An Innovative Aggregation-Induced Emission-Based NIR Fluorescent Probe for Visualizing Carboxylesterases in Living Cells, Zebrafish, and Tumor-Bearing Mice
Abstract
1. Introduction
2. Results and Discussion
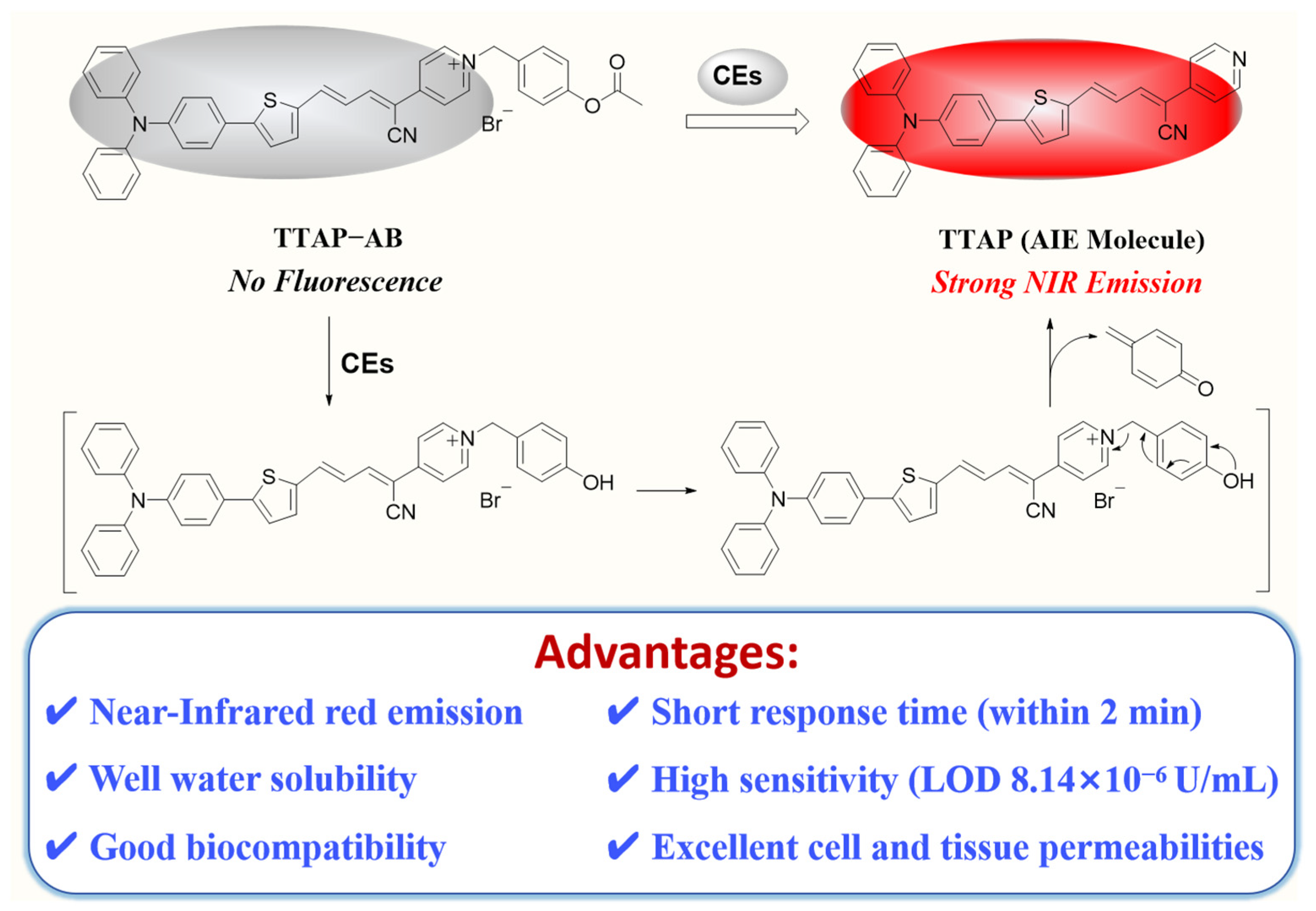
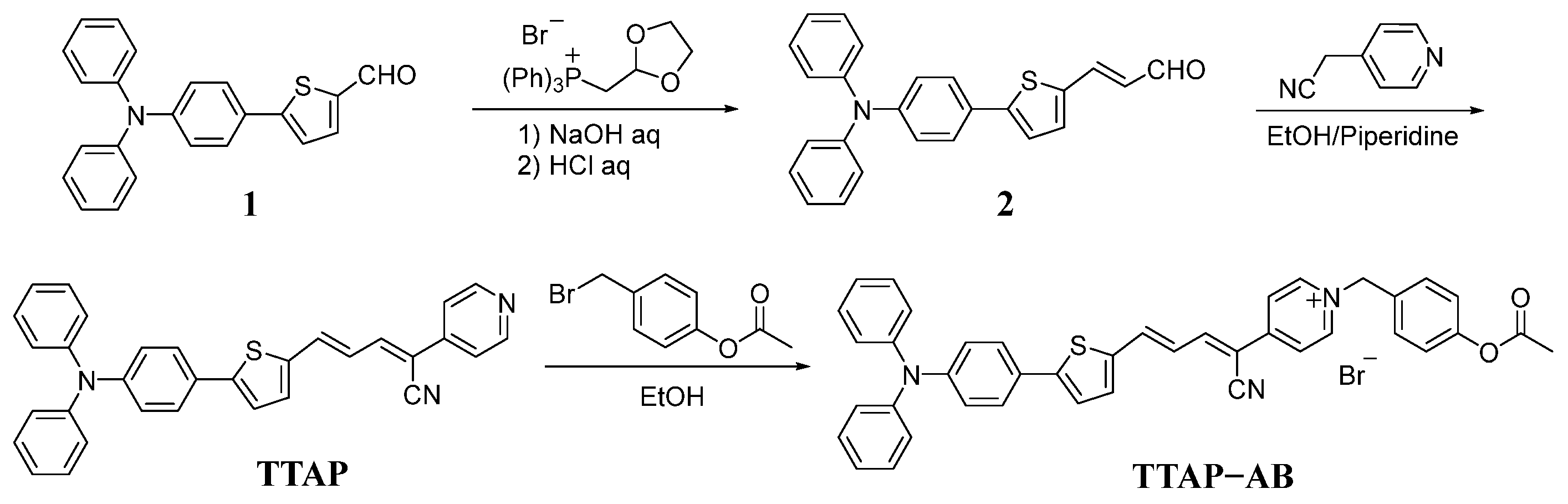
2.1. Synthesis and Characterization of the TTAP−AB Probe
2.2. Spectroscopic Response of TTAP−AB to CEs
2.3. The Studies of Selectivity, Response Time, and pH Effect
2.4. Sensing Mechanism
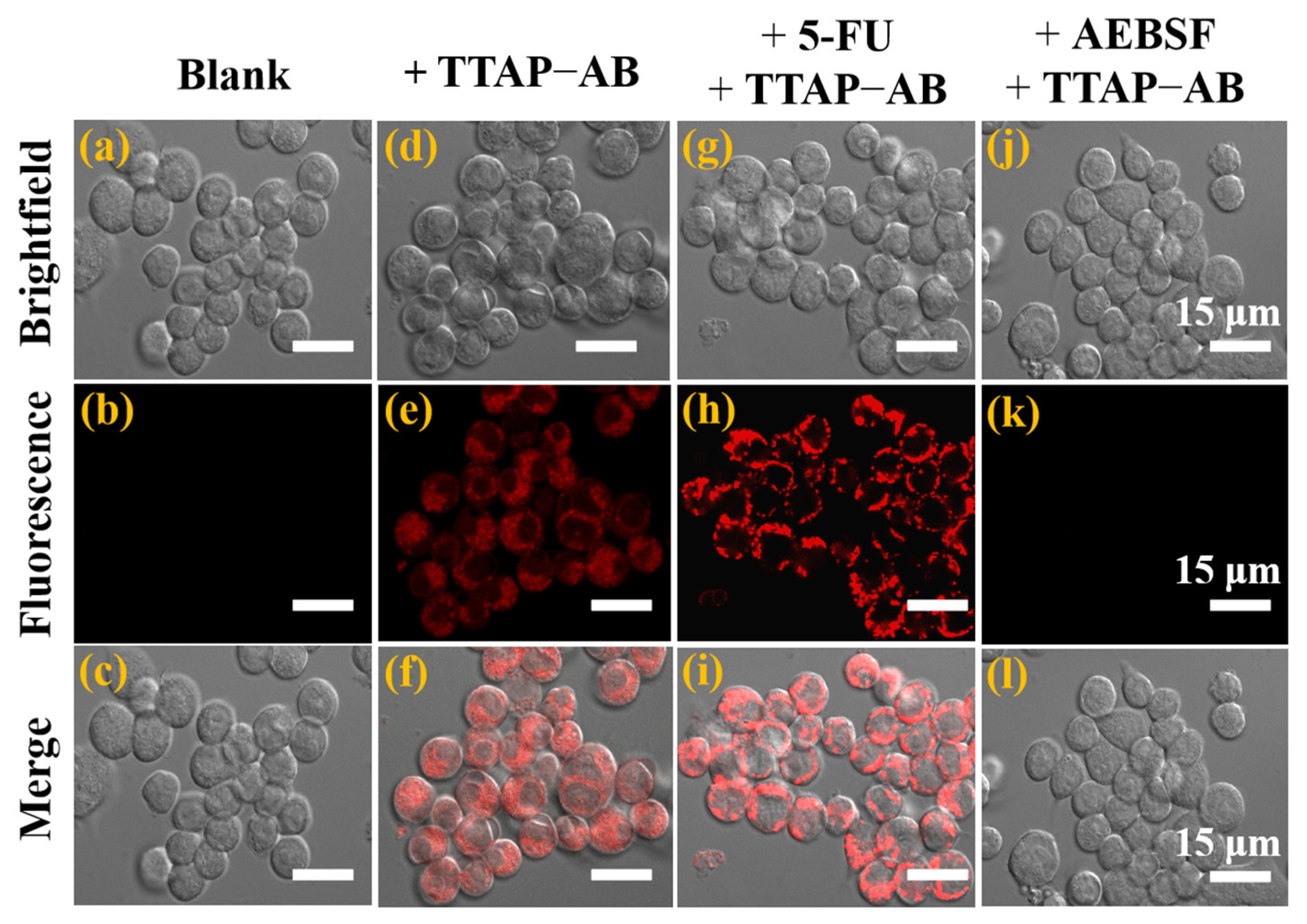
2.5. Cell Imaging of CEs
2.6. Visualization of CEs Levels in Zebrafish
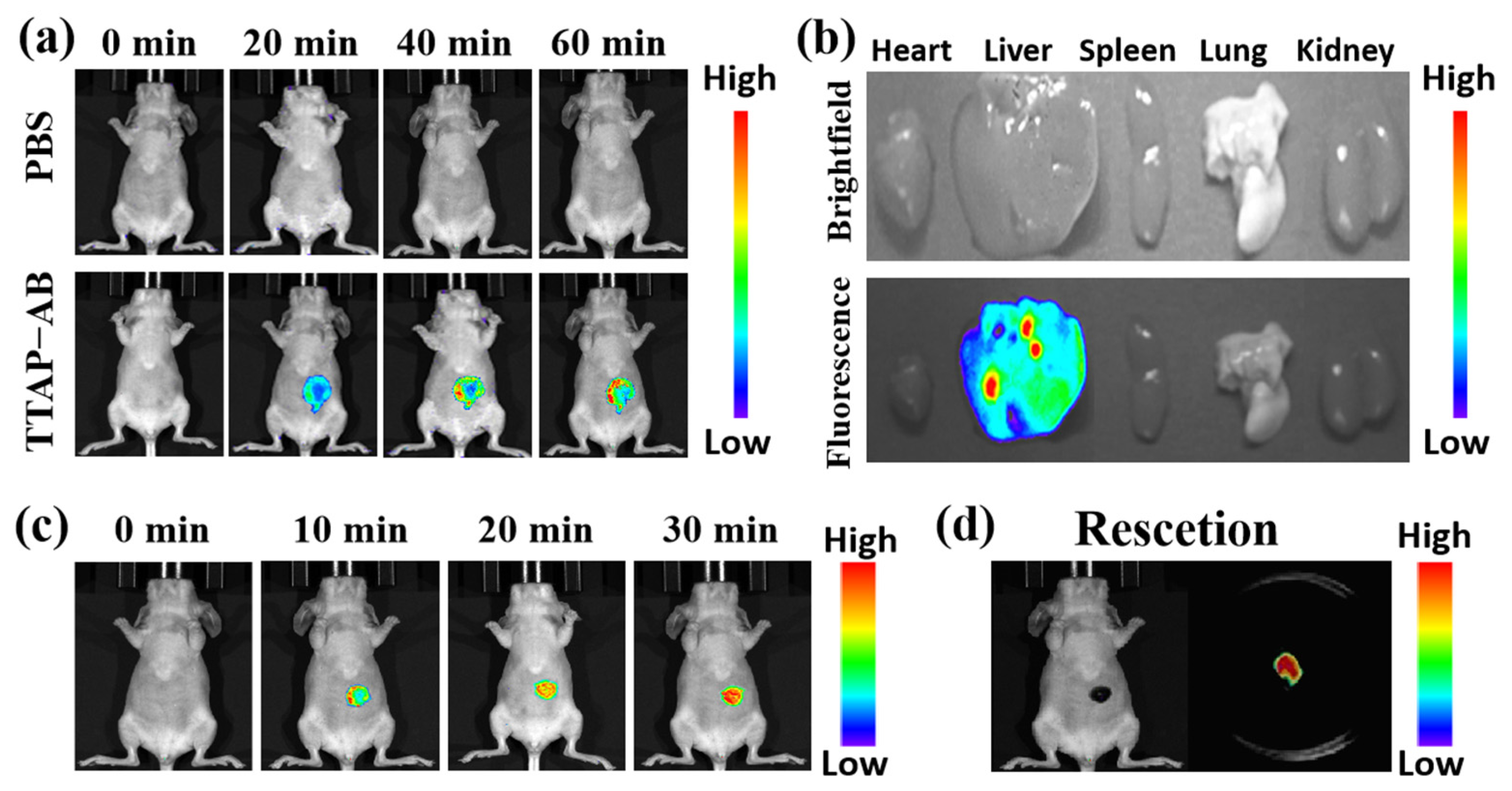
2.7. Bioimaging of CEs in Tumor-Bearing Mice
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Fluorophore TTAP
3.3. Synthesis of the TTAP−AB Probe
3.4. Optical Study
3.5. Living Cell Imaging
3.6. Zebrafish Imaging
3.7. Fluorescence Imaging in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laizure, S.C.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The role of human carboxylesterases in drug metabolism: Have we overlooked their importance? Pharmacotherapy 2013, 33, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Nelson, R.; Lehner, R. Carboxylesterases in lipid metabolism: From mouse to human. Protein Cell 2018, 9, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, E.Y.; Lee, H.J.; Kim, K.Y.; Lee, H.; Jeong, S.K.; Jeong, A.S.; Cho, S.Y.; Kim, S.A.; Song, S.Y.; et al. Human plasma carboxylesterase 1, a novel serologic biomarker candidate for hepatocellular carcinoma. Proteomics 2009, 9, 3989–3999. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, Y.; Huang, W.; Kong, H.; Li, Y.; Cheng, H.; Li, L. A magnetic SERS immunosensor for highly sensitive and selective detection of human carboxylesterase 1 in human serum samples. Anal. Chim. Acta 2020, 1097, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Flinders, C.; Mumenthaler, S.M.; Hummon, A.B. MALDI mass spectrometry imaging for evaluation of therapeutics in colorectal tumor organoids. J. Am. Soc. Mass Spectrom. 2018, 29, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Jin, Q.; Hou, J.; Feng, L.; Li, N.; Li, S.Y.; Zhou, Q.; Zou, L.W.; Ge, G.B.; Wang, J.G.; et al. Highly sensitive and selective detection of human carboxylesterase 1 activity by liquid chromatography with fluorescence detection. J. Chromatogr. B 2016, 1008, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, T.; Yu, F.; Guo, L.; Wu, B. E4bp4 regulates carboxylesterase 2 enzymes through repression of the nuclear receptor Rev-erbα in mice. Biochem. Pharmacol. 2018, 152, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gao, M.; Beck, M.W. Human carboxylesterases and fluorescent probes to image their activity in live cells. RSC Med. Chem. 2021, 12, 1142–1153. [Google Scholar] [CrossRef]
- Wang, K.; Wang, R.; Yan, Z.; Li, Y.; Shi, Y.; Ge, J.Y.; Bai, Y.; Chen, Z.; Zhang, L. Rational design of a highly sensitive carboxylesterase probe and its application in high-throughput screening for uncovering carboxylesterase inhibitors. J. Org. Chem. 2024. [Google Scholar] [CrossRef]
