Dynamic and Static Regulation of Nicotinamide Adenine Dinucleotide Phosphate: Strategies, Challenges, and Future Directions in Metabolic Engineering
Abstract
:1. Introduction
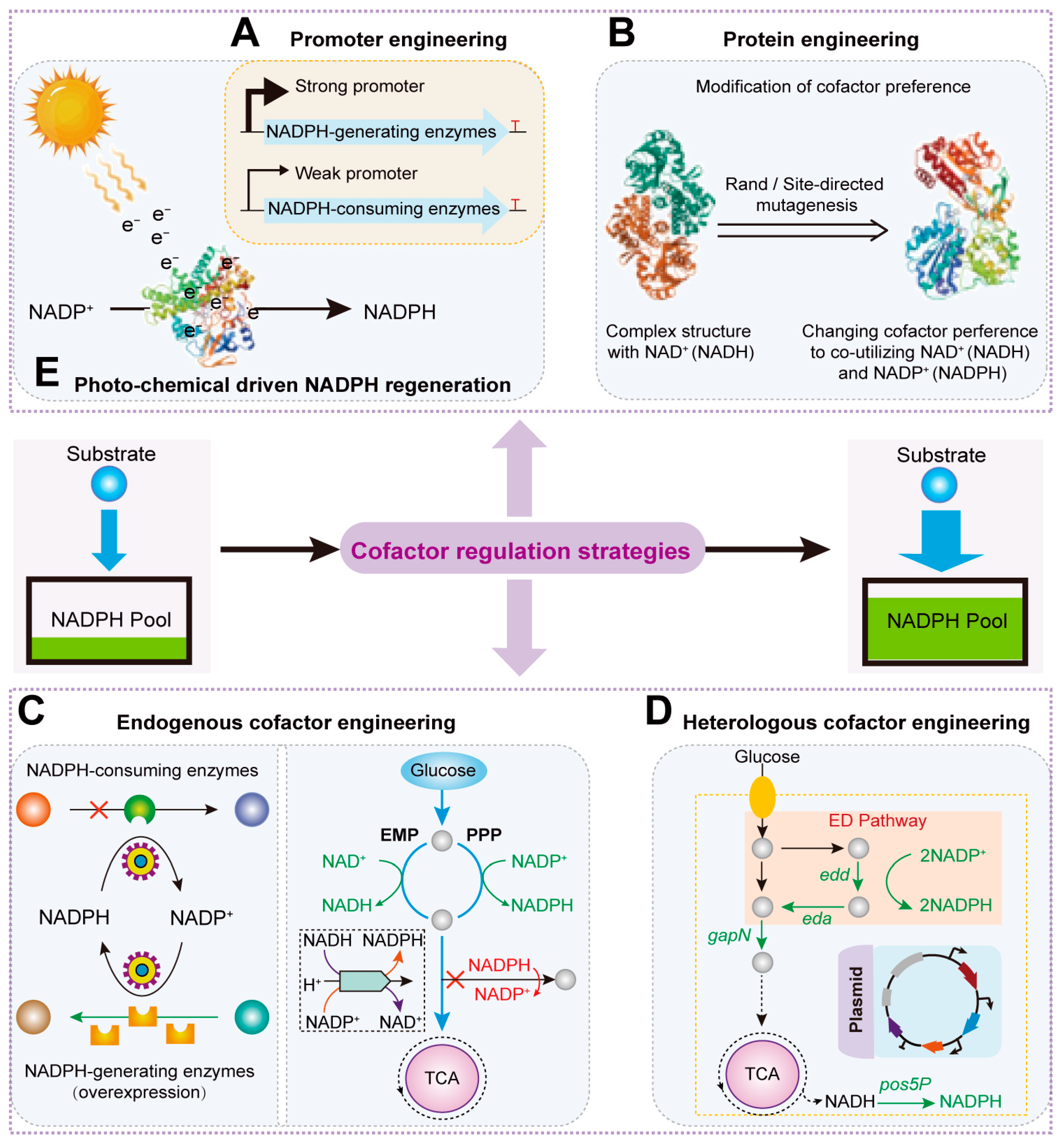
2. Static Regulation Strategies for NADPH Regeneration
2.1. Promoter and RBS Engineering Strategies to Enhance NADPH Regeneration
2.2. Protein Engineering Strategies to Enhance NADPH Levels
2.3. Endogenous Cofactor Engineering Strategies to Regulate NADPH Consumption and Regeneration
2.4. Heterologous Cofactor Engineering Strategies to Supplement NADPH Regeneration Systems
2.5. Photo- or Electrochemical Methods Driven NADPH Regeneration
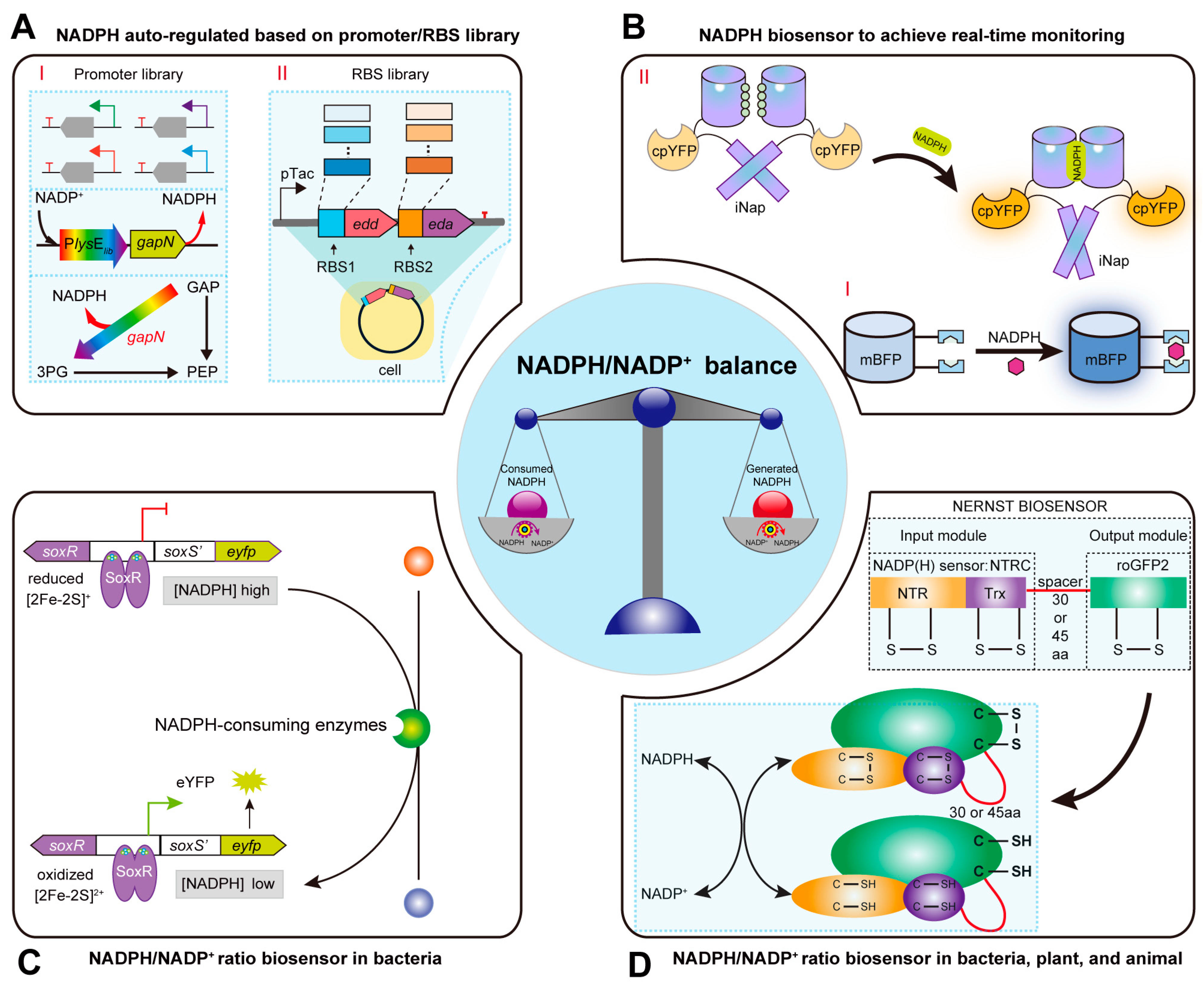
3. Strategies for Dynamic Regulation of NADPH/NADP+ Ratio Based on Regulatory Element Libraries and Genetically Encoded Biosensors
3.1. Constructing Promoter and RBS Libraries for Dynamic Regulation of the NADPH Pool
3.2. Constructing Biosensors for Real-Time Monitoring of Intracellular NADP(H) Levels
3.3. Constructing NADPH/NADP+ Ratio Biosensors for Dynamic Regulation of Redox State
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Lindner, S.N.; Ramirez, L.C.; Krüsemann, J.L.; Yishai, O.; Belkhelfa, S.; He, H.; Bouzon, M.; Döring, V.; Bar-Even, A. NADPH-auxotrophic E. coli: A sensor strain for testing in vivo regeneration of NADPH. ACS Synth. Biol. 2018, 7, 2742–2749. [Google Scholar] [CrossRef]
- Cracan, V.; Titov, D.V.; Shen, H.; Grabarek, Z.; Mootha, V.K. A genetically encoded tool for manipulation of NADP+/NADPH in living cells. Nat. Chem. Biol. 2017, 13, 1088–1095. [Google Scholar] [CrossRef]
- Spielmann, A.; Baumgart, M.; Bott, M. NADPH-related processes studied with a SoxR-based biosensor in Escherichia coli. MicrobiologyOpen 2018, 8, e785. [Google Scholar] [CrossRef]
- Li, S.; Ye, Z.; Moreb, E.A.; Hennigan, J.N.; Castellanos, D.B.; Yang, T.; Lynch, M.D. Dynamic control over feedback regulatory mechanisms improves NADPH flux and xylitol biosynthesis in engineered E. coli. Metab. Eng. 2021, 64, 26–40. [Google Scholar] [CrossRef]
- Zhu, J.J.; Schwörer, S.; Berisa, M.; Kyung, Y.J.; Ryu, K.W.; Yi, J.M.; Jiang, X.J.; Cross, J.R.; Thompson, C.B. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science 2021, 372, 968–972. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef]
- Molinari, P.E.; Krapp, A.R.; Weiner, A.; Beyer, H.M.; Kondadi, A.K.; Blomeier, T.; López, M.; Bustos-Sanmamed, P.; Tevere, E.; Weber, W.; et al. NERNST: A genetically-encoded ratiometric non-destructive sensing tool to estimate NADP(H) redox status in bacterial, plant and animal systems. Nat. Commun. 2023, 14, 3277. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuoka, Y. Redox rebalance against genetic perturbations and modulation of central carbon metabolism by the oxidative stress regulation. Biotechnol. Adv. 2019, 37, 107441. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Wang, N.; Fan, X.; He, M.; Hu, Z.; Tang, C.; Zhang, S.; Lin, D.; Gan, P.; Wang, J.; Huang, X.; et al. Transcriptional repression of TaNOX10 by TaWRKY19 compromises ROS generation and enhances wheat susceptibility to stripe rust. Plant Cell 2022, 34, 1784–1803. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuoka, Y. Feedback regulation and coordination of the main metabolism for bacterial growth and metabolic engineering for amino acid fermentation. Biotechnol. Adv. 2022, 55, 107887. [Google Scholar] [CrossRef]
- Becker, J.; Rohles, C.M.; Wittmann, C. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab. Eng. 2018, 50, 122–141. [Google Scholar] [CrossRef]
- Yayo, J.; Rydzak, T.; Kuil, T.; Karlsson, A.; Harding, D.J.; Guss, A.M.; van Maris, A.J.A. The roles of nicotinamide adenine dinucleotide phosphate reoxidation and ammonium assimilation in the secretion of amino acids as byproducts of Clostridium thermocellum. Appl. Environ. Microbiol. 2023, 89, e01753-22. [Google Scholar] [CrossRef]
