The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside
Abstract
1. Introduction
2. Results
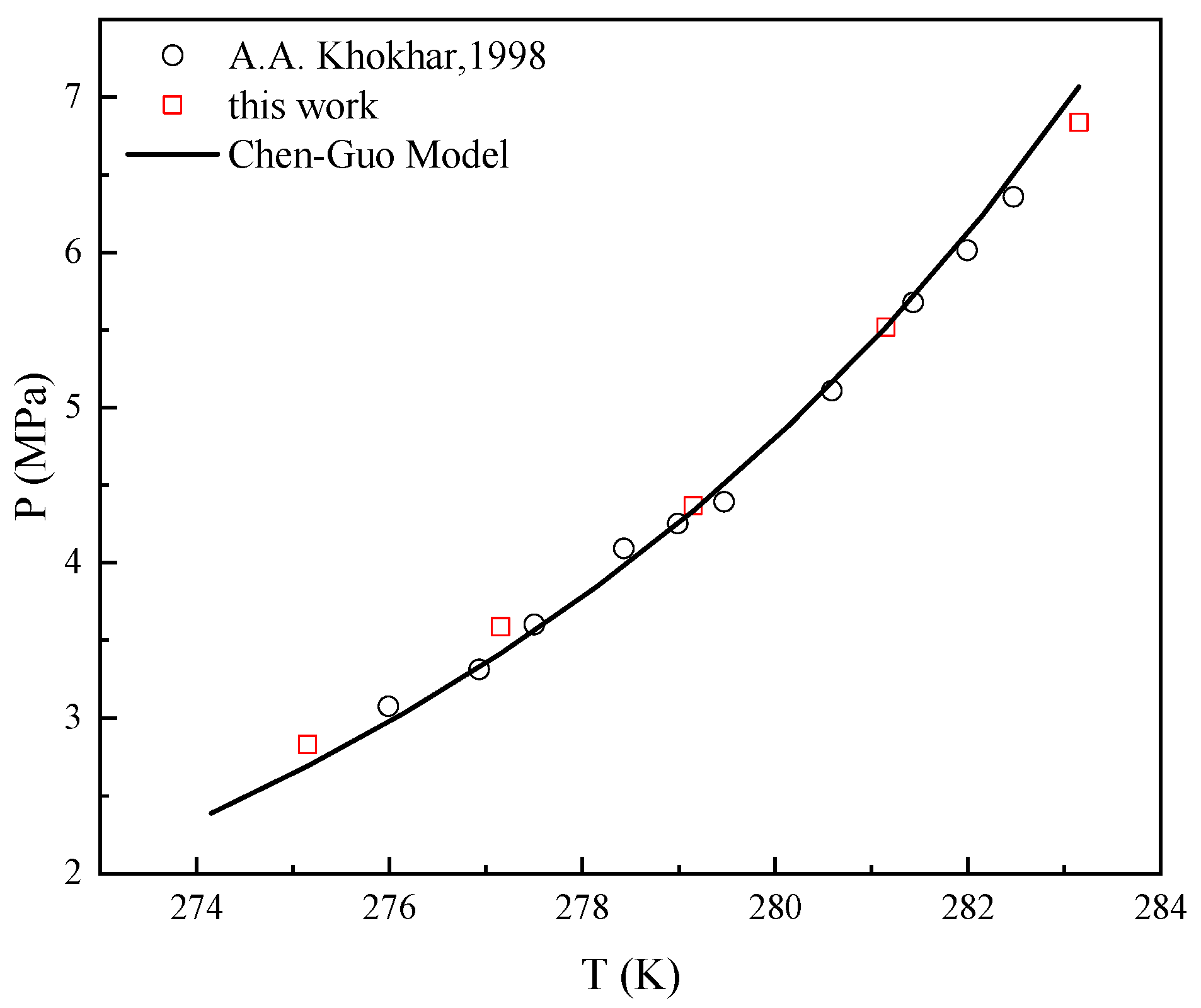
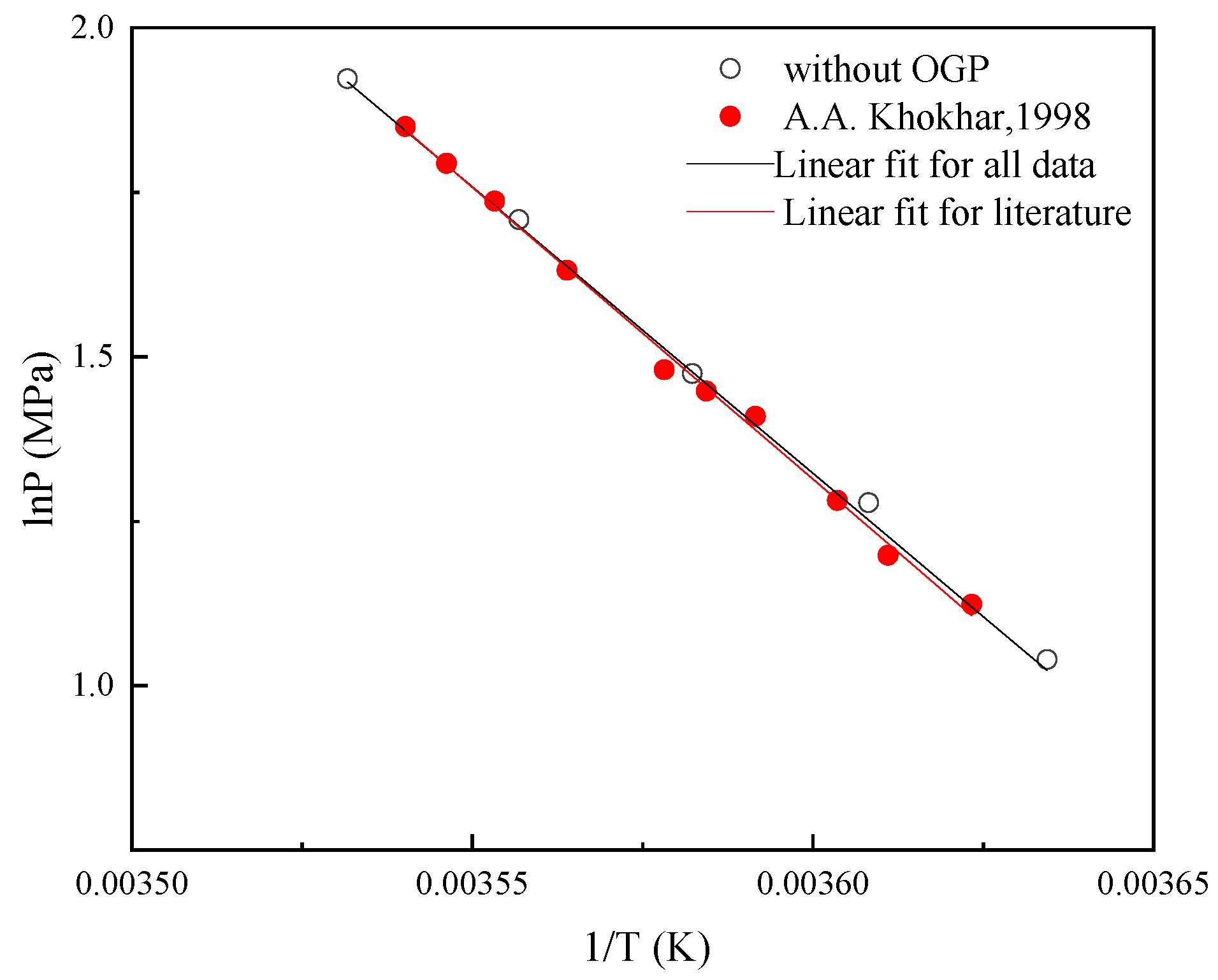
2.1. Thermodynamic Consistency
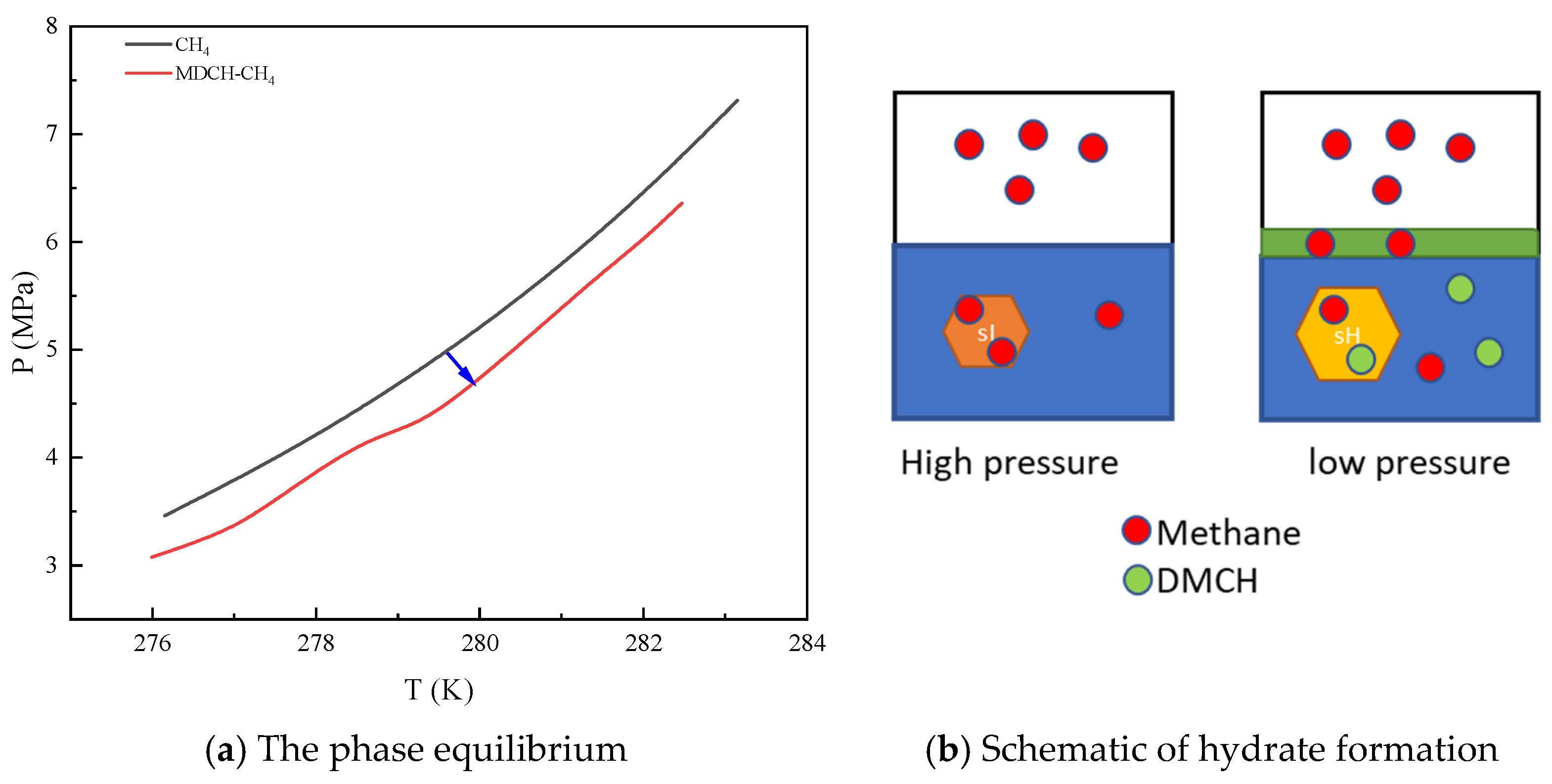
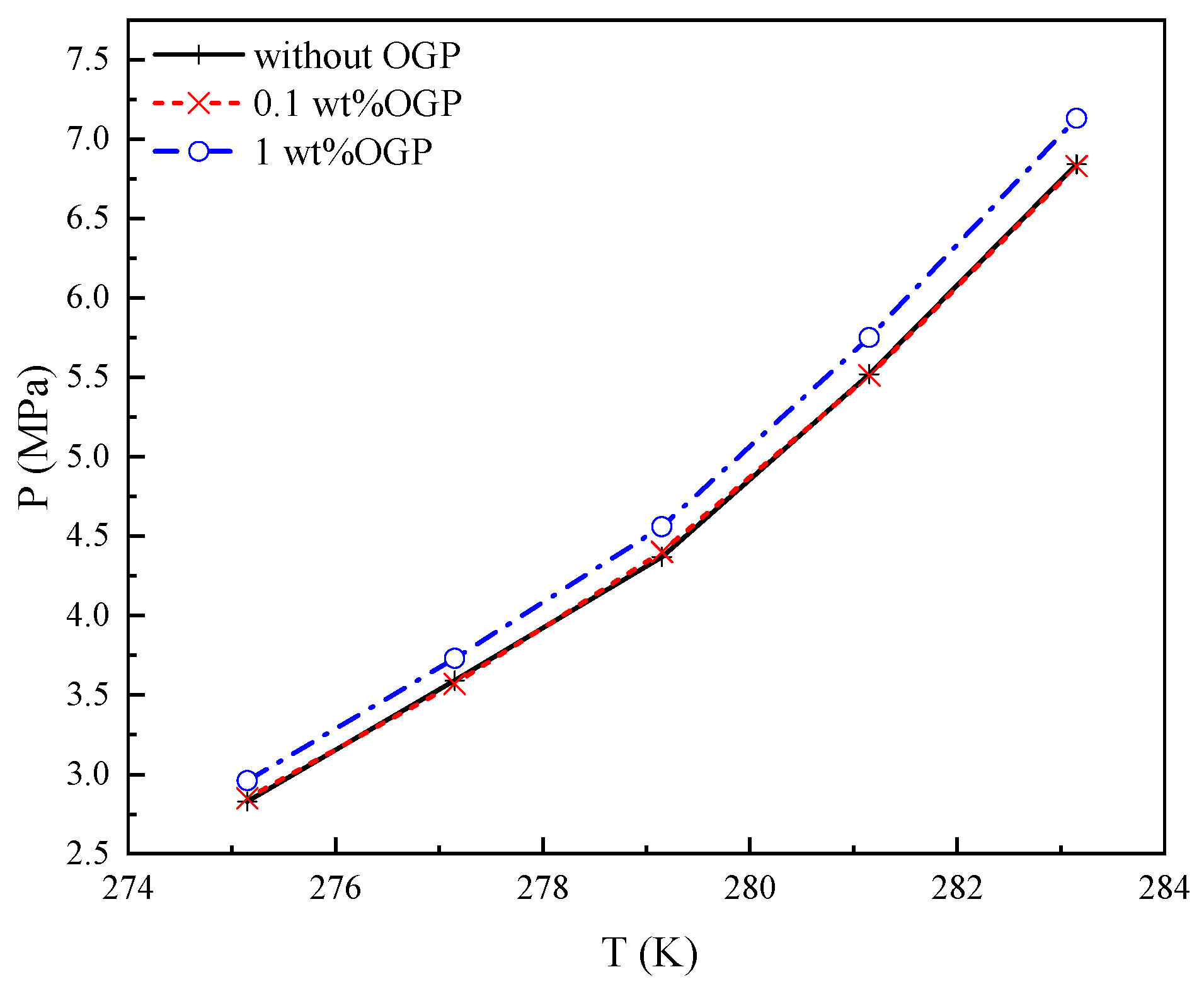
2.2. Thermodynamic Effect on Peq
3. Discussion
3.1. Thermodynamic Effect on Peq
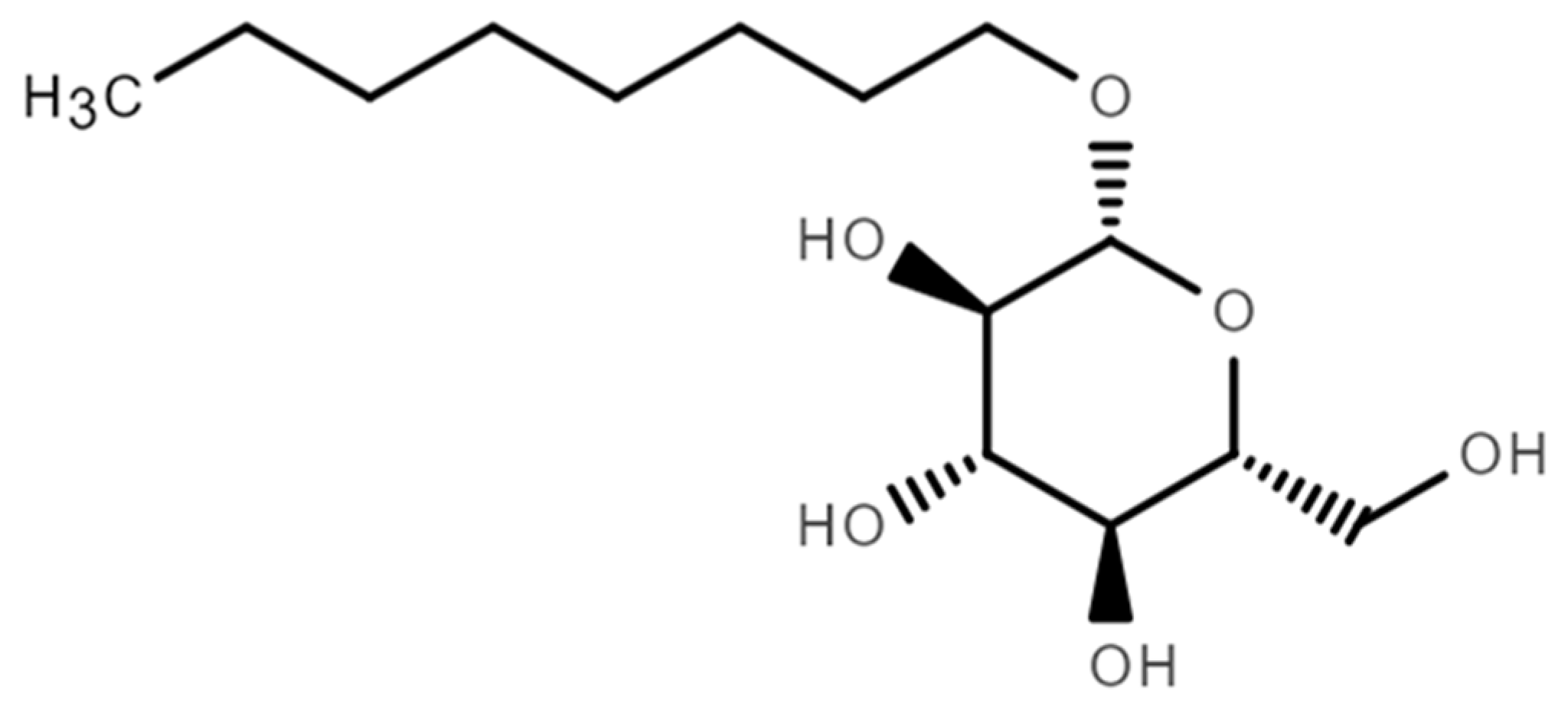
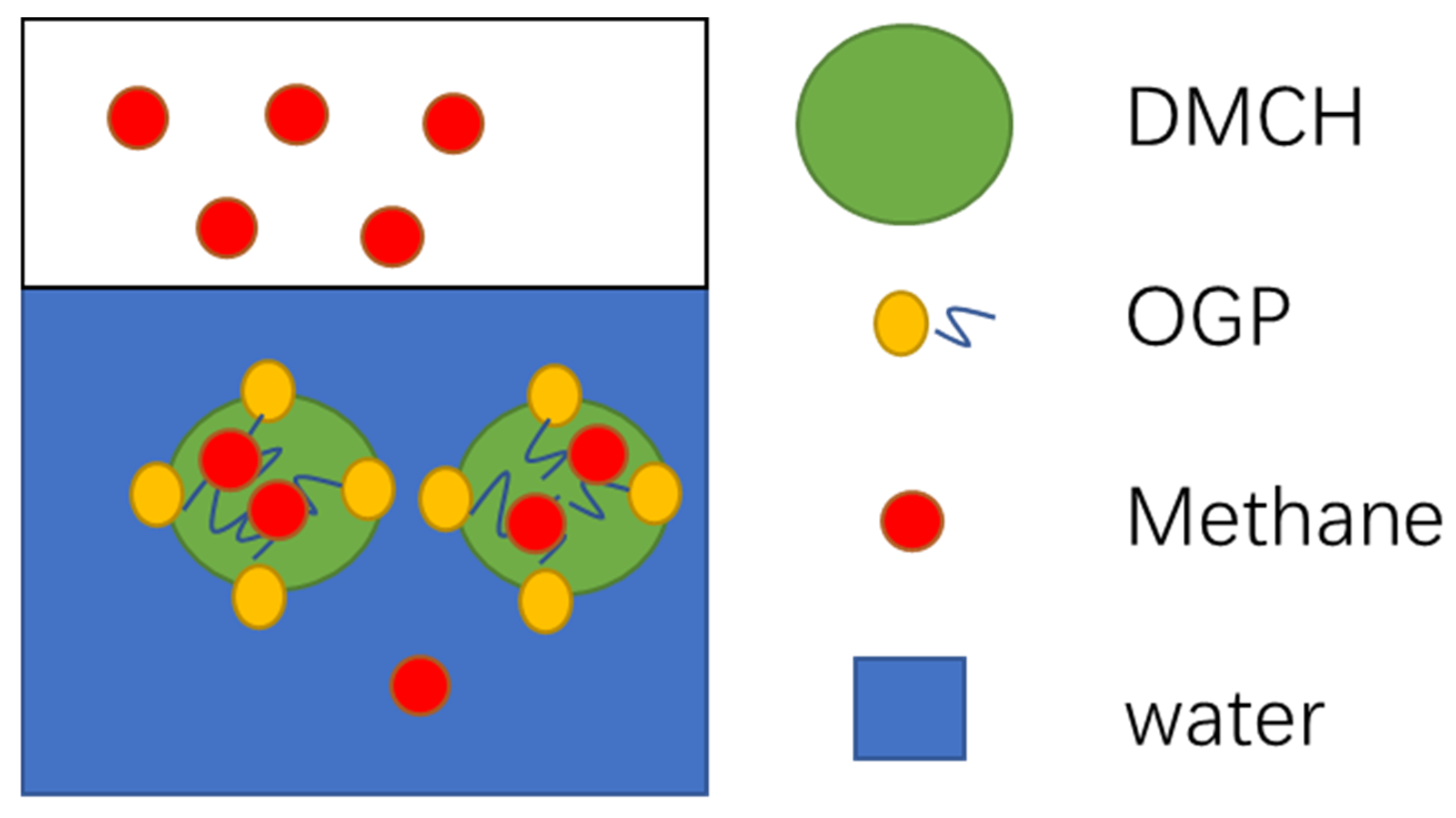
3.2. Mechanism of OGP in Hydrate Formation
4. Materials and Methods