- Lin, X.; Liu, M.; Yi, Q.; Zhou, Y.; Su, J.; Qing, B.; Lu, Y.; Pu, C.; Lan, W.; Zou, L.; et al. Design, synthesis, and evaluation of a carboxylesterase detection probe with therapeutic effects. Talanta 2024, 274, 126060. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.I.; Wang, H.R.; Chen, L.L.; Yang, B.; Yang, Y.S.; He, Z.X.; Zhu, H.L. Multifunctional fluorescent probe for simultaneously detecting microviscosity, micropolarity, and carboxylesterases and its application in bioimaging. Anal. Chem. 2022, 94, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qin, S.; Wang, N.; Lu, X.; Jiao, J.; Zhang, J.; Zhao, W. Diketopyrrolopyrrole-based fluorescent probe for visualizing over-expressed carboxylesterase in fever via ratiometric imaging. Talanta 2024, 266, 124971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qi, C.; Wang, X.; Fu, Z.; Zhang, J.; Zhou, Y.; Wang, Y. An ultrasensitive and selective near-infrared fluorescent probe for tracking carboxylesterases with large Stokes shift in living cells and mice. Spectrochim. Acta A 2024, 308, 123708. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, W.; Liu, R.; Chen, Q.; Yang, J.; Ni, Z.; Yin, X.; Zhou, Q.; Tang, Z. An innovative near-infrared fluorescent probe designed to track the evolution of carboxylesterase in drug-induced liver injury. Sens. Actuators B 2024, 402, 135133. [Google Scholar] [CrossRef]