- Hao, Y.; Pan, X.; You, J.; Li, G.; Xu, M.; Rao, Z. Microbial production of branched chain amino acids: Advances and perspectives. Bioresour. Technol. 2024, 397, 130502. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Li, R.; Liu, Q.; Shao, X.; Zhu, L.; Kang, M.K.; Wei, G.; Kim, S.W.; Wang, C. Reassessing acetyl-CoA supply and NADPH availability for mevalonate biosynthesis from glycerol in Escherichia coli. Biotechnol. Bioeng. 2022, 119, 2868–2877. [Google Scholar] [CrossRef]
- Nowrouzi, B.; Rios-Solis, L. Redox metabolism for improving whole-cell P450-catalysed terpenoid biosynthesis. Crit. Rev. Biotechnol. 2021, 42, 1213–1237. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, X.; He, Q.; Yang, S. Systematic metabolic engineering of Zymomonas mobilis for β-farnesene production. Front. Bioeng. Biotechnol. 2024, 12, 1392556. [Google Scholar] [CrossRef]
- Chang, L.; Lu, H.; Chen, H.; Tang, X.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Lipid metabolism research in oleaginous fungus Mortierella alpina: Current progress and future prospects. Biotechnol. Adv. 2022, 54, 107794. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Zhao, G.; Caiyin, Q.; Qiao, J. Redox cofactor engineering in industrial microorganisms: Strategies, recent applications and future directions. J. Ind. Microbiol. Biotechnol. 2018, 45, 313–327. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Wang, T.; Xu, Q.; Zhang, C.; Fan, X.; Ma, Q.; Chen, N.; Xie, X. Current status on metabolic engineering for the production of l-aspartate family amino acids and derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, R.; Yan, X.; Wang, J.; Wang, X.; Chen, S.; Bai, F.; He, Q.; Yang, S. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB). Green Chem. 2022, 24, 2588–2601. [Google Scholar] [CrossRef]
- Lee, H.-D.; Yoo, S.-K.; Yoo, H.-S.; Yun, C.-H.; Kim, G.-J. Expression and characterization of monomeric recombinant isocitrate dehydrogenases from Corynebacterium glutamicum and Azotobacter vinelandii for NADPH regeneration. Int. J. Mol. Sci. 2022, 23, 15318. [Google Scholar] [CrossRef]
- Shen, Y.-P.; Liao, Y.-L.; Lu, Q.; He, X.; Yan, Z.-B.; Liu, J.-Z. ATP and NADPH engineering of Escherichia coli to improve the production of 4-hydroxyphenylacetic acid using CRISPRi. Biotechnol. Biofuels 2021, 14, 100. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Upp, D.M.; Lewis, J.C.; Zhang, Y.-H.P.J. A high-throughput method for directed evolution of NAD(P)+-dependent dehydrogenases for the reduction of biomimetic nicotinamide analogues. ACS Catal. 2019, 9, 11709–11719. [Google Scholar] [CrossRef]
- Ding, X.; Yang, W.; Du, X.; Chen, N.; Xu, Q.; Wei, M.; Zhang, C. High-level and -yield production of L-leucine in engineered Escherichia coli by multistep metabolic engineering. Metab. Eng. 2023, 78, 128–136. [Google Scholar] [CrossRef]
- Qin, N.; Li, L.; Ji, X.; Li, X.; Zhang, Y.; Larsson, C.; Chen, Y.; Nielsen, J.; Liu, Z. Rewiring central carbon metabolism ensures increased provision of acetyl-CoA and NADPH required for 3-OH-propionic acid production. ACS Synth. Biol. 2020, 9, 3236–3244. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Guo, L.; Hu, G.; Luo, Q.; Liu, J.; Nielsen, J.; Chen, J.; Liu, L. DCEO biotechnology: Tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals. Chem. Rev. 2017, 118, 4–72. [Google Scholar] [CrossRef]
- Yang, H.; Jia, X.; Han, Y. Microbial redox coenzyme engineering and applications in biosynthesis. Trends Microbiol. 2022, 30, 318–321. [Google Scholar] [CrossRef]
