Concise Synthesis of Pseudane IX, Its N-Oxide, and Novel Carboxamide Analogs with Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
- 3-Ethylamino-2-(2-nitrobenzoyl)-dodec-2-enoic acid phenylamide (3a): yellowish oil; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.83 (t, J = 7.0, 3H, CH3), 1.12–1.24 (m, 12H, 6 × CH2), 1.27 (t, J = 7.2, 3H, NHCH2CH3), 1.60 (m, 2H, βCH2), 2.45 (m, 2H, αCH2), 3.50 (m, 2H, NHCH2CH3), 6.94 (m, 1H, ArH), 7.15 (m, 2H, ArH), 7.27 (m, 2H, ArH), 7.50 (m, 2H, ArH), 7.65 (td, J = 7.5, J = 1.2, 1H, ArH), 7.96 (dd, J = 8.2, J = 1.0, 1H, ArH), 9.70 (br s, 1H, CONH), 11.62 (t, J = 5.6, 1H, NHCH2CH3); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.38, 15.77, 22.53, 27.94, 28.81, 29.05, 29.16, 29.51, 29.73, 31.65, 38.04, 107.58, 119.62, 123.62, 124.27, 128.78, 128.83, 129.84, 133.90, 137.70, 139.47, 146.46, 167.14, 169.33, 186.00; HRMS m/z (ES+): calcd. for C27H36N3O4+ [M+H]+ 466.2700, found 466.2704.
- 3-Ethylamino-2-(2-nitrobenzoyl)-dodec-2-enoic acid 4-methoxyphenylamide (3b): yellowish oil; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.82 (t, J = 7.0, 3H, CH3), 1.12–1.24 (m, 12H, 6 × CH2), 1.26 (t, J = 7.2, 3H, NHCH2CH3), 1.60 (m, 2H, βCH2), 2.44 (m, 2H, αCH2), 3.49 (m, 2H, NHCH2CH3), 3.66 (s, 3H, OCH3), 6.73 (m, 2H, ArH), 7.15 (m, 2H, ArH), 7.50 (m, 2H, ArH), 7.66 (td, J = 7.5, J = 1.2, 1H, ArH), 7.96 (dd, J = 8.2, J = 0.9, ArH), 9.52 (br s, 1H, CONH), 11.60 (t, J = 5.5, 1H, NHCH2CH3); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.37, 15.78, 22.54, 27.95, 28.83, 29.08, 29.18, 29.54, 29.69, 31.67, 38.01, 55.52, 113.98, 121.24, 124.25, 128.82, 129.81, 132.60, 133.85, 137.72, 146.46, 155.72, 166.70, 169.20, 185.94; HRMS m/z (ES+): calcd. for C28H38N3O5+ [M+H]+ 496.2806, found 496.2801.
- 3-Ethylamino-2-(2-nitrobenzoyl)-dodec-2-enoic acid 4-chlorophenylamide (3c): yellowish oil; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.82 (t, J = 7.1, 3H, CH3), 1.10–1.22 (m, 12H, 6 × CH2), 1.27 (t, J = 7.2, 3H, NHCH2CH3), 1.59 (m, 2H, βCH2), 2.44 (m, 2H, αCH2), 3.50 (m, 2H, NHCH2CH3), 7.21 (m, 2H, ArH), 7.32 (m, 2H, ArH), 7.49 (m, 2H, ArH), 7.65 (m, 1H, ArH), 7.96 (m, 1H, ArH), 9.87 (br s, 1H, CONH), 11.62 (t, J = 5.6, 1H, NHCH2CH3); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.37, 15.77, 22.54, 27.86, 28.74, 29.07, 29.14, 29.43, 29.64, 31.65, 38.07, 120.91, 124.28, 127.19, 128.78, 129.88, 133.93, 137.57, 138.46, 146.40, 167.28, 186.02; HRMS m/z (ES+): calcd. for C27H35ClN3O4+ [M+H]+ 500.2311, found 500.2318.
