One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening †
Abstract
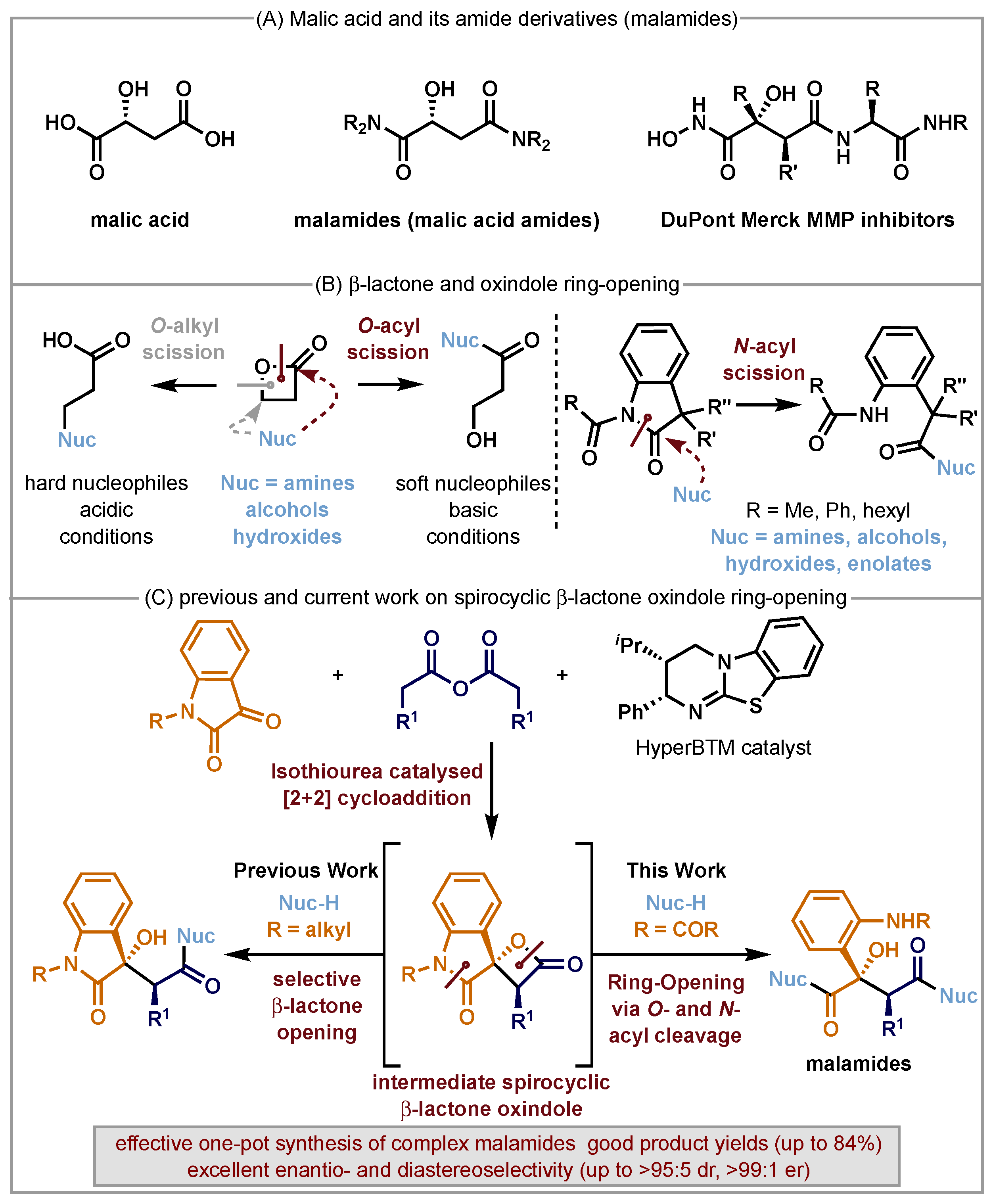
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- General procedure: The appropriate anhydride (1.5 equiv.), protected isatin (1.0 equiv.) and (2S,3R)-HyperBTM (5 mol%) were dissolved in anhydrous CH2Cl2 (0.04 M) and cooled to 0 °C. The reaction was started by the addition of iPr2NEt (1.25 equiv.) and was stirred at 0 °C for 3 h. Then, the appropriate amine (3.0 equiv.) was added, and the mixture was allowed to warm to room temperature to stir overnight. Once complete, the solvent was removed and reduced pressure and the crude reaction mixture was directly subjected to flash silica column chromatography.
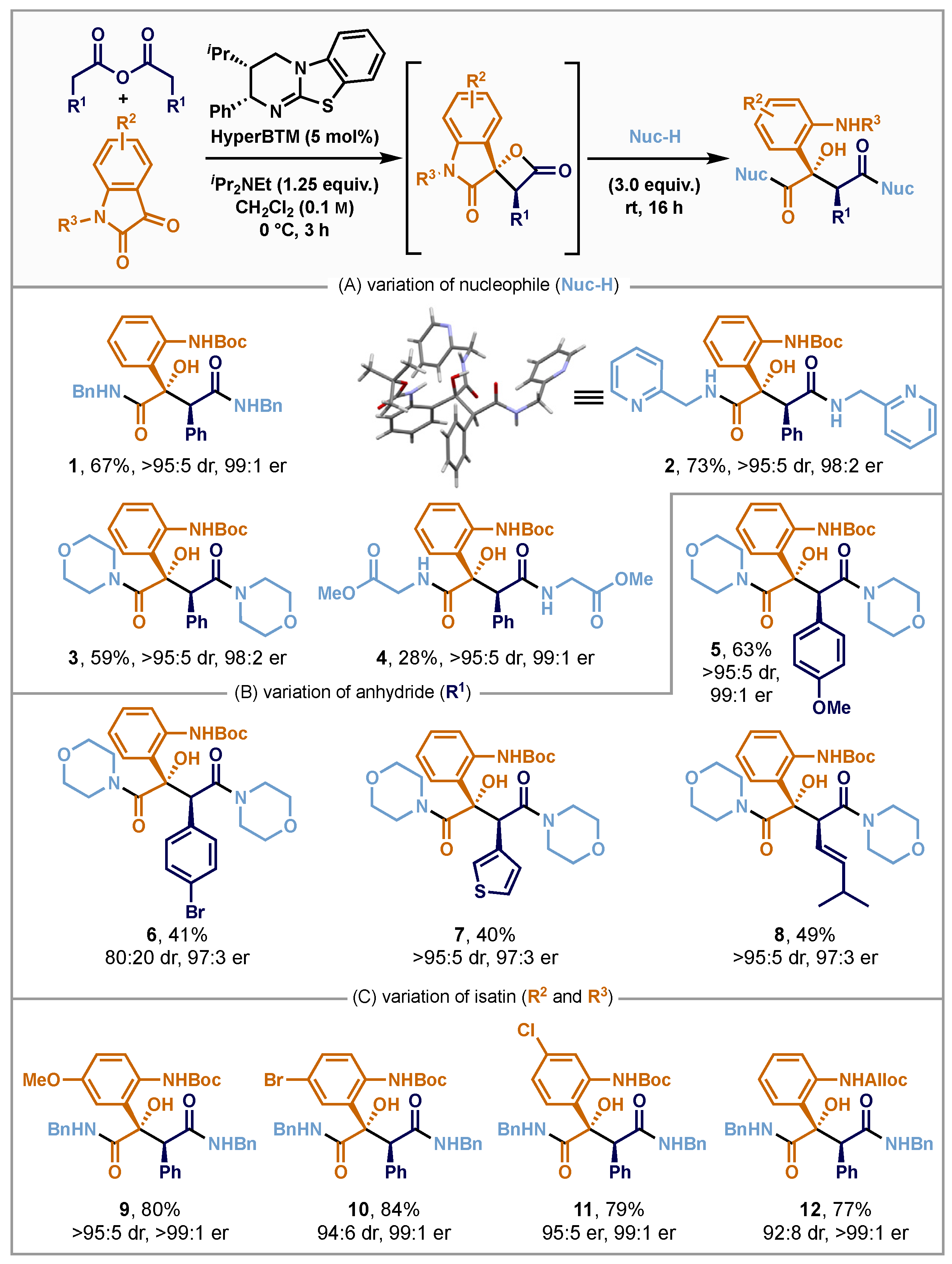
- Preparation of tert-butyl (2-((2S,3S)-1,4-bis(benzylamino)-2-hydroxy-1,4-dioxo-3-phenylbutan-2-yl)phenyl)carbamate (1): Following the general procedure, phenylacetic anhydride (95.3 mg, 0.38 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (61.8 mg, 0.25 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (3.9 mg, 0.012 mmol, 5 mol%) and iPr2NEt (54 μL, 0.31 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by benzylamine (82 μL, 0.75 mmol, 3.0 equiv.) gave crude product that was purified by flash silica column chromatography (hexane:EtOAc 90:10 to 40:60) to give 1 as a pale yellow glass (91 mg, 77%). −127.0 (c 2.3 in CHCl3); Chiral HPLC analysis (Supplementary Materials), Chiralpak IA (95:5 n-hexane: iPrOH, flow rate 2.0 mL·min−1, 211 nm and 40 °C) tR (2S,3S)-1 23.8 min, tR (2R,3R)-1 28.1 min and >99:1 er; IR νmax (film) 3397, 3316, 3088, 3065, 3030, 2978, 2928, 2249, 1717, 1651, 1639, 1587, 1522, 1497, 1452, 1441, 1391, 1366, 1308, 1231, 1157, 1121, 1045, 1026, 961, 908, 831 and 754; 1H NMR (400 MHz, CDCl3) δH: 1.46 (9H, s, OC(CH3)3), 4.25 (1H, dd, J 15.1, 5.4, NCH2), 4.35 (1H, dd, J 15.2, 5.5, NCH2), 4.47 (1H, dd, J 15.1, 6.2, NCH2), 4.50 (1H, dd, J 15.2, 6.1, NCH2), 4.73 (1H, br s, C(3)H), 6.86 (1H, dd, J 8.0, 7.2, ArH), 7.05 (2H, d, J 7.4, ArH), 7.11–7.39 (16H, m, ArH and OH), 7.54 (1H, d, J 8.0, ArH), 7.99 (1H, s, NH), 8.01 (1H, s, NH) and 9.15 (1H, br s, NH); 13C{1H} NMR (101 MHz, CDCl3) δC: 28.5 (OC(CH3)3), 43.5 (NCH2), 43.6 (NCH2), 55.8 (C(2)), 79.4 (OC(CH3)3), 85.0 (C(3)H), 122.0 (ArCH), 126.3 (ArCH), 127.3 (ArCH), 127.4 (ArCH), 127.4 (ArCH), 127.5 (ArCH), 128.0 (ArCH), 128.4 (ArCH), 128.7 (ArCH), 128.9 (ArCH), 129.3 (ArCH), 133.5 (ArC), 137.5 (ArC), 137.6 (ArC), 138.8 (ArC), 152.5 (C=O), 174.4 (C=O) and 175.2 (C=O); HRMS (ESI+) C25H37N3O5Na found 602.2622, requires 602.2625 (−0.5 ppm).