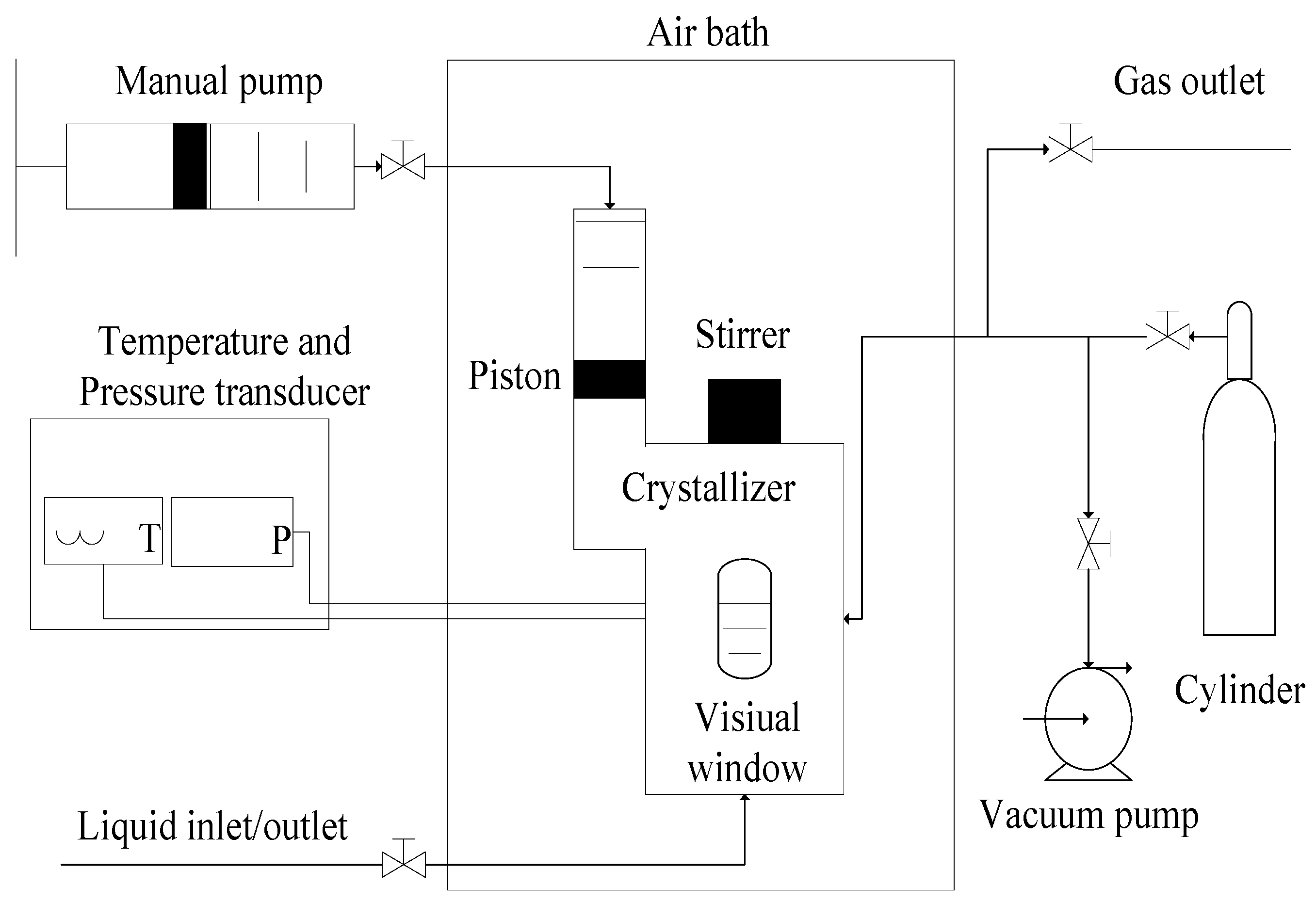
4.1. Materials and Apparatus
4.2. Experimental Method
- The crystallizer was washed with deionized water and then washed with the target liquid at least three times.
- The crystallizer was purged at least three times using methane to completely remove air from the system.
- The 40 mL target liquid was added to the crystallizer.
- The crystallizer volume was set at its maximum value (465.0 mL) when the gas was introduced into the crystallizer.
- The air bath was switched on to maintain the experimental temperature in the crystallizer.
