Advances and Applications of Hybrid Graphene-Based Materials as Sorbents for Solid Phase Microextraction Techniques
Abstract
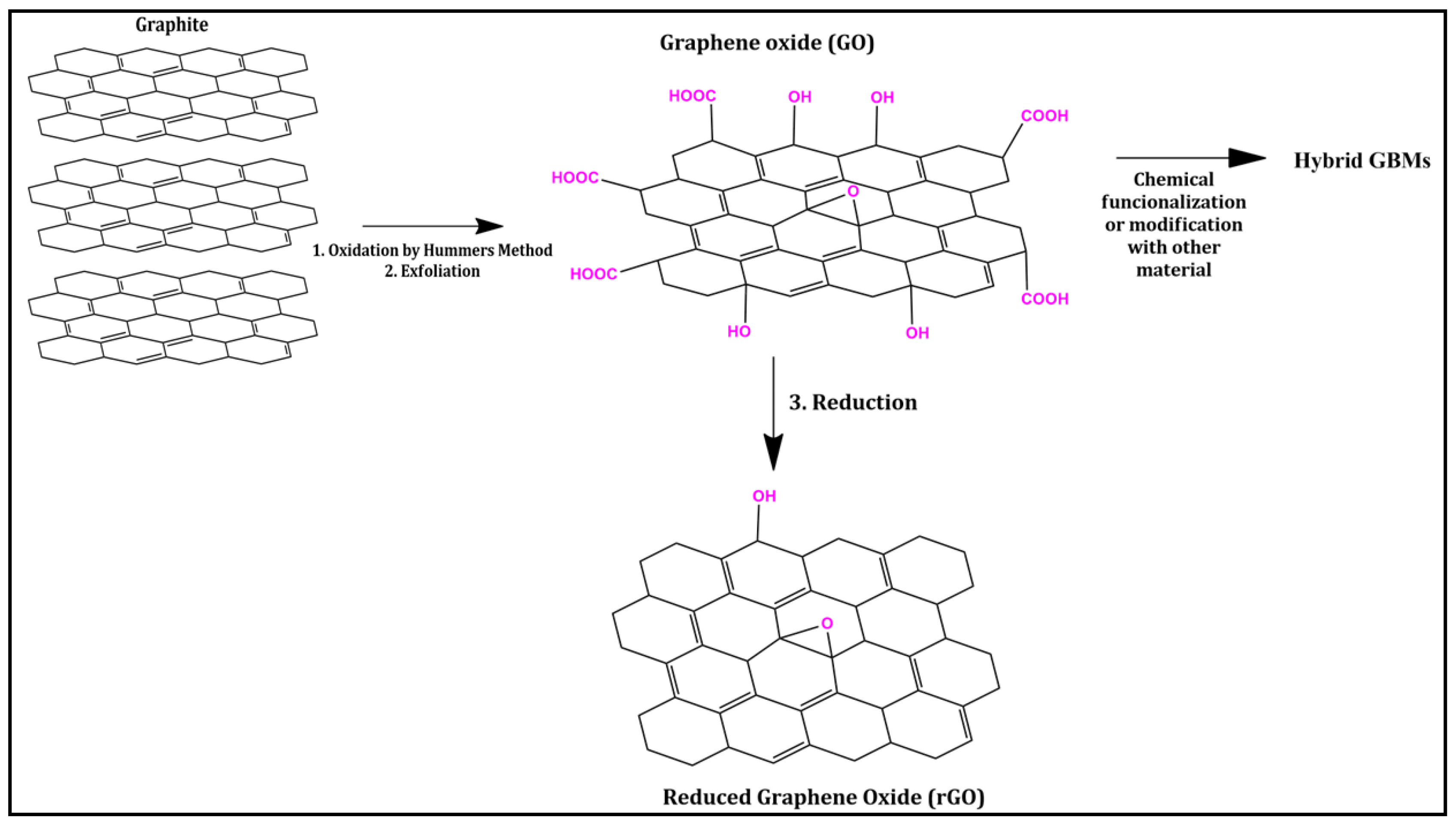
:1. Introduction
2. Hybrid Graphene-Based Materials (Hybrid GBMs)
2.1. Silica Derivatives
2.2. Ionic Liquids
2.3. Magnetic Materials
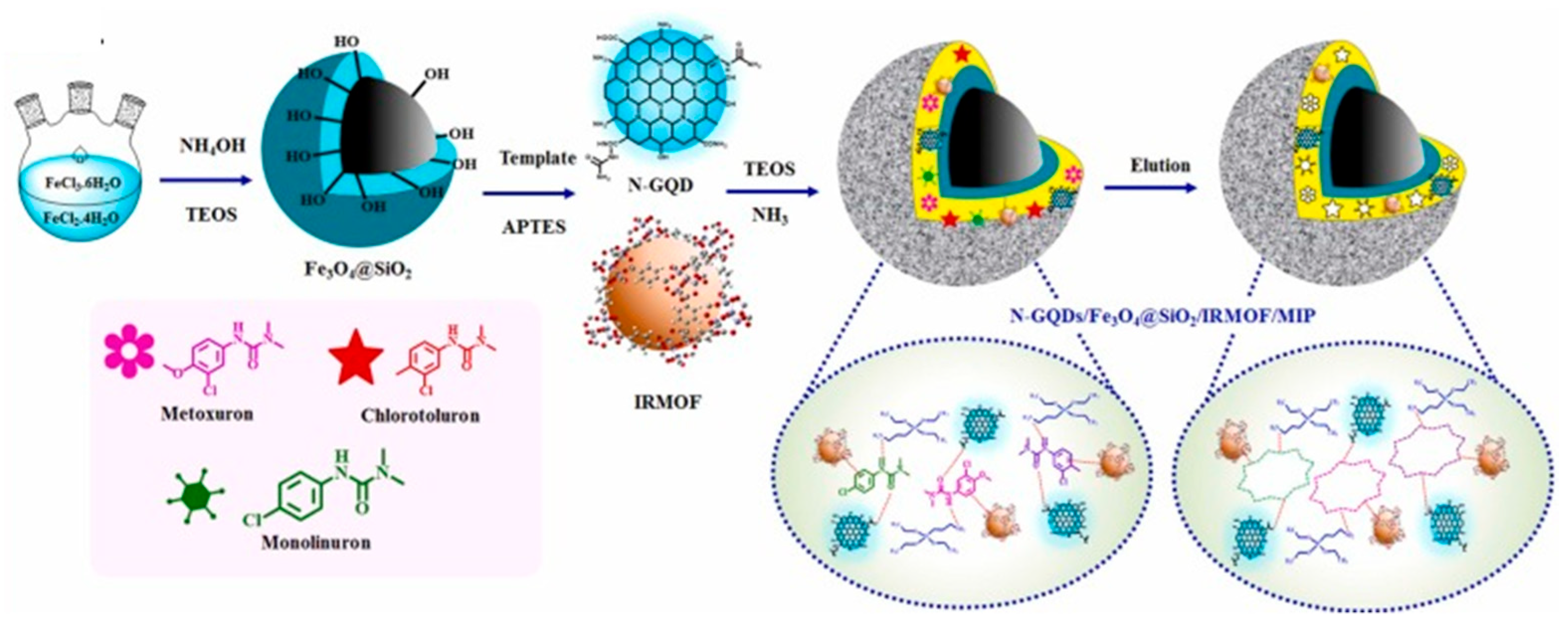
2.4. Molecularly Imprinted Polymers
2.5. Graphene-Based Biosorbents
2.5.1. Graphene-Based Materials Immobilized with Chitosan
2.5.2. Graphene-Based Materials Immobilized with Cyclodextrin
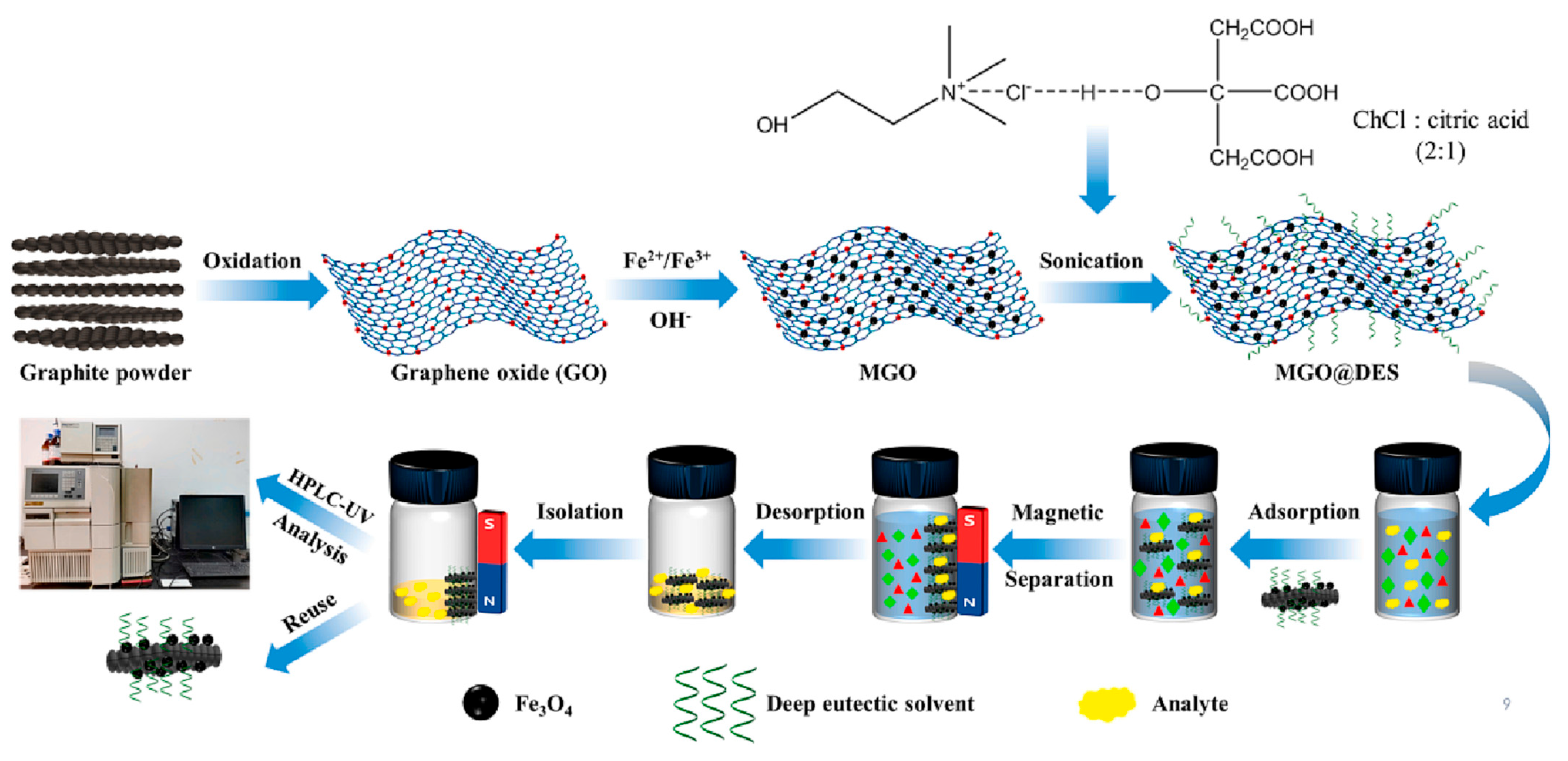
2.6. Deep Eutetic Solvents
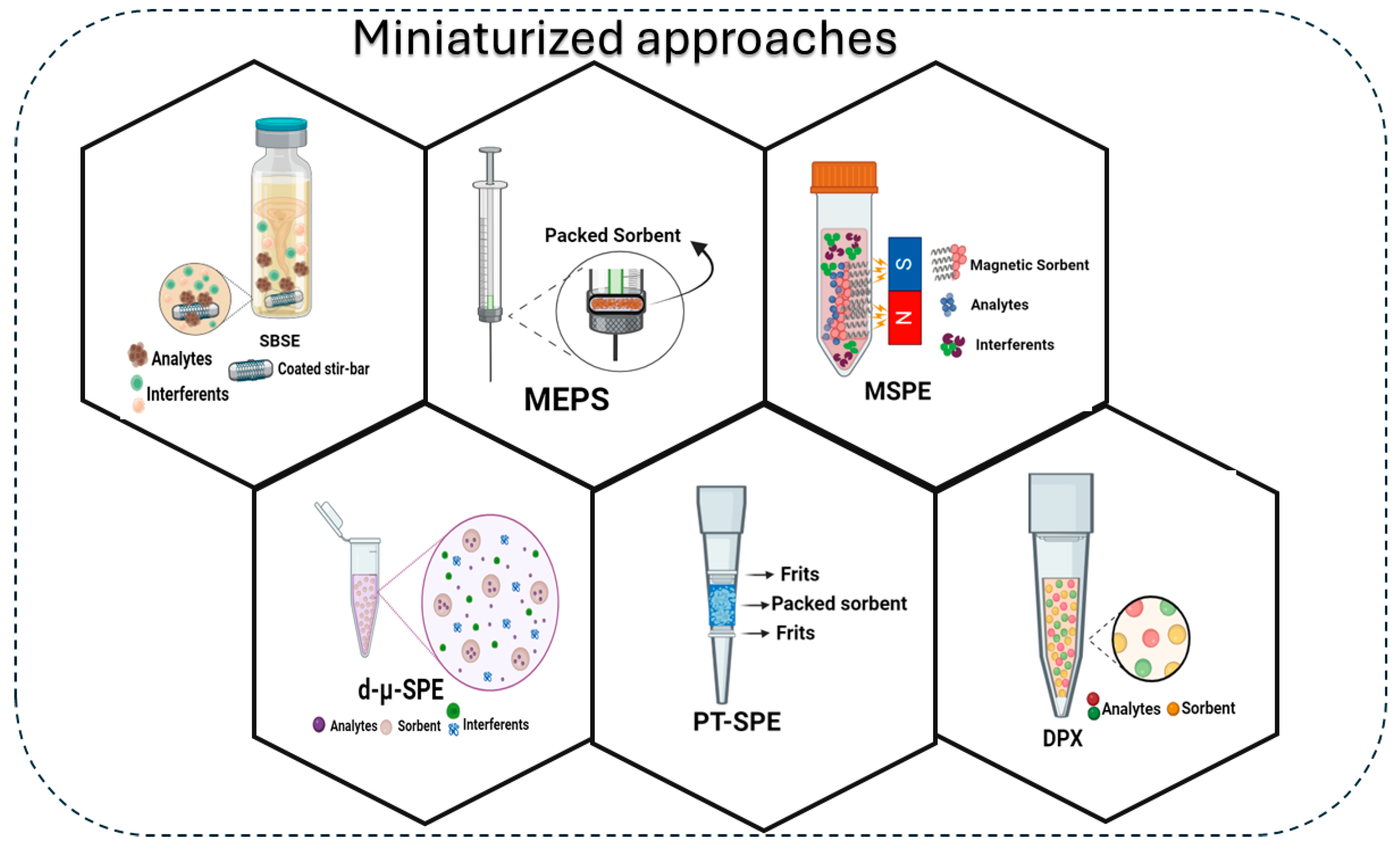
3. Selected Applications of GBMs in Key Miniaturized Techniques
3.1. Stir Bar Sorptive Extraction
3.2. Microextraction by Packed Sorbent
3.3. Pipette-Tip Solid-Phase Extraction and Disposable Pipette Extraction
3.4. Dispersive Micro Solid-Phase Extraction
3.5. Magnetic Solid-Phase Extraction
4. Concluding Remarks and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valor. 2022, 13, 1385–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zhang, X. The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules 2024, 29, 1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, H.K. Plunger-in-Needle Solid-Phase Microextraction with Graphene-Based Sol-Gel Coating as Sorbent for Determination of Polybrominated Diphenyl Ethers. J. Chromatogr. A 2011, 1218, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.B.; Shi, Z.G.; Gao, Q.; Feng, Y.Q. Magnetic Retrieval of Graphene: Extraction of Sulfonamide Antibiotics from Environmental Water Samples. J. Chromatogr. A 2011, 1218, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.B.; Cheng, J.S.; Ma, Q.; Feng, Y.Q.; Li, J.H. Graphene-Polymer Composite: Extraction of Polycyclic Aromatic Hydrocarbons from Water Samples by Stir Rod Sorptive Extraction. Anal. Methods 2011, 3, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.S.; Mejía-Carmona, K.; Jordan-Sinisterra, M.; da Silva, L.F.; Vargas Medina, D.A.; Lanças, F.M. The Current Role of Graphene-Based Nanomaterials in the Sample Preparation Arena. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Ratandeep; Dutt, M.; Kaur, B.; Punia, S.; Sharma, S.; Sahu, P.K.; Pooja; Saya, L. Graphene-Based Nanomaterials as Potential Candidates for Environmental Mitigation of Pesticides. Talanta 2024, 272, 125748. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC-Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Lu, X.; Tang, S.; Wang, L.; Guo, Y. Graphene Oxide Reinforced Ionic Liquid-Functionalized Adsorbent for Solid-Phase Extraction of Phenolic Acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, C.; Yan, H.; Han, Y.; Han, D. An Integrated Solid Phase Extraction with Ionic Liquid-Thiol-Graphene Oxide as Adsorbent for Rapid Isolation of Fipronil Residual in Chicken Eggs. J. Chromatogr. A 2020, 1631, 461568. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.C.; Lanças, F.M. Determination of Selected Herbicides in Sugarcane-Derived Foods by Graphene-Oxide Based Disposable Pipette Extraction Followed by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2023, 1687, 463690. [Google Scholar] [CrossRef] [PubMed]
- Fumes, B.H.; Lanças, F.M. Use of Graphene Supported on Aminopropyl Silica for Microextraction of Parabens from Water Samples. J. Chromatogr. A 2017, 1487, 64–71. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Rahman, I. Dispersive Solid Phase Microextraction of Fenoxaprop-p-Ethyl Herbicide from Water and Food Samples Using Magnetic Graphene Composite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1716–1725. [Google Scholar] [CrossRef]
- Akamine, L.A.; Vargas Medina, D.A.; Lanças, F.M. Magnetic Solid-Phase Extraction of Gingerols in Ginger Containing Products. Talanta 2021, 222, 121683. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gao, Y.; Tan, K.; Wei, W.; Liu, X. Preparation of a Magnetic Molecularly Imprinted Graphene Composite Highly Adsorbent for 4-Nitrophenol in Aqueous Medium. ACS Sustain. Chem. Eng. 2016, 4, 3316–3326. [Google Scholar] [CrossRef]
