Stability of Nitrogen-Doped Activated Carbon as an Electrocatalyst for the Oxygen Reduction Reaction in Various Storage Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study on the Stability of N-Doped Activated Carbons in Air Storage
2.1.1. Catalytic ORR Performance of Samples
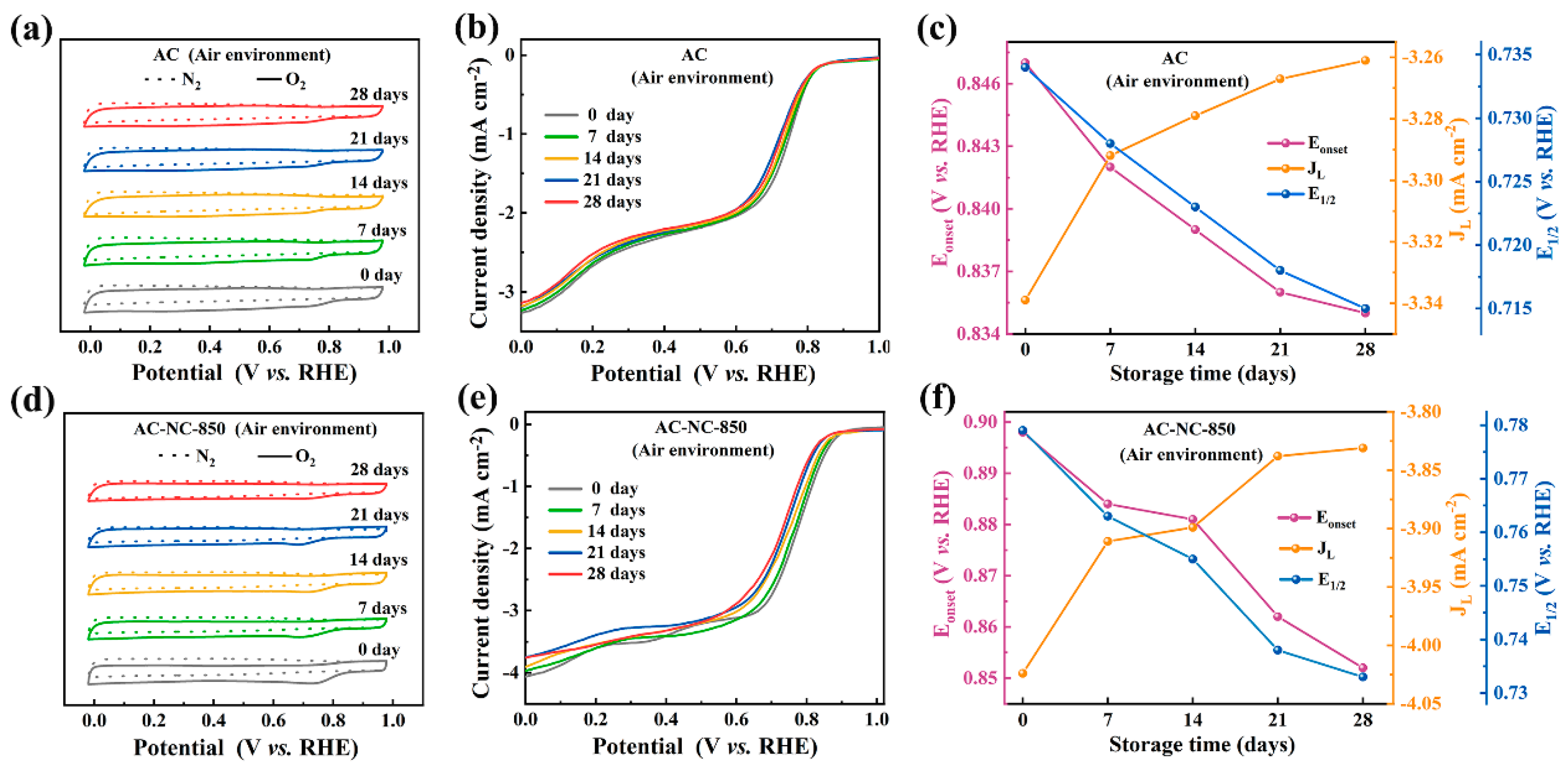
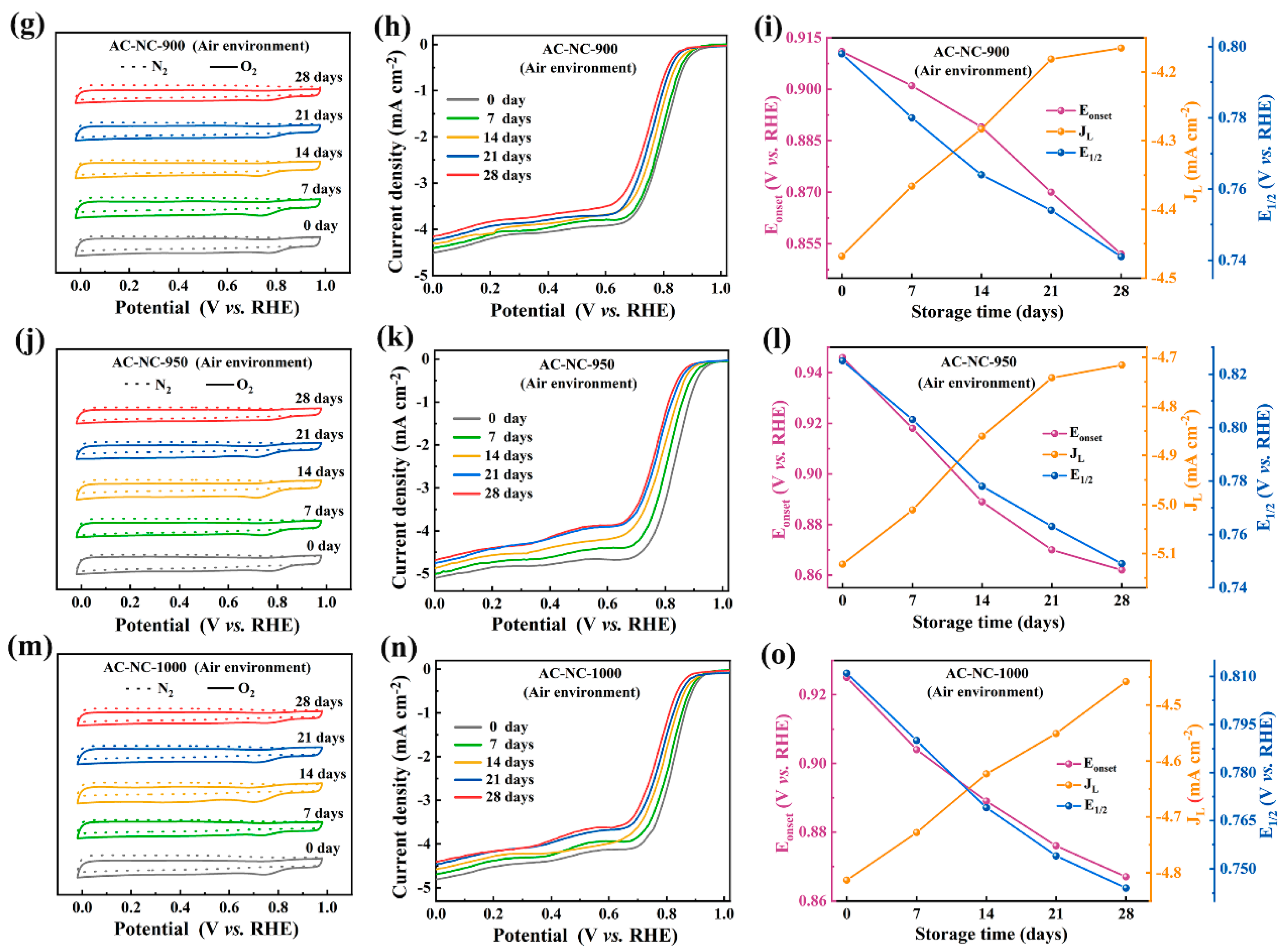
2.1.2. Storage in an Air Environment
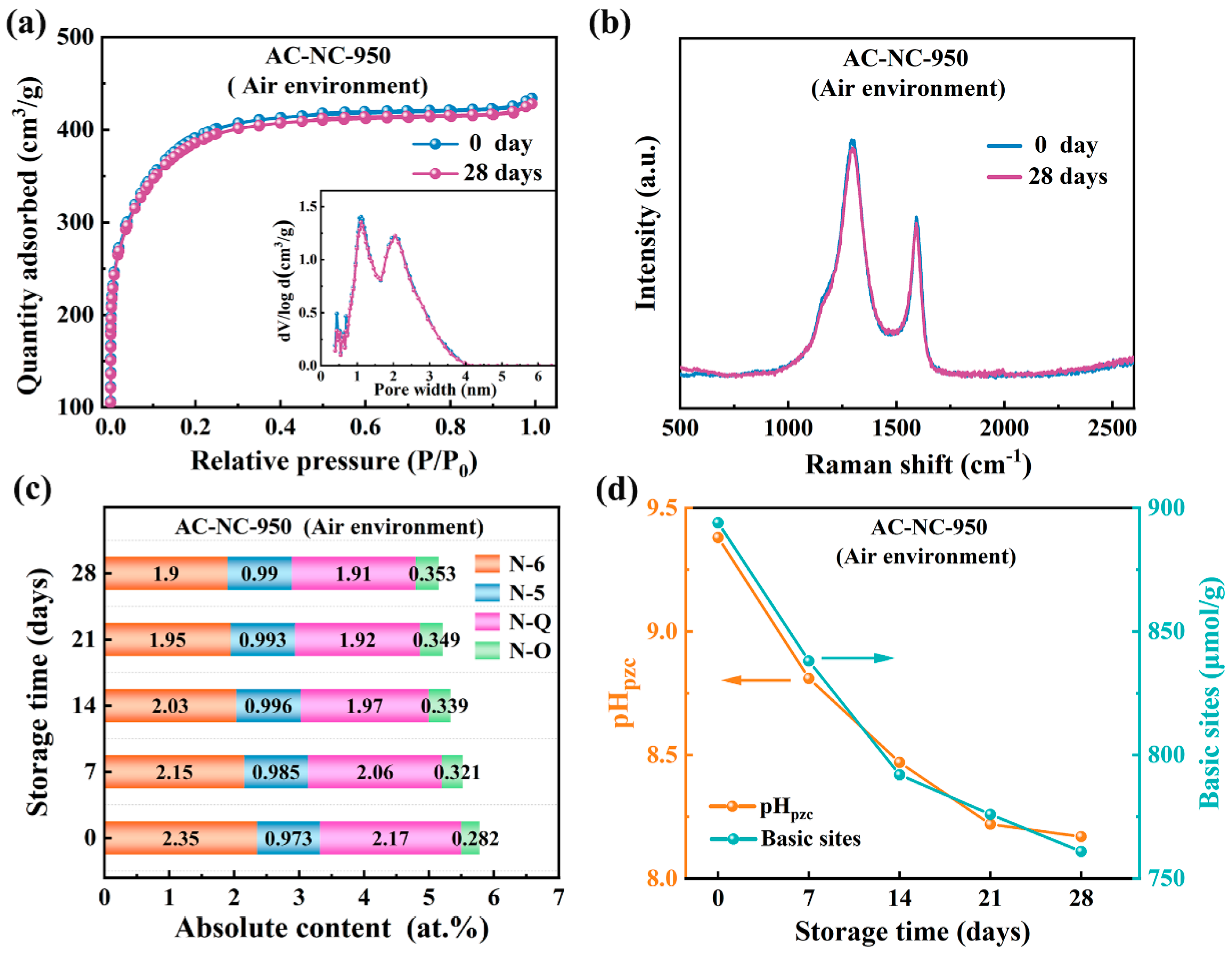
2.2. Evolution of Surface Chemical Properties of N-Doped Activated Carbon Upon Exposure to Air
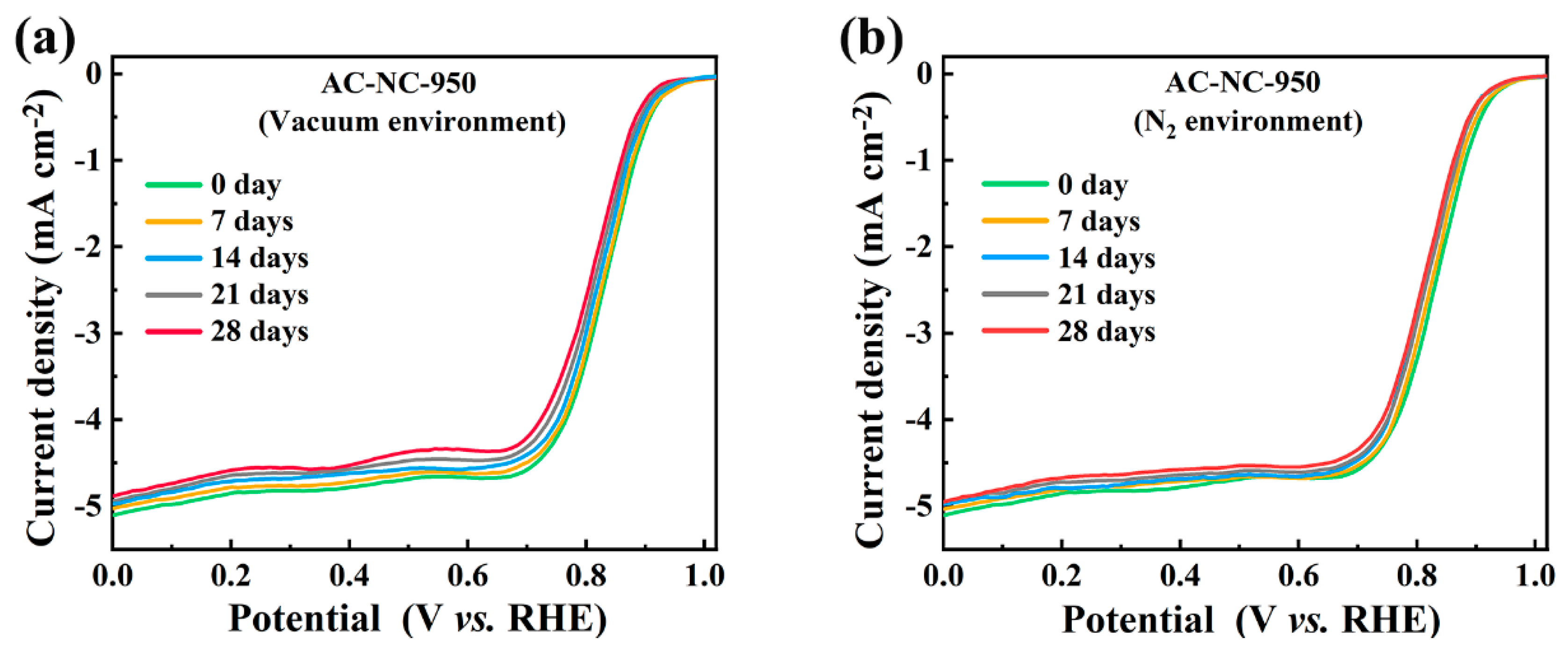
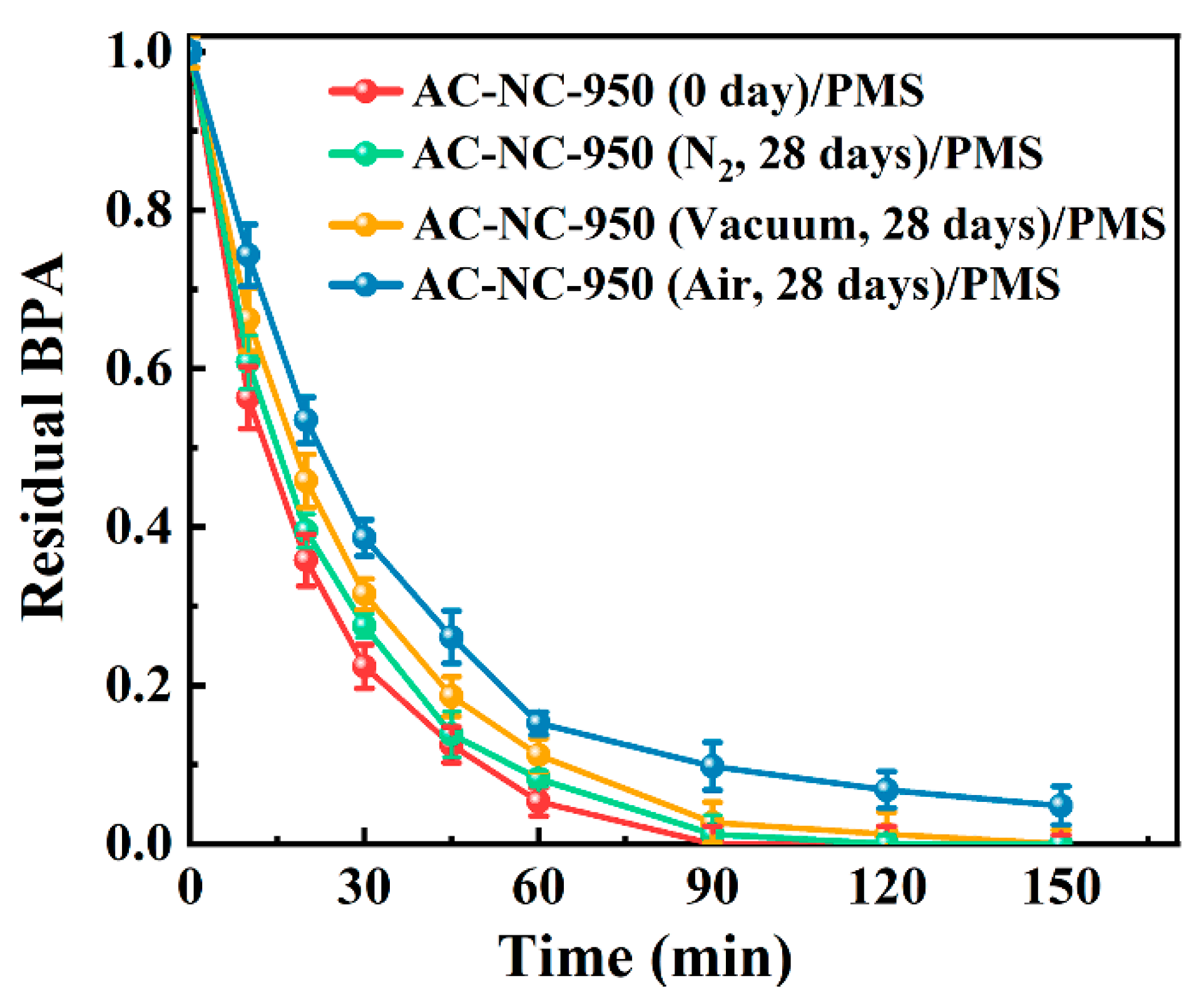
2.3. Stability of N-Doped Activated Carbon under Vacuum and N2 Environment
2.4. BPA Degradation Experiment
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation and Storage Methods for Nitrogen-Doped Activated Carbon
3.2.1. Preparation of Nitrogen-Doped Activated Carbon
3.2.2. Storage Conditions of Carbon Samples
3.3. Electrode Preparation and Electrochemical Measurements
3.4. BPA Degradation Experiments
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samantaray, S.; Mohanty, D.; Satpathy, S.K.; Hung, I.M. Exploring Recent Developments in Graphene-Based Cathode Materials for Fuel Cell Applications: A Comprehensive Overview. Molecules 2024, 29, 2937. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Byeon, A.; Yun, W.C.; Kim, J.M.; Lee, J.W. Recent progress in heteroatom-doped carbon electrocatalysts for the two-electron oxygen reduction reaction. Chem. Eng. J. 2023, 456, 141042. [Google Scholar] [CrossRef]