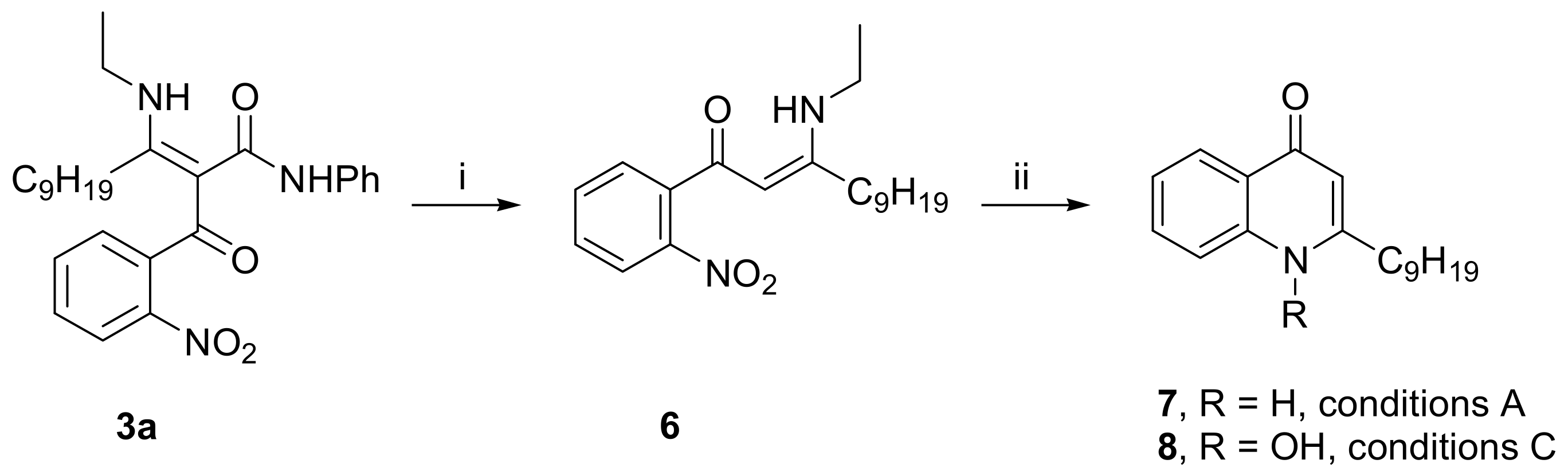
- Synthesis of 3-Ethylamino-1-(2-nitrophenyl)-dodec-2-en-1-one (6): Intermediate 3a (466 mg, 1 mmol) was mixed with anhydrous H3PO4 (5–6 g) in a glass vial. The mixture was stirred intensely for two hours at 60 °C, then the vial was cooled to r.t. with tap water, and the contents were rinsed and poured into a separatory funnel with 50–70 mL of water. The product was extracted in CH2Cl2 (2 × 40 mL), the combined organic layers were dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product obtained this way was sufficiently clean to be used directly in the next stage, without further purification. An analytically pure sample can be obtained by column chromatography on silica gel with Et2O:Petrol (1:1) as the eluent, increasing polarity to Et2O:Petrol (2:1). Yield: 298 mg yellowish oil (86%). The 1H NMR spectra of 6 in DMSO-d6 indicate a Z/E isomeric mixture in a ratio of 85:15. Only NMR signals corresponding to the major Z isomer are listed below. 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.85 (t, J = 7.0, 3H, CH3), 1.19 (t, J = 7.2, 3H, NHCH2CH3), 1.23–1.39 (m, 12H, 6 × CH2), 1.53 (m, 2H, βCH2), 2.34 (m, 2H, αCH2), 3.39 (m, 2H, NHCH2CH3), 5.35 (s, 1H, CH), 7.57–7.70 (m, 3H, ArH), 7.81 (m, 1H, ArH), 10.94 (t, J = 5.6, 1H, NHCH2CH3); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.39, 15.72, 22.56, 27.78, 29.12, 29.17, 29.24, 29.35, 31.74, 37.61, 92.26, 124.12, 129.11, 130.65, 132.79, 136.94, 148.93, 170.38, 184.65; HRMS m/z (ES+): calcd. for C20H31N2O3+ [M+H]+ 347.2329, found 347.2331.
