LaMnO3-Type Perovskite Nanofibers as Effective Catalysts for On-Cell CH4 Reforming via Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Results
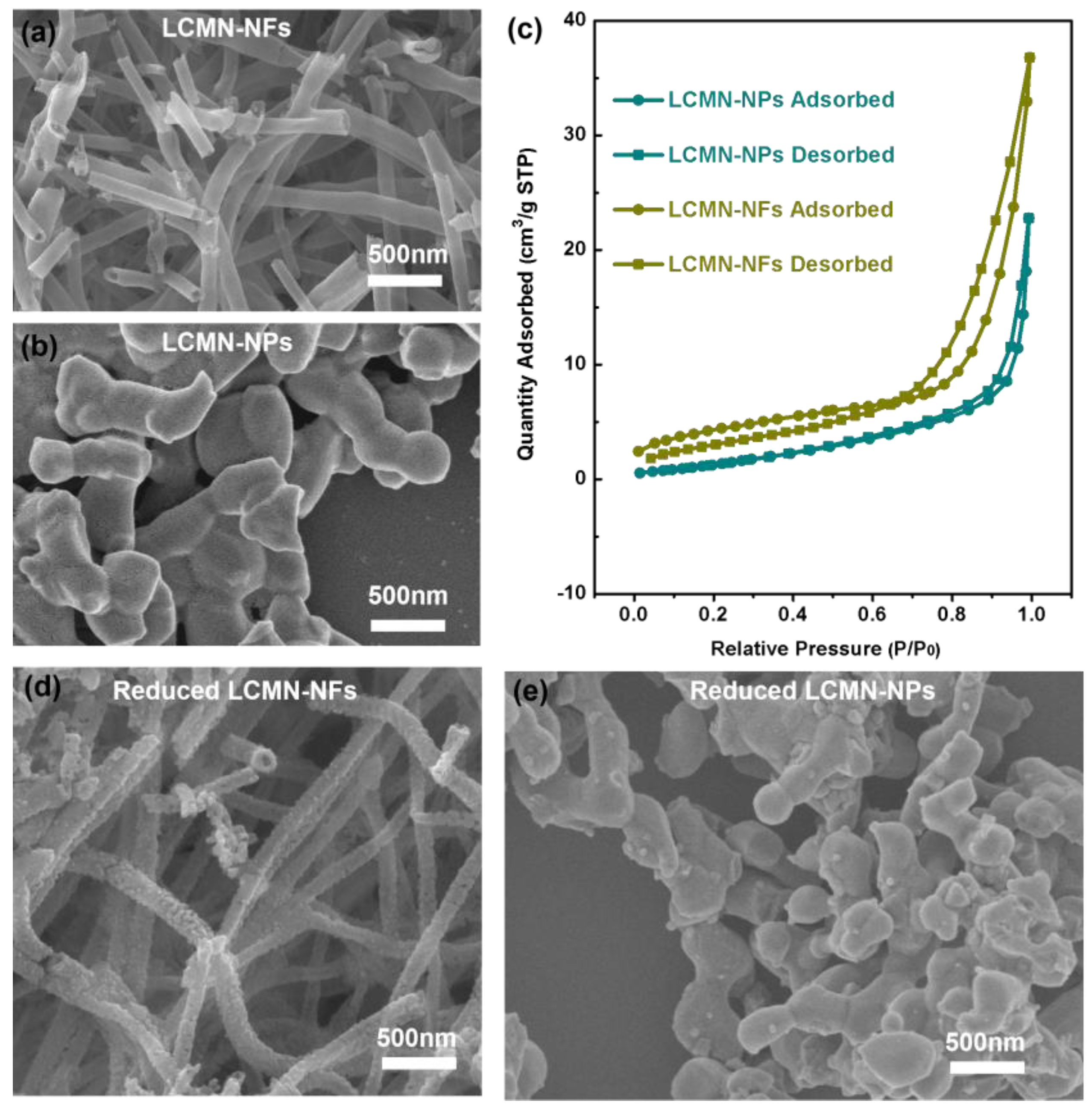
2.1. Material Characteristics
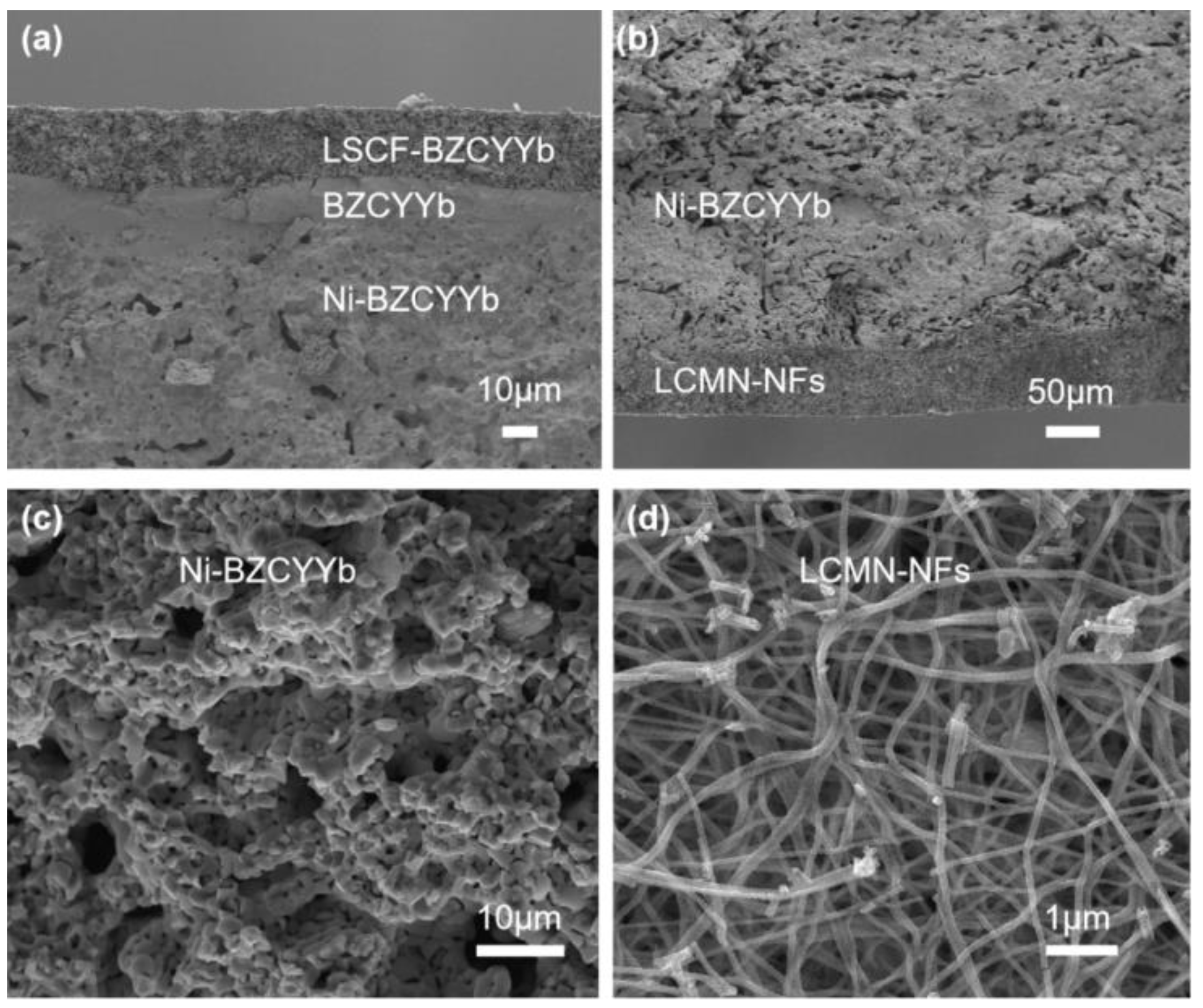
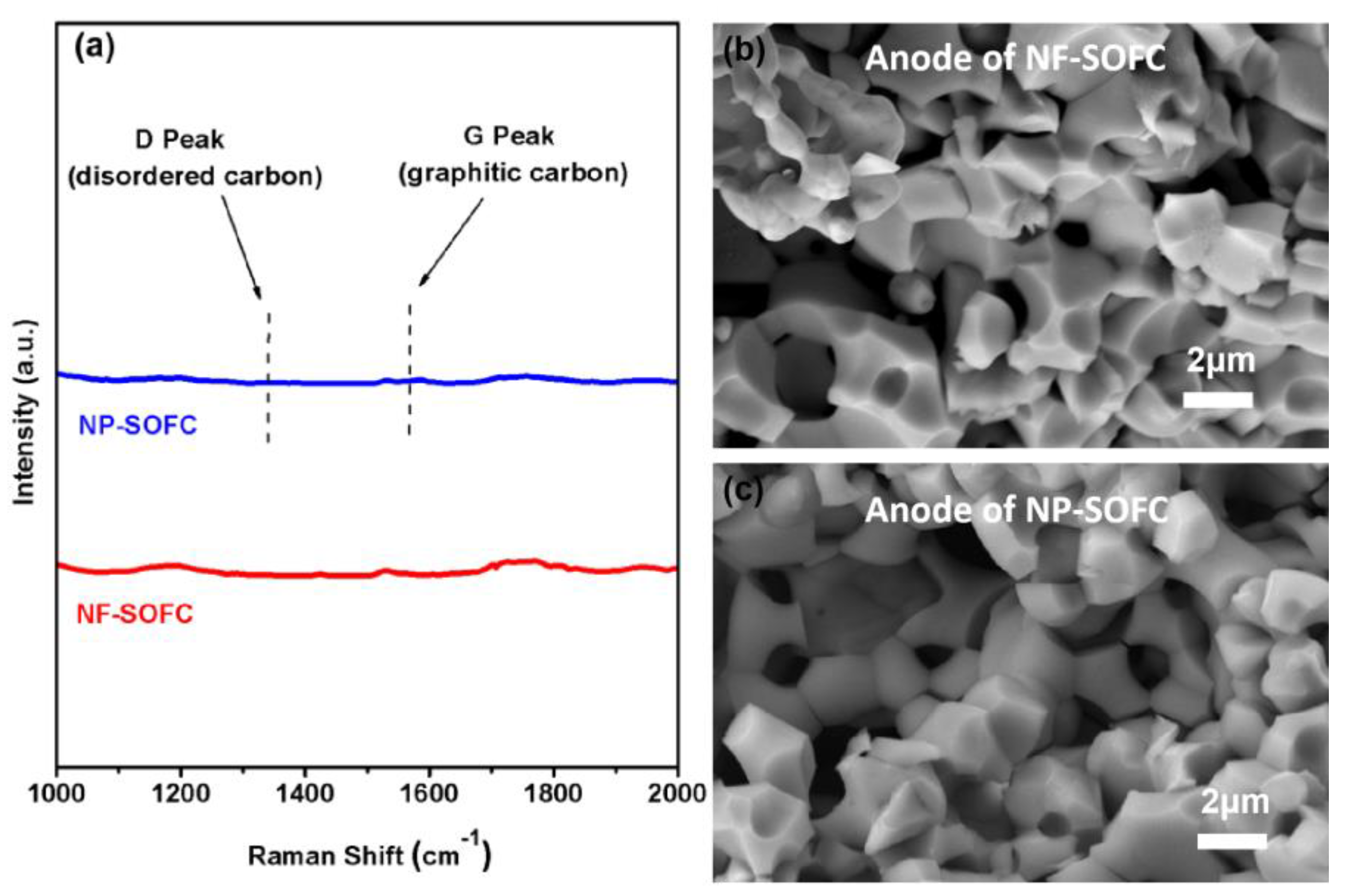
2.2. Cell Microstructures
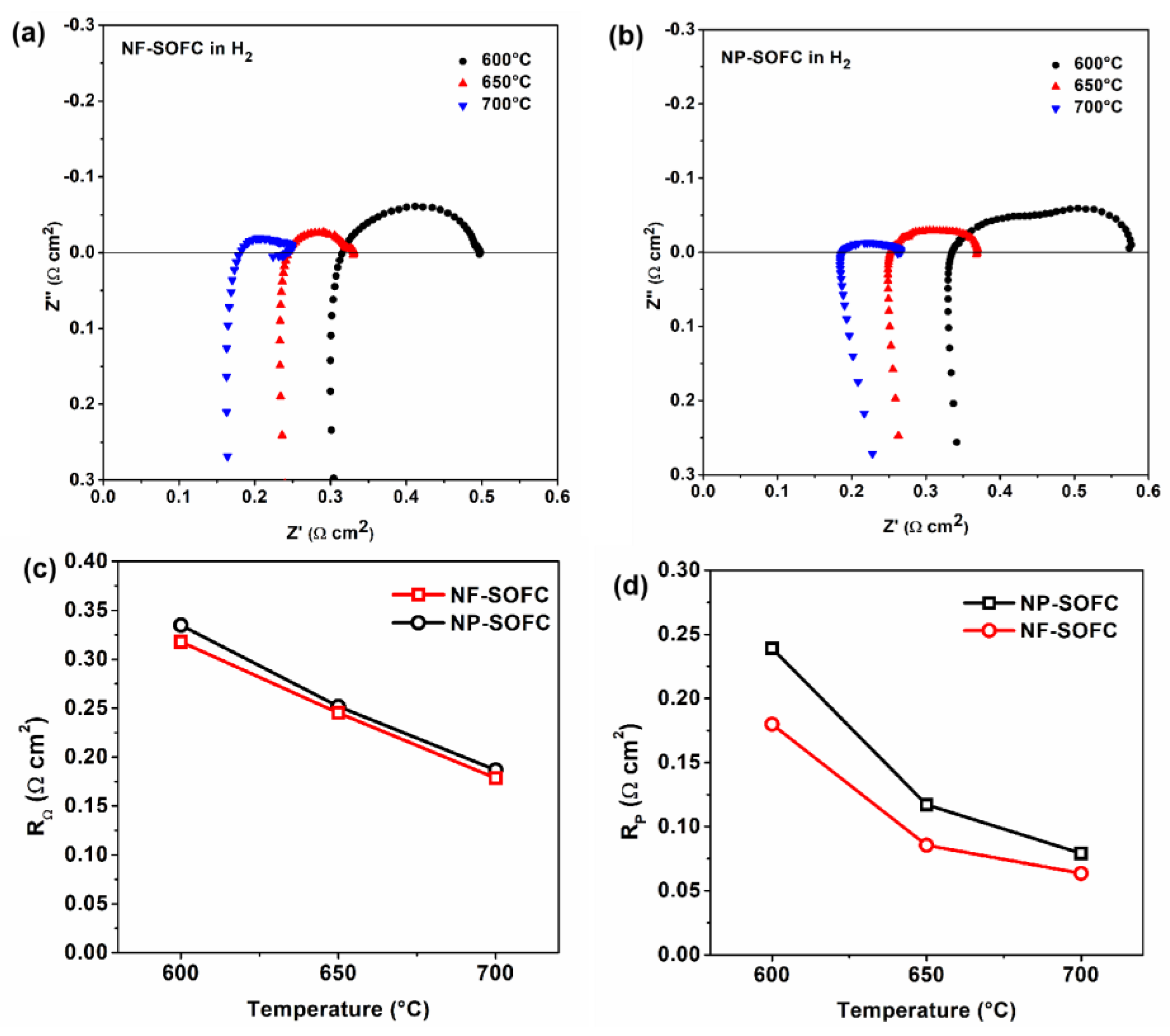
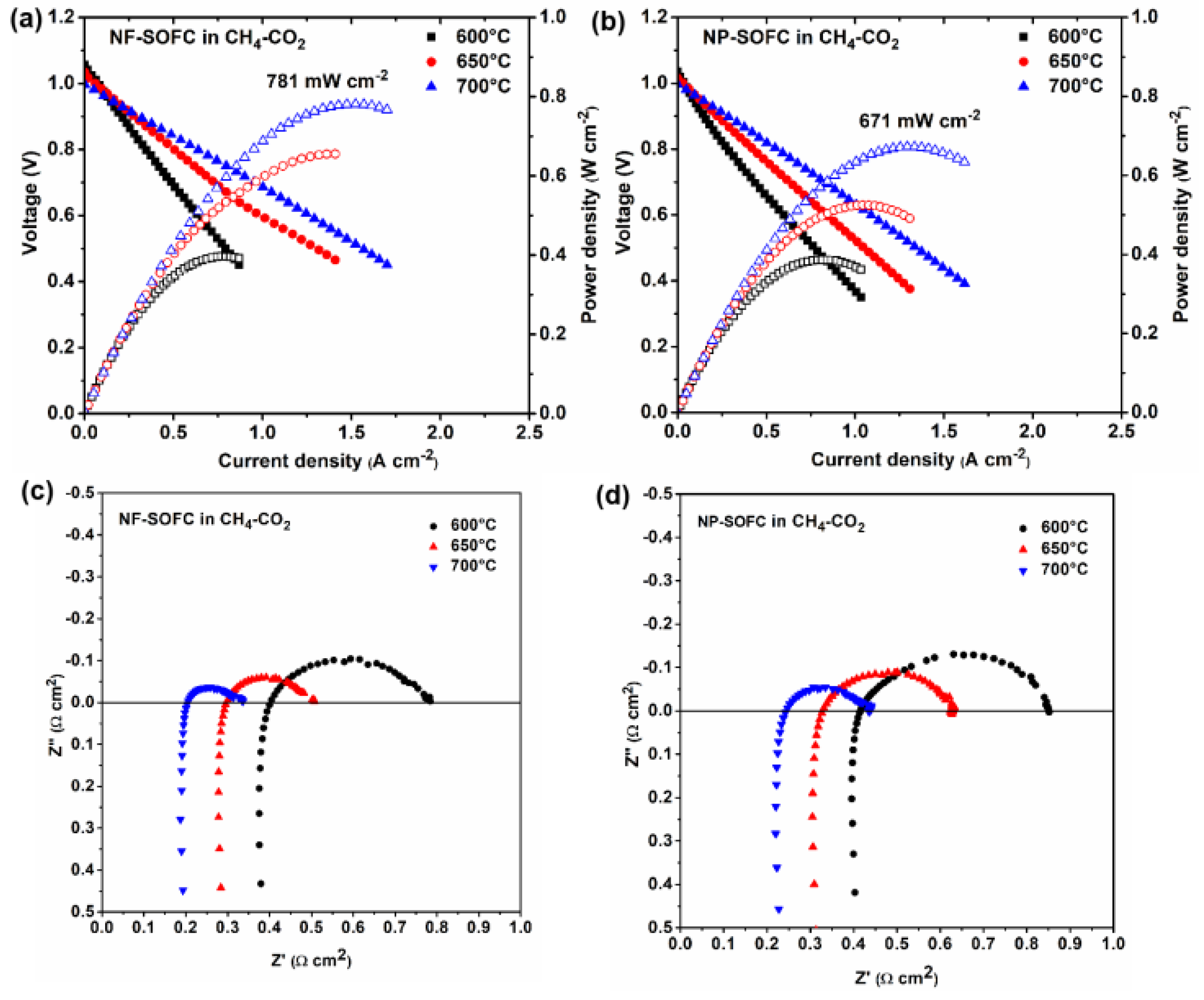
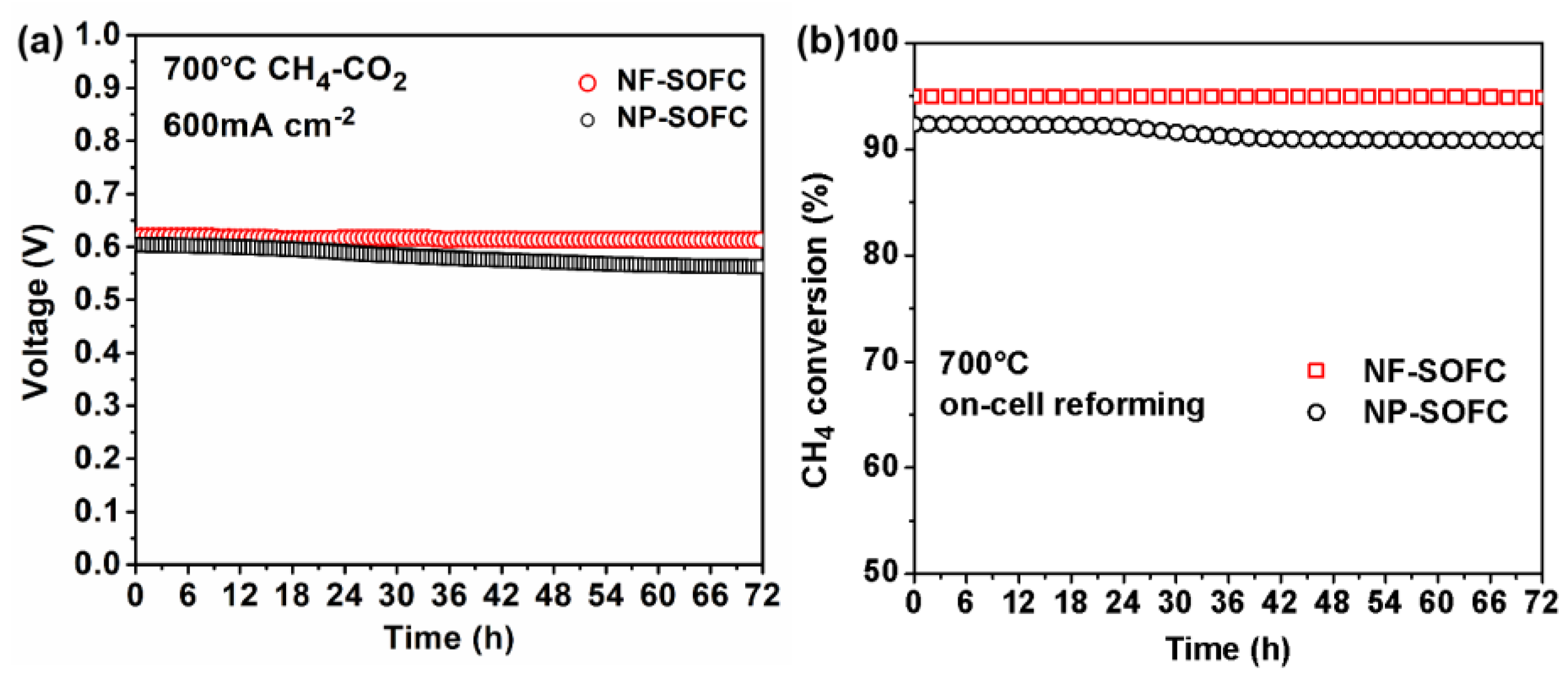
2.3. Cell Performances
3. Materials and Methods
3.1. Material Preparation
3.2. Cell Fabrication
3.3. Material Characterization
3.4. Electrochemical and Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, P.; Zhang, Y.; Yang, C.; Chen, J.; Liu, Z.; Deng, C.; Yang, S. Thermodynamic performance comparison of a SOFC system integrated with steam reforming and dry reforming by utilizing different fuels. Energy Convers. Manag. 2024, 300, 117981. [Google Scholar] [CrossRef]