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Gu, X.; Wu, N.; Zhang, R.; Shen, Y.; Zheng, B.; Wu, J.; Zhang, W.; Li, S.; et al. One-step turning leather wastes into heteroatom doped carbon aerogel for performance enhanced capacitive deionization. Microporous Mesoporous Mater. 2020, 303, 110303. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Bi, X.; Zhao, Y.; Wu, M. Efficient electrocatalytic reduction of CO2 to CO enhanced by the synergistic effect of N,P on carbon aerogel. Chem. Commun. 2024, 60, 6439–6442. [Google Scholar] [CrossRef]
- Carrillo-Rodríguez, J.C.; Garay-Tapia, A.M.; Escobar-Morales, B.; Escorcia-García, J.; Ochoa-Lara, M.T.; Rodríguez-Varela, F.J.; Alonso-Lemus, I.L. Insight into the performance and stability of N-doped Ordered Mesoporous Carbon Hollow Spheres for the ORR: Influence of the nitrogen species on their catalytic activity after ADT. Int. J. Hydrogen Energy 2021, 46, 26087–26100. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Li, J.; Xia, Z.; Wang, X.; Feng, C.; Zhang, Q.; Chen, X.A.; Yang, Y.; Wang, S.; Jin, H. Distinguished Roles of Nitrogen-Doped Sp2 and Sp3 Hybridized Carbon on Extraordinary Supercapacitance in Acidic Aqueous Electrolyte. Adv. Mater. 2024, 36, 2310422. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Duan, X.; Liu, Y.; Wang, S. Density Functional Theory Calculations for Insight into the Heterocatalyst Reactivity and Mechanism in Persulfate-Based Advanced Oxidation Reactions. ACS Catal. 