- Synthesis of compounds 4, 5, 7, and 8 by reductive cyclization of intermediates 3 and 6. General procedure A (preparation of products 4a–c and 7): Zn powder (2 g, prewashed with 1% HCl, water, and acetone) was added to the corresponding nitro-intermediate 3 or 6 (1 mmol), dissolved in a mixture of CH2Cl2 (30 mL) and acetic acid (4 mL). The heterogeneous mixture was magnetically stirred for 24 h at r.t., and then the solids were filtered off with suction and rinsed thoroughly with CH2Cl2. The dichloromethane filtrate was transferred to a separatory funnel and was extracted with water (50 mL) and, then, with a saturated aqueous solution of NaHCO3 (25 mL). The organic phase was dried with anhydrous sodium sulfate, the drying agent was filtered off, and the solvent was removed under reduced pressure. The products crystallized upon trituration with Et2O. Where necessary, further purification can be performed by column chromatography on silica gel with Et2O as the eluent, increasing polarity to Et2O:CH3OH 20:1.
- General procedure B (preparation of products 5a and 5b): To the corresponding nitro-intermediate 3 (100 mg) in CH3OH (10–15 mL), HCOONH4 (300 mg) and Pd on charcoal (10 mg, 10 w% Pd) were added. The mixture was magnetically stirred for 90 min. at r.t., and the catalyst was removed by vacuum filtration through a pad of celite on a sintered glass funnel. The celite was rinsed thoroughly with methanol, and the solvent was removed from the filtrate under reduced pressure. Then, water (50 mL) was added to the solid residue and the product was extracted in CH2Cl2 (3 × 20 mL). The combined organic layers were dried with anhydrous sodium sulfate, the drying agent was filtered off, and the solvent was removed under reduced pressure. The products crystallized upon trituration with Et2O. Where necessary, further purification can be performed by column chromatography on silica gel with Et2O as the eluent, increasing polarity to Et2O:CH3OH 20:1.
- General procedure C (preparation of products 5c and 8): The corresponding nitro-intermediate 3 or 6 (0.5 mmol) was dissolved in isopropanol (IPA) (2 mL). Then, DMSO (0.013 g, 0.012 mL), n-butylamine (0.037 g, 0.050 mL), and 5 wt% Pt/Al2O3 (0.010 g) were added to the solution. The air in the reaction vessel was evacuated and replaced with H2 with the help of a three-way stopper. The H2 atmosphere was kept with a balloon for the next 24 h, while the reaction mixture was magnetically stirred at 25 °C. Then, the catalyst was filtered off through a pad of celite on a sintered glass funnel, with thorough rinsing with IPA. The IPA was removed from the filtrate on a rotary evaporator under reduced pressure. To remove any residual n-butylamine and DMSO, dilute aqueous HCl was added to the residue and the product was extracted in CH2Cl2 (2 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and the oily residue solidified upon trituration with diethyl ether, providing practically clean products. Analytically pure samples were obtained by column chromatography on a short silica gel plug, using Et2O as the eluent.
- 2-Nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid phenylamide (4a): white solid, mp 192–193 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.83 (t, J = 6.9, 3H, CH3), 1.17–1.33 (m, 10H, 5 × CH2), 1.39 (m, 2H, γCH2), 1.72 (m, 2H, βCH2), 3.16 (m, 2H, αCH2), 7.06 (m, 1H, ArH), 7.33 (m, 2H, ArH), 7.43 (m, 1H, ArH), 7.64–7.76 (m, 4H, ArH), 8.23 (dd, J = 8.2, J = 1.1, 1H, ArH), 12.17 (br s, 1H, NH), 12.20 (s, 1H, NH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.40, 22.56, 29.14, 29.31, 29.52, 29.88, 31.74, 33.59, 112.38, 118.61, 120.06, 123.49, 124.84, 125.15, 125.88, 129.22, 133.08, 138.85, 139.81, 158.87, 164.58, 176.70; HRMS m/z (ES+): calcd. for C25H31N2O2+ [M+H]+ 391.2380, found 391.2387.
- 2-Nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-methoxyphenylamide (4b): white solid, mp 150–151 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.82 (t, J = 6.9, 3H, CH3), 1.17–1.33 (m, 10H, 5 × CH2), 1.38 (m, 2H, γCH2), 1.72 (m, 2H, βCH2), 3.15 (m, 2H, αCH2), 3.74 (s, 3H, OCH3), 6.90 (m, 2H, ArH), 7.42 (m, 1H, ArH), 7.63 (m, 3H, ArH), 7.73 (m, 1H, ArH), 8.21 (dd, J = 8.1, J = 1.1, 1H, ArH), 12.03 (s, 1H, NH), 12.12 (br s, 1H, NH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.40, 22.56, 29.15, 29.17, 29.33, 29.52, 29.91, 31.75, 33.56, 55.61, 112.54, 114.34, 118.60, 121.47, 124.75, 125.14, 125.85, 132.99, 133.03, 138.88, 155.55, 158.63, 164.16, 176.62; HRMS m/z (ES+): calcd. for C26H33N2O3+ [M+H]+ 421.2486, found 421.2481.
