Injecting Sustainability into Epoxy-Based Composite Materials by Using Bio-Binder from Hydrothermal Liquefaction Processing of Microalgae
Abstract
:1. Introduction
 ) in epoxy resin is added and reacts with epoxy resin at an elevated temperature, forming a 3D crosslinked molecular network. It is noteworthy that the production of conventional epoxy resin and its curing agent requires a large amount of fossil fuel that can significantly contribute to greenhouse gas emissions and climate change. Furthermore, the epoxy products are not biodegradable, and they can stay in our environment for hundreds of years without significant degradation, posing a risk of contamination to air, soil, and water.
) in epoxy resin is added and reacts with epoxy resin at an elevated temperature, forming a 3D crosslinked molecular network. It is noteworthy that the production of conventional epoxy resin and its curing agent requires a large amount of fossil fuel that can significantly contribute to greenhouse gas emissions and climate change. Furthermore, the epoxy products are not biodegradable, and they can stay in our environment for hundreds of years without significant degradation, posing a risk of contamination to air, soil, and water.2. Results and Discussion
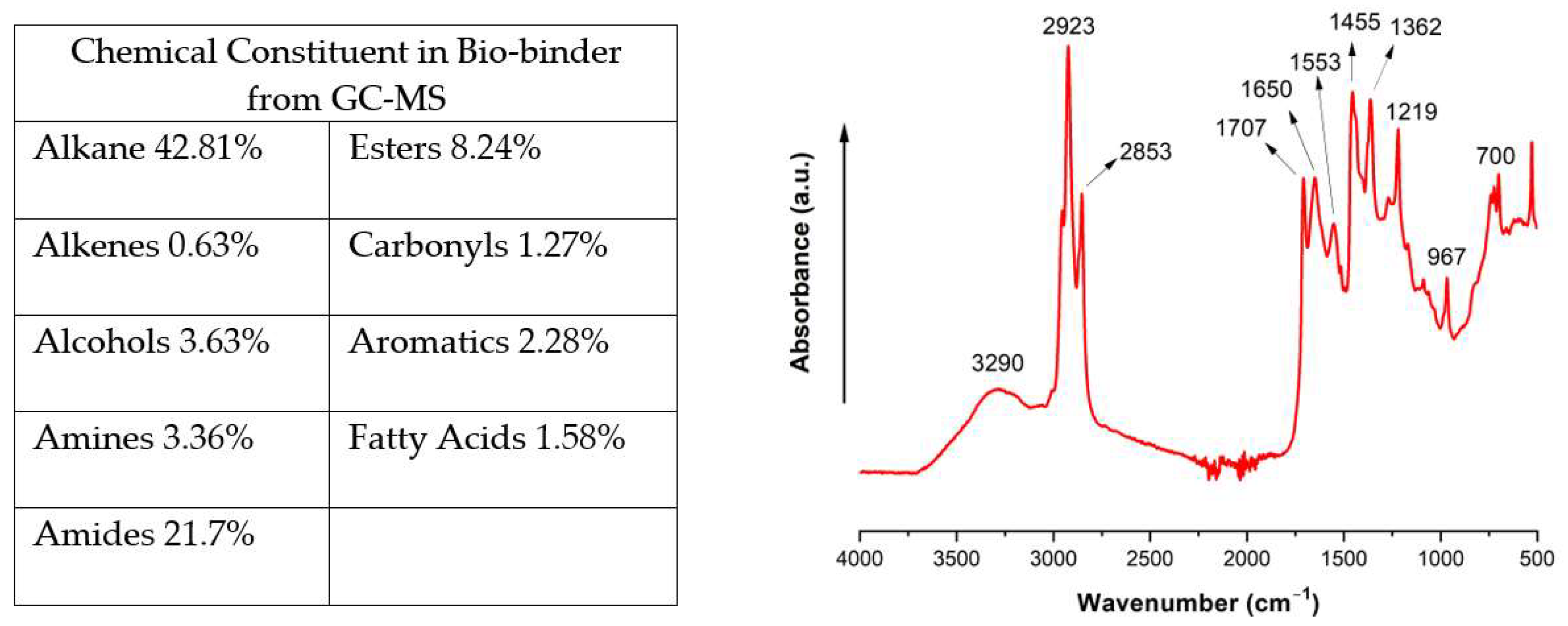
2.1. Composition and Curing
