S.; Almessiere, M.A.; Baykal, A.; Yasin, G. Preparation of cerium and yttrium doped ZnO nanoparticles and tracking their structural, optical, and photocatalytic performances. J. Rare Earths 2023, 41, 682–688. [Google Scholar] [CrossRef]
- Bao, L.-L.; Li, Y.; Xi, Z.; Wang, X.-Y.; Afzal, M.; Alarifi, A.; Srivastava, D.; Prakash, O.; Kumar, A.; Jin, J.-C. A new 2D Zn(II)-based coordination polymer as photocatalyst for photodegradation of methyl orange in water: Effect of photocatalyst dosage and dye concentration. J. Mol. Struct. 2023, 1292, 136103. [Google Scholar] [CrossRef]
- Gilani, S.A.B.; Naseeb, F.; Kiran, A.; Ihsan, M.U.; Iqbal, J.; Javed, H.M.A.; Bhatti, H.N.; Karami, A.M.; Hussain, S.; ShabirMahr, M. pH dependent synthesis of ceria nanoparticles for efficient sunlight-driven photocatalysis of methyl orange containing wastewater. Opt. Mater. 2024, 148, 114871. [Google Scholar] [CrossRef]
- Dimitropoulos, M.; Aggelopoulos, C.; Sygellou, L.; Tsantis, S.; Koutsoukos, P.; Yannopoulos, S. Unveiling the photocorrosion mechanism of zinc oxide photocatalyst: Interplay between surface corrosion and regeneration. J. Environ. Chem. Eng. 2024, 12, 112102. [Google Scholar] [CrossRef]
- Elahi, B.; Mirzaee, M.; Darroudi, M.; Oskuee, R.K.; Sadri, K.; Amiri, M.S. Preparation of cerium oxide nanoparticles in Salvia Macrosiphon Boiss seeds extract and investigation of their photo-catalytic activities. Ceram. Int. 2019, 45, 4790–4797. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Su, Q.; Lv, C.; Jin, X.; Wen, X. UV-induced photodegradation of naproxen using a nano γ-FeOOH composite: Degradation kinetics and photocatalytic mechanism. Front. Chem. 2019, 7, 847. [Google Scholar] [CrossRef]
- Yang, H.; Shan, C.; Li, F.; Han, D.; Zhang, Q.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 26, 3880–3882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Han, B.; Li, B.; Wang, L.; Wang, J.; Wang, X.; Zhu, L. Controllable preparation of chiral oxazoline-Cu(II) catalyst as nanoreactor for highly asymmetric Henry reaction in water. Catal. Lett. 2022, 152, 106–115. [Google Scholar] [CrossRef]
- George, A.; Sharma, S.K.; Chawla, S.; Malik, M.M.; Qureshi, M. Detailed of X-Ray diffraction and photoluminescence studies of Ce doped ZnO nanocrystals. J. Alloys Compd. 2011, 509, 5942–5946. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, C.; Li, S.; Wang, Q.; Zhang, B.; Wang, W.; Zhang, J.; Zhu, H. Enhancing blue luminescence from Ce-doped ZnO nanophosphor by Li doping. Nanoscale Res. Lett. 2014, 9, 480. [Google Scholar] [CrossRef]
- Hussain, A.; Maqsood, S.; Ji, R.; Zhang, Q.; Farooq, M.U.; Boota, M.; Umer, M.; Hashim, M.; Naeem, H.; Toor, Z.S.; et al. Investigation of transition metal-doped graphitic carbon nitride for MO dye degradation. Diam. Relat. Mater. 2023, 132, 109648. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Z.; Guo, L.; Zhang, H.; Zhang, L.-C.; Wang, W. Role of maze like structure and Y2O3 on Al-based amorphous ribbon surface in MO solution degradation. J. Mol. Liq. 2020, 318, 114318. [Google Scholar] [CrossRef]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007, 102, 093515. [Google Scholar] [CrossRef]


| Entries | PN64(IL)8 (g) | n(Ce3+) (mmol) | n(Zn2+) (mmol) | Citric Acid (mmol) |
|---|---|---|---|---|
| ZnO | 0.01 | 0 | 1 | 0.750 |
| ZnO-Ce-2‰ | 0.01 | 0.002 | 0.998 | 0.735 |
| ZnO-Ce-6‰ | 0.01 | 0.006 | 0.996 | 0.720 |
| ZnO-Ce-10‰ | 0.01 | 0.010 | 0.990 | 0.705 |
| ZnO-Ce-14‰ | 0.01 | 0.014 | 0.986 | 0.690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, W.; Zhu, Z.; Zhang, L.; Peng, W. Temperature-Sensitive Template for Preparation of ZnO/CeO2 Composite Photocatalytic Materials and Its Catalytic Performance. Molecules 2024, 29, 3589. https://doi.org/10.3390/molecules29153589
Zhang Y, Yang W, Zhu Z, Zhang L, Peng W. Temperature-Sensitive Template for Preparation of ZnO/CeO2 Composite Photocatalytic Materials and Its Catalytic Performance. Molecules. 2024; 29(15):3589. https://doi.org/10.3390/molecules29153589
Chicago/Turabian StyleZhang, Yaoyao, Wenjie Yang, Zhengyuan Zhu, Lin Zhang, and Wenju Peng. 2024. "Temperature-Sensitive Template for Preparation of ZnO/CeO2 Composite Photocatalytic Materials and Its Catalytic Performance" Molecules 29, no. 15: 3589. https://doi.org/10.3390/molecules29153589