- Cheng, L.; Pan, S.; Ding, C.; He, J.; Wang, C. Dispersive Solid-Phase Microextraction with Graphene Oxide Based Molecularly Imprinted Polymers for Determining Bis(2-Ethylhexyl) Phthalate in Environmental Water. J. Chromatogr. A 2017, 1511, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Wang, M.; Cao, J.; Yan, H. Green Synthesis of Superhydrophilic Resin/Graphene Oxide for Efficient Analysis of Multiple Pesticide Residues in Fruits and Vegetables. Food Chem. 2024, 450, 139341. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel Ibukun, A.; Yahaya, N.; Husaini Mohamed, A.; Semail, N.F.; Abd Hamid, M.A.; Nadhirah Mohamad Zain, N.; Anuar Kamaruddin, M.; Hong Loh, S.; Kamaruzaman, S. Recent Developments in Synthesis and Characterisation of Graphene Oxide Modified with Deep Eutectic Solvents for Dispersive and Magnetic Solid-Phase Extractions. Microchem. J. 2024, 199, 110111. [Google Scholar] [CrossRef]
- Miyardan, F.N.; Afshar Mogaddam, M.R.; Farajzadeh, M.A.; Nemati, M. Combining Modified Graphene Oxide-Based Dispersive Micro Solid Phase Extraction with Dispersive Liquid–Liquid Microextraction in the Extraction of Some Pesticides from Zucchini Samples. Microchem. J. 2022, 182, 107884. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Vargas Medina, D.A.; Lanças, F.M. Automated Needle-Sleeve Based Online Hyphenation of Solid-Phase Microextraction and Liquid Chromatography. Talanta 2021, 221, 121608. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.F.; Lanças, F.M. β-Cyclodextrin Coupled to Graphene Oxide Supported on Aminopropyl Silica as a Sorbent Material for Determination of Isoflavones. J. Sep. Sci. 2020, 43, 4347–4355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, P.; Lu, Y.; Cao, J.; Yan, H. Development of a Three-Dimensional Porous Ionic Liquid-Chitosan-Graphene Oxide Aerogel for Efficient Extraction and Detection of Polyhalogenated Carbazoles in Sediment Samples. Talanta 2024, 271, 125711. [Google Scholar] [CrossRef] [PubMed]
- Vargas Medina, D.A.; Cardoso, A.T.; Maciel, E.V.S.; Lanças, F.M. Current Materials for Miniaturized Sample Preparation: Recent Advances and Future Trends. TrAC-Trends Anal. Chem. 2023, 165, 117120. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, S.; Sun, M. Recent Advances on Graphene and Graphene Oxide as Extraction Materials in Solid-Phase (Micro)Extraction. TrAC-Trends Anal. Chem. 2023, 168, 117283. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Vargas-Medina, D.A.; Lancas, F.M. Analyzes of β-Lactam Antibiotics by Direct Injection of Environmental Water Samples into a Functionalized Graphene Oxide-Silica Packed Capillary Extraction Column Online Coupled to Liquid Chromatography Tandem Mass Spectrometry. Talanta Open 2023, 7, 100185. [Google Scholar] [CrossRef]
- Soares Maciel, E.V.; de Toffoli, A.L.; da Silva Alves, J.; Lanças, F.M. Multidimensional Liquid Chromatography Employing a Graphene Oxide Capillary Column as the First Dimension: Determination of Antidepressant and Antiepileptic Drugs in Urine. Molecules 2020, 25, 1092. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.G.; Santos, D.; Vasconcelos, E.; Maciel, S.; Mejía-Carmona, K.; Lanças, F.M. Multidimensional Capillary Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Multiclass Pesticides in “Sugarcane Spirits” (Cachaças). Anal. Bioanal. Chem. 2020, 412, 7789–7797. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Huang, J.; Wu, C.; Zhao, X.; Feng, Y.; Gao, Y. Adaptive Polarity of Graphene Oxide Anchored Silica Doped with C18 for Effective Enrichment of Aflatoxins from Foodstuff. Microchem. J. 2024, 197, 109728. [Google Scholar] [CrossRef]
- Oviedo, M.N.; Botella, M.B.; Fiorentini, E.F.; Pacheco, P.; Wuilloud, R.G. A Simple and Green Dispersive Micro-Solid Phase Extraction Method by Combined Application of Graphene Oxide and a Magnetic Ionic Liquid for Selective Determination of Inorganic Antimony Species in Water, Tea and Honey Samples. Spectrochim. Acta Part B At. Spectrosc. 2023, 199, 106591. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Guo, H.; Bian, Y.; Li, J.; Zhang, F. Magnetic Graphene Oxide−based Covalent Organic Frameworks as Novel Adsorbent for Extraction and Separation of Triazine Herbicides from Fruit and Vegetable Samples. Anal. Chim. Acta 2022, 1219, 339984. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, P.; Sadegh, N.; Asfaram, A. Nanostructured Magnetic Graphene Oxide/UIO-66 Sorbent for Ultrasound-Assisted Dispersive Solid-Phase Microextraction of Food Colorants in Soft Drinks, Candies, and Pastilles Prior to HPLC Analysis. Microchem. J. 2023, 184, 108149. [Google Scholar] [CrossRef]