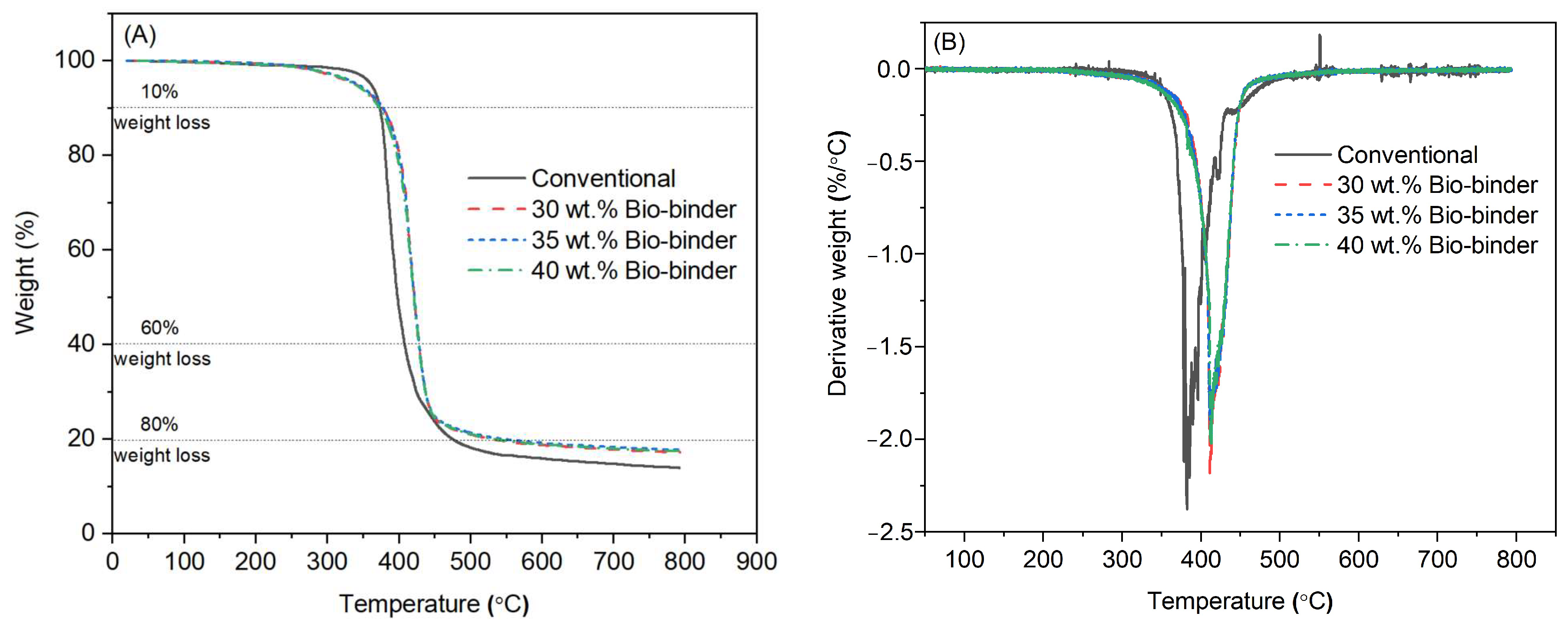
2.2. Thermal Stability
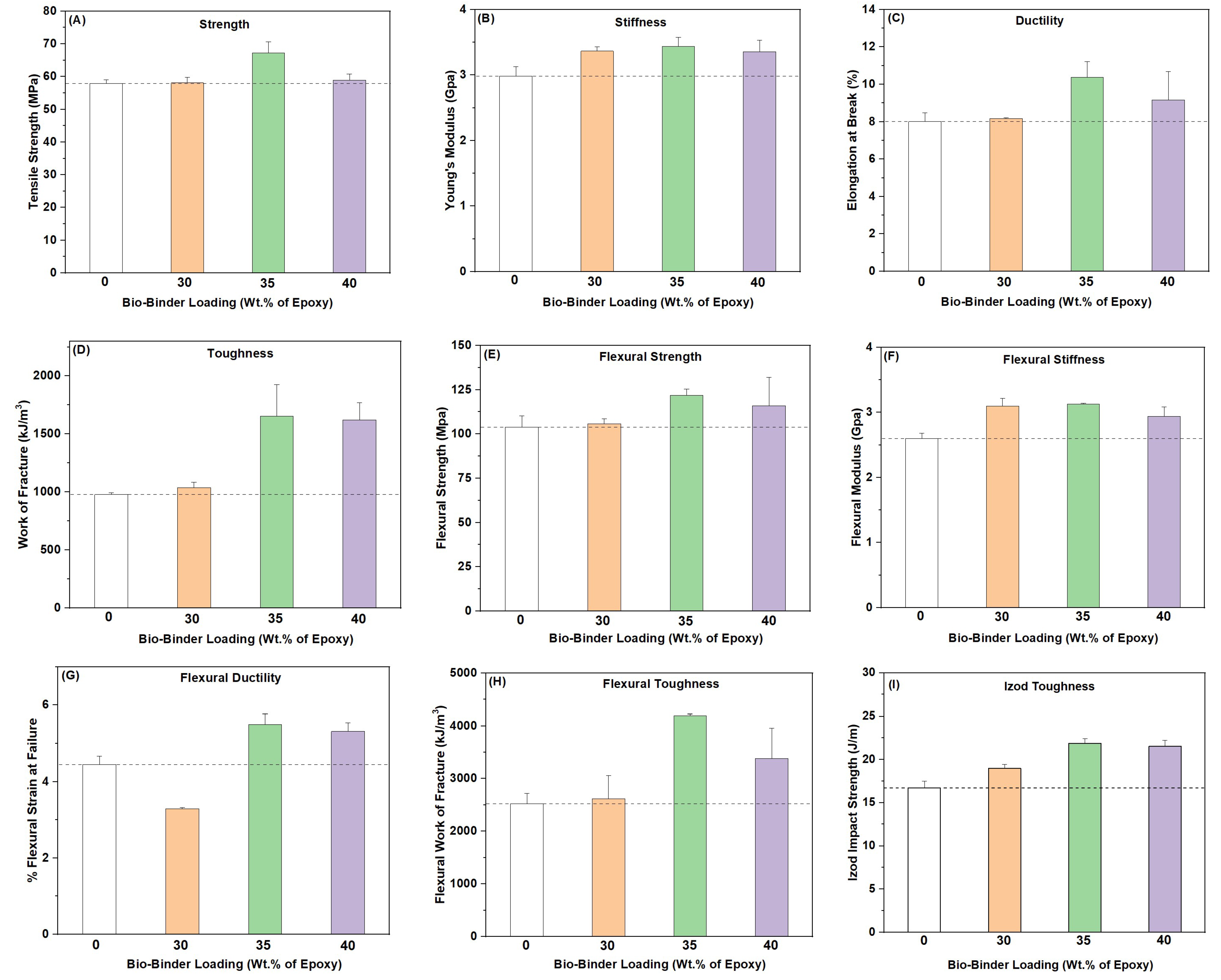
2.3. Mechanical Performance
3. Material and Methods
3.1. Materials
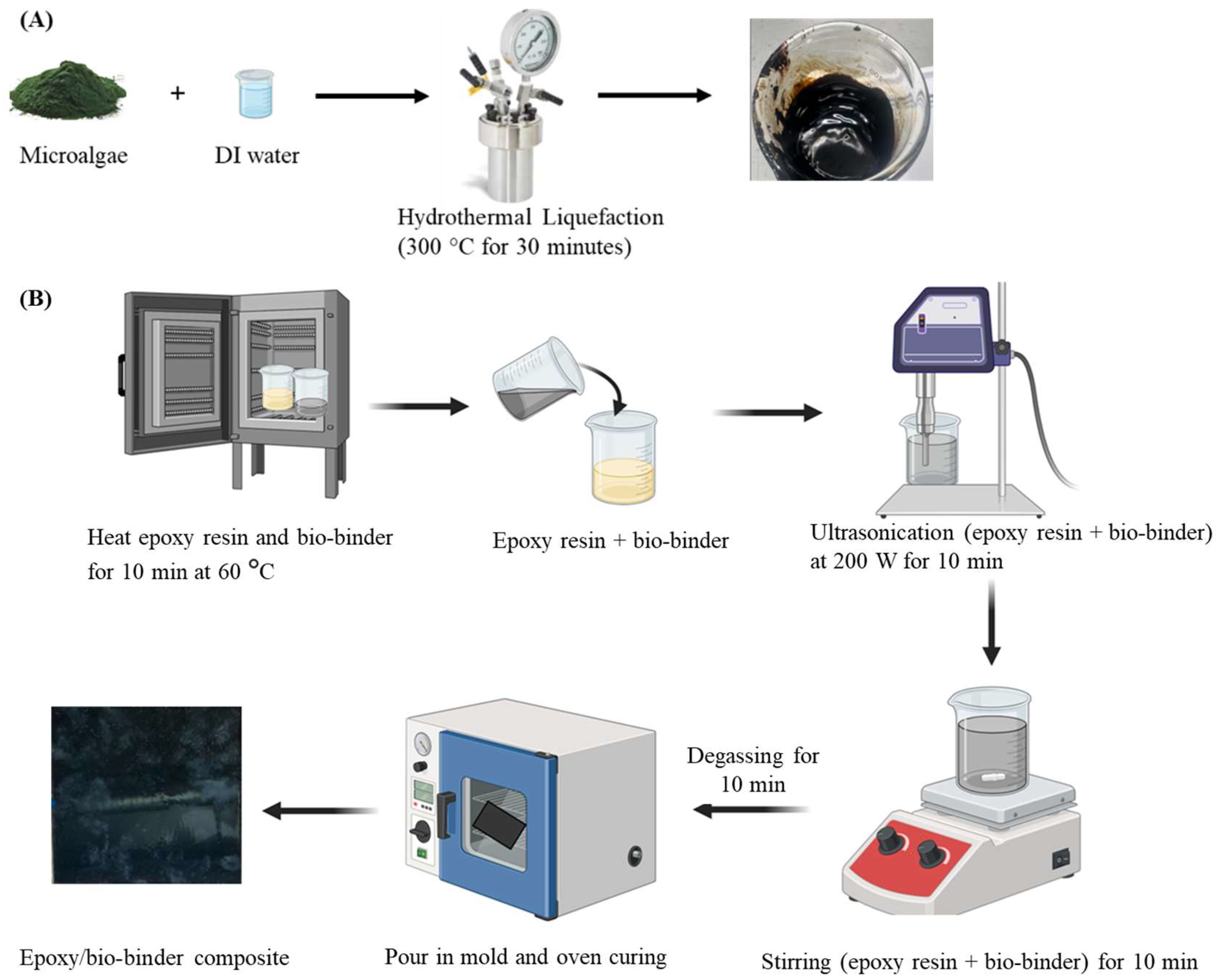
3.2. Bio-Binder Production
3.3. Fabrication of Epoxy/Bio-Binder Composite
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappa, C.; Feghali, E.; Vanbroekhoven, K.; Triantafyllidis, K.S. Recent advances in epoxy resins and composites derived from lignin and related bio-oils. Curr. Opin. Green Sustain. Chem. 2022, 38, 100687. [Google Scholar] [CrossRef]
- Celikbag, Y.; Meadows, S.; Barde, M.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L.; Via, B.K. Synthesis and characterization of bio-oil-based self-curing epoxy resin. Ind. Eng. Chem. Res. 2017, 56, 9389–9400. [Google Scholar] [CrossRef]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw-Hill: New York, NY, USA, 2006; pp. 1–26. [Google Scholar]
- Agbo, P.; Mali, A.; Deng, D.; Zhang, L. Bio-oil-based epoxy resins from thermochemical processing of sustainable resources: A short review. J. Compos. Sci. 2023, 7, 374. [Google Scholar] [CrossRef]
- Goncalves, F.A.M.M.; Santos, M.; Cernadas, T.; Ferreira, P.; Alves, P. Advances in the development of biobased epoxy resins: Insight into more sustainable materials and future applications. Int. Mater. Rev. 2022, 67, 119–149. [Google Scholar] [CrossRef]