2021, 11, 11129–11159. [Google Scholar] [CrossRef]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-Doped and Oxygen-Functionalized Nanocarbons for High-Performance Supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Z.; Liu, Z.; Wei, X.; Cai, W. Nitrogen-doped carbon particles with distinctive ethylene adsorption selectivity for efficient ethylene/acetylene separation. Chem. Eng. J. 2023, 477, 147220. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, S. Drying enables multiple reuses of activated carbon without regeneration. Environ. Sci. Pollut. Res. 2023, 30, 45097–45111. [Google Scholar] [CrossRef]
- Di, L.; Wang, T.; Lu, Q.; Lu, J.; Zhang, Y.; Zhou, Y.; Zhou, Y. Efficient PMS activation toward degradation of bisphenol A by metal-free nitrogen-doped hollow carbon spheres. Sep. Purif. Technol. 2024, 339, 126740. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.-G.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-Doped Carbon as CO2 Adsorbent with High CO2 Selectivity from Rationally Designed Polypyrrole Precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, L.; Chen, X.; Cai, W.; Wang, L.; Wang, B. Metal–Organic Framework-Derived N-Doped Carbon with Controllable Mesopore Sizes for Low-Pt Fuel Cells. Adv. Funct. Mater. 2023, 33, 2302582. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Liao, Y.; Huang, K.; Lian, C.; Zhou, X.; Zhang, Z.; Kauppinen, E.I.; Wu, Z.-S. Floating Catalyst Chemical Vapor Deposition Patterning Nitrogen-Doped Single-Walled Carbon Nanotubes for Shape Tailorable and Flexible Micro-Supercapacitors. Adv. Funct. Mater. 2023, 33, 2301103. [Google Scholar] [CrossRef]