- When the temperature in the crystallizer was stabilized at the experimental temperature for at least 15 min, the pressure in the crystallizer was increased gradually by adjusting the manual pump until a small trace of hydrate was observed.
- The pressure was set at the estimated value.
- If the trace of hydrate disappeared in 4 h, this indicated that the estimated value was lower than Peq. The estimated pressure value was increased, and the experiment was repeated from Step 5.
- If the amount of hydrate increased, indicating that the estimated value was higher than the equilibrium pressure at the experimental temperature, the pressure was decreased to completely dissociate the hydrate. The estimated value of the pressure was decreased, and the experiment was repeated from Step 6.
- If the trace of hydrate crystals lasted for more than 4 h (indicating that the estimated value was equal to the equilibrium pressure at the experimental temperature), the data for equilibrium hydrate formation were recorded.
- Peq under each experimental condition was measured at least three times. The uncertainty of Peq is ±0.01 MPa.
4.3. Thermodynamic Framework
5. Conclusions
- (1)
- The kinetic promotion effects of OGP on Peq were described as an emulsifying effect, and that effect was the main effect in the 0.1 wt% OGP system, which had no significant thermodynamic effect on Peq because of the low concentration.
- (2)
- The thermodynamic effect on Peq was the main effect in the 1 wt% OGP system, which showed a negative effect because of the hydroxyl groups of OGP bonding to water by hydrogen bonds.
- (3)
- The Clausius-Clapeyron equation was used to explore the thermodynamic effects of DMCH-methane hydrates in the presence of OGP on ΔHdiss. The results show that ΔHdiss of the methane-DMCH-water system was close to that of the methane-DMCH-OGP-water system (around 71.0 kJ mol−1) and it remains constant, which confirms that OGP had the same effect on Peq as other kinetic promoters, and OGP was only presented in the liquid (water or oil) phase and was not in the hydrate phase.