- Yang, B.; Ding, X.; Li, J.; Lai, J.; Zhang, Z.; Xu, X.; Liu, Z.; Song, Z.; Wang, X.; Wang, B. Dihydroxanthene-derived fluorescent probe with near-infrared excitation and emission maxima for detecting human carboxylesterase-2 and bioimaging. Sens. Actuators B 2023, 395, 134503. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zou, X.; Guo, Y.; Gao, X. A highly sensitive and selective enzyme activated fluorescent probe for in vivo profiling of carboxylesterase 2. Anal. Chim. Acta 2022, 1221, 340126. [Google Scholar] [CrossRef]
- Shu, Y.; Huang, C.; Liu, H.; Hu, F.; Wen, H.; Liu, J.; Wang, X.; Shan, C.; Li, W. A hemicyanine-based fluorescent probe for simultaneous imaging of carboxylesterases and histone deacetylases in hepatocellular carcinoma. Spectrochim. Acta A 2022, 281, 121529. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jing, N.; Zhang, M.; Huo, F.; Li, Z.; Yin, C. A space-dependent ‘enzyme-substrate’ type probe based on ‘carboxylesterase-amide group’ for ultrafast fluorescent imaging orthotopic hepatocellular carcinoma. Adv. Sci. 2023, 10, 2206681. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Sun, L.; Zhao, X.; He, Y.; Gao, G.; Han, W.; Zhou, J. Lysosome-targeting red fluorescent probe for broad carboxylesterases detection in breast cancer cells. Chin. Chem. Lett. 2022, 33, 4229–4232. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, J.; Chen, Y.; Zheng, S.; Chen, X.; Wang, Y.; Wang, F. The visualized fluorescent probes based on benzothiazole used to detect esterase. Dye. Pigment. 2021, 191, 109349. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, Y.; Hou, Y.; Zhong, G.; Gao, R.; Wu, J.; Shen, B.; Zhang, X. Detection of carboxylesterase 1 and carbamates with a novel fluorescent protein chromophore based probe. Dye. Pigment. 