- Preparation of tert-butyl (2-((2S,3S)-2-hydroxy-1,4-dioxo-3-phenyl-1,4-bis((pyri-din-2-ylmethyl)amino)butan-2-yl)phenyl)carbamate (2): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and i-Pr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by 2-picolylamine (104 μL, 1.00 mmol, 3.0 equiv.), gave crude product that was purified by flash silica column chromatography (CH2Cl2:10% NH4OH in MeOH 99:1 to 93:7) to give 2 as a clear glass (142 mg, 73%). −138.5 (c 1.4 in CHCl3); Chiral HPLC analysis, Chiralpak IA (80:20 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 270 nm, 30 °C) tR (2R,3R)-2 15.9 min, tR (2S,3S)-2 18.5 min and 98:2 er; IR νmax (film) 3345, 2972, 1722, 1651, 1589, 1518, 1476, 1437, 1366, 1306, 1233, 1157, 1045, 1024 and 951; 1H NMR (500 MHz, CDCl3) δH: 1.50 (9H, s, OC(CH3)3), 4.48 (1H, dd, J 16.3, 4.9, NCH2), 4.52 (1H, dd, J 16.3, 5.1, NCH2), 4.60 (1H, dd, J 16.5, 5.3, NCH2), 4.67 (1H, dd, J 16.5, 5.9, NCH2), 4.76 (1H, s, C(3)H), 6.90 (1H, ddd, J 8.3, 7.2, 1.4, ArH), 7.01 (2H, m, ArH), 7.11–7.20 (9H, m, ArH), 7.23 (1H, d, J 7.8, ArH), 7.35 (1H, t, J 4.2, NH), 7.53 (1H, dd, J 8.1, 1.6, ArH), 7.59 (1H, ddd, J 7.9, 7.7, 1.9, ArH), 7.62 (1H, ddd, J 7.9, 7.8, 1.9, ArH), 7.92 (1H, br s, NH), 8.47 (1H, ddd, J 4.9, 1.8, 0.9, ArH), 8.52 (1H, ddd, J 4.9, 1.8, 1.0, ArH) and 9.22 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.5 (C(CH3)3), 44.4 (NCH2), 44.8 (NCH2), 79.4 (OC(CH3)3), 121.2 (ArCH), 121.6 (ArCH), 122.2 (ArCH), 122.4 (ArCH), 122.6 (ArC), 126.3 (ArCH), 127.8 (ArCH), 128.1 (ArCH), 128.7 (ArCH), 129.3 (ArCH), 133.5 (ArC), 136.7 (ArCH), 136.8 (ArCH), 138.9 (ArC), 148.9 (ArCH), 149.1 (ArCH), 155.4 (ArC), 156.5 (ArC), 174.8 (C=O) and 175.0 (C=O); HRMS (ESI+) C33H35N5O5Na found 604.2522, requires 604.2530 (−1.4 ppm).
- Preparation of tert-butyl (2-((2S,3S)-2-hydroxy-1,4-dimorpholino-1,4-dioxo-3-phenylbutan-2-yl)phenyl) carbamate (3): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by morpholine (87 μL, 1.0 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (CH2Cl2:10% NH4OH in MeOH 99:1 to 95:5) to give 3 as a clear glass (106 mg, 59%). −176.3 (c 0.4 in CHCl3); Chiral HPLC analysis, Chiralpak IA (98:2 n-hexane: iPrOH, flow rate 1.5 mL·min−1, 254 nm, 40 °C) tR (2S,3S)-3 10.9 min, tR (2R,3R)-3 21.3 min and 98:2 er; IR νmax (film) 3339, 2855, 1722, 1632, 1614, 1587, 1529, 1439, 1366, 1304, 1242, 1227, 1157, 1111 and 1045; 1H NMR (400 MHz, CDCl3) δH: 1.41 (9H, s, C(CH3)3), 2.98–3.06 (2H, m, OCH2), 3.26–3.33 (2H, m, NCH2), 3.44 (1H, ddd, J 11.4, 5.8, 2.9, OCH2), 3.49–3.67 (7H, m, NCH2 and OCH2), 3.71–3.86 (4H, m, NCH2 and OCH2), 4.41 (1H, s, C(3)H), 6.82 (2H, d, J 7.1, ArH), 6.95 (1H, ddd, J 8.1, 7.2, 1.3, ArH), 7.08 (1H, dd, J 7.9, 1.6, ArH), 7.13–7.22 (4H, m, ArH), 8.03 (1H, d, J 8.4, ArH), 8.63 (1H, s, OH) and 8.91 (1H, s, NH); 13C{1H} NMR (101 MHz, CDCl3) δC: 28.4 (C(CH3)3), 42.3 (NCH2), 43.5 (NCH2), 46.2 (NCH2), 47.2 (NCH2), 55.1 (C(3)H), 66.0 (OCH2), 66.5 (OCH2), 66.6 (OCH2), 79.2 (OC(CH3)3), 86.6 (C(2)), 119.2 (ArCH), 121.4 (ArCH), 123.0 (ArC), 125.7 (ArCH), 128.0 (ArCH), 128.3 (ArCH), 128.9 (ArCH), 129.6 (ArCH), 132.6 (ArC), 139.3 (ArC), 151.9 (C=O), 171.8 (C=O) and 174.5 (C=O); HRMS (ESI+) C29H37N3O5Na found 562.2515, requires 562.2524 (−1.6 ppm).
- Preparation of dimethyl 2,2’-(((2S,3S)-2-(2-((tert-butoxycarbonyl)amino)phenyl)-2-hydroxy-3-phenylsuccinyl)bis(azanediyl))diacetate (4): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by glycine methyl ester hydrochloride (126 mg, 1.00 mmol, 3.0 equiv.) and iPr2NEt (170 μL, 1.00 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 100:0 to 0:100) to give 4 as a colourless glass (50 mg, 28%). −106.9 (c 1.5 in CHCl3); Chiral HPLC analysis, Chiralpak IA (70:30 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 254 nm, 30 °C) tR (2S,3S)-4 6.7 min, tR (2R,3R)-4 12.7 min and 99:1 er; IR νmax (film) 3335, 2978, 1749, 1728, 1655, 1589, 1526, 1443, 1368, 1308, 1234, 1209, 1163, 1047, 1026 and 756; 1H NMR (500 MHz, CDCl3) δH: 1.46 (9H, s, OC(CH3)3), 3.69 (3H, s, OCH3), 3.70 (3H, s, OCH3), 3.92–4.08 (4H, m, NCH2), 4.64 (1H, br s, OH), 6.72 (1H, br s, NH), 6.84 (1H, ddd, J 8.2, 7.2, 1.3, ArH), 6.94–7.00 (2H, m, ArH), 7.09–7.19 (4H, m, ArH), 7.37 (1H, br s, ArH), 7.42 (1H, dd, J 8.1, 1.5, ArH), 7.89 (1H, br s, NH) and 9.01 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.6 (C(CH3)3), 41.5 (NCH2), 41.7 (NCH2), 52.4 (OCH3), 52.5 (OCH3), 79.5 (OC(CH3)3), 120.9 (ArC), 122.2 (ArCH), 126.4 (ArCH), 128.1 (ArCH), 128.2 (ArCH), 128.9 (ArCH), 129.4 (ArCH), 133.0 (ArCH), 138.5 (ArC), 152.6 (ArC), 169.5 (C=O), 169.6 (C=O), 174.7 (C=O) and 175.2 (C=O); HRMS (ESI+) C27H33N3O9Na found 566.2108, requires 566.2109 (−0.2 ppm).