- Aznam, I.; Muchtar, A.; Somalu, M.R.; Baharuddin, N.A.; Rosli, N.A.H. Advanced materials for heterogeneous catalysis: A comprehensive review of spinel materials for direct internal reforming of methane in solid oxide fuel cell. Chem. Eng. J. 2023, 471, 144751. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Gür, T.M. Comprehensive review of methane conversion in solid oxide fuel cells: Prospects for efficient electricity generation from natural gas. Prog. Energy Combust. Sci. 2016, 54, 1–64. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Requies, J.; Cambra, J.F.; Guemez, M.B.; Arias, P.L. Tri-reforming: A new biogas process for synthesis gas and hydrogen production. Int. J. Hydrog. Energy 2013, 38, 7623–7631. [Google Scholar] [CrossRef]
- Shi, N.; Xie, Y.; Yang, Y.; Xue, S.; Li, X.; Zhu, K.; Huan, D.; Peng, R.; Xia, C.; Lu, Y. Review of anodic reactions in hydrocarbon fueled solid oxide fuel cells and strategies to improve anode performance and stability. Mater. Renew. Sustain. Energy 2020, 9, 6. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °c with chemically stable proton-conducting electrolytes. Adv. Mater. 2012, 24, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Rioja-Monllor, L.; Bernuy-Lopez, C.; Fontaine, M.-L.; Grande, T.; Einarsrud, M.-A. Processing of high performance composite cathodes for protonic ceramic fuel cells by exsolution. J. Mater. Chem. A 2019, 7, 8609–8619. [Google Scholar] [CrossRef]
- Danilov, N.A.; Starostina, I.A.; Starostin, G.N.; Kasyanova, A.V.; Medvedev, D.A.; Shao, Z. Fundamental Understanding and Applications of Protonic Y- and Yb-Coped Ba(Ce,Zr)O3 Perovskites: State-of-the-Art and Perspectives. Adv. Energy Mater. 2023, 13, 2302175. [Google Scholar] [CrossRef]
- Chen, Y.; de Glee, B.; Tang, Y.; Wang, Z.; Zhao, B.; Wei, Y.; Zhang, L.; Yoo, S.; Pei, K.; Kim, J.H.; et al. A robust fuel cell operated on nearly dry methane at 500 °C enabled by synergistic thermal catalysis and electrocatalysis. Nat. Energy 2018, 3, 1042–1050. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Luo, J.-L. Alternative Fuel Cell Technologies for Cogenerating Electrical Power and Syngas from Greenhouse Gases. ACS Energy Lett. 2017, 2, 1789–1796. [Google Scholar] [CrossRef]
- Wang, W.; Ran, R.; Shao, Z. Lithium and lanthanum promoted Ni-Al2O3 as an active and highly coking resistant catalyst layer for solid-oxide fuel cells operating on methane. J. Power Sources 2011, 196, 90–97. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, X.; Zhou, W.; Blakey, I.; Liu, S.; Zhu, Z. Proton-Conducting La-Doped Ceria-Based Internal Reforming Layer for Direct Methane Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 33758–33765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Yang, W.; Peng, R.; Lu, Y. Carbon-tolerant solid oxide fuel cells using NiTiO3 as an anode internal reforming layer. J. Power Sources 2014, 255, 404–409. [Google Scholar] [CrossRef]
- Zhan, Z.; Barnett, S.A. An Octane-Fueled Solid Oxide Fuel Cell. Science 2005, 308, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Yan, N.; Li, M.; Zhang, Y.-Q.; Sun, Y.-F.; Li, J.; Etsell, T.; Sarkar, P.; Chuang, K.; Luo, J.-L. Novel layered solid oxide fuel cells with multiple-twinned Ni0.8Co0.2 nanoparticles: The key to thermally independent CO2 utilization and power-chemical cogeneration. Energy Environ. Sci. 2016, 9, 207–215. [Google Scholar] [CrossRef]
- Lyu, Z.; Wang, Y.; Zhang, Y.; Han, M. Solid oxide fuel cells fueled by simulated biogas: Comparison of anode modification by infiltration and reforming catalytic layer. Chem. Eng. J. 2020, 393, 124755. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Barnett, S.A. Improving the stability of direct-methane solid oxide fuel cells using anode barrier layers. J. Power Sources 2006, 158, 1313–1316. [Google Scholar] [CrossRef]
- Dong, J.; Fu, Q.; Li, H.; Xiao, J.; Yang, B.; Zhang, B.; Bai, Y.; Song, T.; Zhang, R.; Gao, L.; et al. Reaction-Induced Strong Metal−Support Interactions between Metals and Inert Boron Nitride Nanosheets. J. Am. Chem. Soc. 2020, 142, 17167–17174. [Google Scholar] [CrossRef]