- Soylak, M.; Jagirani, M.S.; Uzcan, F. Metal-Doped Magnetic Graphene Oxide Nanohybrid for Solid-Phase Microextraction of Copper from Environmental Samples. Iran. J. Sci. Technol. Trans. A Sci. 2022, 46, 807–817. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Zhang, Y.; Yin, J.; Han, Y.; Han, D.; Yan, H. Miniaturized Centrifugation Accelerated Pipette-Tip Matrix Solid-Phase Dispersion Based on Poly(Deep Eutectic Solvents) Surface Imprinted Graphene Oxide Composite Adsorbent for Rapid Extraction of Anti-Adipogenesis Markers from Solidago decurrens Lour. J. Chromatogr. A 2024, 1715, 464599. [Google Scholar] [CrossRef] [PubMed]
- Sa-nguanprang, S.; Phuruangrat, A.; Bunkoed, O. A Magnetic Adsorbent of Nitrogen-Doped Graphene Quantum Dots, Zinc Metal-Organic Framework and Molecularly Imprinted Polymer to Extract Phenylureas. J. Food Compos. Anal. 2024, 126, 105911. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, L.; Cheng, J.; Huang, X.; Yan, L.; Li, H. Molecularly Imprinted Polymers Immobilized on Graphene Oxide Film for Monolithic Fiber Solid Phase Microextraction and Ultrasensitive Determination of Triphenyl Phosphate. Anal. Chim. Acta 2020, 1133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jian, Y.; Cheng, J.; Yan, L.; Huang, X. Preparation and Application of Graphene Oxide-Based Surface Molecularly Imprinted Polymer for Monolithic Fiber Array Solid Phase Microextraction of Organophosphate Flame Retardants in Environmental Water. J. Chromatogr. A 2020, 1623, 461200. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, Y.; Ouyang, S.; Huang, J.; Shi, Y.; Tong, Y.J.; Zhao, X.; Li, N.; Zheng, J.; Zheng, J.; et al. Efficient Solid Phase Microextraction of Organic Pollutants Based on Graphene Oxide/Chitosan Aerogel. Anal. Chim. Acta 2022, 1195, 339462. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Jafari, Z.; Raoof, J.B. Porous Agarose/Chitosan/Graphene Oxide Composite Coupled with Deep Eutectic Solvent for Thin Film Microextraction of Chlorophenols. J. Chromatogr. A 2023, 1694, 463899. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Xu, Y.; Bao, J.; Wang, W.; Wang, A.J. β-Cyclodextrin-Functionalized Magnetic Graphene Oxide for the Efficient Enrichment of Bisphenols in Milk and Milk Packaging. J. Chromatogr. A 2023, 1692, 463854. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Jiang, X.; Fu, J. Polydimethylsiloxane/Graphene Oxide/β-Cyclodextrin Sponge as a Solid-Phase Extraction Sorbent Coupled with Gas Chromatography-Mass Spectrometry for Rapid Adsorption and Sensitive Determination of Lavender Essential Oil. J. Sep. Sci. 2022, 45, 1904–1917. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Han, Y.; Yang, C.; Han, D.; Yan, H. Deep Eutectic Solvent Functionalized Graphene Oxide Composite Adsorbent for Miniaturized Pipette-Tip Solid-Phase Extraction of Toluene and Xylene Exposure Biomarkers in Urine Prior to Their Determination with HPLC-UV. Microchim. Acta 2020, 187, 387. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhou, W.; Wang, X.; Liu, Y.; Di, X. Carboxyl-Based Deep Eutectic Solvent Modified Magnetic Graphene Oxide as a Novel Adsorbent for Fast Enrichment and Extraction of Estrogens in Milk Prior to HPLC-UV Analysis. Microchem. J. 2023, 193, 109050. [Google Scholar] [CrossRef]
- De Toffoli, A.L.; Maciel, E.V.S.; Fumes, B.H.; Lanças, F.M. The Role of Graphene-Based Sorbents in Modern Sample Preparation Techniques. J. Sep. Sci. 2018, 41, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.S.; Borsatto, J.V.B.; Mejia-Carmona, K.; Lanças, F.M. Application of an In-House Packed Octadecylsilica-Functionalized Graphene Oxide Column for Capillary Liquid Chromatography Analysis of Hormones in Urine Samples. Anal. Chim. Acta 2023, 1239, 340718. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Carmona, K.; Lanças, F.M. Modified Graphene-Silica as a Sorbent for in-Tube Solid-Phase Microextraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry. Determination of Xanthines in Coffee Beverages. J. Chromatogr. A 2020, 1621, 461089. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liang, C.; Majeed, Z.; Tian, M.; Zhao, C.; Luo, M.; Li, C. Advances of Imidazolium Ionic Liquids for the Extraction of Phytochemicals from Plants. Separations 2023, 10, 151. [Google Scholar] [CrossRef]