2021, 192, 109444. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Reja, S.I.; Minoshima, M.; Hori, Y.; Kikuchi, K. Near-infrared fluorescent probes: A next-generation tool for protein-labeling applications. Chem. Sci. 2020, 12, 3437–3447. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Huang, Y.; Tian, H. Progress and trends in AIE-based bioprobes: A brief overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.Y.; Mei, L.J.; Tian, R.; Li, C.; Wang, Y.L.; Xiang, S.L.; Zhu, M.Q.; Tang, B.Z. Recent advances in super-resolution optical imaging based on aggregation-induced emission. Chem. Soc. Rev. 2024, 53, 3350–3383. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hou, M.; Xu, Z.; Tan, L.; Zhong, C.; Zhu, L. A new red fluorophore with aggregation enhanced emission by an unexpected “One-step” protocol. RSC Adv. 2018, 8, 18327–18333. [Google Scholar] [CrossRef]
- Shen, X.Y.; Wang, Y.J.; Zhao, E.; Yuan, W.Z.; Liu, Y.; Lu, P.; Qin, A.; Ma, Y.; Sun, J.Z.; Tang, B.Z. Effects of substitution with donor−acceptor groups on the properties of tetraphenylethene trimer: Aggregation-induced emission, solvatochromism, and mechanochromism. J. Phys. Chem. C 2013, 117, 7334–7347. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, W.; Gong, S.; Jiang, S.; Feng, G. Selective visualization of tumor cell membranes and tumors with a viscosity-sensitive plasma membrane probe. Anal. Chem. 2023, 95, 7254–7261. [Google Scholar] [CrossRef] [PubMed]
