Utilization of Lead Nitrate to Enhance the Impact of Hydroxamic Acids on the Hydrophobic Aggregation and Flotation Behavior of Cassiterite
Abstract
1. Introduction
2. Results
2.1. The Effect of Lead Nitrate on the Flotation Behavior of Fine Cassiterite
2.2. The Effect of Lead Nitrate on the Hydrophobic Aggregation of Fine Cassiterite
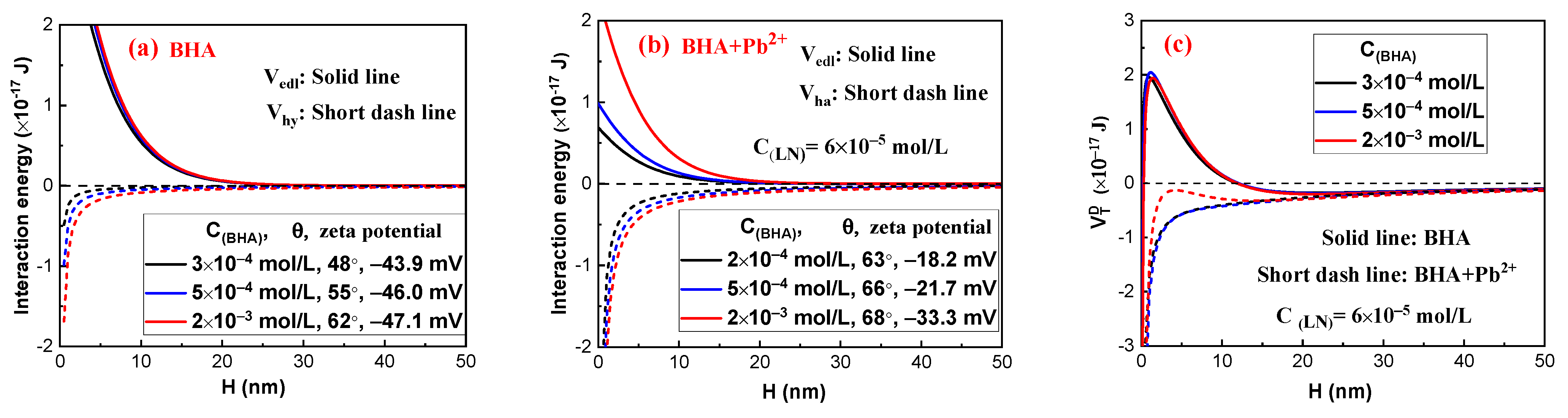
2.2.1. Effect of Lead Nitrate on the Hydrophobic Flocculation of Fine Cassiterite Using BHA
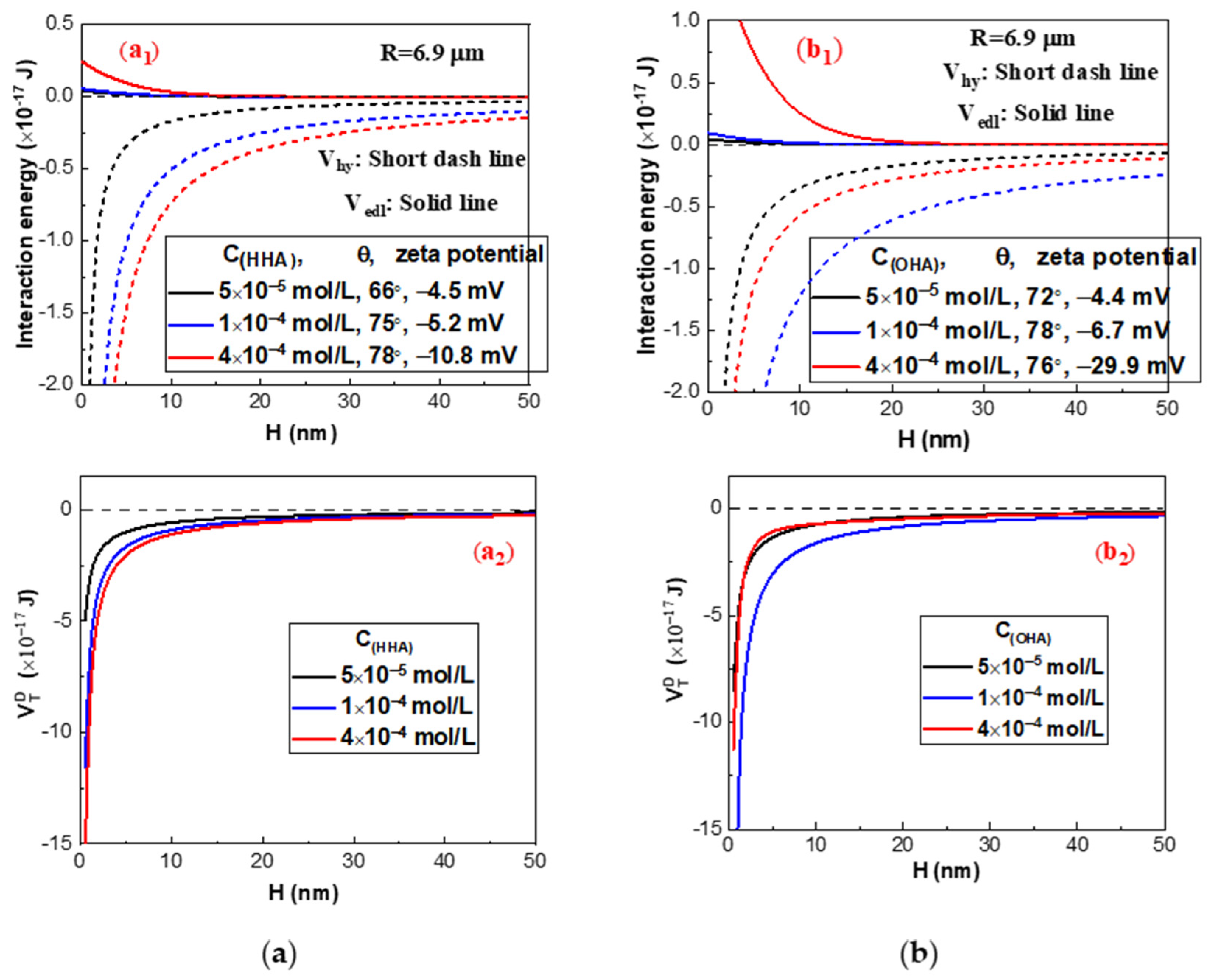
2.2.2. Effect of Lead Nitrate on the Hydrophobic Flocculation of Cassiterite Using HHA
2.2.3. Effect of Lead Nitrate on Hydrophobic Flocculation of Cassiterite Using OHA
2.3. Interaction Energy Estimation by the Extended DLVO Theory
3. Material and Methods
3.1. Single Cassiterite Sample and Reagents
3.2. Micro-Flotation Tests
3.3. Zeta Potential and Contact Angle Measurements
3.4. Focused Beam Reflectance Measurement (FBRM) and Particle Video Microscope (PVM) Observation
4. Conclusions
- (1)
- Micro-flotation tests suggested that the addition of LN could improve the flotation recovery of fine cassiterite and the activation effect of LN on BHA as a collector was the most effective, followed by HHA and OHA.
- (2)
- FBRM and PVM results indicated that lead nitrate could facilitate the formation of hydrophobic aggregates of fine cassiterite using BHA and HHA as the collector, and reduce the necessary concentration of OHA to induce the formation of hydrophobic aggregates. In the presence of lead nitrate, when the amount of BHA was too large (2 × 10−3 mol/L), fine cassiterite particle could not form hydrophobic aggregates, and when the amount of OHA was too large (4 × 10−4 mol/L), the formed hydrophobic aggregates could be broken quickly.