- 2-Nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-chlorophenylamide (4c): white solid, mp 172–173 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.83 (t, J = 6.9, 3H, CH3), 1.17–1.32 (m, 10H, 5 × CH2), 1.38 (m, 2H, γCH2), 1.71 (m, 2H, βCH2), 3.13 (m, 2H, αCH2), 7.37 (m, 2H, ArH), 7.43 (m, 1H, ArH), 7.65 (m, 1H, ArH), 7.74 (m, 3H, ArH), 8.21 (dd, J = 8.2, J = 1.1, 1H, ArH), 12.19 (br s, 1H, NH), 12.30 (s, 1H, NH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.39, 22.56, 29.14, 29.32, 29.49, 29.84, 31.74, 33.54, 112.24, 118.65, 121.54, 124.89, 125.11, 125.84, 126.96, 129.09, 133.12, 138.74, 138.87, 158.89, 164.73, 176.64; HRMS m/z (ES+): calcd. for C25H30ClN2O2+ [M+H]+ 425.1990, found 425.1989.
- 1-Hydroxy-2-nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid phenylamide (5a): white solid, mp 162–163 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.84 (t, J = 7.0, 3H, CH3), 1.17–1.32 (m, 10H, 5 × CH2), 1.39 (m, 2H, γCH2), 1.76 (m, 2H, βCH2), 3.12 (m, 2H, αCH2), 7.07 (m, 1H, ArH), 7.33 (m, 2H, ArH), 7.49 (m, 1H, ArH), 7.71 (m, 2H, ArH), 7.83 (m, 1H, ArH), 7.93 (m, 1H, ArH), 8.25 (dd, J = 8.1, J = 1.2, 1H, ArH), 11.30 (s, 1H, NH), 12.10 (br s, 1H, OH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.41, 22.56, 28.44, 29.00, 29.15, 29.25, 29.58, 31.73, 115.05, 115.66, 119.90, 123.61, 124.96, 125.52, 125.97, 129.16, 133.33, 139.86, 139.95, 155.74, 164.60, 173.62; HRMS m/z (ES+): calcd. for C25H31N2O3+ [M+H]+ 407.2329, found 407.2324.
- 1-Hydroxy-2-nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-methoxyphenylamide (5b): white solid, mp 163–164 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.84 (t, J = 6.9, 3H, CH3), 1.15–1.32 (m, 10H, 5 × CH2), 1.38 (m, 2H, γCH2), 1.75 (m, 2H, βCH2), 3.11 (m, 2H, αCH2), 3.74 (s, 3H, OCH3), 6.91 (m, 2H, ArH), 7.47 (m, 1H, ArH), 7.63 (m, 2H, ArH), 7.82 (m, 1H, ArH), 7.92 (m, 1H, ArH), 8.24 (m, 1H, ArH), 11.15 (s, 1H, NH), 12.13 (br s, 1H, OH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.40, 22.57, 28.44, 29.01, 29.17, 29.27, 29.59, 31.74, 55.61, 114.28, 115.16, 115.64, 121.32, 124.87, 125.51, 125.95, 133.09, 133.23, 139.95, 155.63, 164.13, 173.57; HRMS m/z (ES+): calcd. for C26H33N2O4+ [M+H]+ 437.2435, found 437.2434.