- Dinu, R.; Lafont, U.; Damiano, O.; Mija, A. High glass transition materials from sustainable epoxy resins with potential applications in the aerospace and space sector. ACS Appl. Polym. Mater. 2022, 4, 3636–3646. [Google Scholar] [CrossRef]
- Chong, K.L.; Lai, J.C.; Rahman, R.A.; Adrus, N.; Al-Saffar, Z.H.; Hassan, A.; Lim, T.H.; Wahit, M.U. A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils. Ind. Crops Prod. 2022, 189, 115857. [Google Scholar] [CrossRef]
- Rapi, Z.; Szolnoki, B.; Bako, P.; Niedermann, P.; Toldy, A.; Bodzay, B.; Keglevich, G.; Morosi, G. Synthesis and Characterization of biobased epoxy monomers derived from D-glucose. Eur. Polym. J. 2015, 67, 375–382. [Google Scholar] [CrossRef]
- Marriam, F.; Irshad, A.; Umer, I.; Asghar, M.A.; Atif, M. Vegetable oils as bio-based precursors for epoxies. Sustain. Chem. Pharm. 2023, 31, 100935. [Google Scholar] [CrossRef]
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully biobased epoxy resins from fatty acids and lignin. Molecules 2020, 25, 1158. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, L.; Turchetta, S.; Parodo, G.; Papa, R.; Toto, E.; Santonicola, M.G.; Laurenzi, S. RIFT process analysis for the production of green composites in flax fibers and bio-based epoxy resin. Materials 2022, 15, 8173. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Bouscher, R.F.; Nwosu, J.; Soucek, M.D. Sustainable thermosets and composites based on the epoxides of norbornylized seed oils and biomass fillers. ACS Sustain. Chem. Eng. 2022, 10, 12342–12354. [Google Scholar] [CrossRef]
- Hidalgo, P.; Salgado, L.; Ibacache, N.; Hunter, R. Influence of biochar and bio-oil loading on the properties of epoxy resin composites. Polymers 2023, 15, 1895. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.M.; Zhang, B.; Wang, L.; Joseph, G.; Shahbazi, A. A combined fermentation and ethanol-assisted liquefaction process to produce biofuel from Nannochloropsis sp. Fuel 2019, 238, 159–165. [Google Scholar] [CrossRef]
- Chen, W.-T.; Qian, W.; Zhang, Y.; Mazur, Z.; Kuo, C.-T.; Scheppe, K.; Schideman, L.C.; Sharma, B.K. Effect of ash on hydrothermal liquefaction of high-ash content algal biomass. Algal Res. 2017, 25, 297–306. [Google Scholar] [CrossRef]
- Muppaneni, T.; Reddy, H.K.; Selvaratnam, T.; Dandamudi, K.P.R.; Dungan, B.; Nirmalakhandan, N.; Schaub, T.; Holguin, F.O.; Voorhies, W.; Lammers, P. Hydrothermal liquefaction of Cyanidioschyzon merolae and the influence of catalysts on products. Bioresour. Technol. 2017, 223, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res. 2015, 8, 15–22. [Google Scholar] [CrossRef]