- Prepration of tert-butyl (2-((2S,3S)-2-hydroxy-3-(p-anisyl)-1,4-dimorpholino-1,4-dioxobutan-2-yl) phenyl)carbamate (5): Following the general procedure, p-anisylacetic anhydride (157 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by morpholine (87 μL, 1.0 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 70:30 to 20:80) to give 5 as clear glass (120 mg, 63%). −108.0 (c 1.4 in CHCl3); Chiral HPLC analysis, Chiralpak IA (98:2 n-hexane: iPrOH, flow rate 1.5 mL·min−1, 254 nm, 40 °C) tR (2S,3S)-5 12.3 min, tR (2R,3R)-5 28.1 min and 99:1 er; IR νmax (film) 3337, 2972, 2856, 1722, 1632, 1611, 1589, 1512, 1439, 1366, 1302, 1242, 1159, 1113, 1034 and 910; 1H NMR (400 MHz, CDCl3) δH: 1.41 (9H, s, C(CH3)3), 2.99 (1H, ddd, J 10.9, 7.5, 2.8, OCH2), 3.05 (1H, ddd, J 11.1, 7.81, 2.9, OCH2), 3.24–3.34 (2H, m, NCH2), 3.40–3.64 (10H, m, NCH2 and OCH2), 3.72 (3H, s, OCH3), 3.74–3.86 (2H, m, NCH2), 4.35 (1H, s, C(3)H), 6.64–6.74 (4H, m, ArH), 6.94 (1H, ddd, J 8.1, 7.2, 1.3, ArH), 7.07 (1H, dd, J 7.9, 1.5, ArH), 7.14–7.22 (1H, m, ArH), 8.07 (1H, d, J 8.5, ArH), 8.52 (1H, s, OH) and 8.91 (1H, s, NH); 13C{1H} NMR (101 MHz, CDCl3) δC: 28.3 (C(CH3)3), 42.3 (NCH2), 43.5 (NCH2), 46.2 (NCH2), 47.2 (NCH2), 54.3 (C(3)H), 55.0 (OCH3), 66.0 (OCH2), 66.1 (OCH2), 66.5 (OCH2), 66.6 (OCH2), 79.2 (OC(CH3)3), 86.6 (C(2)), 113.8 (ArCH), 119.2 (ArCH), 121.4 (ArCH), 123.2 (ArC), 124.3 (ArC), 125.8 (ArCH), 128.9 (ArCH), 130.7 (ArCH), 139.4 (ArC), 152.1 (C=O), 159.1 (ArC), 171.9 (C=O) and 174.8 (C=O); HRMS (ESI+) C30H39N3O8Na found 592.2624, requires 592.2629 (−0.9 ppm).
- Preparation of tert-butyl (2-((2S,3S)-1,4-bis(benzylamino)-3-(4-bromophenyl)-2-hydroxy-1,4-dioxobutan-2-yl)phenyl)carbamate (6): Following the general procedure, 2-(4-bromophenyl)acetic anhydride (206 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M) followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.) gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 100:0 to 70:30) to give 6 as an inseparable mixture of anti and syn diastereoisomers (80:20 dr) (88 mg, 41%) as a pale yellow glass. −56.2 (c 1.3 in CHCl3); Chiral HPLC analysis, Chiralpak AD-H (70:30 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 211 nm, 30 °C) tR (2R,3R)-6 9.7 min, tR (2S,3S)-6 19.7 min and 97:3 er; tR (2S,3R)-6 11.4 min, tR (2R,3S)-6 15.0 min and 77:23 er; IR νmax (film) 3312, 3030, 2976, 2930, 2357, 2320, 1717, 1647, 1587, 1522, 1489, 1443, 1366, 1308, 1234, 1161, 1047, 1026 and 1013; HRMS () C35H36O5N3BrNa found 680.1712, requires 680.1731 (−2.8 ppm); NMR data for major diastereoisomer: 1H NMR (500 MHz, CDCl3) δH: 1.46 (9H, s, C(CH3)3), 4.16–4.53 (4H, m, CH2N), 4.66 (1H, br s, OH), 6.77–6.90 (4H, m, ArH), 7.06–7.29 (14H, m, ArH), 7.99 (2H, br s, 2 NH) and 9.12 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.6 (C(CH3)3), 43.6 (CH2N), 43.6 (CH2N), 55.4 (C(3)H), 79.7 (C(CH3)3), 86.6 (C(2)), 122.3 (ArCH), 126.2 (ArCH), 127.1 (ArCH), 127.3 (ArCH), 127.3 (ArCH), 127.4 (ArCH), 127.6 (ArCH), 127.7 (ArCH), 128.7 (ArCH), 128.8 (ArCH), 129.3 (ArC), 131.0 (ArCH), 131.3 (ArCH), 131.7(ArC), 132.0 (ArC), 132.7 (ArC), 136.9 (ArC), 137.3 (ArC), 137.5 (ArC), 152.5 (C=O), 174.4 (C=O) and 174.8 (C=O); NMR data for minor diastereoisomer: 1H NMR (500 MHz, CDCl3) (selected) δH: 1.52 (9H, s, C(CH3)3), 3.81–3.88 (1H, m, NCH2), 4.00–4.07 (1H, m, NCH2), 4.97–4.97 (1H, m, NCH2), 6.57–6.62 (2H, m, ArH), 7.34–7.39 (1H, m, ArH), 7.65–7.70 (1H, m, ArH), 7.74–7.78 (1H, m, ArH) and 10.03 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) (selected) δC: 28.7 (C(CH3)3), 43.1 (CH2N), 43.7 (CH2N), 80.4 (C(CH3)3), 86.6 (C(2)), 122.7 (ArCH), 125.1 (ArCH), 128.7 (ArCH), 133.1 (ArC), 136.2 (ArC), 155.0 (C=O), 171.8 (C=O) and 172.4 (C=O).
- Preparation of tert-butyl (2-((2S,3S)-2-hydroxy-1,4-dimorpholino-1,4-dioxo-3-(thiophen-3-yl)butan-2-yl)phenyl) carbamate (7): Following the general procedure, (thiophen-3-yl)acetic anhydride (133 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by morpholine (87 μL, 1.0 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 50:50 to 10:90) to give 7 as clear glass (73 mg, 40%). −151.8 (c 0.9 in CHCl3); Chiral HPLC analysis, Chiralpak IA (98:2 n-hexane: iPrOH, flow rate 1.5 mL·min−1, 211 nm, 40 °C) tR (2S,3S)-7 13.1 min, tR (2R,3R)-7 26.5 min and 98:2 er; IR νmax (film) 3335, 2857, 1721, 1632, 1614, 1587, 1530, 1439, 1365, 1233, 1157, 1111, 1045 and 995; 1H NMR (400 MHz, CDCl3) δH: 1.45 (9H, s, OC(CH3)3), 3.00 (1H, ddd, J 10.8, 7.4, 2.8, OCH2), 3.12 (1H, ddd, J 11.1, 7.8, 2.9, OCH2), 3.26–3.36 (2H, m, NCH2), 3.41–3.46 (1H, m, OCH2), 3.50–3.63 (6H, m, NCH2 and OCH2), 3.67–3.87 (5H, m, NCH2 and OCH2), 4.55 (1H, s, C(3)H), 6.43 (1H, dd, J 5.0, 1.3, ArH), 6.82 (1H, dd, J 3.1, 1.3, ArH), 6.95 (1H, ddd, J 8.1, 7.2, 1.3, ArH), 7.05 (1H, dd, J 7.9, 1.5, ArH), 7.12 (1H, dd, J 4.9, 3.0, ArH), 7.21 (1H, ddd, J 8.5, 7.1, 1.5, ArH), 8.12 (1H, d, J 8.4, ArH), 8.66 (1H, s, OH) and 9.06 (1H, s, NH); 13C{1H} NMR (101 MHz, CDCl3) δC: 28.4 (C(CH3)3), 42.3 (NCH2), 43.5 (NCH2), 46.3 (NCH2), 47.1 (NCH2), 50.5 (C(3)H), 66.0 (OCH2), 66.1 (OCH2), 66.5 (OCH2), 66.6 (OCH2), 79.4 (OC(CH3)3), 86.3 (C(2)), 119.3 (ArCH), 121.5 (ArCH), 123.3 (ArC), 124.5 (ArCH), 125.4 (ArCH), 125.6 (ArCH), 128.3 (ArCH), 129.0 (ArCH), 132.7 (ArC), 139.4 (ArC), 152.3 (C=O), 171.6 (C=O) and 174.6 (C=O); HRMS (ESI+) C27H35N3O7SNa found 568.2083, requires 568.2088 (−0.9 ppm).