- Li, J.; Bao, A. Self-sacrificing template synthesis of nitrogen-doped hierarchical porous carbons as an effective adsorbent for CO2 capture. J. Porous Mater. 2022, 29, 1895–1908. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Rydzek, G.; Pakdel, A.; Oruganti, A.; Hasegawa, K.; Mitome, M.; Golberg, D.; Hill, J.P.; Abe, H.; Ariga, K. Nanostructured polymeric yolk–shell capsules: A versatile tool for hierarchical nanocatalyst design. J. Mater. Chem. A 2016, 4, 9850–9857. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, S.; Zhao, L.; Kong, L.; Zhan, J.; Zhao, R.-S. Porous 3D superstructure of nitrogen doped carbon decorated with ultrafine cobalt nanodots as peroxymonosulfate activator for the degradation of sulfonamides. Chem. Eng. J. 2022, 428, 131329. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lisak, G.; Webster, R.D.; Liang, Y.-N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.-W.; Lim, T.-T. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites. Appl. Catal. B Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Wang, S.; Xia, Y.; Tan, L.; Wu, S.; Yu, Y.; Yu, X.; Guan, Z.; Chen, H.; Jiang, F. Unraveling the instability of Nitrogen-Doped carbon during BPA treatment by peroxymonosulfate Activation: Effect of free radical grafting. Sep. Purif. Technol. 2023, 317, 123873. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. On the deactivation of N-doped carbon materials active sites during oxygen reduction reaction. Carbon 2022, 189, 548–560. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Choi, C.H.; Lim, H.