- 1-Hydroxy-2-nonyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid 4-chlorophenylamide (5c): white solid, mp 182–183 °C; 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.83 (t, J = 7.0, 3H, CH3), 1.14–1.30 (m, 10H, 5 × CH2), 1.38 (m, 2H, γCH2), 1.75 (m, 2H, βCH2), 3.09 (m, 2H, αCH2), 7.38 (m, 2H, ArH), 7.49 (m, 1H, ArH), 7.75 (m, 2H, ArH), 7.83 (m, 1H, ArH), 7.93 (m, 1H, ArH), 8.24 (dd, J = 8.1, J = 1.2, 1H, ArH), 11.40 (s, 1H, NH), 12.13 (br s, 1H, OH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.40, 22.57, 28.37, 28.96, 29.16, 29.24, 29.50, 31.73, 114.93, 115.70, 121.36, 125.02, 125.50, 125.94, 127.14, 129.07, 133.39, 138.80, 139.97, 155.68, 164.77, 173.53; HRMS m/z (ES+): calcd. for C25H30ClN2O3+ [M+H]+ 441.1939, found 441.1932.
- 2-Nonyl-1H-quinolin-4-one (7): white solid, mp 135–136 °C (Lit. [2] mp 138–139 °C, Lit. [3] mp 134 °C); 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.83 (t, J = 6.7, 3H, CH3), 1.18–1.36 (m, 12H, 6 × CH2), 1.66 (m, 2H, βCH2), 2.58 (m, 2H, αCH2), 5.92 (s, 1H, C3-H), 7.27 (m, 1H, ArH), 7.54 (m, 1H, ArH), 7.61 (m, 1H, ArH), 8.04 (m, 1H, ArH), 11.50 (br s, 1H, NH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.39, 22.55, 28.81, 28.97, 29.12, 29.19, 29.35, 31.72, 33.72, 108.09, 118.33, 123.16, 125.10, 125.22, 131.87, 140.62, 154.02, 177.33; HRMS m/z (ES+): calcd. for C18H26NO+ [M+H]+ 272.2009, found 272.2007.
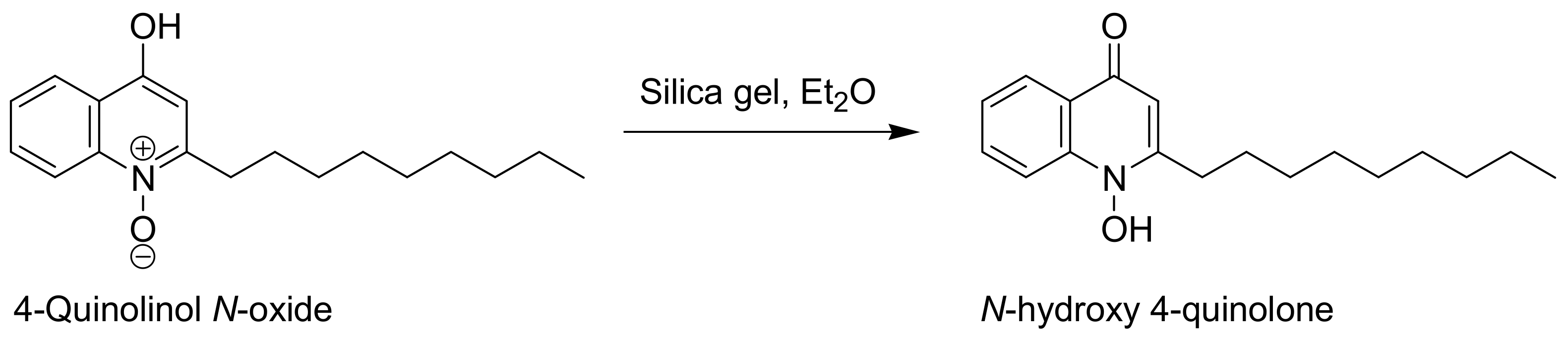
- 2-Nonyl-1-oxyquinolin-4-ol (8, quinolinol tautomer): white solid, mp 103–104 °C (Lit. [10] mp 148–149 °C, Lit. [3] mp 132 °C); 1H-NMR (400 MHz, DMSO-d6, δ ppm, J Hz): 0.84 (t, J = 6.8, 3H, CH3), 1.17–1.43 (m, 12H, 6 × CH2), 1.75 (m, 2H, βCH2), 3.09 (m, 2H, αCH2), 7.08 (s, 1H, C3-H), 7.77 (m, 1H, ArH), 8.07 (m, 1H, ArH), 8.25 (m, 1H, ArH), 8.30 (m, 1H, ArH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 14.40, 22.55, 27.55, 29.13, 29.15, 29.29, 31.73, 31.75, 105.48, 117.31, 121.35, 124.25, 127.70, 134.82, 140.03, 158.60, 167.31; HRMS m/z (ES+): calcd. for C18H26NO2+ [M+H]+ 288.1958, found 288.1963.