- Preparation of tert-butyl (2-((2S,3S,E)-1-(benzylamino)-3-(benzylcarbamoyl)-2-hydroxy-6-methyl-1-oxohept-4-en-2-yl)phenyl)carbamate (8): Following the general procedure, (E)-5-methylhex-3-enoic anhydride (119 mg, 0.50 mmol, 1.5 equiv.), tert- butyl 2,3-dioxoindoline-1-carboxylate (81.6 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M) followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.) gave crude product that was purified by flash silica column chromatography (hexane:EtOAc 100:0 to 70:30) to give 8 (93 mg, 49%) as a colourless solid. mp 80–82 °C; −89.7 (c 0.7 in CHCl3); Chiral HPLC analysis, Chiralpak IA (97:3 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 211 nm, 40 °C) tR (2S,3S)-8 30.6 min, tR (2R,3R)-8 34.4 min and 97:3 er; IR νmax (film) 3312, 2961, 2928, 2359, 2344, 1732, 1717, 1636, 1589, 1558, 1522, 1437, 1364, 1306, 1234, 1161, 1045, 1026 and 972; 1H NMR (400 MHz, CDCl3) δH: 0.75 (3H, d, J 6.7, CHCH3), 0.80 (3H, d, J 6.8, CHCH3), 1.53 (9H, s, C(CH3)3), 2.06–2.16 (1H, m, CH(CH3)2), 4.08–4.17 (1H, m, C(3)H), 4.25–4.38 (2H, m, CHAHBN and CHAHBN), 4.46 (1H, dd, J 15.1, 6.3, CHAHBN), 4.56 (1H, dd, J 15.1, 6.3, CHAHBN), 5.34 (1H, dd, J 15.5, 6.5, C(5)H), 5.45 (1H, dd, J 15.5, 8.7, C(4)H), 6.68 (1H, br s, NH), 6.93 (1H, t, J 7.4, ArH), 7.11–7.16 (2H, m, ArH), 7.21–7.36 (9H, m, ArH), 7.40 (1H, br s, NH), 7.57 (2H, d, J 8.1, ArH), 8.07 (1H, br s, OH) and 9.76 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 21.8 (CHCH3), 22.1 (CHCH3), 28.6 (C(CH3)3), 31.2 (CH(CH3)2), 43.4 (CH2N), 43.5 (CH2N), 53.6 (C(3)H), 79.9 (C(CH3)3), 83.1 (C(2)OH), 119.4 (C(4)H), 121.7 (ArC), 122.6 (2 × ArCH), 126.4 (ArCH), 127.3 (ArCH), 127.4 (ArCH), 127.5 (ArCH), 127.7 (ArCH), 128.6 (ArCH), 128.7 (ArCH), 128.8 (ArCH), 137.5 (ArC), 137.6 (ArC), 137.9 (ArC), 144.5 (C(5)H), 153.2 (C=O), 174.6 (C=O) and 175.5 (C=O); HRMS () C34H41O5N3Na found 594.2928, requires 594.2938 (−1.7 ppm).
- Preparation of tert-butyl (2-((2S,3S)-1,4-bis(benzylamino)-2-hydroxy-1,4-dioxo-3-phenylbutan-2-yl)-4-methoxyphenyl)carbamate (9): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 5-methoxy-2,3-dioxoindoline-1-carboxylate (91.5 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M) followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.) gave crude product that was purified by flash silica column chromatography (hexane:EtOAc 95:5 to 50:50) to give 9 (160 mg, 80%) as a colourless solid. mp 78–80 °C; −116.8 (c 1.0 in CHCl3); Chiral HPLC analysis, Chiralpak IA (90:10 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 211 nm, 30 °C) tR (2S,3S)-9 29.0 min, tR (2R,3R)-9 46.5 min, >99:1 er; IR νmax (film) 3318, 3063, 3030, 2976, 2932, 1719, 1653, 1522, 1454, 1412, 1366, 1288, 1227, 1163, 1042, 1026 and 810; 1H NMR (400 MHz, CDCl3) δH: 1.45 (9H, s, C(CH3)3), 3.63 (3H, s, OCH3), 4.25 (1H, dd, J 15.1, 5.4, CHAHBN), 4.34 (1H, dd, J 15.1, 5.5, CHAHBN), 4.40–4.53 (2H, m, CH2N), 4.76 (1H, br s, OH), 6.72 (1H, dd, J 9.1, 3.0, ArH), 7.05–7.19 (10H, m, ArH), 7.19–7.29 (7H, m, ArH), 7.29–7.38 (1H, m, NH), 7.81 (1H, br s, NH) and 8.95 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.6 (C(CH3)3), 43.5 (CH2N), 43.5 (CH2N), 55.4 (OCH3), 77.4 (CH), 79.3 (C(CH3)3), 83.6 (COH), 112.0 (ArCH), 113.7 (ArCH), 122.4 (ArC), 127.3 (ArCH), 127.4 (ArCH), 127.5 (ArCH), 127.5 (ArCH), 128.0 (ArCH), 128.2 (ArCH), 128.7 (ArCH), 128.7 (ArCH), 129.4 (ArCH), 131.4 (ArC), 133.5 (ArC), 137.4 (ArC), 137.6 (ArC), 152.9 (C=O), 154.8 (ArC), 174.6 (C=O) and 175.1 (C=O); HRMS () C36H39O6N3Na found 632.2721, requires 632.2731 (−1.6 ppm).
- Preparation of tert-butyl (2-((2S,3S)-1,4-bis(benzylamino)-2-hydroxy-1,4-dioxo-3-phenylbutan-2-yl)-4-bromophenyl)carbamate (10): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 5-bromo-2,3-dioxoindoline-1-carboxylate (108 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 100:0 to 70:30) to give 10 as an inseparable mixture of anti and syn diastereoisomers (94:6 dr) (182 mg, 84%) and as a colourless solid. mp 77–79 °C; −88.4 (c 0.9 in CHCl3); Chiral HPLC analysis, Chiralpak IA (90:10 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 211 nm, 30 °C) tR (2S,3S)-10 12.2 min, tR (2R,3R)-10 30.7 min and 99:1 er; IR νmax (film) 3316, 3030, 2978, 2928, 2357, 2320, 1717, 1647, 1454, 1393, 1366, 1300, 1233, 1161, 1049, 1026 and 824; 1H NMR (500 MHz, CDCl3) δH: 1.44 (9H, s, C(CH3)3), 4.21–4.44 (3H, m, CH2N), 4.51 (1H, dd, J 15.1, 6.2, CHAHBN), 4.67 (1H, br s, C(3)H), 7.04–7.33 (17H, m, ArH), 7.68 (1H, d, J 2.4, ArH), 7.90 (1H, br s, NH), 8.07 (1H, br s, NH) and 9.03 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.5 (C(CH3)3), 43.5 (CH2N), 43.6 (CH2N), 56.5 (C(3)H), 79.7 (C(CH3)3), 84.9 (COH), 114.8 (ArC), 121.3 (ArCH), 127.4 (2 × ArCH), 127.6 (ArCH), 127.6 (ArCH), 128.2 (ArCH), 128.4 (ArCH), 128.7 (ArCH), 128.8 (ArCH), 129.4 (ArCH), 131.8 (ArC), 133.2 (ArC), 137.3 (ArC), 137.5 (ArC), 152.2 (C=O), 174.0 (C=O) and 175.0 (C=O); HRMS () C35H36O5N3BrNa found 680.1723, requires 680.1731 (−1.2 ppm).
- Preparation of tert-butyl (2-((2S,3S)-1,4-bis(benzylamino)-2-hydroxy-1,4-dioxo-3-phenylbutan-2-yl)-5-chlorophenyl)carbamate (11): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), tert-butyl 6-chloro-2,3-dioxoindoline-1-carboxylate (93.0 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M), followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 100:0 to 70:30) to give 11 as an inseparable mixture of anti and syn diastereoisomers (95:5 dr) (160 mg, 79%) and as a colourless solid. mp 83–85 °C; −109.0 (c 0.7 in CHCl3); Chiral HPLC analysis, Chiralpak ID (90:10 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 254 nm, 30 °C) tR (2S,3S)-11 10.1 min, tR (2R,3R)-11 28.6 min and 99:1 er; IR νmax (film) 3319, 3063, 3030, 2978, 2930, 1730, 1717, 1653, 1578, 1522, 1454, 1414, 1366, 1281, 1233, 1161, 1051, 1028, 860; 1H NMR (500 MHz, CDCl3) δH: 1.46 (9H, s, C(CH3)3), 4.27 (1H, dd, J 15.0, 5.5, CHAHBN), 4.36 (1H, dd, J 15.1, 5.7, CHAHBN), 4.41–4.52 (2H, m, CH2N), 4.61 (1H, br s, CH), 6.83 (1H, dd, J 8.6, 2.2, ArH), 6.99–7.07 (2H, m, ArH), 7.11–7.18 (4H, m, ArH), 7.18–7.36 (10H, m, ArH), 7.44–7.50 (1H, m, ArH), 8.12 (2H, br s, NH) and 9.14 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 28.5 (C(CH3)3), 43.5 (CH2N), 43.6 (CH2N), 56.4 (CH), 79.8 (C(CH3)3), 84.8 (COH), 121.8 (ArCH), 127.3 (ArCH), 127.4 (ArCH), 127.6 (ArCH), 127.6 (ArCH), 128.2 (ArCH), 128.4 (ArCH), 128.7 (2 ArCH), 129.3 (ArCH), 133.2 (ArC), 134.8 (ArC), 137.3 (ArC), 137.5 (ArC), 140.2 (ArC), 152.1 (C=O), 174.0 (C=O) and 175.0 (C=O); HRMS () C35H36O5N3ClNa found 636.2232, requires 636.2236 (−0.6 ppm).