- Conway, T. The Entner-Doudoroff pathway: History, physiology and molecular biology. FEMS Microbiol. Lett. 1992, 103, 1–27. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Xue, Y.; Yu, B. Engineering Pseudomonas putida KT2440 for dipicolinate production via the Entner-Doudoroff pathway. J. Agric. Food Chem. 2024, 72, 6500–6508. [Google Scholar] [CrossRef] [PubMed]
- Satanowski, A.; Dronsella, B.; Noor, E.; Vogeli, B.; He, H.; Wichmann, P.; Erb, T.J.; Lindner, S.N.; Bar-Even, A. Awakening a latent carbon fixation cycle in Escherichia coli. Nat. Commun. 2020, 11, 5812. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Jojima, T.; Suda, M.; Inui, M. Isobutanol production in Corynebacterium glutamicum: Suppressed succinate by-production by pckA inactivation and enhanced productivity via the Entner-Doudoroff pathway. Metab. Eng. 2020, 59, 24–35. [Google Scholar] [CrossRef]
- Yang, H.; He, Y.; Zhou, S.; Deng, Y. Dynamic regulation and cofactor engineering of escherichia coli to enhance production of glycolate from corn stover hydrolysate. Bioresour. Technol. 2024, 398, 130531. [Google Scholar] [CrossRef]
- de Oliveira, R.D.; Novello, V.; da Silva, L.F.; Gomez, J.G.C.; Le Roux, G.A.C. Glucose metabolism in Pseudomonas aeruginosa is cyclic when producing Polyhydroxyalkanoates and Rhamnolipids. J. Biotechnol. 2021, 342, 54–63. [Google Scholar] [CrossRef]
- Kohlstedt, M.; Wittmann, C. GC-MS-based 13C metabolic flux analysis resolves the parallel and cyclic glucose metabolism of Pseudomonas putida KT2440 and Pseudomonas aeruginosa PAO1. Metab. Eng. 2019, 54, 35–53. [Google Scholar] [CrossRef]
- Cardinali-Rezende, J.; Di Genova, A.; Nahat, R.; Steinbuchel, A.; Sagot, M.F.; Costa, R.S.; Oliveira, H.C.; Taciro, M.K.; Silva, L.F.; Gomez, J.G.C. The relevance of enzyme specificity for coenzymes and the presence of 6-phosphogluconate dehydrogenase for polyhydroxyalkanoates production in the metabolism of Pseudomonas sp. LFM046. Int. J. Biol. Macromol. 2020, 163, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Castro, I.; Binger, D.; Rodrigues, A.; Becker, J.; dos Santos, V.A.P.M.; Wittmann, C. In-silico-driven metabolic engineering of Pseudomonas putida for enhanced production of poly-hydroxyalkanoates. Metab. Eng. 2013, 15, 113–123. [Google Scholar] [CrossRef]
- Sohn, S.B.; Kim, T.Y.; Park, J.M.; Lee, S.Y. In silico genome-scale metabolic analysis of Pseudomonas putida KT2440 for polyhydroxyalkanoate synthesis, degradation of aromatics and anaerobic survival. Biotechnol. J. 2010, 5, 739–750. [Google Scholar] [CrossRef]
- Oberhardt, M.A.; Puchalka, J.; dos Santos, V.A.P.M.; Papin, J.A. Reconciliation of genome-scale metabolic reconstructions for comparative systems analysis. PLoS Comput. Biol. 2011, 7, e1001116. [Google Scholar] [CrossRef]
- Olavarria, K.; Marone, M.P.; da Costa Oliveira, H.; Roncallo, J.C.; da Costa Vasconcelos, F.N.; da Silva, L.F.; Gomez, J.G. Quantifying NAD(P)H production in the upper Entner-Doudoroff pathway from Pseudomonas putida KT2440. FEBS Open Bio 2015, 5, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Volke, D.C.; Olavarría, K.; Nikel, P.I. Cofactor specificity of glucose-6-phosphate dehydrogenase isozymes in Pseudomonas putida reveals a general principle underlying glycolytic strategies in bacteria. Msystems 2021, 6, e00014-21. [Google Scholar] [CrossRef]
- Choe, M.; Titov, D.V. Genetically encoded tools for measuring and manipulating metabolism. Nat. Chem. Biol. 2022, 18, 451–460. [Google Scholar] [CrossRef]
- Tschirhart, T.; Kim, E.; McKay, R.; Ueda, H.; Wu, H.-C.; Pottash, A.E.; Zargar, A.; Negrete, A.; Shiloach, J.; Payne, G.F.; et al. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat. Commun. 2017, 8, 14030. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kawaguchi, H.; Shirai, T.; Ninomiya, K.; Takahashi, K.; Kondo, A.; Tsuge, Y. Automatic redirection of carbon flux between glycolysis and pentose phosphate pathway using an oxygen-responsive metabolic switch in Corynebacterium glutamicum. ACS Synth. Biol. 2020, 9, 814–826. [Google Scholar] [CrossRef]