- (4)
- Furthermore, these effects can guide the number of kinetic promoters used for hydrate formation for gas storage, gas separation, and other natural gas hydrate research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkhel, R.; Sahu, C.; Kumar, R.; Sangwai, J.S. Effects of sodium hydroxide and calcium hydroxide on the phase equilibria of methane hydrates. J. Chem. Thermodyn. 2023, 177, 106935. [Google Scholar] [CrossRef]
- Chami, N.; Salehy, Y.; Burgner, D.; Clain, P.; Dalmazzone, D.; Delahaye, A.; Fournaison, L. Rheological study of mixed cyclopentane + CO2 hydrate slurry in a dynamic loop for refrigeration systems. Energy 2023, 263, 125661. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Nouri, S.; Aghajanloo, M.; Mohammadi, A.H.; Mohammadi, S. Experimental measurements and thermodynamic modeling of hydrate dissociation conditions for CO2 +THF + MgCl2 + water systems. Fluid Phase Equilib. 2023, 564, 113626. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; He, Y.; Zhou, X.; Liang, D. Experimental investigation of hydrate formation kinetics and microscopic properties by a synthesized ternary gas mixture with combination additives. Energy 2022, 259, 125006. [Google Scholar] [CrossRef]
- Seyfi Mazraeno, M.; Naeiji, P.; Varaminian, F. Kinetic studies of structure-h hydrate using a Langmuir adsorption model: Effects of methyl cyclohexane and methyl cyclopentane as large-molecule guest substances on methane hydrate. J. Mol. Liq. 2021, 343, 117213. [Google Scholar] [CrossRef]
- Khokhar, A.A.; Gudmundsson, J.S.; Sloan, E.D. Gas storage in structure h hydrates. Fluid Phase Equilib. 1998, 150, 383–392. [Google Scholar] [CrossRef]
- Qin, Y.; Bao, R.; Zhou, L.; Yang, X.; Liu, C.; Wan, W.; Chen, Y.; Guo, Y. Enhanced formation kinetics of hydrates using 1,3-dioxolane, l-leucine, and β-cyclodextrin. Fuel 2023, 332, 126165. [Google Scholar] [CrossRef]
- Zheng, R.; Fan, Z.; Li, X.; Negahban, S. Phase behavior of high-pressure CH4-CO2 hydrates in nacl solutions. Fuel 2020, 280, 118549. [Google Scholar] [CrossRef]
- Mehta, A.P.; Sloan, E.D. Structure H hydrate phase equilibria of paraffins, naphthenes, and olefins with methane. J. Chem. Eng. Data 1994, 39, 887–890. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Shariati, A.; Peters, C.J. Phase equilibrium measurements of structure sh hydrogen clathrate hydrates with various promoters. J. Chem. Eng. Data 2009, 54, 1628–1632. [Google Scholar] [CrossRef]
- Sugahara, T.; Hara, T.; Hashimoto, S.; Ohgaki, K. Icosahedron cage occupancy of structure-h hydrate helped by xe -1,1-, cis -1,2-, trans-1,2-, and cis-1,4-dimethylcyclohexanes. Chem. Eng. Sci. 2005, 60, 1783–1786. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, Y.; Liu, Z.; Xu, T.; Sun, Q.; Liu, A.; Yang, L.; Gong, J.; Guo, X. The hydrate-based separation of hydrogen and ethylene from fluid catalytic cracking dry gas in presence of n-octyl-β-d-glucopyranoside. Int. J. Hydrog. Energy 2022, 47, 31350–31369. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liu, Z.; Ma, R.; Sun, Q.; Liu, A.; Yang, L.; Gong, J.; Guo, X. The formation of structure I hydrate in presence of n-octyl-β-d-glucopyranoside. Fluid Phase Equilib. 2021, 556, 113373. [Google Scholar] [CrossRef]
- Hu, P.; Wu, G.; Zi, M.; Li, L.; Chen, D. Effects of modified metal surface on the formation of methane hydrate. Fuel 2019, 255, 115720. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, C.; Liu, Q.; Chen, J. Separation of c3h8 and c3h6 from butyl alcohol–octyl alcohol vent gas mixture via hydrate formation in the presence of SDS and THF in tap-water system. J. Chem. Eng. Data 2019, 64, 1244–1249. [Google Scholar] [CrossRef]
- Sun, Z.; Dai, M.; Zhu, M.; Li, J. Improving THF hydrate formation in the presence of nonanoic acid. J. Mol. Liq. 2020, 299, 112188. [Google Scholar] [CrossRef]
- Babaee, S.; Hashemi, H.; Javanmardi, J.; Eslamimanesh, A.; Mohammadi, A.H. Thermodynamic model for prediction of phase equilibria of clathrate hydrates of hydrogen with different alkanes, alkenes, alkynes, cycloalkanes or cycloalkene. Fluid Phase Equilib. 2012, 336, 71–78. [Google Scholar] [CrossRef]
- Zheng, R.; Li, X.; Negahban, S. Phase boundary of gas hydrates in single and mixed electrolyte solutions: Using a novel unified equation of state. J. Mol. Liq. 2022, 345, 117825. [Google Scholar] [CrossRef]
- Chen, G.; Sun, C.; Guo, T. Modelling of the formation conditions of structure-h hydrates. Fluid Phase Equilib. 2003, 204, 107–117. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Q.; Wang, Y.; Luo, Y.; Yue, G.; Guo, X.; Li, X.; Yang, L. Experimental and modelling study on the effect of maltose as a green additive on methane hydrate. J. Chem. Thermodyn. 2020, 144, 105980. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Kan, J.; Liu, A.; Sun, Q.; Chen, J.; Guo, X. Experimental measurement and model prediction on methane hydrate equilibrium conditions in the presence of organic carboxylic sodium salts. J. Chem. Thermodyn. 2023, 180, 107005. [Google Scholar] [CrossRef]