- Preparation of allyl (2-((2S,3S)-1,4-bis(benzylamino)-2-hydroxy-1,4-dioxo-3-phenylbutan-2-yl)phenyl)carbamate (12): Following the general procedure, phenylacetic anhydride (127 mg, 0.50 mmol, 1.5 equiv.), allyl 2,3-dioxoindoline-1-carboxylate (76.3 mg, 0.33 mmol, 1.0 equiv.), (2S,3R)-HyperBTM (5.1 mg, 0.017 mmol, 5 mol%) and iPr2NEt (75 μL, 0.43 mmol, 1.3 equiv.) in CH2Cl2 (0.04 M) followed by benzylamine (109 μL, 1.00 mmol, 3.0 equiv.), gave a crude product that was purified by flash silica column chromatography (hexane:EtOAc 90:10 to 70:30) to give 12 as an inseparable mixture of anti and syn diastereoisomers (92:8 dr) (143 mg, 77%) and as a colourless solid. mp 73–75 °C; −115.6 (c 1.0 in CHCl3); Chiral HPLC analysis, Chiralpak ID (60:40 n-hexane: iPrOH, flow rate 1.0 mL·min−1, 254 nm, 40 °C) tR (2S,3S)-12 6.4 min, tR (2R,3R)-12 12.3 min, and >99:1 er; IR νmax (film) 3316, 3063, 3030, 1732, 1717, 1647, 1589, 1526, 1447, 1308, 1219 and 1045; 1H NMR (400 MHz, CDCl3) δH: 4.26 (1H, dd, J 15.2, 5.5, CHAHBN), 4.33 (1H, dd, J 15.2, 5.9, CHAHBN), 4.40–4.58 (4H, m, CH2O and CH2N), 4.72 (1H, br s, CH), 5.22–5.28 (1H, m, CH=CHAHB), 5.31–5.38 (1H, m, CH=CHAHB), 5.88–6.02 (1H, m, CH=CH2), 6.87–6.93 (1H, m, ArH), 7.00–7.06 (2H, m, ArH), 7.11–7.31 (14H, m, ArH), 7.33–7.46 (1H, m, NH), 7.52–7.59 (1H, m, ArH), 7.89–7.96 (1H, m, ArH), 8.09 (1H, br s, NH) and 9.38 (1H, br s, NH); 13C{1H} NMR (126 MHz, CDCl3) δC: 43.4 (CH2N), 43.5 (CH2N), 56.2 (CH), 65.3 (CH2O), 85.0 (COH), 117.6 (CH=CH2), 120.6 (ArCH), 122.6 (ArCH), 126.3 (ArC), 127.3 (ArCH), 127.4 (ArCH), 127.5 (ArCH), 127.5 (ArCH), 127.8 (ArCH), 128.3 (ArCH), 128.7 (2 ArCH), 129.0 (ArCH), 129.3 (ArCH), 133.0 (CH=CH2), 133.5 (ArC), 137.4 (ArC), 137.6 (ArC), 152.9 (C=O), 174.3 (C=O) and 175.1 (C=O); HRMS () C34H33O5N3Na found 586.2306, requires 586.2312 (−1.1 ppm).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klimek-Szzykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Ghai, M.; Agnihotri, N.; Kumar, V.; Agnihotri, R.; Kumar, A.; Sahu, K. Global organic acids production and their industrial applications. Phys. Sci. Rev. 2023. [Google Scholar] [CrossRef]
- Zou, X.; Cheng, C.; Feng, J.; Song, X.; Lin, M.; Yang, S.-T. Biosynthesis of polymalic acid in fermentation: Advances and prospects for industrial application. Crit. Rev. Biotechnol. 2019, 39, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, B.; Selvakumari, I.A.E.; Jayamuthunagai, J.; Kumar, R.P.; Varjani, S.; Pandey, A.; Gnansounou, E. Biochemical conversion of biodiesel by-product into malic acid: A way towards sustainability. Sci. Total Environ. 2020, 709, 136206. [Google Scholar] [CrossRef]
- Luo Shipeng, H.P. Malic acid—A Versatile Chiral Building Block in the Enantioselective Total Synthesis of Natural Products and in Synthetic Methodologies. Prog. Chem. 2020, 32, 1846–1868. [Google Scholar]
- Wu, D.; Chang, H.; Wang, Y.; Xin, M. Isolation, structure determination and antibacterial activities of succinamide conjugate diacid from Acinetobacter sp. BJ-L. Microbiol. Res. 2011, 166, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, J.-J.; Linget-Morice, C.; Hoh, F.; Collinson, S.K.; Kyslík, P.; Page, W.J.; Dell, A.; Abdallah, M.A. Bacterial siderophores: Structure elucidation, and 1H, 13C and 15N two-dimensional NMR assignments of azoverdin and related siderophores synthesized by Azomonas macrocytogenes ATCC 12334. Biometals 1996, 9, 107–120. [Google Scholar] [CrossRef]
- Michalke, R.; Taraz, K.; Budzikiewiez, H. Azoverdin -an Isopyoverdin. Z. Naturforsch. C 1996, 51, 772–780. [Google Scholar] [CrossRef]
- Demange, P.; Bateman, A.; Mertz, C.; Dell, A.; Piemont, Y.; Abdallah, M.A. Bacterial siderophores: Structures of pyoverdins Pt, siderophores of Pseudomonas tolaasii NCPPB 2192, and pyoverdins Pf, siderophores of Pseudomonas fluorescens CCM 2798. Identification of an unusual natural amino acid. Biochemistry 1990, 29, 11041–11051. [Google Scholar] [CrossRef]
- Buyer, J.S.; Wright, J.M.; Leong, J. Structure of pseudobactin A214, a siderophore from a bean-deleterious Pseudomonas. Biochemistry 1986, 25, 5492–5499. [Google Scholar] [CrossRef]
- Sudo, M.; Sakamoto, H. Pharmaceutical Agents for Treating HCV Infections. US008183005B1, 22 May 2012. [Google Scholar]
- Sato, M.; Wada, H.; Amada, H. Tetraamine Derivative. JP2006008533A, 2006. [Google Scholar]
- Moreno, M.; Sani, M.; Raos, G.; Meille, S.V.; Belotti, D.; Giavazzi, R.; Bellosta, S.; Volonterio, A.; Zanda, M. Stereochemically pure α-trifluoromethyl-malic hydroxamates: Synthesis and evaluation as inhibitors of matrix metalloproteinases. Tetrahedron 2006, 62, 10171–10181. [Google Scholar] [CrossRef]
- Nonoyama, K.; Mori, W.; Nakajima, K.; Nonoyama, M. Copper(II) complexes of optically active binucleating ligands, N,N′-bis [2-pyridylmethyl and 2-(2-pyridyl)ethyl]-(S)-malamide and related amides. Polyhedron 1997, 16, 3815–3826. [Google Scholar] [CrossRef]
- Cohen, S.M.; Jacobson, F.E.; Lewis, J.A. Metalloprotein Inhibitors Containing Nitrogen Based Ligands. US20120135959A1, 31 May 2012. [Google Scholar]
- Alpegiani, M.; Palladino, M.; Corigli, R.; Jabes, D.; Perrone, E.; Abrate, F.; Bissolino, P.; Lombroso, M. Matrix Metalloproteinase Inhibitors. WO 98/33788, 6 August 1998. [Google Scholar]
- Jacobson, I.C.; Reddy, P.G.; Wasserman, Z.R.; Hardman, K.D.; Covington, M.B.; Arner, E.C.; Copeland, R.A.; Decicco, C.P.; Magolda, R.L. Structure-based design and synthesis of a series of hydroxamic acids with a quaternary-hydroxy group in P1 as inhibitors of matrix metalloproteinases. Bioorg. Med. Chem. Lett. 1998, 8, 837–842. [Google Scholar] [CrossRef]
- Jeanpetit, C.; Pringent, D.; Settembre, P.-A.; Trancart, M.-M. Derives D’Amino-Acides Inhibiteurs des Metalloproteases de la Matrice Extracellulaire et de la Liberation du TNF. WO 98/47863, 29 October 1998. [Google Scholar]
- Jacobson, I.C.; Decicco, C.P.; Cherney, R.J. Hydroxamic and Carbocyclic Acids as Metalloprotease Inhibitors. US005691381A, 25 November 1997. [Google Scholar]
- Jacobson, I.C.; Decicco, C.P.; Cherney, R.J. Hydroxamic and Carboxlic Acids as Metalloprotease Inhibitors. WO 96/33166, 24 October 1996. [Google Scholar]
- Wiles, J.A.; Karra, S.; Grau, D.; Ray, S. Mannose 6-Phosphate or ASGPR Receptor Binding Compounds for the Degradation of Extracellular Proteins. WO2023028338, 2 March 2023. [Google Scholar]
- Molteni, M.; Pesenti, C.; Sani, M.; Volonterio, A.; Zanda, M. Fluorinated peptidomimetics: Synthesis, conformational and biological features. J. Fluorine Chem. 2004, 125, 1735–1743. [Google Scholar] [CrossRef]
- Sani, M.; Belotti, D.; Giavazzi, R.; Panzeri, W.; Volonterio, A.; Zanda, M. Synthesis and evaluation of stereopure α-trifluoromethyl-malic hydroxamates as inhibitors of matrix metalloproteinases. Tetrahedron Lett. 2004, 45, 1611–1615. [Google Scholar] [CrossRef]
- Böttcher, T.; Sieber, S.A. β-Lactams and β-lactones as activity-based probes in chemical biology. MedChemComm 2012, 3, 408–417. [Google Scholar] [CrossRef]
- Atta, H.; Alzahaby, N.; Hamdy, N.M.; Emam, S.H.; Sonousi, A.; Ziko, L. New trends in synthetic drugs and natural products targeting 20S proteasomes in cancers. Bioorg. Chem. 2023, 133, 106427. [Google Scholar] [CrossRef]
- Groll, M.; Potts, B.C. Proteasome Structure, Function, and Lessons Learned from Beta-Lactone Inhibitors. Curr. Top. Med. Chem. 2011, 11, 2850–2878. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef]
- Tomoda, H.; Omura, S. Lactacystin, a Proteasome Inhibitor: Discovery and its Application in Cell Biology. Yakugaku Zasshi 2000, 120, 935–949. [Google Scholar] [CrossRef]