- Ražić, S.; Gadžurić, S.; Trtić-Petrović, T. Ionic Liquids in Green Analytical Chemistry—Are They That Good and Green Enough? Anal. Bioanal. Chem. 2024, 416, 2023–2029. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Yuan, Y.; Han, D.; Qiao, F.; Yan, H. An Ionic Liquid Functionalized Graphene Adsorbent with Multiple Adsorption Mechanisms for Pipette-Tip Solid-Phase Extraction of Auxins in Soybean Sprouts. Food Chem. 2018, 265, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.T.; Martins, R.O.; Lanças, F.M.; Chaves, A.R. Molecularly Imprinted Polymers in Online Extraction Liquid Chromatography Methods: Current Advances and Recent Applications. Anal. Chim. Acta 2023, 1284, 341952. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Passos, H.; Ferreira, A.M.; Kuroda, K.; Pereira, J.L.; Gonçalves, F.J.M.; Coutinho, J.A.P.; Ventura, S.P.M. Zwitterionic Compounds Are Less Ecotoxic than Their Analogous Ionic Liquids. Green. Chem. 2021, 23, 3683–3692. [Google Scholar] [CrossRef]
- Jordan-Sinisterra, M.; Vargas Medina, D.A.; Lanças, F.M. Microextraction by Packed Sorbent of Polycyclic Aromatic Hydrocarbons in Brewed Coffee Samples with a New Zwitterionic Ionic Liquid-Modified Silica Sorbent. J. Food Compos. Anal. 2022, 114, 104832. [Google Scholar] [CrossRef]
- Faraji, M.; Shirani, M.; Rashidi-Nodeh, H. The Recent Advances in Magnetic Sorbents and Their Applications. TrAC Trends Anal. Chem. 2021, 141, 116302. [Google Scholar] [CrossRef]
- Suliman, M.A.; Sajid, M.; Nazal, M.K.; Islam, M.A. Carbon-Based Materials as Promising Sorbents for Analytical Sample Preparation: Recent Advances and Trends in Extraction of Toxic Metal Pollutants from Various Media. TrAC Trends Anal. Chem. 2023, 167, 117265. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Chisvert, A. Magnetic Sorbents: Synthetic Pathways and Application in Dispersive (Micro)Extraction Techniques for Bioanalysis. TrAC Trends Anal. Chem. 2024, 171, 117486. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.L.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.S.; Lin, J.M. Recent Advances in Graphene-Based Magnetic Composites for Magnetic Solid-Phase Extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Oliveira, D.M.; Assis, L.K.C.S.; Rodrigues, A.R.; Guzzo, P.L.; Almeida, L.C.; Padrón-Hernández, E. Synthesis of Nanocomposites Based on Fe3O4 Nanoparticles and Nitrogen-Doped Reduced Graphene Oxide Aerogel by Ex-Situ Approach and Their Magnetic Properties. J. Alloys Compd. 2023, 968, 172038. [Google Scholar] [CrossRef]
- Thi Mong Thy, L.; Tan Tai, L.; Duy Hai, N.; Quang Cong, C.; Minh Dat, N.; Ngoc Trinh, D.; Truong Son, N.; Thi Yen Oanh, D.; Thanh Phong, M.; Huu Hieu, N. Comparison of In-Situ and Ex-Situ Methods for Synthesis of Iron Magnetic Nanoparticles-Doped Graphene Oxide: Characterization, Adsorption Capacity, and Fenton Catalytic Efficiency. FlatChem 2022, 33, 100365. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Tavakkoli, O.; Mesbah, M.; Bhutto, J.K.; Khademi, T.; Kirpichnikova, I.; Ahmad, A.; ALJohani, A.A. A Review on Carbon-Based Molecularly-Imprinted Polymers (CBMIP) for Detection of Hazardous Pollutants in Aqueous Solutions. Chemosphere 2022, 308, 136471. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.O.; Bernardo, R.A.; Machado, L.S.; Batista Junior, A.C.; Maciel, L.Í.L.; de Aguiar, D.V.A.; Sanches Neto, F.O.; Oliveira, J.V.A.; Simas, R.C.; Chaves, A.R. Greener Molecularly Imprinted Polymers: Strategies and Applications in Separation and Mass Spectrometry Methods. TrAC Trends Anal. Chem. 2023, 168, 117285. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Soylak, M. Green Sorbents for the Solid Phase Extraction of Trace Species. Curr. Opin. Green Sustain. Chem. 2024, 47, 100899. [Google Scholar] [CrossRef]