- Li, Y.; Xian, H.; Xu, Y.; Zhu, Y.; Sun, Z.; Wang, Q.; Qi, Q. Fine tuning the glycolytic flux ratio of EP-bifido pathway for mevalonate production by enhancing glucose-6-phosphate dehydrogenase (Zwf) and CRISPRi suppressing 6-phosphofructose kinase (PfkA) in Escherichia coli. Microb. Cell Factories 2021, 20, 32. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, H.; Liu, R.; Yu, H.; Zhuo, M.; Zhou, T.; Li, S. Tuning a bi-enzymatic cascade reaction in Escherichia coli to facilitate NADPH regeneration for ε-caprolactone production. Bioresour. Bioprocess. 2021, 8, 32. [Google Scholar] [CrossRef]
- Wang, M.; Chen, B.; Fang, Y.; Tan, T. Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnol. Adv. 2017, 35, 1032–1039. [Google Scholar] [CrossRef]
- Cheng, F.; Li, Q.H.; Zhang, H.Y.; Wei, L.; Zheng, Y.G. Simultaneous directed evolution of coupled enzymes for efficient asymmetric synthesis of l-phosphinothricin. Appl. Environ. Microbiol. 2020, 87, e02563-20. [Google Scholar] [CrossRef] [PubMed]
- Dmytruk, O.V.; Dmytruk, K.V.; Abbas, C.A.; Voronovsky, A.Y.; Sibirny, A.A. Engineering of xylose reductase and overexpression of xylitol dehydrogenase and xylulokinase improves xylose alcoholic fermentation in the thermotolerant yeast Hansenula polymorpha. Microb. Cell Factories 2008, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, F.; Dong, F.; Wang, Q.; Yang, J.; Jiang, Y.; Yang, S. De novo engineering of Corynebacterium glutamicum for l-proline production. ACS Synth. Biol. 2020, 9, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, X.; Liu, Y.; Yang, M.; Wang, L.; Li, Y.; Wang, J. Increased NADPH supply enhances glycolysis metabolic flux and L-methionine production in Corynebacterium glutamicum. Foods 2022, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-Y.; Yang, Z.-Y.; Chen, Q.; Zong, M.-H.; Li, N. Engineering promiscuous alcohol dehydrogenase activity of a reductive aminase AspRedAm for selective reduction of biobased furans. Front. Chem. 2021, 9, 610091. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.R.; Weusthuis, R.A.; Huang, W.E. Growth-coupled enzyme engineering through manipulation of redox cofactor regeneration. Biotechnol. Adv. 2023, 63, 108102. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Motmaen, A.; Dauparas, J.; Baek, M.Y.; Abedi, M.H.; Baker, D.; Bradley, P. Peptide-binding specificity prediction using fine-tuned protein structure prediction networks. Proc. Natl. Acad. Sci. USA 2023, 120, e2216697120. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, 439–444. [Google Scholar] [CrossRef]
- Calzadiaz-Ramirez, L.; Calvó-Tusell, C.; Stoffel, G.M.M.; Lindner, S.N.; Osuna, S.; Erb, T.J.; Garcia-Borràs, M.; Bar-Even, A.; Acevedo-Rocha, C.G. In vivo selection for formate dehydrogenases with high efficiency and specificity toward NADP+. ACS Catal. 2020, 10, 7512–7525. [Google Scholar] [CrossRef]
- Schwendenwein, D.; Ressmann, A.K.; Doerr, M.; Höhne, M.; Bornscheuer, U.T.; Mihovilovic, M.D.; Rudroff, F.; Winkler, M. Random mutagenesis-driven improvement of carboxylate reductase activity using an amino benzamidoxime-mediated high-throughput assay. Adv. Synth. Catal. 2019, 361, 2544–2549. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Lai, Y.; Mo, Q.; Yuan, J. High-yielding terpene-based biofuel production in Rhodobacter capsulatus. ACS Synth. Biol. 2021, 10, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, P.; Li, Y.; Liu, X.; Wang, Z.; Chen, T.; Zhao, X. Enhancing β-Carotene production in Escherichia coli by perturbing central carbon metabolism and improving the NADPH supply. Front. Bioeng. Biotechnol. 2020, 8, 585. [Google Scholar] [CrossRef]