- Patel, N.C.; Teja, A.S. A new cubic equation of state for fluids and fluid mixtures. Chem. Eng. Sci. 1982, 463–473. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Liu, Y.; Sun, Q.; Xu, Z.; Liu, A.; Wang, Y.; Guo, X. Thermodynamic effects of the interaction of multiple solutes and dodecahedral-cage deformation on the semi-clathrate hydrate formation with CH4-CO2. Chem. Eng. Sci. 2023, 269, 118468. [Google Scholar] [CrossRef]
- Sa, J.; Hu, Y.; Sum, A.K. Assessing thermodynamic consistency of gas hydrates phase equilibrium data for inhibited systems. Fluid Phase Equilib. 2018, 473, 294–299. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, Z.; Li, Y.; Sun, Q.; Liu, A.; Yang, L.; Gong, J.; Guo, X. The thermodynamic and kinetic effects of sodium lignin sulfonate on ethylene hydrate formation. Energies 2021, 14, 3291. [Google Scholar] [CrossRef]
- Das, R.; Mallik, N.; Adhikari, A.; Bhattarai, A. A comprehensive review on the creation, description, and utilization of surfactants containing multiple hydroxyl groups. Int. J. Polym. Sci. 2024, 2024, 6120535. [Google Scholar] [CrossRef]
- Gaudin, T.; Lu, H.; Fayet, G.; Berthauld-Drelich, A.; Rotureau, P.; Pourceau, G.; Wadouachi, A.; Van Hecke, E.; Nesterenko, A.; Pezron, I. Impact of the chemical structure on amphiphilic properties of sugar-based surfactants: A literature overview. Adv. Colloid. Interface. Sci. 2019, 270, 87–100. [Google Scholar] [CrossRef]
- Stubenrauch, C. Sugar surfactants—Aggregation, interfacial, and adsorption phenomena. Curr. Opin. Colloid Interface Sci. 2001, 6, 160–170. [Google Scholar] [CrossRef]
- Kanduč, M.; Schneck, E.; Stubenrauch, C. Intersurfactant h-bonds between head groups of n-dodecyl-β-d-maltoside at the air-water interface. J. Colloid. Interface. Sci. 2021, 586, 588–595. [Google Scholar] [CrossRef]
- Siddiqui, U.S.; Aslam, J.; Ansari, W.H.; Kabir-Ud-Din. Micellization and aggregation behavior of a series of cationic gemini surfactants (m-s-m type) on their interaction with a biodegradable sugar-based surfactant (octyl-β-d-glucopyranoside). Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 164–172. [Google Scholar] [CrossRef]
- Ahmadian, S.; Mohammadi, M.; Ehsani, M.R. Thermodynamic modeling of methane hydrate formation in the presence of imidazolium-based ionic liquids using two-step hydrate formation theory and CPA EOS. Fluid Phase Equilib. 2018, 474, 32–42. [Google Scholar] [CrossRef]
- Chen, G.; Guo, T. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Voutsas, E.C.; Yakoumis, I.V.; Tassios, D.P. An equation of state for associating fluids. Ind. Eng. Chem. Res. 1996, 35, 4310–4318. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Corvisier, J.; Coquelet, C.; Paricaud, P.; Houriez, C.; Ahmar, E.E. Thermodynamic study of the CO2–H2O–NaCl system: Measurements of CO2 solubility and modeling of phase equilibria using soreide and whitson, electrolyte CPA and sit models. Int. J. Greenh. Gas Control 2019, 91, 102825. [Google Scholar] [CrossRef]
- Palma, A.M.; Queimada, A.J.; Coutinho, J.A.P. Modeling of hydrate dissociation curves with a modified cubic-plus-association equation of state. Ind. Eng. Chem. Res. 2019, 58, 14476–14487. [Google Scholar] [CrossRef]
| OGP Concentrations (wt%) | Calculated Dissociation Enthalpies (kJ∙mol−1) |
|---|---|
| 0 | 71.1 |
| 0.1 | 71.3 |
| 1 | 71.0 |
| (Pa) | (K) | (K) | |
|---|---|---|---|
| 2.3048 × 10−11 | 2752.29 | 23.01 | |
| 1.433 × 10−10 | 2625.04 | 19.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Chen, M.; Pang, W.; Liu, Z.; Xu, Z.; Lei, X. The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside. Molecules 2024, 29, 3604. https://doi.org/10.3390/molecules29153604
Fu Q, Chen M, Pang W, Liu Z, Xu Z, Lei X. The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside. Molecules. 2024; 29(15):3604. https://doi.org/10.3390/molecules29153604
Chicago/Turabian StyleFu, Qiang, Mingqiang Chen, Weixin Pang, Zengqi Liu, Zhen Xu, and Xin Lei. 2024. "The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside" Molecules 29, no. 15: 3604. https://doi.org/10.3390/molecules29153604
APA StyleFu, Q., Chen, M., Pang, W., Liu, Z., Xu, Z., & Lei, X. (2024). The Phase Equilibria of Natural Gas Hydrate in the Presence of 1,3-Dimethylcyclohexane and Octyl-β-D-glucopyranoside. Molecules, 29(15), 3604. https://doi.org/10.3390/molecules29153604