- Yang, Q.; Chai, F.; Ma, C.; Sun, C.; Shi, S.; Chen, L. Enhanced Coking Tolerance of MgO-modified Ni Cermet Anode for Hydrocarbon Fueled Solid Oxide Fuel Cells. J. Mater. Chem. A 2016, 4, 18031–18036. [Google Scholar] [CrossRef]
- Tomiyama, S.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Preparation of Ni/SiO2 catalyst with high thermal stability for CO2-refroming of CH4. Appl. Catal. A 2003, 241, 349–361. [Google Scholar] [CrossRef]
- Ross, J.R.H.; Steel, M.C.F.; Zeini-Isfahani, A. Evidence for the participation of surface nickel aluminate sites in the steam reforming of methane over nickel/alumina catalysts. J. Catal. 1978, 52, 280–290. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3-δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Jia, L.; Zheng, H.; Chi, B.; Pu, J.; Li, J. LaMnO3 -based perovskite with in-situ exsolved Ni nanoparticles: A highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4. Appl. Catal. A 2018, 564, 199–207. [Google Scholar] [CrossRef]
- Alonso, J.A.; Martinez-Lopez, M.J.; Casais, M.T.; MacManus-Driscoll, J.L.; de Silva, P.S.I.P.N.; Cohen, L.F.; Fernandez-Diaz, M.T. Non-stoichiometry, structural defects and properties of LaMnO3+d with high d values (0.11<d<0.29). J. Mater. Chem. 1997, 7, 2139–2144. [Google Scholar]
- Wei, T.; Pan, X.; Wang, S.; Qiu, P.; Du, X.; Liu, B.; Wang, Z.; Jia, L. Ce-enhanced LaMnO3 perovskite catalyst with exsolved Ni particles for H2 production from CH4 dry reforming. Sustain. Energy Fuels 2021, 5, 5481–5489. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Armstrong, T.R.; Stevenson, J.W.; Pederson, L.R.; Raney, P.E. Dimensional Instability of Doped Lanthanum Chromite. J. Electrochem. Soc. 1996, 143, 2919. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Wei, T.; Shao, Z.; Song, Y.; Huang, X.; Huang, B.; Cao, C.; Zhi, Y. LaMnO3-Type Perovskite