- (3)
- The EDLVO calculation results indicated that lead nitrate could decrease Vedl and increase Vhy between cassiterite particles, eliminating the high energy barriers that existed between the particles and favoring the hydrophobic aggregate using BHA, HHA, and a lower concentration of OHA as the collector.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Xie, X.; Tong, X.; Feng, D.; Song, Q. The activation mechanism of Fe(II) ion-modified cassiterite surface to promote salicylhydroxamic acid adsorption. Miner. Eng. 2021, 160, 106707. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Z.; Zeng, W.; Zhang, Y. Adhesion between nanobubbles and fine cassiterite particles. Int. J. Min. Sci. Technol. 2023, 33, 503–509. [Google Scholar] [CrossRef]
- Wang, P.; Qin, W.; Ren, L.; Wei, Q.; Liu, R.; Yang, C.; Zhong, S. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite. Trans. Nonferrous Met. Soc. China 2013, 23, 1789–1796. [Google Scholar] [CrossRef]
- Jin, S.; Ou, L. Comparison of the Effects of Sodium Oleate and Benzohydroxamic Acid on Fine Scheelite and Cassiterite Hydrophobic Flocculation. Minerals 2022, 12, 687. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Ou, L. Hydrophobic flocculation of fine cassiterite using alkyl hydroxamic acids with different carbon chain lengths as collectors. Molecules 2023, 28, 3911. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; He, J.; Hu, Y.; Sun, W.; Yuan, D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Sun, W.; Han, H.; Hu, Y. Activation role of lead ions in benzohydroxamic acid flotation of oxide minerals: New perspective and new practice. J. Colloid Interface Sci. 2018, 529, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; Wang, L.; Li, Y.; Ji, B.; Hu, Y. Effects of the preassembly of benzohydroxamic acid with Fe (III) ions on its adsorption on cassiterite surface. Miner. Eng. 2018, 127, 32–41. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Y.; Cheng, Y.; Xiao, Y.; Zhang, Y.; Bai, J. Effect of Pb(II) on the flotation behavior of scheelite using sodium oleate as collector. Miner. Eng. 2019, 136, 161–167. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Fu, W. Separation of wolframite ore by froth flotation using a novel “crab” structure sebacoyl hydroxamic acid collector without Pb(NO3)2 activation. Powder Technol. 2021, 389, 96–103. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, T.; Ren, S.; Qiu, X. Research on flotation mechanism of wolframite activated by Pb(II) in neutral solution. Appl. Surf. Sci. 2020, 530, 147036. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Jia, T.; Hu, Y. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, X.; Feng, Q.; Wen, S. Activation mechanism of lead ion in the flotation of stibnite. Miner. Eng. 2018, 119, 173–182. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite—Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Liu, J.; Ejtemaei, M.; Nguyen, A.V.; Wen, S.; Zeng, Y. Surface chemistry of Pb-activated sphalerite. Miner. Eng. 2020, 145, 106058. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Ou, L.; Zhang, Y.; Cheng, J. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Xia, J.; Ni, Y. Surface Activity and Detergent Chemistry and Technology; China Light Industry Press Ltd.: Bingjing, China, 1997. [Google Scholar]
- Fu, J.; Han, H.; Wei, Z.; Sun, W.; Yue, T. Recovery of ultrafine scheelite particles by magnetic seeding flocculation and its mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127266. [Google Scholar] [CrossRef]
- Yoon, R.-H.; Flinn, D.H.; Rabinovich, Y.I. Hydrophobic interactions between dissimilar surfaces. J. Colloid Interface Sci. 1997, 185, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, M.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]

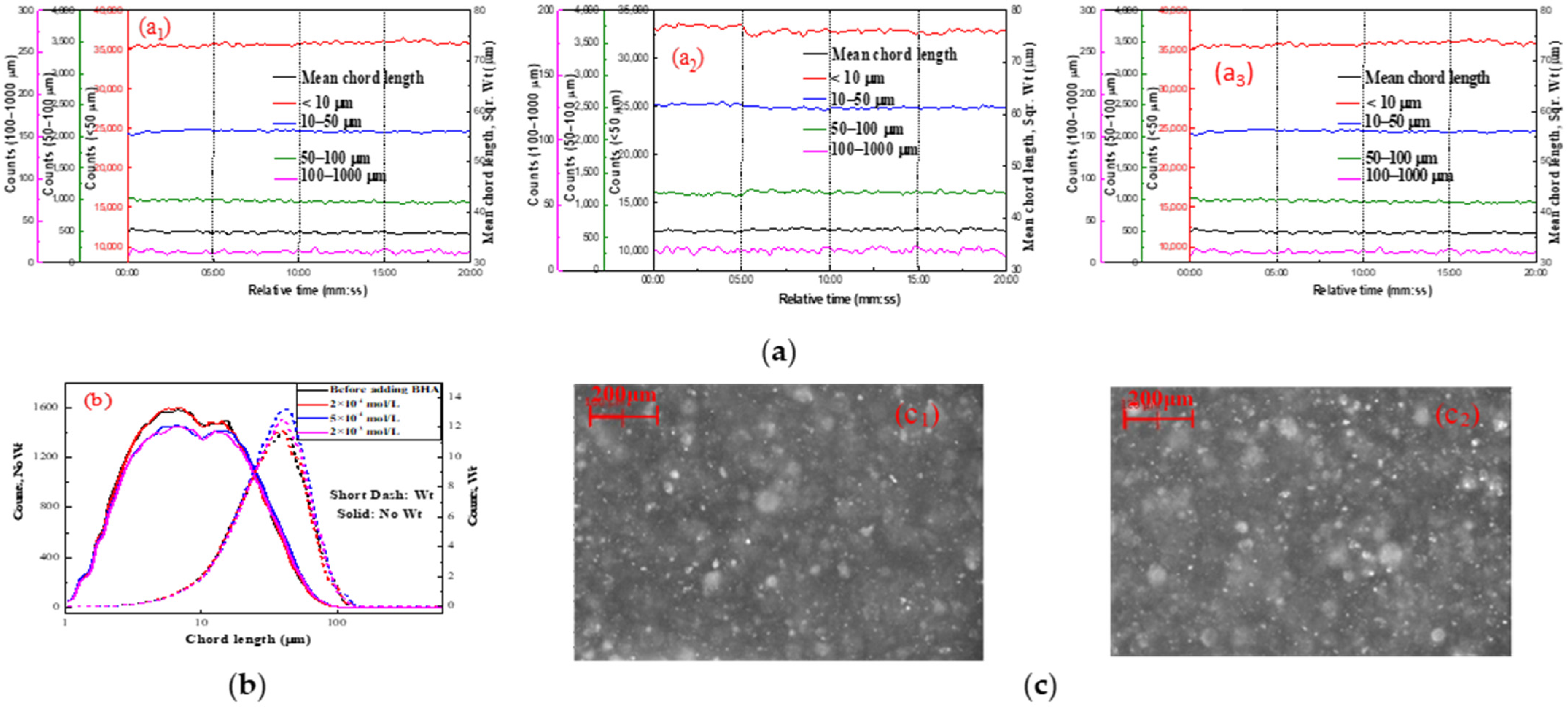