- Koel, M. Developments in Analytical Chemistry Initiated from Green Chemistry. Sustain. Chem. Environ. 2024, 5, 100078. [Google Scholar] [CrossRef]
- Werner, J.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Frankowski, R. Biopolymers-Based Sorbents as a Future Green Direction for Solid Phase (Micro)Extraction Techniques. TrAC Trends Anal. Chem. 2024, 173, 117659. [Google Scholar] [CrossRef]
- Da Silva Alves, D.C.; Healy, B.; Yu, T.; Breslin, C.B. Graphene-Based Materials Immobilized within Chitosan: Applications as Adsorbents for the Removal of Aquatic Pollutants. Materials 2021, 14, 3655. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiao, D.; Zhao, S.; Liu, P.; Xie, F.; Zhang, B. Biofunctional Chitosan–Biopolymer Composites for Biomedical Applications. Mater. Sci. Eng. R Rep. 2024, 159, 100775. [Google Scholar] [CrossRef]
- Li, C.; Li, F.; Wang, K.; Wang, Q.; Liu, H.; Sun, X.; Xie, D. Synthesis, Characterizations, and Release Mechanisms of Carboxymethyl Chitosan-Graphene Oxide-Gelatin Composite Hydrogel for Controlled Delivery of Drug. Inorg. Chem. Commun. 2023, 155, 110965. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of Chitosan/Polyacrylate/Graphene Oxide Composite Physical Hydrogel by Semi-Dissolution/Acidification/Sol-Gel Transition Method and Its Simultaneous Cationic and Anionic Dye Adsorption Properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef] [PubMed]
- Han Lyn, F.; Tan, C.P.; Zawawi, R.M.; Nur Hanani, Z.A. Enhancing the Mechanical and Barrier Properties of Chitosan/Graphene Oxide Composite Films Using Trisodium Citrate and Sodium Tripolyphosphate Crosslinkers. J. Appl. Polym. Sci. 2021, 138, 50618. [Google Scholar] [CrossRef]
- Ayazi, Z.; Farshineh Saei, S.; Pashayi Sarnaghi, S. A Novel Self-Supportive Thin Film Based on Graphene Oxide Reinforced Chitosan Nano-Biocomposite for Thin Film Microextraction of Fluoxetine in Biological and Environmental Samples. J. Pharm. Biomed. Anal. 2023, 236, 115678. [Google Scholar] [CrossRef] [PubMed]
- Marapureddy, S.G.; Thareja, P. Synergistic Effect of Chemical Crosslinking and Addition of Graphene-Oxide in Chitosan—Hydrogels, Films, and Drug Delivery. Mater. Today Commun. 2022, 31, 103430. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Abdiryim, T.; Liu, X. Cyclodextrin-Derived Materials: From Design to Promising Applications in Water Treatment. Coord. Chem. Rev. 2024, 502, 215613. [Google Scholar] [CrossRef]
- Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.S.; Pereira dos Santos, N.G.; Medina, D.A.V.; Lanças, F.M. Cyclodextrins-Based Sorbents for Sustainable Sample Preparation Focusing on Food Analysis. Green Anal. Chem. 2023, 7, 100077. [Google Scholar] [CrossRef]
- Gentili, A. Cyclodextrin-Based Sorbents for Solid Phase Extraction. J. Chromatogr. A 2020, 1609, 460654. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, H.; Xu, S.; Dong, P.; Long, A.; Xiao, L.; Feng, S.; Chen, C.P. Cellulose Nanocrystal Regulated Ultra-Loose, Lightweight, and Hierarchical Porous Reduced Graphene Oxide Hybrid Aerogel for Capturing and Determining Organic Pollutants from Water. Carbon 2023, 204, 94–101. [Google Scholar] [CrossRef]
- Tabish, M.S.; Hanapi, N.S.M.; Wan Ibrahim, W.N.; Saim, N.; Yahaya, N. Alginate-Graphene Oxide Biocomposite Sorbent for Rapid and Selective Extraction of Non-Steroidal Anti-Inflammatory Drugs Using Micro-Solid Phase Extraction. Indones. J. Chem. 2019, 19, 684–695. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P.; Gębicki, J. Sorbents Modified by Deep Eutectic Solvents in Microextraction Techniques. TrAC-Trends Anal. Chem. 2024, 172, 117577. [Google Scholar] [CrossRef]