- Oushiki, D.; Kojima, H.; Terai, T.; Arita, M.; Hanaoka, K.; Urano, Y.; Nagano, T. Development and application of a near-infrared fluorescence probe for oxidative stress based on differential reactivity of linked cyanine dyes. J. Am. Chem. Soc. 2010, 132, 2795–2801. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Chen, D.-D.; Liu, H.-W.; Ma, M.-L.; Zhang, L.; Cui, H.-R. An Innovative Aggregation-Induced Emission-Based NIR Fluorescent Probe for Visualizing Carboxylesterases in Living Cells, Zebrafish, and Tumor-Bearing Mice. Molecules 2024, 29, 3660. https://doi.org/10.3390/molecules29153660
Gao C, Chen D-D, Liu H-W, Ma M-L, Zhang L, Cui H-R. An Innovative Aggregation-Induced Emission-Based NIR Fluorescent Probe for Visualizing Carboxylesterases in Living Cells, Zebrafish, and Tumor-Bearing Mice. Molecules. 2024; 29(15):3660. https://doi.org/10.3390/molecules29153660
Chicago/Turabian StyleGao, Chao, Dan-Dan Chen, Hu-Wei Liu, Ming-Lan Ma, Lin Zhang, and Hai-Rong Cui. 2024. "An Innovative Aggregation-Induced Emission-Based NIR Fluorescent Probe for Visualizing Carboxylesterases in Living Cells, Zebrafish, and Tumor-Bearing Mice" Molecules 29, no. 15: 3660. https://doi.org/10.3390/molecules29153660
APA StyleGao, C., Chen, D.-D., Liu, H.-W., Ma, M.-L., Zhang, L., & Cui, H.-R. (2024). An Innovative Aggregation-Induced Emission-Based NIR Fluorescent Probe for Visualizing Carboxylesterases in Living Cells, Zebrafish, and Tumor-Bearing Mice. Molecules, 29(15), 3660. https://doi.org/10.3390/molecules29153660