- Wang, X.; Goh, E.-B.; Beller, H.R. Engineering E. coli for simultaneous glucose–xylose utilization during methyl ketone production. Microb. Cell Factories 2018, 17, 12. [Google Scholar] [CrossRef]
- Satowa, D.; Fujiwara, R.; Uchio, S.; Nakano, M.; Otomo, C.; Hirata, Y.; Matsumoto, T.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of E. coli for improving mevalonate production to promote NADPH regeneration and enhance acetyl-CoA supply. Biotechnol. Bioeng. 2020, 117, 2153–2164. [Google Scholar] [CrossRef]
- Wang, H.-D.; Xu, J.-Z.; Zhang, W.-G. Reduction of acetate synthesis, enhanced arginine export, and supply of precursors, cofactors, and energy for improved synthesis of L-arginine by Escherichia coli. Appl. Microbiol. Biotechnol. 2023, 107, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, Y.; Shao, J.; Liu, H.; Wang, Y. Ferulic acid production by metabolically engineered Escherichia coli. Bioresour. Bioprocess. 2021, 8, 70. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Y.; Yuan, Z.Z.; Liu, G.Q.; Sun, Z.J.; Du, S.Y.; Liu, H.; Li, Y.T.; Liu, H.L.; Zhou, Z.M. Evaluation of metabolic engineering strategies on 2-ketoisovalerate production by Escherichia coli. Appl. Environ. Microbiol. 2022, 88, e00976-22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cai, M.; Liu, Y.; Hu, M.; Yang, F.; Zhu, R.; Xu, M.; Rao, Z. Genomics and transcriptomics-guided metabolic engineering Corynebacterium glutamicum for l-arginine production. Bioresour. Technol. 2022, 364, 128054. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Xu, J.; Ruan, H.; Zhang, W. Deciphering the crucial roles of AraC-type transcriptional regulator Cgl2680 on NADPH metabolism and l-lysine production in Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2020, 36, 82. [Google Scholar] [CrossRef]
- Yuan, L.; Qin, Y.-L.; Zou, Z.-C.; Appiah, B.; Huang, H.; Yang, Z.-H.; Qun, C. Enhancing intracellular NADPH bioavailability through improving pentose phosphate pathway flux and its application in biocatalysis asymmetric reduction reaction. J. Biosci. Bioeng. 2022, 134, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zheng, Z.; Zhao, G.; Wang, L.; Wang, H.; Yang, Q.; Zhang, M.; Li, L.; Wang, P. Bottom-up synthetic biology approach for improving the efficiency of menaquinone-7 synthesis in Bacillus subtilis. Microb. Cell Factories 2022, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Qiao, J.; Qi, Y.; Li, L.; Xu, N.; Shao, L.; Wei, L.; Liu, J. Reprogramming the sulfur recycling network to improve l-cysteine production in Corynebacterium glutamicum. Green Chem. 2023, 25, 3152–3165. [Google Scholar] [CrossRef]
- Chen, S.-L.; Liu, T.-S.; Zhang, W.-G.; Xu, J.-Z. Cofactor engineering for efficient production of α-farnesene by rational modification of NADPH and ATP regeneration pathway in Pichia pastoris. Int. J. Mol. Sci. 2023, 24, 1767. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhao, J.; Chen, C.; Hu, X.; Wang, X. Enhancing the carbon flux and NADPH supply to increase L-isoleucine production in Corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 2014, 19, 132–142. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Li, B.; Xue, Y.; Wang, X.; Liu, S.; Hu, S.; Tang, J.; Yan, B.; Li, T.; et al. NADP+-dependent isocitrate dehydrogenase as a novel target for altering carbon flux to lipid accumulation and enhancing antioxidant capacity in Tetradesmus obliquus. Bioresour. Technol. 2024, 395, 130365. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-C.; Zhang, J.-L.; Song, H.; Cao, Y.-X. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering. Microb. Cell Factories 2019, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.L.; Jungmann, L.; Schiefelbein, S.; Peyriga, L.; Cahoreau, E.; Portais, J.