- Werner, J.; Zgoła-Grześkowiak, A.; Płatkiewicz, J.; Płotka-Wasylka, J.; Jatkowska, N.; Kalyniukova, A.; Zaruba, S.; Andruch, V. Deep Eutectic Solvents in Analytical Sample Preconcentration Part B: Solid-Phase (Micro)Extraction. Microchem. J. 2023, 191, 108898. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Pérez-Nava, A.; Valle-Sánchez, M.; Delgado-Rangel, L.H. Deep Eutectic Solvents Applications Aligned to 2030 United Nations Agenda for Sustainable Development. Chem. Eng. Process. 2024, 199, 109751. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhang, X.; Mo, C.E.; Huang, Y.P.; Liu, Z.S. Preparation of Graphene Oxide Incorporated Monolithic Chip Based on Deep Eutectic Solvents for Solid Phase Extraction. Anal. Chim. Acta 2020, 1096, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.S.; Ger, T.R.; Hsiao, C.-D. Toxicity Studies on Graphene-Based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef] [PubMed]
- Ghulam, A.N.; Dos Santos, O.A.L.; Hazeem, L.; Backx, B.P.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Kanu, A.B. Recent Developments in Sample Preparation Techniques Combined with High-Performance Liquid Chromatography: A Critical Review. J. Chromatogr. A 2021, 1654, 4. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Y.; Zhang, Q.; Zang, L.; Chen, B.; Hu, B. Stir Bar Sorptive Extraction and Its Application. J. Chromatogr. A 2021, 1637, 461810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; You, L.; Chen, B.; He, M.; Hu, B. Reduced Graphene Oxide Coated Nickel Foam for Stir Bar Sorptive Extraction of Benzotriazole Ultraviolet Absorbents from Environmental Water. Talanta 2021, 231, 122332. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.T.; Rezaei, B.; Bahrami, H. Zirconium Dioxide-Reduced Graphene Oxide Nanocomposite-Coated Stir-Bar Sorptive Extraction Coupled with Ion Mobility Spectrometry for Determining Ethion. Talanta 2018, 182, 285–291. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Giokas, D.L.; Chisvert, A.; Salvador, A. Fundamentals and Applications of Stir Bar Sorptive Dispersive Microextraction: A Tutorial Review. Anal. Chim. Acta 2021, 1153, 338271. [Google Scholar] [CrossRef] [PubMed]
- Madej, K.; Jonda, A.; Borcuch, A.; Piekoszewski, W.; Chmielarz, L.; Gil, B. A Novel Stir Bar Sorptive-Dispersive Microextraction in Combination with Magnetically Modified Graphene for Isolation of Seven Pesticides from Water Samples. Microchem. J. 2019, 147, 962–971. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Chisvert, A.; Salvador, A. Reduced Graphene Oxide-Based Magnetic Composite for Trace Determination of Polycyclic Aromatic Hydrocarbons in Cosmetics by Stir Bar Sorptive Dispersive Microextraction. J. Chromatogr. A 2020, 1624, 461229. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M. New Trend in Sample Preparation: On-Line Microextraction in Packed Syringe for Liquid and Gas Chromatography Applications: I. Determination of Local Anaesthetics in Human Plasma Samples Using Gas Chromatography–Mass Spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Granados-Guzmán, G.; Díaz-Hernández, M.; Alvarez-Román, R.; Cavazos-Rocha, N.; Portillo-Castillo, O.J. A Brief Review of the Application of Microextraction by Packed Sorbent for Antibiotics Analysis from Biological, Food, and Environmental Samples. Rev. Anal. Chem. 2023, 42, 20230057. [Google Scholar] [CrossRef]
- Martins, R.O.; de Araújo, G.L.; de Freitas, C.S.; Silva, A.R.; Simas, R.C.; Vaz, B.G.; Chaves, A.R. Miniaturized Sample Preparation Techniques and Ambient Mass Spectrometry as Approaches for Food Residue Analysis. J. Chromatogr. A 2021, 1640, 461949. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Said, R.; Abdel-Rehim, M. Sorbent, Device, Matrix and Application in Microextraction by Packed Sorbent (MEPS): A Review. J. Chromatogr. B 2017, 1043, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Moein, M.M.; Madrakian, T.; Afkhami, A.; Bahar, S.; Abdel-Rehim, M. Reduced Graphene Oxide as an Efficient Sorbent in Microextraction by Packed Sorbent: Determination of Local Anesthetics in Human Plasma and Saliva Samples Utilizing Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2018, 1095, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Karimiyan, H.; Uheida, A.; Hadjmohammadi, M.; Moein, M.M.; Abdel-Rehim, M. Polyacrylonitrile/Graphene Oxide Nanofibers for Packed Sorbent Microextraction of Drugs and Their Metabolites from Human Plasma Samples. Talanta 2019, 201, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos Soares Maciel, E.; Henrique Fumes, B.; Lúcia de Toffoli, A.; Mauro Lanças, F. Graphene Particles Supported on Silica as Sorbent for Residue Analysis of Tetracyclines in Milk Employing Microextraction by Packed Sorbent. Electrophoresis 2018, 39, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Seidi, S.; Tajik, M.; Baharfar, M.; Rezazadeh, M. Micro Solid-Phase Extraction (Pipette Tip and Spin Column) and Thin Film Solid-Phase Microextraction: Miniaturized Concepts for Chromatographic Analysis. TrAC-Trends Anal. Chem. 2019, 118, 810–827. [Google Scholar] [CrossRef]