- Fenteany, G.; Standaert, R.F.; Lane, W.S.; Choi, S.; Corey, E.J.; Schreiber, S.L. Inhibition of Proteasome Activities and Subunit-Specific Amino-Terminal Threonine Modification by Lactacystin. Science 1995, 268, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, Y.; Kasten, K.; Dunne, J.; Hartley, W.C.; Young, C.M.; Cordes, D.B.; Slawin, A.M.Z.; Ng, S.; Smith, A.D. Isothiourea-Catalyzed [2 + 2] Cycloaddition of C(1)-Ammonium Enolates and N-Alkyl Isatins. Org. Lett. 2022, 24, 5444–5449. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Shirai, T.; Yatsuzuka, K.; Shirai, R. Hydrolytic dynamic kinetic resolution of racemic 3-phenyl-2-oxetanone to chiral tropic acid. RSC Adv. 2024, 14, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liang, Z.-Q.; Chen, K.-Q.; Dai, L.; Ye, S. Enantioselective N-heterocyclic carbene-catalyzed rearrangement of enol ε-lactones. Org. Chem. Front. 2023, 10, 799–805. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Wang, Y.-N.; Li, S.-J.; Wei, D.; Lan, Y. Origins of Catalyst-Controlled Chemoselectivity in Transition-Metal-Catalyzed Divergent Epoxide Conversion. ACS Catal. 2023, 13, 7616–7626. [Google Scholar] [CrossRef]
- Atkin, L.; Robertson, A.; White, J.M.; Rizzacasa, M.A. Total Synthesis of Viridiofungins A and B. Org. Lett. 2021, 23, 3557–3560. [Google Scholar] [CrossRef]
- Fung, J.; Duong, T.-V.; Braceros, K.C.A.; Brooks, M.L.; Schloesser-Lingscheit, K.; Tagawa, T.K.S.; Wilson, J.M.; Jones, K.K.; Valente, E.J. Arylpyran Pseudoacid Racemization: Rate Estimation and Structural Influences. J. Chem. Crystallogr. 2021, 51, 14–41. [Google Scholar] [CrossRef]
- Barrios Antúnez, D.-J.; Greenhalgh, M.D.; Brueckner, A.C.; Walden, D.M.; Elías-Rodríguez, P.; Roberts, P.; Young, B.G.; West, T.H.; Slawin, A.M.Z.; Ha-Yeon Cheong, P.; et al. Catalytic enantioselective synthesis of perfluoroalkyl-substituted β-lactones via a concerted asynchronous [2 + 2] cycloaddition: A synthetic and computational study. Chem. Sci. 2019, 10, 6162–6173. [Google Scholar] [CrossRef]
- Khalil, A.; Cammas-Marion, S.; Coulembier, O. Organocatalysis applied to the ring-opening polymerization of β-lactones: A brief overview. J. Polym. Sci. A Polym. Chem. 2019, 57, 657–672. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, F.; Liao, M.; Hu, R.; Tang, B.Z. Room temperature multicomponent polymerizations of alkynes, sulfonyl azides, and N-protected isatins toward oxindole-containing poly(N-acylsulfonamide)s. Polym. Chem. 2018, 9, 1674–1683. [Google Scholar] [CrossRef]
- Malapit, C.A.; Caldwell, D.R.; Sassu, N.; Milbin, S.; Howell, A.R. Pd-Catalyzed Acyl C–O Bond Activation for Selective Ring-Opening of α-Methylene-β-lactones with Amines. Org. Lett. 2017, 19, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mukherjee, S.; Das, T.K.; Gonnade, R.; Biju, A.T. N-Heterocyclic Carbene-Catalyzed Aldol-Lactonization of Ketoacids via Dynamic Kinetic Resolution. ACS Catal. 2017, 7, 3995–3999. [Google Scholar] [CrossRef]
- Yang, S.; Sankar, K.; Skepper, C.K.; Barker, T.J.; Lukesh Iii, J.C.; Brody, D.M.; Brütsch, M.M.; Boger, D.L. Total synthesis of a key series of vinblastines modified at C4 that define the importance and surprising trends in activity. Chem. Sci. 2017, 8, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mondal, M.; Ibrahim, A.A.; Wheeler, K.A.; Kerrigan, N.J. Asymmetric Synthesis of Dipropionate Derivatives through Catalytic Hydrogenation of Enantioenriched E-Ketene Heterodimers. Synthesis 2016, 48, 2619–2626. [Google Scholar] [CrossRef]
- Cheng, D.; Ling, F.; Zheng, C.; Ma, C. Tuning of Copper-Catalyzed Multicomponent Reactions toward 3-Functionalized Oxindoles. Org. Lett. 2016, 18, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, E.N.; Mandl, F.A.; Blank, I.D.; Ochsenfeld, C.; Ofial, A.R.; Sieber, S.A. Kinetic and Theoretical Studies of Beta-Lactone Reactivity—A Quantitative Scale for Biological Application. ChemPlusChem 2015, 80, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, X.; Li, W.; Tan, F.; Chu, Y.; Zhao, X.; Lin, L.; Feng, X. Chiral Lewis Acid Catalyzed Asymmetric Cycloadditions of Disubstituted Ketenes for the Synthesis of β-Lactones and δ-Lactones. Org. Lett. 2014, 16, 134–137. [Google Scholar] [CrossRef]
- Douglas, J.; Taylor, J.E.; Churchill, G.; Slawin, A.M.Z.; Smith, A.D. NHC-Promoted Asymmetric β-Lactone Formation from Arylalkylketenes and Electron-Deficient Benzaldehydes or Pyridinecarboxaldehydes. J. Org. Chem. 2013, 78, 3925–3938. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Jeon, N.Y.; Park, A.-R.; Min, B.; Kim, B.T.; Park, S.; Lee, H. Experimental and Computation Studies on Candida antarctica Lipase B-Catalyzed Enantioselective Alcoholysis of 4-Bromomethyl-β-lactone Leading to Enantiopure 4-Bromo-3-hydroxybutanoate. Adv. Synth. Catal. 2013, 355, 1808–1816. [Google Scholar] [CrossRef]
- Rousseau, G.; Robin, S. Four-Membered Heterocycles: Structure and Reactivity. In Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011; pp. 163–268. [Google Scholar]
- Frébault, F.; Luparia, M.; Oliveira, M.T.; Goddard, R.; Maulide, N. A Versatile and Stereoselective Synthesis of Functionalized Cyclobutenes. Angew. Chem. Int. Ed. 2010, 49, 5672–5676. [Google Scholar] [CrossRef]
- Kull, T.; Cabrera, J.; Peters, R. Catalytic Asymmetric Synthesis of trans-Configured β-Lactones: Cooperation of Lewis Acid and Ion Pair Catalysis. Chem. Eur. J. 2010, 16, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Coulembier, O.; Degée, P.; Hedrick, J.L.; Dubois, P. From controlled ring-opening polymerization to biodegradable aliphatic polyester: Especially poly(β-malic acid) derivatives. Prog. Polym. Sci. 2006, 31, 723–747. [Google Scholar] [CrossRef]
- Groll, M.; Huber, R.; Potts, B.C.M. Crystal Structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in Complex with the 20S Proteasome Reveal Important Consequences of β-Lactone Ring Opening and a Mechanism for Irreversible Binding. J. Am. Chem. Soc. 2006, 128, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Tomooka, K.; Okinaga, T.; Suzuki, K.; Tsuchihashi, G.-I. Lactols in stereoselection 3: Highly anti-cram selective 1,2-asymmetric induction. Tetrahedron Lett. 1989, 30, 1563–1566. [Google Scholar] [CrossRef]
- Tomooka, K.; Okinaga, T.; Suzuki, K.; Tsuchihashi, G.-I. Lactols in stereoselection 1. Highly selective 1,4- and 1,5-asymmetric induction. Tetrahedron Lett. 1987, 28, 6335–6338. [Google Scholar] [CrossRef]
- Christenson, J.K.; Robinson, S.L.; Engel, T.A.; Richman, J.E.; Kim, A.N.; Wackett, L.P. OleB from Bacterial Hydrocarbon Biosynthesis Is a β-Lactone Decarboxylase That Shares Key Features with Haloalkane Dehalogenases. Biochemistry 2017, 56, 5278–5287. [Google Scholar] [CrossRef] [PubMed]
- Morao, I.; Lecea, B.; Arrieta, A.; Cossío, F.P. Structural and Solvent Effects on the Mechanism of the Thermal Decarboxylation of 2-Oxetanones. A Limiting Case between Concerted and Stepwise Pathways in Pericyclic Reactions. J. Am. Chem. Soc. 1997, 119, 816–825. [Google Scholar] [CrossRef]
- Moyano, A.; Pericas, M.A.; Valenti, E. A theoretical study on the mechanism of the thermal and the acid-catalyzed decarboxylation of 2-oxetanones (β-lactones). J. Org. Chem. 1989, 54, 573–582. [Google Scholar] [CrossRef]
- Mulzer, J.; Kerkmann, T. α Deprotonation of β-lactones—An example of a forbidden β-elimination. J. Am. Chem. Soc. 1980, 102, 3620–3622. [Google Scholar] [CrossRef]
- Mead, K.T.; Yang, H.-L. A new approach to the preparation of 2-substituted tetrahydrofurans with alpha-syn selectivity. Tetrahedron Lett. 1989, 30, 6829–6832. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Ring Expansions of β-Lactams and β-(thio)lactones. In Synthesis of 4- to 7-Membered Heterocycles by Ring Expansion: Aza-, Oxa- and Thiaheterocyclic Small-Ring Systems; D’hooghe, M., Ha, H.-J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–280. [Google Scholar]