-K.; Chung, M.W.; Park, J.C.; Shin, H.; Kim, H.; Woo, S.I. Long-Range Electron Transfer over Graphene-Based Catalyst for High-Performing Oxygen Reduction Reactions: Importance of Size, N-doping, and Metallic Impurities. J. Am. Chem. Soc. 2014, 136, 9070–9077. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Ren, G.; Li, Y.; Li, Y.; Du, X. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion. Nat. Commun. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Xia, C.; Wang, H. Insights into Practical-Scale Electrochemical H2O2 Synthesis. Trends Chem. 2020, 2, 942–953. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Pérez-Rodríguez, S.; Torres, D.; Canevesi, R.; Morallón, E.; Cazorla-Amorós, D.; Celzard, A.; Fierro, V. Nitrogen sites prevail over textural properties in N-doped carbons for the oxygen reduction reaction. J. Colloid Interface Sci. 2024, 654, 446–453. [Google Scholar] [CrossRef]
- Encalada, J.; Savaram, K.; Travlou, N.A.; Li, W.; Li, Q.; Delgado-Sánchez, C.; Fierro, V.; Celzard, A.; He, H.; Bandosz, T.J. Combined Effect of Porosity and Surface Chemistry on the Electrochemical Reduction of Oxygen on Cellular Vitreous Carbon Foam Catalyst. ACS Catal. 2017, 7, 7466–7478. [Google Scholar] [CrossRef]
- Bandosz, T.J. Revealing the impact of small pores on oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon 2022, 188, 289–304. [Google Scholar] [CrossRef]
- Zhang, D.; Mitchell, E.; Lu, X.; Chu, D.; Shang, L.; Zhang, T.; Amal, R.; Han, Z. Metal-free carbon-based catalysts design for oxygen reduction reaction towards hydrogen peroxide: From 3D to 0D. Mater. Today 2023, 63, 339–359. [Google Scholar] [CrossRef]