- Liu, L.; Gao, S.; Jiang, Z.; Zhang, Y.; Gui, D.; Zhang, S. Amide-funcitonalized ionic liquid as curing agents for epoxy resin: Preparation, characterization, and curing behaviors with TDE-85. Ind. Eng. Chem. Res. 2019, 58, 14088–14097. [Google Scholar] [CrossRef]
- Galehdari, N.A.; Kelkar, A.D. Effect of neutron radiation on the mechanical and thermophysical properties of nanoengineered polymer composites. J. Mater. Res. 2017, 32, 426–434. [Google Scholar] [CrossRef]
- Wei, N.; Via, B.; Wang, Y.; McDonald, T.; Auad, M. Liquefaction and substitution of switchgrass (Panicum virgatum) based bio-oil into epoxy resins. Ind. Crops Prod. 2014, 57, 116–123. [Google Scholar] [CrossRef]


| Bio-Binder Loading (wt.%) | Curing Time (h) | Curing Temperature | ||||
|---|---|---|---|---|---|---|
| 93.3 °C | 121.1 °C | 148.9 °C | 176.7 °C | 204.4 °C | ||
| 0 | 1 | 24.8 | 47.5 | 72.4 | 84.8 | 129.2 |
| 2 | 31.3 | 49.3 | 88.0 | 126.0 | 134.1 | |
| 3 | 36.8 | 56.3 | 97.3 | 135.8 | 141.0 | |
| 4 | 39.6 | 96.6 | 112.9 | 138.5 | 142.0 | |
| 30 | 1 | 26.1 | 26.5 | 28.5 | 299.0 | 30.5 |
| 2 | 26.4 | 28.4 | 28.6 | 32.4 | 33.2 | |
| 3 | 28.0 | 25.9 | 30.1 | 39.2 | 42.4 | |
| 4 | 28.4 | 28.8 | 33.3 | 39.9 | 45.4 | |
| 35 | 1 | 27.1 | 29.3 | 29.4 | 30.4 | 31.2 |
| 2 | 29.2 | 30.4 | 31.4 | 38.5 | 38.6 | |
| 3 | 30.8 | 31.3 | 38.1 | 39.4 | 42.4 | |
| 4 | 35.5 | 37.3 | 41.3 | 48.2 | 55.0 | |