- 1-Hydroxy-2-nonyl-(1H)-quinolin-4-one (8, quinolone tautomer): white solid, mp 146–147 °C (Lit. [10] mp 148–149 °C, Lit. [3] mp 132 °C); 1H-NMR (400 MHz, DMSO-d6, 70 °C, δ ppm, J Hz): 0.87 (t, J = 6.9, 3H, CH3), 1.25–1.43 (m, 12H, 6 × CH2), 1.70 (m, 2H, βCH2), 2.77 (m, 2H, αCH2), 5.97 (s, 1H, C3-H), 7.36 (m, 1H, ArH), 7.72 (m, 1H, ArH), 7.86 (m, 1H, ArH), 8.12 (m, 1H, ArH), 11.43 (br s, 1H, OH); 13C-NMR (100 MHz, DMSO-d6, 70 °C, δ ppm): 14.22, 22.43, 27.77, 29.02, 29.08, 29.12, 29.25, 31.22, 31.66, 107.13, 115.45, 123.72, 125.30, 125.40, 132.12, 141.09, 153.83; HRMS m/z (ES+): calcd. for C18H26NO2+ [M+H]+ 288.1958, found 288.1954.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hays, E.E.; Wells, I.C.; Katzman, P.A.; Cain, C.K.; Jacobs, F.A.; Thayer, S.A.; Doisy, E.A.; Gaby, W.L.; Roberts, E.C.; Muir, R.D.; et al. Antibiotic substances produced by Pseudomonas aeruginosa. J. Biol. Chem. 1945, 159, 725–750. [Google Scholar] [CrossRef]
- Wells, I.C. Antibiotic substances produced by Pseudomonas aeruginosa. Syntheses of Pyo 1b, Pyo 1c, and Pyo III. J. Biol. Chem. 1952, 196, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bultel-Poncé, V.; Berge, J.P.; Debitus, C.; Jean-Louis, N.; Michèle, G. Metabolites from the Sponge-Associated Bacterium Pseudomonas Species. Mar. Biotechnol. 1999, 1, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Supong, K.; Thawai, C.; Supothina, S.; Auncharoen, P.; Pittayakhajonwut, P. Antimicrobial and anti-oxidant activities of quinoline alkaloids from Pseudomonas aeruginosa BCC76810. Phytochem. Lett. 2016, 17, 100–106. [Google Scholar] [CrossRef]
- Kan-Fan, C.; Das, B.C.; Boiteau, P.; Potier, P. Alcaloïdes de Vepris ampody (Rutacées). Phytochemistry 1970, 9, 1283–1291. [Google Scholar] [CrossRef]
- Wahyuni, T.S.; Widyawaruyanti, A.; Lusida, M.I.; Fuad, A.; Soetjipto; Fuchino, H.; Kawahara, N.; Hayashi, Y.; Aoki, C.; Hotta, H. Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia 2014, 99, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Shanahan, R.; Cano, R.; O’Gara, F.; McGlacken, G.P. A structure activity-relationship study of the bacterial signal molecule HHQ reveals swarming motility inhibition in Bacillus atrophaeus. Org. Biomol. Chem. 2015, 13, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Szamosvári, D.; Sylvester, K.; Schmid, P.; Lu, K.-Y.; Derbyshire, E.R.; Böttcher, T. Close the ring to break the cycle: Tandem quinolone-alkyne-cyclisation gives access to tricyclic pyrrolo[1,2-a]quinolin-5-ones with potent anti-protozoal activity. Chem. Commun. 2019, 55, 7009–7012. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Phelan, J.P.; Gallagher, L.; Woods, D.F.; Shanahan, R.M.; Cano, R.; Ó Muimhneacháin, E.; McGlacken, G.P.; O’Gara, F. Exploiting interkingdom interactions for development of small-molecule inhibitors of Candida albicans biofilm formation. Antimicrob. Agents Chemother. 2016, 60, 5894–5905. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, J.W.; James, A.T. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem. J. 1956, 63, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Szamosvári, D.; Prothiwa, M.; Dieterich, C.L.; Böttcher, T. Profiling structural diversity and activity of 2-alkyl-4(1H)-quinolone N-oxides of Pseudomonas and Burkholderia. Chem. Commun. 2020, 56, 6328–6331. [Google Scholar] [CrossRef] [PubMed]
