Product Selectivity Control in the Brønsted Acid-Mediated Reactions with 2-Alkynylanilines
Abstract
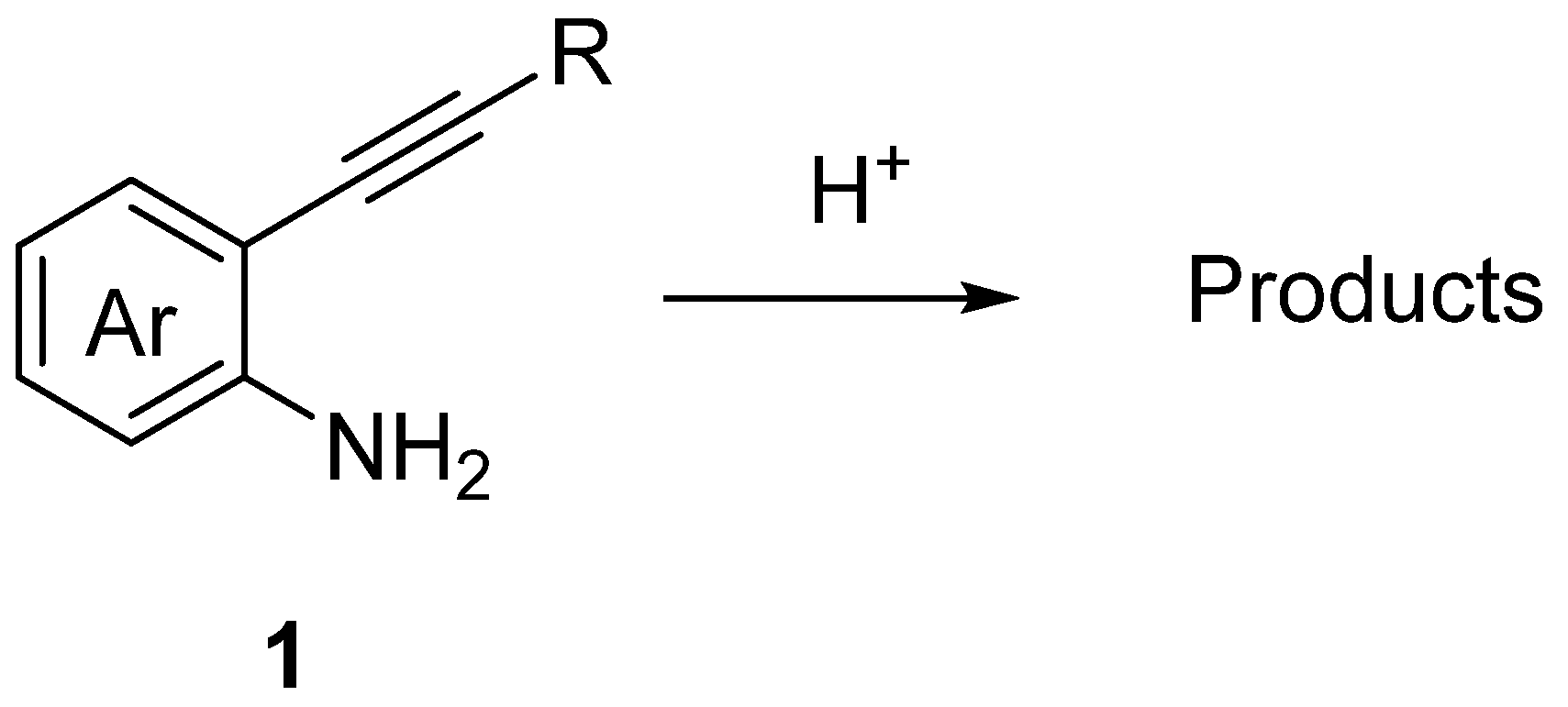
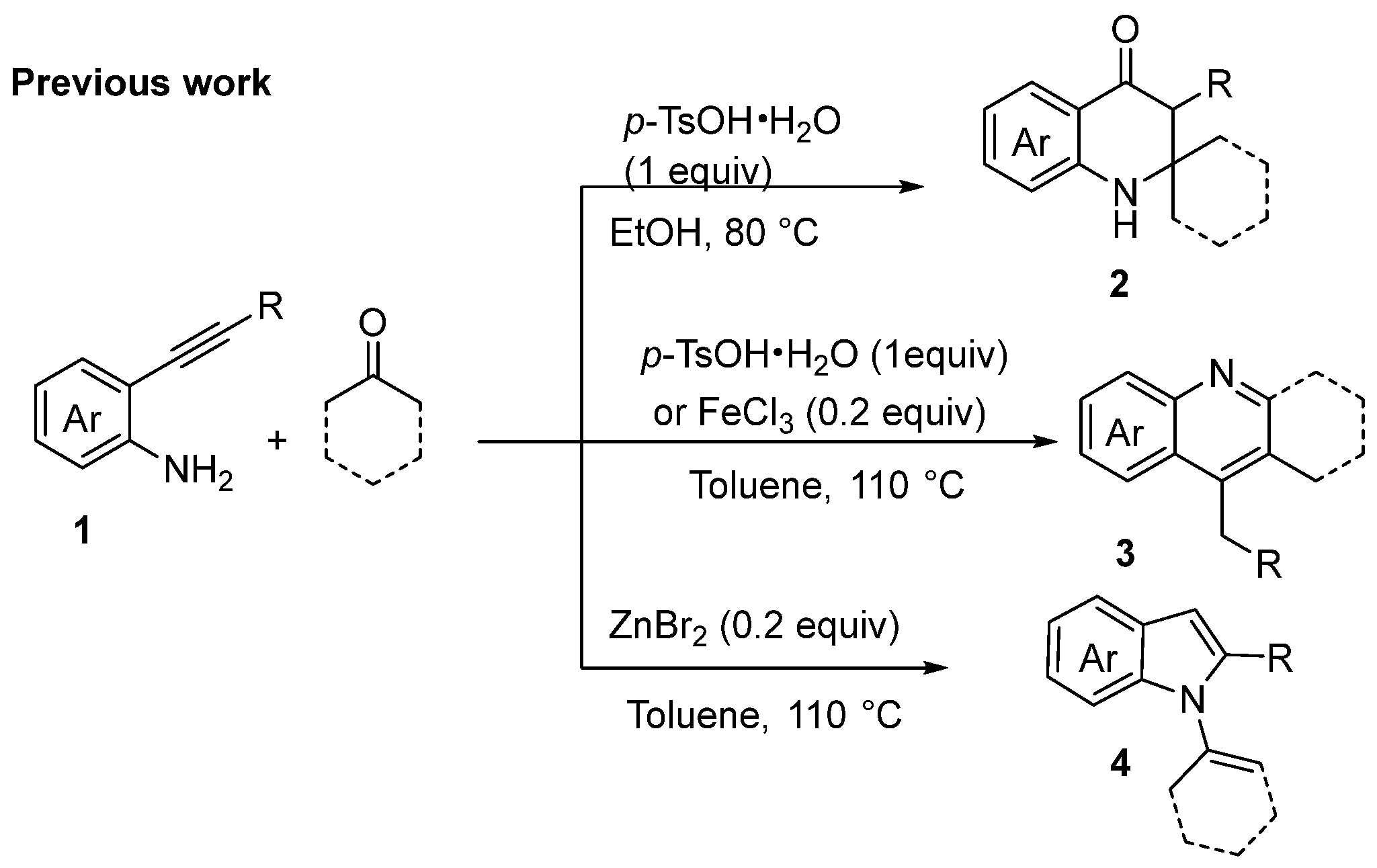
:1. Introduction
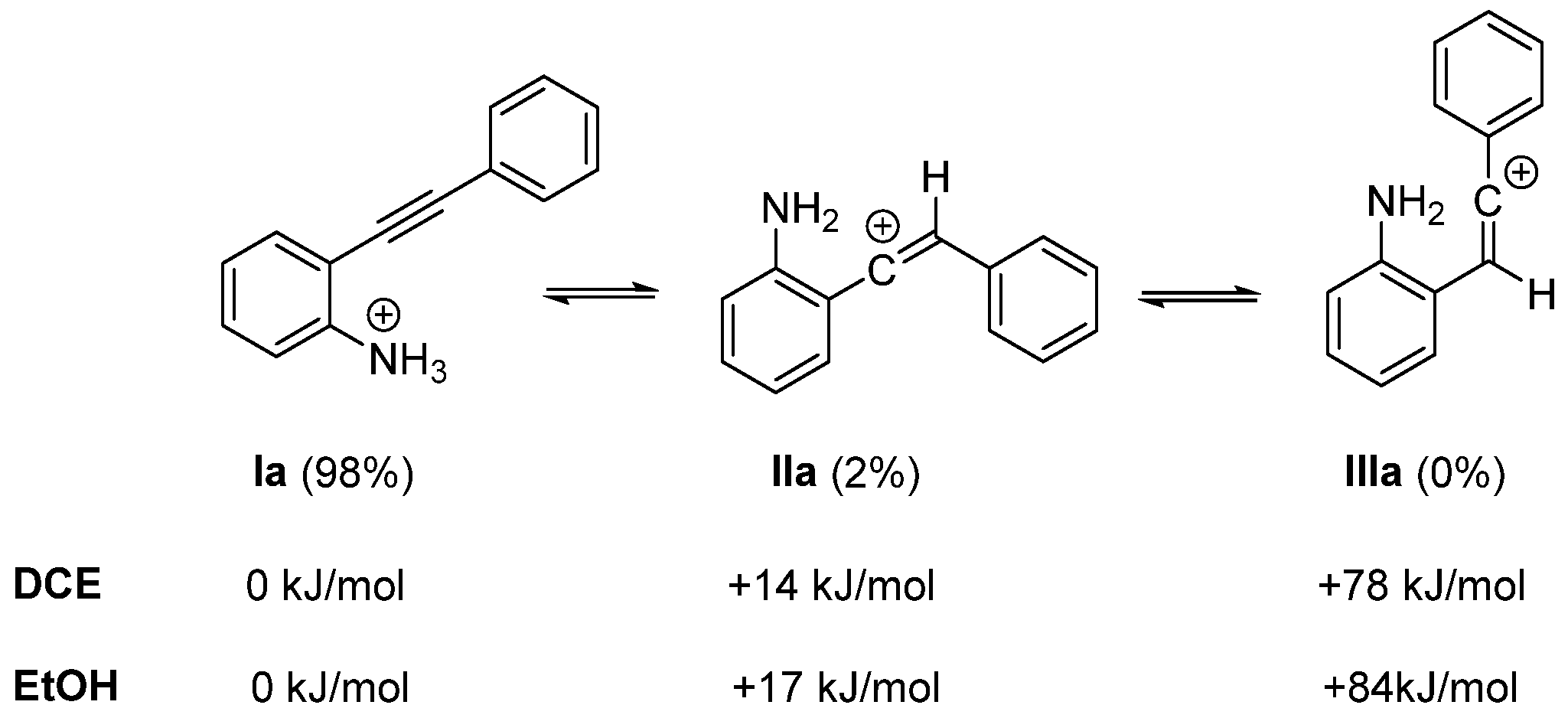
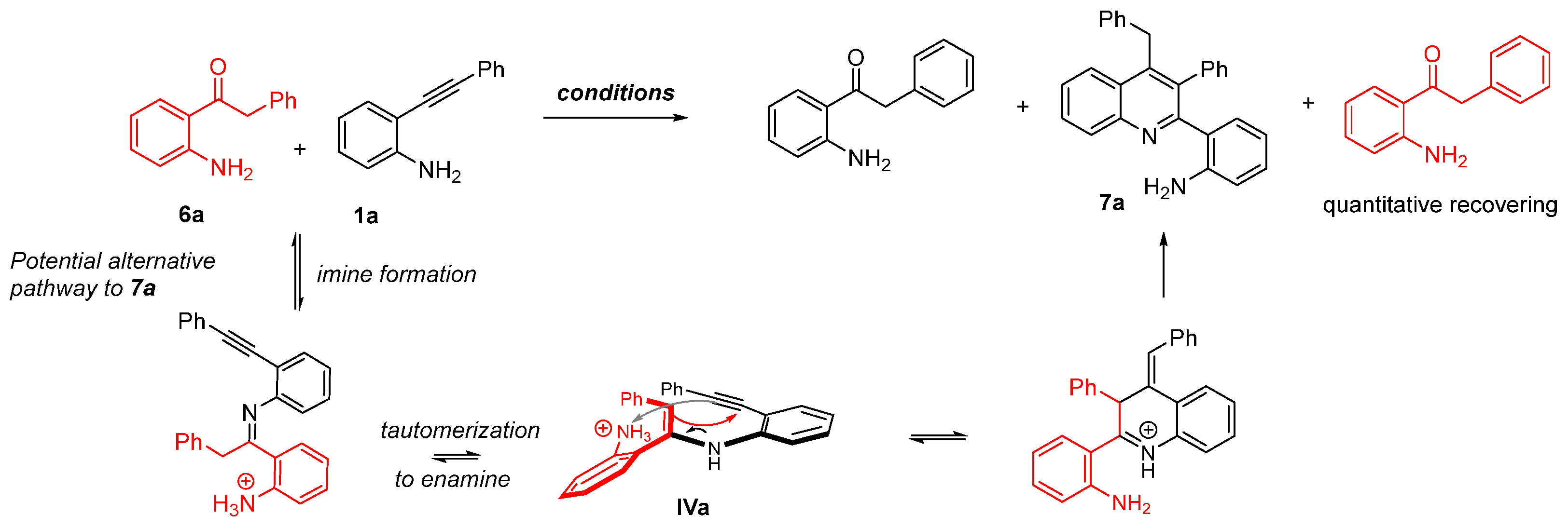
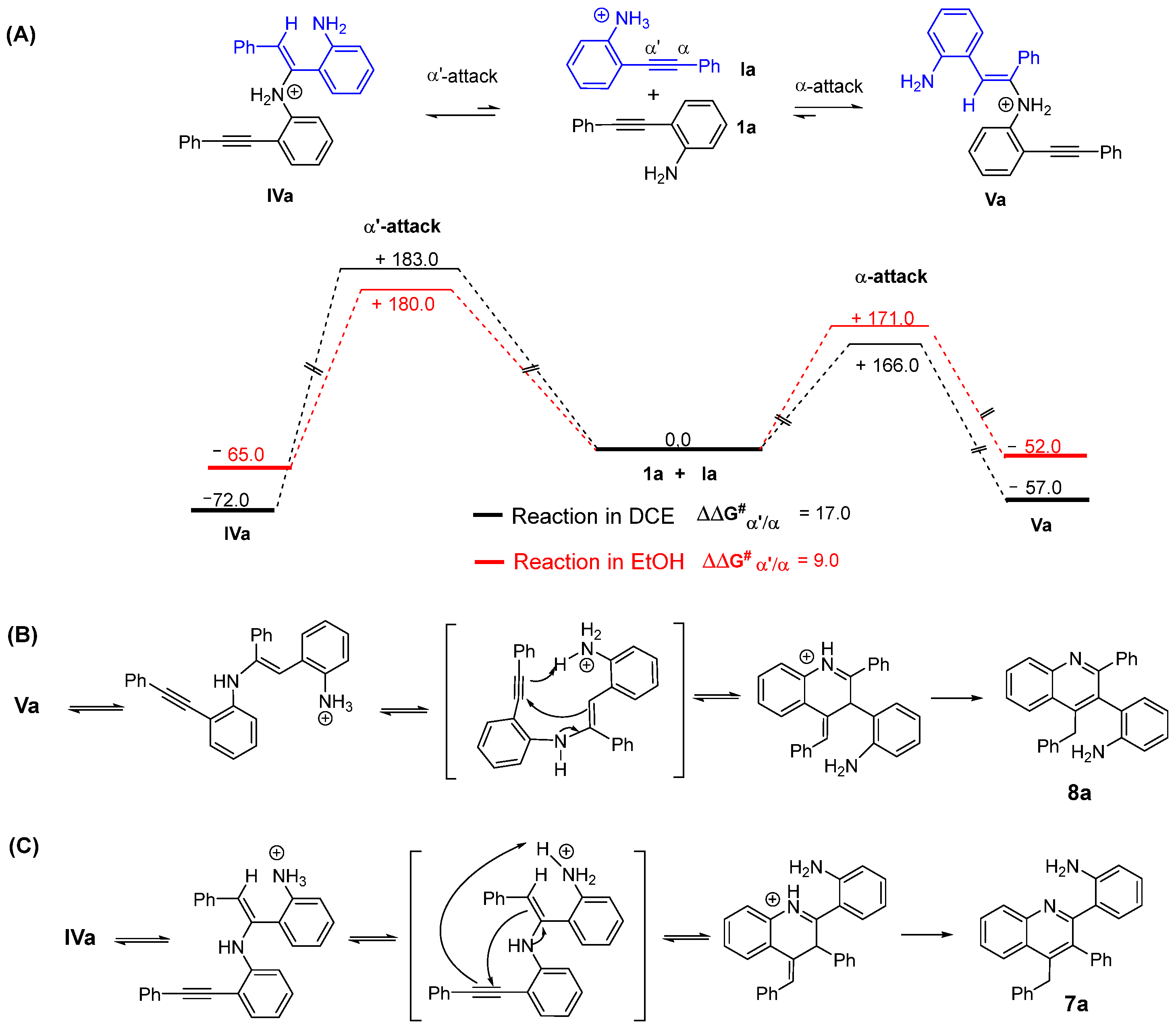
2. Discussion
3. Materials and Methods
Chemistry—General Part
4. Experimental Procedures and Compounds Characterization
Procedure for the Synthesis of 2-Alkynylanilines 1a, 1c-h