- Kislenko, V.A.; Pavlov, S.V.; Nikitina, V.A.; Kislenko, S.A. Revision of the oxygen reduction reaction on N-doped graphenes by grand-canonical DFT. Phys. Chem. Chem. Phys. 2024, 26, 293–303. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zhang, R.; Wang, L.; Wang, Y.; Yang, L.; Li, J.; Guan, F.; Duan, J.; Hou, B. Regulating N Species in N-Doped Carbon Electro-Catalysts for High-Efficiency Synthesis of Hydrogen Peroxide in Simulated Seawater. Adv. Sci. 2023, 10, 2302446. [Google Scholar] [CrossRef]
- Li, B.; Sun, X.; Su, D. Calibration of the basic strength of the nitrogen groups on the nanostructured carbon materials. Phys. Chem. Chem. Phys. 2015, 17, 6691–6694. [Google Scholar] [CrossRef]
- Tan, Z.; Qin, X.; Cao, P.; Chen, S.; Yu, H.; Su, Y.; Quan, X. Enhanced electrochemical-activation of H2O2 to produce •OH by regulating the adsorption of H2O2 on nitrogen-doped porous carbon for organic pollutants removal. J. Hazard. Mater. 2023, 458, 131925. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, S. Nitrogen-doped metal-free granular activated carbons as economical and easily separable catalysts for peroxymonosulfate and hydrogen peroxide activation to degrade bisphenol A. Environ. Sci. Pollut. Res. 2024, 31, 25751–25768. [Google Scholar] [CrossRef]
- Niu, Y.-Q.; Liu, J.-H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.-H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Relationships between texture, surface chemistry and performance of N-doped carbon xerogels in the oxygen reduction reaction. Appl. Surf. Sci. 2021, 548, 149242. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. The influence of structure and surface chemistry of carbon materials on the decomposition of hydrogen peroxide. Carbon 2013, 62, 97–108. [Google Scholar] [CrossRef]
- Vega, E.; Valdés, H. New evidence of the effect of the chemical structure of activated carbon on the activity to promote radical generation in an advanced oxidation process using hydrogen peroxide. Microporous Mesoporous Mater. 2018, 259, 1–8. [Google Scholar] [CrossRef]

| Samples | Eonset (V vs. RHE) | E1/2 (V vs. RHE) | JL (mA cm−2) |
|---|---|---|---|
| AC | 0.847 | 0.734 | −3.339 |
| AC–NC–850 | 0.898 | 0.779 | −4.024 |
| AC–NC–900 | 0.911 | 0.798 | −4.468 |
| AC–NC–950 | 0.946 | 0.827 | −5.122 |
| AC–NC–1000 | 0.925 | 0.811 | −4.813 |
| Pt/C | 0.961 | 0.857 | −5.332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zuo, S. Stability of Nitrogen-Doped Activated Carbon as an Electrocatalyst for the Oxygen Reduction Reaction in Various Storage Media. Molecules 2024, 29, 3611. https://doi.org/10.3390/molecules29153611
Zhang T, Zuo S. Stability of Nitrogen-Doped Activated Carbon as an Electrocatalyst for the Oxygen Reduction Reaction in Various Storage Media. Molecules. 2024; 29(15):3611. https://doi.org/10.3390/molecules29153611
Chicago/Turabian StyleZhang, Tao, and Songlin Zuo. 2024. "Stability of Nitrogen-Doped Activated Carbon as an Electrocatalyst for the Oxygen Reduction Reaction in Various Storage Media" Molecules 29, no. 15: 3611. https://doi.org/10.3390/molecules29153611