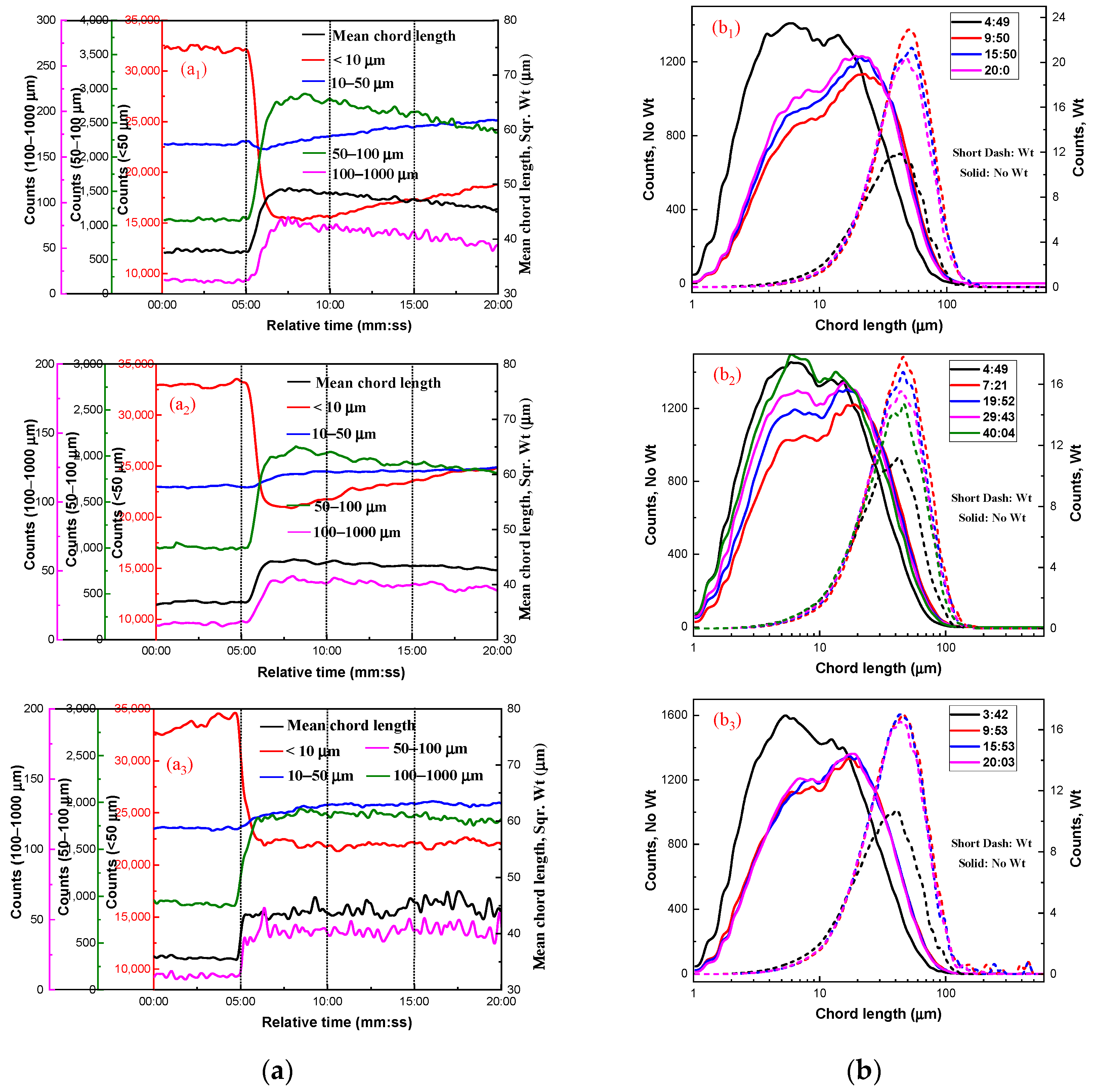
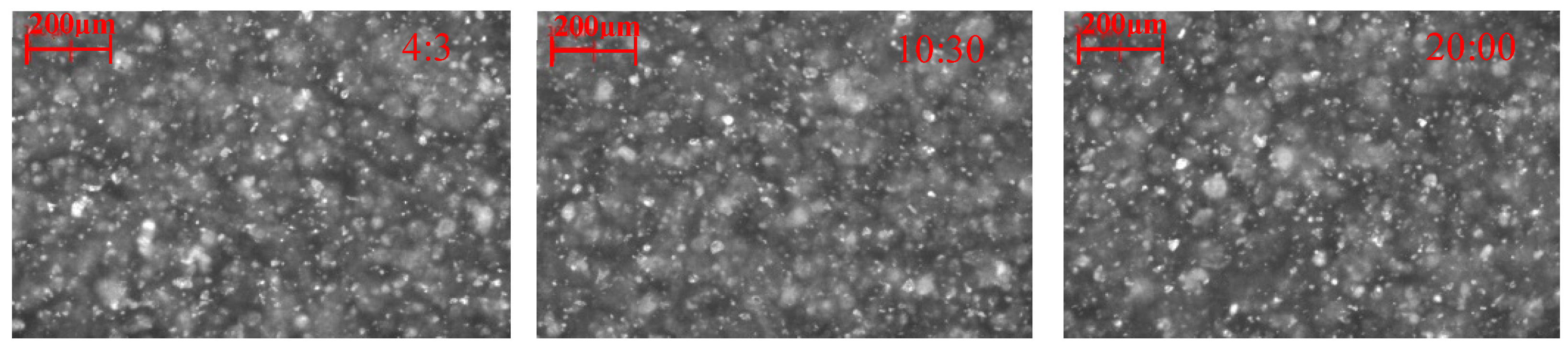
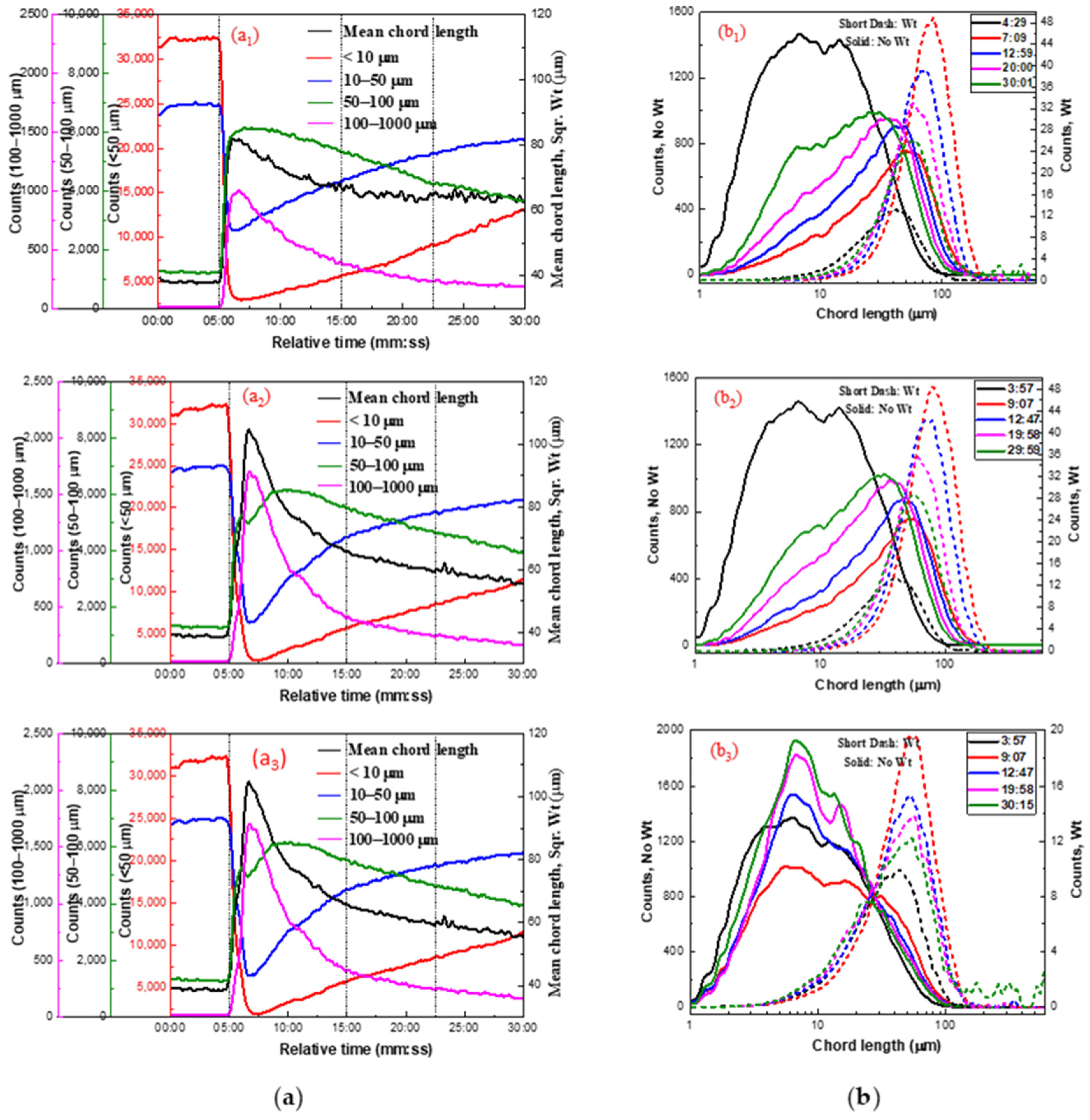
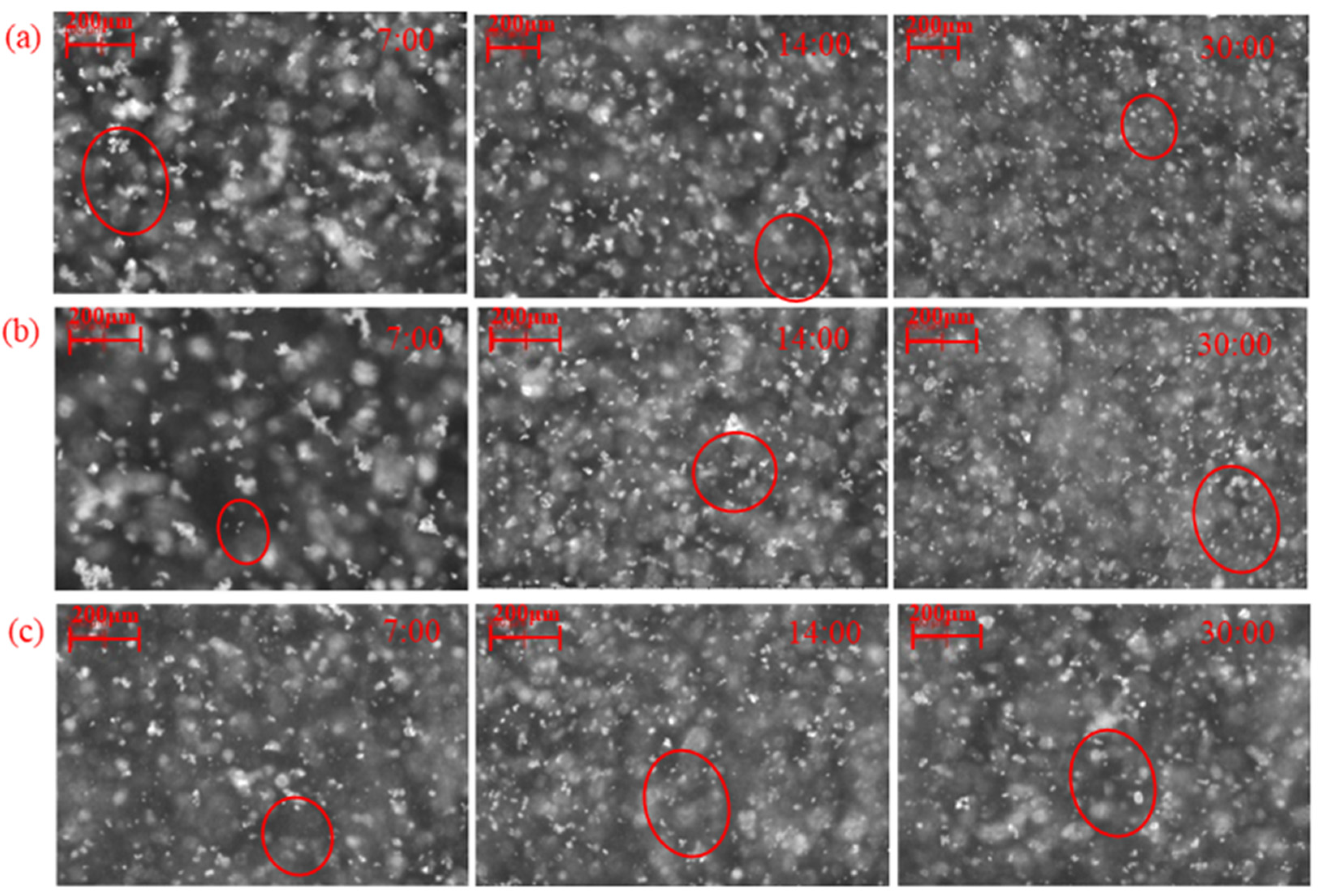
| Reagent | Concentration (mol/L) | d1 (μm) | d2 (μm) | d3 (μm) | R (μm/min) | s (μm/min) |
|---|---|---|---|---|---|---|
| BHA | 3 × 10−4 | 38.3 | 47.1 | 44.5 | 1.76 | 0.26 |
| 5 × 10−4 | 37.5 | 45.4 | 43.7 | 1.60 | 0.17 | |
| 2 × 10−3 | 35.6 | 37.1 | 37.2 | 0.30 | 0.01 | |
| HHA | 5 × 10−5 | 37.8 | 48.4 | 45.6 | 2.12 | 0.28 |
| 1 × 10−4 | 36.8 | 43.9 | 42.8 | 1.43 | 0.11 | |
| 4 × 10−4 | 35.7 | 44.4 | 44.4 | 1.75 | 0.002 | |
| OHA | 5 × 10−5 | 37.2 | 74.7 | 64.9 | 7.30 | 0.98 |
| 1 × 10−4 | 38.8 | 81.4 | 61.4 | 8.51 | 2.00 | |
| 4 × 10−4 | 37.4 | 48.5 | 44.8 | 2.23 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Liu, X.; Feng, Y.; Chen, Y.; Wang, M.; Xiao, Q. Utilization of Lead Nitrate to Enhance the Impact of Hydroxamic Acids on the Hydrophobic Aggregation and Flotation Behavior of Cassiterite. Molecules 2024, 29, 3692. https://doi.org/10.3390/molecules29153692
Jin S, Liu X, Feng Y, Chen Y, Wang M, Xiao Q. Utilization of Lead Nitrate to Enhance the Impact of Hydroxamic Acids on the Hydrophobic Aggregation and Flotation Behavior of Cassiterite. Molecules. 2024; 29(15):3692. https://doi.org/10.3390/molecules29153692
Chicago/Turabian StyleJin, Saizhen, Xiaobo Liu, Yun Feng, Yanfei Chen, Mengtao Wang, and Qingfei Xiao. 2024. "Utilization of Lead Nitrate to Enhance the Impact of Hydroxamic Acids on the Hydrophobic Aggregation and Flotation Behavior of Cassiterite" Molecules 29, no. 15: 3692. https://doi.org/10.3390/molecules29153692
APA StyleJin, S., Liu, X., Feng, Y., Chen, Y., Wang, M., & Xiao, Q. (2024). Utilization of Lead Nitrate to Enhance the Impact of Hydroxamic Acids on the Hydrophobic Aggregation and Flotation Behavior of Cassiterite. Molecules, 29(15), 3692. https://doi.org/10.3390/molecules29153692