| 40 | 1 | 29.0 | 30.5 | 34.8 | 45.4 | 47.7 |
| 2 | 30.1 | 32.7 | 39.5 | 42.9 | 51.2 | |
| 3 | 31.3 | 33.5 | 41.3 | 43.2 | 53.7 | |
| 4 | 38.0 | 47.6 | 48.8 | 49.5 | 56.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbo, P.; Mali, A.; Kelkar, A.D.; Wang, L.; Zhang, L. Injecting Sustainability into Epoxy-Based Composite Materials by Using Bio-Binder from Hydrothermal Liquefaction Processing of Microalgae. Molecules 2024, 29, 3656. https://doi.org/10.3390/molecules29153656
Agbo P, Mali A, Kelkar AD, Wang L, Zhang L. Injecting Sustainability into Epoxy-Based Composite Materials by Using Bio-Binder from Hydrothermal Liquefaction Processing of Microalgae. Molecules. 2024; 29(15):3656. https://doi.org/10.3390/molecules29153656
Chicago/Turabian StyleAgbo, Philip, Abhijeet Mali, Ajit D. Kelkar, Lijun Wang, and Lifeng Zhang. 2024. "Injecting Sustainability into Epoxy-Based Composite Materials by Using Bio-Binder from Hydrothermal Liquefaction Processing of Microalgae" Molecules 29, no. 15: 3656. https://doi.org/10.3390/molecules29153656
APA StyleAgbo, P., Mali, A., Kelkar, A. D., Wang, L., & Zhang, L. (2024). Injecting Sustainability into Epoxy-Based Composite Materials by Using Bio-Binder from Hydrothermal Liquefaction Processing of Microalgae. Molecules, 29(15), 3656. https://doi.org/10.3390/molecules29153656