Nanofibers as Effective Catalysts for On-Cell CH4 Reforming via Solid Oxide Fuel Cells. Molecules 2024, 29, 3654. https://doi.org/10.3390/molecules29153654
Jia Y, Wei T, Shao Z, Song Y, Huang X, Huang B, Cao C, Zhi Y. LaMnO3-Type Perovskite Nanofibers as Effective Catalysts for On-Cell CH4 Reforming via Solid Oxide Fuel Cells. Molecules. 2024; 29(15):3654. https://doi.org/10.3390/molecules29153654
Chicago/Turabian StyleJia, Yangbo, Tong Wei, Zhufeng Shao, Yunpeng Song, Xue Huang, Beila Huang, Chen Cao, and Yufan Zhi. 2024. "LaMnO3-Type Perovskite Nanofibers as Effective Catalysts for On-Cell CH4 Reforming via Solid Oxide Fuel Cells" Molecules 29, no. 15: 3654. https://doi.org/10.3390/molecules29153654
APA StyleJia, Y., Wei, T., Shao, Z., Song, Y., Huang, X., Huang, B., Cao, C., & Zhi, Y. (2024). LaMnO3-Type Perovskite Nanofibers as Effective Catalysts for On-Cell CH4 Reforming via Solid Oxide Fuel Cells. Molecules, 29(15), 3654. https://doi.org/10.3390/molecules29153654