-C.; Becker, J.; Wittmann, C. Lysine production from the sugar alcohol mannitol: Design of the cell factory Corynebacterium glutamicum SEA-3 through integrated analysis and engineering of metabolic pathway fluxes. Metab. Eng. 2018, 47, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xin, Q.; Pan, L.; Wang, B. Improved recombinant expression of maltogenic α-amylase AmyM in Bacillus subtilis by optimizing its secretion and NADPH production. Fermentation 2023, 9, 475. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, W.; Wu, M.; Bao, H.; Sun, X.; Dou, Q.; Jia, H.; Liu, W.; Shen, Y. Construction of an alternative NADPH regeneration pathway improves ethanol production in Saccharomyces cerevisiae with xylose metabolic pathway. Synth. Syst. Biotechnol. 2024, 9, 269–276. [Google Scholar] [CrossRef]
- Hao, Y.; Pan, X.; Xing, R.; You, J.; Hu, M.; Liu, Z.; Li, X.; Xu, M.; Rao, Z. High-level production of L-valine in Escherichia coli using multi-modular engineering. Bioresour. Technol. 2022, 359, 127461. [Google Scholar] [CrossRef]
- Basle, M.; Padley, H.A.W.; Martins, F.L.; Winkler, G.S.; Jäger, C.M.; Pordea, A. Design of artificial metalloenzymes for the reduction of nicotinamide cofactors. J. Inorg. Biochem. 2021, 220, 111446. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Metal oxide mediated extracellular NADPH regeneration improves ethanol production by engineered Synechocystis sp. PCC 6803. Front. Bioeng. Biotechnol. 2019, 7, 148. [Google Scholar] [CrossRef]
- Kadowaki, J.T.; Jones, T.H.; Sengupta, A.; Gopalan, V.; Subramaniam, V.V. Copper oxide-based cathode for direct NADPH regeneration. Sci. Rep. 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, H.; Ning, C.; Deng, S.; Su, E. Integrating a light-driven coenzyme regeneration system by expression of an alcohol dehydrogenase in phototrophic bacteria for synthesis of chiral alcohol. J. Biotechnol. 2017, 259, 120–125. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Demaurex, N. Illuminating redox biology using NADH- and NADPH-specific sensors. Nat. Methods 2017, 14, 671–672. [Google Scholar] [CrossRef]
- Ding, N.; Sun, L.; Zhou, X.; Zhang, L.; Deng, Y.; Yin, L. Enhancing glucaric acid production from myo-inositol in Escherichia coli by eliminating cell-to-cell variation. Appl. Environ. Microbiol. 2024, 90, e00149-24. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Zhao, Y.; Chu, H.; Wang, A.; Zhu, J.; Chen, X.; Zou, Y.; Shi, M.; Liu, R.; Su, N.; et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 2017, 14, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Farasat, I.; Maranas, C.D.; Salis, H.M. Rational design of a synthetic Entner–Doudoroff pathway for improved and controllable NADPH regeneration. Metab. Eng. 2015, 29, 86–96. [Google Scholar] [CrossRef]
- Luo, Z.W.; Kim, W.J.; Lee, S.Y. Metabolic engineering of Escherichia coli for efficient production of 2-pyrone-4,6-dicarboxylic acid from glucose. ACS Synth. Biol. 2018, 7, 2296–2307. [Google Scholar] [CrossRef]
- Liu, J.; Ou, Y.; Xu, J.-Z.; Rao, Z.-M.; Zhang, W.-G. L-lysine production by systems metabolic engineering of an NADPH auto-regulated Corynebacterium glutamicum. Bioresour. Technol. 2023, 387, 129701. [Google Scholar] [CrossRef]
- Goldbeck, O.; Eck, A.W.; Seibold, G.M. Real time monitoring of NADPH concentrations in Corynebacterium glutamicum and Escherichia coli via the genetically encoded sensor mBFP. Front. Microbiol. 2018, 9, 2564. [Google Scholar] [CrossRef]
- Seo, P.-W.; Kim, G.-J.; Kim, J.-S. A short guide on blue fluorescent proteins: Limits and perspectives. Appl. Microbiol. Biotechnol. 2024, 108, 208. [Google Scholar] [CrossRef]