- Romo, D.; Tennyson, R.L.; Wang, Y. β-Lactones as intermediates for natural product total synthesis and new transformations. Heterocycles 2004, 64, 605–658. [Google Scholar] [CrossRef] [PubMed]
- Black, T.H.; Hall, J.A.; Sheu, R.G. Improved, stereospecific synthesis of highly substituted butyrolactones via dyotropic rearrangement. J. Org. Chem. 1988, 53, 2371–2374. [Google Scholar] [CrossRef]
- Black, T.H.; Jianhua, H. A new synthesis of substituted butenolides via cation-initiated ring expansion/elimination of β-lactones. Tetrahedron Lett. 1993, 34, 1411–1412. [Google Scholar] [CrossRef]
- Black, T.H.; Smith, D.C.; Eisenbeis, S.A.; Peterson, K.A.; Harmon, M.S. cis-Fused γ-lactones from simple precursors via β-lactone rearrangements. Chem. Commun. 2001, 8, 753–754. [Google Scholar] [CrossRef]
- Park, H.D.; Dincă, M.; Román-Leshkov, Y. Continuous-Flow Production of Succinic Anhydrides via Catalytic β-Lactone Carbonylation by Co(CO)4⊂Cr-MIL-101. J. Am. Chem. Soc. 2018, 140, 10669–10672. [Google Scholar] [CrossRef]
- Pommier, A.; Pons, J.-M. Recent Advances in β-Lactone Chemistry. Synthesis 1993, 1993, 441–459. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Elsaman, T.; Mohamed, M.S.; Eltayib, E.M.; Abdel-aziz, H.A.; Abdalla, A.E.; Munir, M.U.; Mohamed, M.A. Isatin derivatives as broad-spectrum antiviral agents: The current landscape. Med. Chem. Res. 2022, 31, 244–273. [Google Scholar] [CrossRef]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- Chahal, V.; Sonam; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. MedChemComm 2019, 10, 351–368. [Google Scholar]
- Guo, H. Isatin derivatives and their anti-bacterial activities. Eur. J. Med. Chem. 2019, 164, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, G.-Q.; Liu, X.; Zhang, Z.; Feng, L.-S.; Liu, M.-L. Isatin Derivatives with Potential Antitubercular Activities. J. Heterocycl. Chem. 2018, 55, 1263–1279. [Google Scholar] [CrossRef]
- Vandana, K.; Akash, M.; Thushara, B.S.; Rajitha, K. A Review on Isatin Derivatives with Diverse Biological Activities. World J. Pharm. Res. 2017, 6, 318–332. [Google Scholar]
- Grewal, A.S. Isatin Derivatives with Several Biological Activities. Int. J. Pharm. Res. 2014, 6, 1–7. [Google Scholar]
- Pakravan, P.; Kashanian, S.; Khodaei, M.M.; Harding, F.J. Biochemical and pharmacological characterization of isatin and its derivatives: From structure to activity. Pharmacol. Rep. 2013, 65, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and Anticancer Activities of Isatin and Its Derivatives: A Comprehensive Review from 2000–2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397–414. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Glover, V. Biological targets for isatin and its analogues: Implications for therapy. Biol. Targets Ther. 2007, 1, 151–162. [Google Scholar]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological Activites of Isatin and its Derivatives. Acta Pharm. 2005, 55, 27–46. [Google Scholar]
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Chandra Sekhar, K.V.G. Oxindole and its derivatives: A review on recent progress in biological activities. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, M.; Chadha, N.; Silakari, O. Oxindole: A chemical prism carrying plethora of therapeutic benefits. Eur. J. Med. Chem. 2016, 123, 858–894. [Google Scholar] [CrossRef]
- Badillo, J.J.; Hanhan, N.V.; Franz, A.K. Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery. Curr. Opin. Drug Discov. Dev. 2010, 13, 758–776. [Google Scholar]
- Zhang, S.; Huang, D.; Wu, J.; Wang, Z. Decade Advance of Isatin in Three-component Reactions. Asian J. Org. Chem. 2023, 12, e202200591. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Bayat, M. Synthesis of Heterocyclic Compounds Based on Isatins. Curr. Org. Chem. 2022, 26, 756–770. [Google Scholar] [CrossRef]
- Salvio, R.; Placidi, S.; Sinibaldi, A.; Di Sabato, A.; Buscemi, D.C.; Rossi, A.; Antenucci, A.; Malkov, A.; Bella, M. Organocatalytic Synthesis of Benzazetidines by Trapping Hemiaminals with Protecting Groups. J. Org. Chem. 2019, 84, 7395–7404. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, Ö.; Biswas, N.N.; Gardner, C.R.; Griffith, R.; Rice, S.A.; Manefield, M.; Willcox, M.; Black, D.S.; Kumar, N. Design, synthesis and evaluation of N-aryl-glyoxamide derivatives as structurally novel bacterial quorum sensing inhibitors. Org. Biomol. Chem. 2016, 14, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Ho, K.K.K.; Kimyon, Ö.; Yee, E.; Berry, T.; Manefield, M.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Synthesis and biological evaluation of N-naphthoyl-phenylglyoxamide-based small molecular antimicrobial peptide mimics as novel antimicrobial agents and biofilm inhibitors. Org. Biomol. Chem. 2016, 14, 3623–3637. [Google Scholar] [CrossRef] [PubMed]
- Nardi, D.; Tajana, A.; Portioli, F.; Bonola, G. Nucleophilic opening of the hetero-ring of N-acylisatins by acylhydrazines. Il Farm. Ed. Sci. 1982, 37, 815–823. [Google Scholar]
- Black, D.S.C.; Ian Moss, G.; Wong, L.C.H. A versatile new synthesis of macrocyclic amide complexes. Tetrahedron Lett. 1978, 19, 2837–2838. [Google Scholar] [CrossRef]
- Suarez-Castillo, O.R.; Bautista-Hernandez, C.I.; Sanchez-Zavala, M.; Melenez-Rodriguez, M.; Sierra-Zenteno, A.; Morales-Rios, M.S.; Joseph-Nathan, P. Mircorwave-assisted synthesis of 3,1-benzoxazin-2-ones from 3-hydroxyoxindoles. Heterocycles 2012, 85, 2147–2171. [Google Scholar] [CrossRef]
- Mandai, H.; Shiomoto, R.; Fujii, K.; Mitsudo, K.; Suga, S. Kinetic Resolution of Tertiary Alcohols by Chiral DMAP Derivatives: Enantioselective Access to 3-Hydroxy-3-substituted 2-Oxindoles. Org. Lett. 2021, 23, 1169–1174. [Google Scholar] [CrossRef]
- Papaioannou, N.; Blank, J.T.; Miller, S.J. Enantioselective Synthesis of an Aziridinomitosane and Selective Functionalizations of a Key Intermediate. J. Org. Chem. 2003, 68, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Rodríguez-Acebes, R. Efficient Entry to Diversely Functionalized Spirocyclic Oxindoles from Isatins through Carbonyl-Addition/Cyclization Reaction Sequences. J. Org. Chem. 2006, 71, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Stensland, B. Cyclization of Cyanoethylated Ketones as a Route to 6-Substituted Indole Derivatives. J. Heterocycl. Chem. 2014, 51, 1–10. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, S.; Liu, Y.; Feng, X. Photoinduced Chemo-, Site- and Stereoselective α-C(sp3)−H Functionalization of Sulfides. Angew. Chem. Int. Ed. 2022, 61, e202203374. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Nagaoka, M.; Satoh, T.; Okamoto, A.; Ukon, R.; Ogawa, A. Synthesis of 3-hydroxyindolin-2-one alkaloids, (±)-donaxaridine and (±)-convolutamydines A and E, through enolization–Claisen rearrangement of 2-allyloxyindolin-3-ones. Tetrahedron 2004, 60, 3493–3503. [Google Scholar] [CrossRef]
- Gao, M.; Luo, Y.; Xu, Q.; Zhao, Y.; Gong, X.; Xia, Y.; Hu, L. A Unified Catalytic Asymmetric (4+1) and (5+1) Annulation Strategy to Access Chiral Spirooxindole-Fused Oxacycles. Angew. Chem. Int. Ed. 2021, 60, 19813–19820. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Â.C.; Da Silva, R.S.; Hollins, R.A. A simple synthesis of 1-phenothiazineethanolamines: Potential antimalarials. J. Heterocycl. Chem. 1979, 16, 1085–1086. [Google Scholar] [CrossRef]
- Inoue, M.; Sakazaki, H.; Furuyama, H.; Hirama, M. Total Synthesis of TMC-95A. Angew. Chem. Int. Ed. 2003, 42, 2654–2657. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Takamiya, W.; Okamoto, N.; Nagaoka, M.; Hirayama, T. Silyl-enolization-asymmetric Claisen rearrangement of 2-allyloxyindolin-3-one: Enantioselective total synthesis of 3a-hydroxypyrrolo [2,3-b]indoline alkaloid alline. Tetrahedron Lett. 2006, 47, 5379–5382. [Google Scholar] [CrossRef]