- Mollova-Sapundzhieva, Y.; Angelov, P.; Georgiev, D.; Yanev, P. Synthetic approach to 2-alkyl-4-quinolones and 2-alkyl-4-quinolone-3-carboxamides based on common β-keto amide precursors. Beilstein J. Org. Chem. 2023, 19, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Angelov, P. Enamine-Based Domino Strategy for C-Acylation/Deacetylation of Acetoacetamides: A Practical Synthesis of β-Keto Amides. Synlett 2010, 2010, 1273–1275. [Google Scholar] [CrossRef]
- Mollova-Sapundzhieva, Y.; Alonso, F.; Angelov, P.; Nedialkov, P. Synthesis of 4-Quinolone N-Oxides via Controlled Partial Hydrogenation of 2-Nitrobenzoyl Enamines. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Venkov, A.P.; Angelov, P.A. Synthesis of Unsymmetrical β-Enamino Ketones. Synthesis 2003, 2003, 2221–2225. [Google Scholar] [CrossRef]
- Tantray, J.A.; Mansoor, S.; Wani, R.F.C.; Un Nissa, N. Antibiotic resistance test by agar well diffusion method. In Basic Life Science Methods; Academic Press: Cambridge, MA, USA, 2023; Chapter 46; p. 189. [Google Scholar] [CrossRef]

| Product | R1 | R2 | Conditions | Yield (%) |
|---|---|---|---|---|
| 4a | C6H5 | H | A | 84 |
| 4b | p-MeOC6H4 | H | A | 80 |
| 4c | p-ClC6H4 | H | A | 72 |
| 5a | C6H5 | OH | B | 70 |
| 5b | p-MeOC6H4 | OH | B | 70 |
| 5c | p-ClC6H4 | OH | C | 82 |
| Sterile Zone Diameter (mm) 1 | ||||||
|---|---|---|---|---|---|---|
| Product | E. coli ATCC 25922 | E. coli ATCC 8739 | S. aureus ATCC 25923 | S. aureus ATCC 6538 | Enterococcus faecalis ATCC 29212 | B. subtilis NBIMCC 1208 |
| 4a | - | - | 19 | - | - | - |
| 4b | - | - | 11 | - | - | - |
| 4c | - | - | 9 | - | - | 9 |
| 5a | - | 15 | 25 | - | - | 15 |
| 5b | - | - | 20 | - | 15 | 15 |
| 5c | 15 | 15 | 19 | 16 | 15 | 15 |
| 7 | - | - | 14 | - | - | 9 |
| 8 | 14 | 15 | 27 | 14 | 14 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelov, P.; Mollova-Sapundzhieva, Y.; Alonso, F.; Goranov, B.; Nedialkov, P.; Bachvarova, D. Concise Synthesis of Pseudane IX, Its N-Oxide, and Novel Carboxamide Analogs with Antibacterial Activity. Molecules 2024, 29, 3676. https://doi.org/10.3390/molecules29153676
Angelov P, Mollova-Sapundzhieva Y, Alonso F, Goranov B, Nedialkov P, Bachvarova D. Concise Synthesis of Pseudane IX, Its N-Oxide, and Novel Carboxamide Analogs with Antibacterial Activity. Molecules. 2024; 29(15):3676. https://doi.org/10.3390/molecules29153676
Chicago/Turabian StyleAngelov, Plamen, Yordanka Mollova-Sapundzhieva, Francisco Alonso, Bogdan Goranov, Paraskev Nedialkov, and Denitsa Bachvarova. 2024. "Concise Synthesis of Pseudane IX, Its N-Oxide, and Novel Carboxamide Analogs with Antibacterial Activity" Molecules 29, no. 15: 3676. https://doi.org/10.3390/molecules29153676
APA StyleAngelov, P., Mollova-Sapundzhieva, Y., Alonso, F., Goranov, B., Nedialkov, P., & Bachvarova, D. (2024). Concise Synthesis of Pseudane IX, Its N-Oxide, and Novel Carboxamide Analogs with Antibacterial Activity. Molecules, 29(15), 3676. https://doi.org/10.3390/molecules29153676