- Sun, H.; Feng, J.; Han, S.; Ji, X.; Li, C.; Feng, J.; Sun, M. Recent Advances in Micro- and Nanomaterial-Based Adsorbents for Pipette-Tip Solid-Phase Extraction. Microchem. Acta 2021, 188. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Yang, H.; Li, Y.; Li, S.; Chen, K.; Wang, H.; Wang, H.; Ma, J. Rapid Determination of Antiviral Drugs in Yellow Catfish (Pelteobagrus fulvidraco) Using Graphene/Silica Nanospheres (G/KCC-1) Based Pipette Tip Solid-Phase Extraction with Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1189, 123097. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.; Yin, J.; Wang, M.; Han, Y.; Yan, H. Determination of Alectinib and Its Active Metabolite in Plasma by Pipette-Tip Solid-Phase Extraction Using Porous Polydopamine Graphene Oxide Adsorbent Coupled with High-Performance Liquid Chromatography-Ultraviolet Detection. J. Chromatogr. A 2024, 1714, 464578. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.C.; Pundi, A.; Brindhadevi, K.; Ponnusamy, V.K. Novel Semi-Automated Graphene Nanosheets Based Pipette-Tip Assisted Micro-Solid Phase Extraction as Eco-Friendly Technique for the Rapid Detection of Emerging Environmental Pollutant in Waters. Chemosphere 2021, 276, 130031. [Google Scholar] [CrossRef]
- Carasek, E.; Morés, L.; Huelsmann, R.D. Disposable Pipette Extraction: A Critical Review of Concepts, Applications, and Directions. Anal. Chim. Acta 2022, 1192, 339383. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Syaleyana Md Shukri, D.; Yahaya, N.; Miskam, M.; Yusof, R.; Husaini Mohamed, A.; Kamaruzaman, S.; Nadhirah Mohamad Zain, N.; Semail, N.F. Advances in Dispersive Solid-Phase Extraction Techniques for Analytical Quantification of Fluoroquinolone Antibiotics. Microchem. J. 2023, 193, 109154. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Jatkowska, N.; Paszkiewicz, M.; Caban, M.; Fares, M.Y.; Dogan, A.; Garrigues, S.; Manousi, N.; Kalogiouri, N.; Nowak, P.M.; et al. Miniaturized Solid Phase Extraction Techniques for Different Kind of Pollutants Analysis: State of the Art and Future Perspectives—PART 1. TrAC Trends Anal. Chem. 2023, 162, 117034. [Google Scholar] [CrossRef]
- Ghorbani, M.; Aghamohammadhassan, M.; Ghorbani, H.; Zabihi, A. Trends in Sorbent Development for Dispersive Micro-Solid Phase Extraction. Microchem. J. 2020, 158, 105250. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive Micro-Solid Phase Extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Feist, B. Dispersive Micro-Solid Phase Extraction Using a Graphene Oxide Nanosheet with Neocuproine and Batocuproine for the Preconcentration of Traces of Metal Ions in Food Samples. Molecules 2023, 28, 4140. [Google Scholar] [CrossRef] [PubMed]
- Greda, K.; Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Dispersive Micro-Solid Phase Extraction Based on Graphene Oxide for the Ultrasensitive Determination of Cd by Slurry Sampling Microplasma Optical Emission Spectrometry. Microchem. J. 2024, 196, 109715. [Google Scholar] [CrossRef]
- Nakhonchai, N.; Prompila, N.; Ponhong, K.; Siriangkhawut, W.; Vichapong, J.; Supharoek, S. ang Green Hairy Basil Seed Mucilage Biosorbent for Dispersive Solid Phase Extraction Enrichment of Tetracyclines in Bovine Milk Samples Followed by HPLC Analysis. Talanta 2024, 271, 125645. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef] [PubMed]
- Kalaboka, M.; Sakkas, V. Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS. Molecules 2023, 28, 2277. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Sabzehmeidani, M.M.; Ghaedi, M.; Dashtian, K.; Abbasi-Asl, H. Dispersive Micro-Solid Phase Extraction Coupled with Spectrophotometric Using (MgFe CLDH)/GO Magnetically Separable Sorbent for Pre-Concentration of Anionic Food Dyes in Water Samples. Emerg. Contam. 2024, 10, 100347. [Google Scholar] [CrossRef]