- Virolle, M.-J.; You, S.-H.; Lim, H.-D.; Cheong, D.-E.; Kim, E.-S.; Kim, G.-J. Rapid and sensitive detection of NADPH via mBFP-mediated enhancement of its fluorescence. PLoS ONE 2019, 14, e0212061. [Google Scholar] [CrossRef]
- Roshanzadeh, A.; Kang, H.; You, S.-H.; Park, J.; Khoa, N.D.; Lee, D.-H.; Kim, G.-J.; Kim, E.-S. Real-time monitoring of NADPH levels in living mammalian cells using fluorescence-enhancing protein bound to NADPHs. Biosens. Bioelectron. 2019, 146, 111753. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Jin, X.; Qi, Q.; Liang, Q. Genetically encoded ATP and NAD(P)H biosensors: Potential tools in metabolic engineering. Crit. Rev. Biotechnol. 2022, 43, 1211–1225. [Google Scholar] [CrossRef]
- Lim, S.-L.; Voon, C.P.; Guan, X.; Yang, Y.; Gardeström, P.; Lim, B.L. In planta study of photosynthesis and photorespiration using NADPH and NADH/NAD+ fluorescent protein sensors. Nat. Commun. 2020, 11, 3238. [Google Scholar] [CrossRef]
- Moon, S.J.; Dong, W.; Stephanopoulos, G.N.; Sikes, H.D. Oxidative pentose phosphate pathway and glucose anaplerosis support maintenance of mitochondrial NADPH pool under mitochondrial oxidative stress. Bioeng. Transl. Med. 2020, 5, e10184. [Google Scholar] [CrossRef]
- Zhao, F.-L.; Zhang, C.; Zhang, C.; Tang, Y.; Ye, B.-C. A genetically encoded biosensor for in vitro and in vivo detection of NADP+. Biosens. Bioelectron. 2016, 77, 901–906. [Google Scholar] [CrossRef]
- Cameron, W.D.; Bui, C.V.; Hutchinson, A.; Loppnau, P.; Gräslund, S.; Rocheleau, J.V. Apollo-NADP+: A spectrally tunable family of genetically encoded sensors for NADP+. Nat. Methods 2016, 13, 352–358. [Google Scholar] [CrossRef]
- Watanabe, S.; Kita, A.; Kobayashi, K.; Miki, K. Crystal structure of the [2Fe-2S] oxidative-stress sensor SoxR bound to DNA. Proc. Natl. Acad. Sci. USA 2008, 105, 4121–4126. [Google Scholar] [CrossRef]
- Siedler, S.; Schendzielorz, G.; Binder, S.; Eggeling, L.; Bringer, S.; Bott, M. SoxR as a single-cell biosensor for NADPH-consuming enzymes in Escherichia coli. ACS Synth. Biol. 2013, 3, 41–47. [Google Scholar] [CrossRef]
- Spielmann, A.; Brack, Y.; van Beek, H.; Flachbart, L.; Sundermeyer, L.; Baumgart, M.; Bott, M. NADPH biosensor-based identification of an alcohol dehydrogenase variant with improved catalytic properties caused by a single charge reversal at the protein surface. AMB Express 2020, 10, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Sonnenschein, N.; Pihl, T.P.B.; Pedersen, K.R.; Jensen, M.K.; Keasling, J.D. Engineering an NADPH/NADP+ redox biosensor in Yeast. ACS Synth. Biol. 2016, 5, 1546–1556. [Google Scholar] [CrossRef]
- Liu, X.; Qin, L.; Yu, J.; Sun, W.; Xu, J.; Li, C. Real-time monitoring of subcellular states with genetically encoded redox biosensor system (RBS) in yeast cell factories. Biosens. Bioelectron. 2023, 222, 114988. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, A.; Shi, M.; Chen, X.; Liu, R.; Li, T.; Zhang, C.; Zhang, Z.; Zhu, L.; Ju, Z.; et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat. Protoc. 2018, 13, 2362–2386. [Google Scholar] [CrossRef]
- Liang, M.; Li, Z.; Wang, W.; Liu, J.; Liu, L.; Zhu, G.; Karthik, L.; Wang, M.; Wang, K.-F.; Wang, Z.; et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat. Commun. 2019, 10, 3672. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, T.; Liu, Y.; Tian, R.; Lv, X.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis. Nucleic Acids Res. 2020, 48, 996–1009. [Google Scholar] [CrossRef]