- Kim, S.C.; Gowrisankar, S.; Kim, J.N. Synthesis of 3-aryl-3-hydroxypyrrolidin-2-ones and 2-benzyl-9b-hydroxy-3,3a,5,9b-tetrahydro-2H-pyrrolo[3,4-c]quinoline-1,4-dione derivatives from the Baylis–Hillman adducts of isatins. Tetrahedron Lett. 2006, 47, 3463–3466. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, K.Y.; Gowrisankar, S.; Kim, J.N. Synthesis of 3-Aryl-3-Hydroxypyrrolidin-2-Ones and 2-Benzyl-9b-Hydroxy-3,3a,5,9b-Tetrahydro-2H-Pyrrolo[3,4-c]Quinoline-1,4-Dione Derivatives from the Baylis-Hillman Adducts of Isatins. Bull. Korean Chem. Soc. 2006, 27, 1133–1139. [Google Scholar]
- Chen, W.-B.; Du, X.-L.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Highly enantioselective aldol reaction of acetaldehyde and isatins only with 4-hydroxydiarylprolinol as catalyst: Concise stereoselective synthesis of (R)-convolutamydines B and E, (−)-donaxaridine and (R)-chimonamidine. Tetrahedron 2010, 66, 1441–1446. [Google Scholar] [CrossRef]
- Wu, H.; Xue, F.; Xiao, X.; Qin, Y. Total Synthesis of (+)-Perophoramidine and Determination of the Absolute Configuration. J. Am. Chem. Soc. 2010, 132, 14052–14054. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, D.; Richy, N.; Vidal, J. Synthesis of Lactams by Isomerization of Oxindoles Substituted at C-3 by an ω-Amino Chain. J. Org. Chem. 2014, 79, 10945–10955. [Google Scholar] [CrossRef]
- Subba Reddy, U.V.; Chennapuram, M.; Seki, K.; Seki, C.; Anusha, B.; Kwon, E.; Okuyama, Y.; Uwai, K.; Tokiwa, M.; Takeshita, M.; et al. A Diamino Alcohol Catalyzed Enantioselective Crossed Aldol Reaction of Acetaldehyde with Isatins—A Concise Total Synthesis of Antitumor Agents. Eur. J. Org. Chem. 2017, 2017, 3874–3885. [Google Scholar] [CrossRef]
- Richy, N.; Sarraf, D.; Maréchal, X.; Janmamode, N.; Le Guével, R.; Genin, E.; Reboud-Ravaux, M.; Vidal, J. Structure-based design of human immuno- and constitutive proteasomes inhibitors. Eur. J. Med. Chem. 2018, 145, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.; Wolf, C. Catalytic Enantioselective Ynamide Additions to Isatins: Concise Access to Oxindole Alkaloids. Angew. Chem. Int. Ed. 2019, 58, 3402–3406. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P.; Vaithiyanathan, V.; Viswambharan, B. A Facile and Efficient Synthesis of Functionalized -Butyrolactones from BaylisHillman Adducts of Isatin. Aust. J. Chem. 2007, 60, 296–301. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wang, B.-L.; Cao, J.-J.; Chen, L.; Zhang, Y.-X.; Wang, C.; Zhou, J. Organocatalytic Asymmetric Synthesis of Substituted 3-Hydroxy-2-oxindoles via Morita−Baylis−Hillman Reaction. J. Am. Chem. Soc. 2010, 132, 15176–15178. [Google Scholar] [CrossRef]
- Dong, Z.; Yan, C.; Gao, Y.; Dong, C.; Qiu, G.; Zhou, H.-B. Tunable Bifunctional Phosphine–Squaramide Promoted Morita–Baylis–Hillman Reaction of N-Alkyl Isatins with Acrylates. Adv. Synth. Catal. 2015, 357, 2132–2142. [Google Scholar] [CrossRef]
- Sakla, A.P.; Kansal, P.; Shankaraiah, N. Syntheses and reactivity of spiro-epoxy/aziridine oxindole cores: Developments in the past decade. Org. Biomol. Chem. 2020, 18, 8572–8596. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Singha Roy, S.; Biswas, A.; Saleh, S.A. Catalyst-Free Ring Opening of Spiroaziridine Oxindoles by Heteronucleophiles: An Approach to the Synthesis of Enantiopure 3-Substituted Oxindoles. J. Org. Chem. 2018, 83, 3633–3644. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Hazra, A.; Mandal, P. Stereocontrolled Nucleophilic Fluorination at the Tertiary sp3-Carbon Center for Enantiopure Synthesis of 3-Fluorooxindoles. Org. Lett. 2018, 20, 6471–6475. [Google Scholar] [CrossRef] [PubMed]
- Sakla, A.P.; Kansal, P.; Shankaraiah, N. Syntheses and Applications of Spirocyclopropyl Oxindoles: A Decade Review. Eur. J. Org. Chem. 2021, 2021, 757–772. [Google Scholar] [CrossRef]
- Conboy, A.; Goodfellow, A.S.; Kasten, K.; Dunne, J.; Cordes, D.B.; Bühl, M.; Smith, A.D. De-epimerizing DyKAT of β-lactones generated by isothiourea-catalysed enantioselective [2 + 2] cycloaddition. Chem. Sci. 2024, 15, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Gleiter, R.; Werz, D.B.; Köppel, H. Theoretical Investigations on Heteronuclear Chalcogen−Chalcogen Interactions: On the Nature of Weak Bonds between Chalcogen Centers. Inorg. Chem. 2007, 46, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, X.; Birman, V.B.; Houk, K.N. Origin of Enantioselectivity in Benzotetramisole-Catalyzed Dynamic Kinetic Resolution of Azlactones. Org. Lett. 2012, 14, 3288–3291. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.R.T.; Walden, D.M.; Fallan, C.; Greenhalgh, M.D.; Cheong, P.H.-Y.; Smith, A.D. Non-bonding 1,5-S⋯O interactions govern chemo- and enantioselectivity in isothiourea-catalyzed annulations of benzazoles. Chem. Sci. 2016, 7, 6919–6927. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef]
- Greenhalgh, M.D.; Smith, S.M.; Walden, D.M.; Taylor, J.E.; Brice, Z.; Robinson, E.R.T.; Fallan, C.; Cordes, D.B.; Slawin, A.M.Z.; Richardson, H.C.; et al. A C=O⋅⋅⋅Isothiouronium Interaction Dictates Enantiodiscrimination in Acylative Kinetic Resolutions of Tertiary Heterocyclic Alcohols. Angew. Chem. Int. Ed. 2018, 57, 3200–3206. [Google Scholar] [CrossRef]
- Breugst, M.; Koenig, J.J. σ-Hole Interactions in Catalysis. Eur. J. Org. Chem. 2020, 2020, 5473–5487. [Google Scholar] [CrossRef]
- Kolb, S.; Oliver, G.A.; Werz, D.B. Chemistry Evolves, Terms Evolve, but Phenomena Do Not Evolve: From Chalcogen–Chalcogen Interactions to Chalcogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 22306–22310. [Google Scholar] [CrossRef] [PubMed]
- Young, C.M.; Elmi, A.; Pascoe, D.J.; Morris, R.K.; McLaughlin, C.; Woods, A.M.; Frost, A.B.; de la Houpliere, A.; Ling, K.B.; Smith, T.K.; et al. The Importance of 1,5-Oxygen⋅⋅⋅Chalcogen Interactions in Enantioselective Isochalcogenourea Catalysis. Angew. Chem. Int. Ed. 2020, 59, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Wille, G.; Steglich, W. A Short Synthesis of the Bacterial Pigments Violacein and Deoxyviolacein. Synthesis 2001, 2001, 759–762. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlisPro, v1.171.42.94a; Rigaku Oxford Diffraction; Rigaku Corporation: Tokyo, Japan, 2023.
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

 | ||||
|---|---|---|---|---|
| Entry | R | NH2Bn (equiv.) | Product Yield [%] b | dr/er c |
| 1 a | Ph | 1.5 | 0 | - |
| 2 | C(O)NHPh | 3.0 | 0 | - |
| 3 | Ts | 3.0 | Complex mixture | - |
| 4 | Boc | 3.0 | 67 | >95:5/99:1 |
| 5 | Boc | 1.0 | 32 | >95:5/98:2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimmo, A.J.; Kasten, K.; White, G.; Roeterdink, J.; McKay, A.P.; Cordes, D.B.; Smith, A.D. One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening. Molecules 2024, 29, 3635. https://doi.org/10.3390/molecules29153635
Nimmo AJ, Kasten K, White G, Roeterdink J, McKay AP, Cordes DB, Smith AD. One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening. Molecules. 2024; 29(15):3635. https://doi.org/10.3390/molecules29153635
Chicago/Turabian StyleNimmo, Alastair J., Kevin Kasten, George White, Julia Roeterdink, Aidan P. McKay, David B. Cordes, and Andrew David Smith. 2024. "One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening" Molecules 29, no. 15: 3635. https://doi.org/10.3390/molecules29153635
APA StyleNimmo, A. J., Kasten, K., White, G., Roeterdink, J., McKay, A. P., Cordes, D. B., & Smith, A. D. (2024). One-Pot Access to Functionalised Malamides via Organocatalytic Enantioselective Formation of Spirocyclic β-Lactone-Oxindoles and Double Ring-Opening. Molecules, 29(15), 3635. https://doi.org/10.3390/molecules29153635