- Cao, S.; Chen, J.; Lai, G.; Xi, C.; Li, X.; Zhang, L.; Wang, G.; Chen, Z. A High Efficient Adsorbent for Plant Growth Regulators Based on Ionic Liquid and β-Cyclodextrin Functionalized Magnetic Graphene Oxide. Talanta 2019, 194, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, P. A Novel Magnetic β-Cyclodextrin-Modified Graphene Oxide and Chitosan Composite as an Adsorbent for Trace Extraction of Four Bisphenol Pollutants from Environmental Water Samples and Food Samples. Molecules 2024, 29, 867. [Google Scholar] [CrossRef] [PubMed]

| Modifier Material | Type of GBM | Analyte | Matrix | Microextraction Technique 1 | Recovery (%) | Reusability Rate (cycles) | Ref. |
|---|---|---|---|---|---|---|---|
| Silica Derivatives | GO@SiO2 | Benzylpenicillin, Cefalexin, Cefoperazone, and Ceftiofur | Wastewater | Column-switching | >60 | 100 | [28] |
| GO@SiO2 | Carbamazepine, Citalopram, Desipramine, Sertraline and Clomipramine | Urine | Multidimensional LC-MS/MS | - | 250 | [29] | |
| SiGO-C18 | Simazine, Atrazine, Carbofuran, Tebuthiuron, Diuron, Ametryn, Clomazone, Thiacloprid, Hexazinone, and Imidacloprid | Sugarcane spirits | In-tube SPME | >80 | - | [30] | |
| SiGO-C18 | Aflatoxins G2, G1, B2 and B1 | Food | PT-SPE | >70 | 10 | [31] | |
| Si@GO@βCD | Daidzein, Genistein, Formononetin and Biochanin A | Urine | Online SPME-LC | - | 200 | [23] | |
| Ionic Liquids | IL-TGO | Fipronil | Chicken eggs | PT-SPE and DSPE | >90 | 15 | [13] |
| IL–CS–GOA | 3-bromocarbazole, 2,7-dibromocarbazole and 1,3,6,8-tetrabromocarbazole | Sediment | Glass dropper Extraction | >80 | 6 | [25] | |
| GO@MIL | Inorganic antimony (Sb III and Sb IV) | Water, Tea And Honey | d-μ-SPE | >97 | - | [32] | |
| Magnetic Materials | MGO | Triazine Herbicides | Fruit and vegetable | MSPE | >70 | 8 | [33] |
| MGO@UIO-66 | Food colorants | Soft drinks, candies, and pastilles | UA-DSPE | >95 | 6 | [34] | |
| rGO@MNS | Copper(II) | Environmental waters | SPME | >95 | - | [35] | |
| Molecularly imprinted polymers | PDESs-MIP/GO: | Anti-adipogenic drugs | Solidago decurrens | CPT-MSPD | >94 | - | [36] |
| N-GQDs/Fe3O4 @SiO2/IRMOF-1/MIP | Phenylureas | Cucumber, tomato, and radish | d-MSPE | >80 | 4 | [37] | |
| TPhP-MIPs/GO | Triphenyl phosphate | Environmental water | DI-SPME | >70 | 110 | [38] | |
| GO/MIP-FA | Organophosphate flame retardants | Environmental water | SPME | >70 | 110 | [39] | |
| Carbon-based biosorbents | GO/CS | Organic pollutants | Water | SPME | >90 | 100 | [40] |
| ACGO | Chlorophenols | Food and environmental samples | TFME | >80 | 48 | [41] | |
| NiFe2O4 @GO@ β-CD | Bisphenols | Milk and Milk packing | MPSE | >78 | 12 | [42] | |
| PDMS/GO/β-CD sponge | Lavander essential oil | Lavender | HS-SPME | - | 6 | [43] | |
| β-CD@GO@Si | Isoflavones | Soy-based juice | MEPS | >90 | 50 | [24] | |
| Deep eutectic solvents | GO@DES | Chlorpyrifos, diazinon, Tebuconazole, Deltamethrin, Permethrin, Haloxyfop-methyl, Penconazole, and Cyhalothrin | Zucchini | d-μ-SPE | >70% | - | [22] |
| DFG | Hippuric acid and Methylhippuric acid | Urine | PT-SPE | >90% | - | [44] | |
| MGO@DES | Estrone, 17β-estradiol and 17α-ethinylestradiol | Milk | MSPE | >90% | 7 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, A.T.; Martins, R.O.; Lanças, F.M. Advances and Applications of Hybrid Graphene-Based Materials as Sorbents for Solid Phase Microextraction Techniques. Molecules 2024, 29, 3661. https://doi.org/10.3390/molecules29153661
Cardoso AT, Martins RO, Lanças FM. Advances and Applications of Hybrid Graphene-Based Materials as Sorbents for Solid Phase Microextraction Techniques. Molecules. 2024; 29(15):3661. https://doi.org/10.3390/molecules29153661
Chicago/Turabian StyleCardoso, Alessandra Timóteo, Rafael Oliveira Martins, and Fernando Mauro Lanças. 2024. "Advances and Applications of Hybrid Graphene-Based Materials as Sorbents for Solid Phase Microextraction Techniques" Molecules 29, no. 15: 3661. https://doi.org/10.3390/molecules29153661