- Brophy, J.A.N.; Voigt, C.A. Antisense transcription as a tool to tune gene expression. Mol. Syst. Biol. 2016, 12, 854. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Liu, Y.; Zhu, Y.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 2020, 16, 1261–1268. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Yang, H.-K.; Zhang, W.-G. NADPH metabolism: A survey of its theoretical characteristics and manipulation strategies in amino acid biosynthesis. Crit. Rev. Biotechnol. 2018, 38, 1061–1076. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Z.; Zhao, Z.; Wu, H.; Xu, M.; Rao, Z. Microbial production of L-methionine and its precursors using systems metabolic engineering. Biotechnol. Adv. 2023, 69, 108260. [Google Scholar] [CrossRef]
- Han, T.; Nazarbekov, A.; Zou, X.; Lee, S.Y. Recent advances in systems metabolic engineering. Curr. Opin. Biotechnol. 2023, 84, 103004. [Google Scholar] [CrossRef]
- Gong, Z.; Chen, J.; Jiao, X.; Gong, H.; Pan, D.; Liu, L.; Zhang, Y.; Tan, T. Genome-scale metabolic network models for industrial microorganisms metabolic engineering: Current advances and future prospects. Biotechnol. Adv. 2024, 72, 108319. [Google Scholar] [CrossRef]
- Chen, S.-L.; Liu, T.-S.; Zhang, W.-G.; Xu, J.-Z. An NADPH-auxotrophic Corynebacterium glutamicum recombinant strain and used it to construct L-leucine high-yielding strain. Int. Microbiol. 2022, 26, 11–24. [Google Scholar] [CrossRef]
- Cheng, Y.; Bi, X.; Xu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Artificial intelligence technologies in bioprocess: Opportunities and challenges. Bioresour. Technol. 2023, 369, 128451. [Google Scholar] [CrossRef]
- Wang, H.; Fu, T.; Du, Y.; Gao, W.; Huang, K.; Liu, Z.; Chandak, P.; Liu, S.; Van Katwyk, P.; Deac, A.; et al. Scientific discovery in the age of artificial intelligence. Nature 2023, 620, 47–60. [Google Scholar] [CrossRef]
- Ding, N.; Zhou, S.; Deng, Y. Transcription-factor-based biosensor engineering for applications in synthetic biology. ACS Synth. Biol. 2021, 10, 911–922. [Google Scholar] [CrossRef]
- Xue, Q.; Miao, P.; Miao, K.; Yu, Y.; Li, Z. An online automatic sorting system for defective Ginseng Radix et Rhizoma Rubra using deep learning. Chin. Herb. Med. 2023, 15, 447–456. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, G.; Zhang, L.; Shen, Z.; Yin, L.; Zhou, S.; Deng, Y. Engineering an AI-based forward-reverse platform for the design of cross-ribosome binding sites of a transcription factor biosensor. Comput. Struct. Biotechnol. J. 2023, 21, 2929–2939. [Google Scholar] [CrossRef]
- Ding, N.; Yuan, Z.; Zhang, X.; Chen, J.; Zhou, S.; Deng, Y. Programmable cross-ribosome-binding sites to fine-tune the dynamic range of transcription factor-based biosensor. Nucleic Acids Res. 2020, 48, 10602–10613. [Google Scholar] [CrossRef]
- Yu, H.; Deng, H.; He, J.; Keasling, J.D.; Luo, X. UniKP: A unified framework for the prediction of enzyme kinetic parameters. Nat. Commun. 2023, 14, 8211. [Google Scholar] [CrossRef]
- Wei, Z.; Hua, K.; Wei, L.; Ma, S.; Jiang, R.; Zhang, X.; Li, Y.; Wong, W.H.; Wang, X. NeuronMotif: Deciphering cis-regulatory codes by layer-wise demixing of deep neural networks. Proc. Natl. Acad. Sci. USA 2023, 120, e2216698120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, N.; Yuan, Z.; Sun, L.; Yin, L. Dynamic and Static Regulation of Nicotinamide Adenine Dinucleotide Phosphate: Strategies, Challenges, and Future Directions in Metabolic Engineering. Molecules 2024, 29, 3687. https://doi.org/10.3390/molecules29153687
Ding N, Yuan Z, Sun L, Yin L. Dynamic and Static Regulation of Nicotinamide Adenine Dinucleotide Phosphate: Strategies, Challenges, and Future Directions in Metabolic Engineering. Molecules. 2024; 29(15):3687. https://doi.org/10.3390/molecules29153687
Chicago/Turabian StyleDing, Nana, Zenan Yuan, Lei Sun, and Lianghong Yin. 2024. "Dynamic and Static Regulation of Nicotinamide Adenine Dinucleotide Phosphate: Strategies, Challenges, and Future Directions in Metabolic Engineering" Molecules 29, no. 15: 3687. https://doi.org/10.3390/molecules29153687