- Representative procedure: preparation of the 2-alkynylanilines 1a [46]. A 50 mL round-bottomed flask containing a magnetic stir bar was charged with 2-iodoaniline (1.900 g, 8.674 mmol, 1.0 equiv) and 5 mL of piperidine. The resulting solution was added with Pd (PPh3)4 (100.00 mg, 0.0870 mmol, 0.01 equiv), followed by CuI (16.0 mg, 0.87 mmol, 0.01 equiv). After degassing, phenylacetylene (1.14 mL, 1.060 g, 10.440 mmol, 1.2 equiv) was added to the reaction mixture. The resulting mixture was allowed to stir at room temperature under N2 for 5 h. Upon completion, the mixture was diluted by the addition of EtOAc and aqueous HCl (0.1 M). The separated aqueous phase was extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude material, which was purified by silica gel column chromatography eluting with n-hexane/EtOAc 99/1 to provide 2-(phenylethynyl)aniline (1a) (1.404 g, 85% yield). White solid. 1H NMR (400 MHz, CDCl3) δ: 7.53–7.52 (m, 2H), 7.37–7.33 (m, 4H), 7.16–7.12 (m, 1H), 6.73–6.71 (m, 2H), 4.27 (br, 2H) ppm.
- Preparation of the 2-(p-Tolylethynyl)aniline (1b) [46]. A 50-mL round-bottomed flask containing a magnetic stir bar was charged with 2-iodoaniline (2.240 g, 10.2 mmol, 1.0 equiv), 7.1 mL of Et3N, and 3 mL of DMF. The resulting reaction mixture was then added with Pd (PPh3)4 (294.5 mg, 0.255 mmol, 0.025 equiv) and CuI (96.9 mg, 0.510 mmol, 0.050 equiv). The heterogeneous mixture was degassed by passing through a steady stream of nitrogen before the addition of ethynyltrimethylsilane (2.20 mL, 1.500 g, 15.300 mmol, 1.5 equiv) via a syringe. The reaction mixture was allowed to stir at room temperature under nitrogen overnight (18–20 h). Upon completion, the mixture was diluted by the addition of aqueous HCl (0.1 M). The mixture was extracted with EtOAc, and the combined organic phases were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude material. The crude product was taken up in 10 mL of MeOH, followed by the addition of K2CO3 (282.0 mg, 2.040 mmol, 0.2 equiv). The resulting mixture was stirred at room temperature for 2 h. Then, the reaction mixture was diluted with aqueous HCl (0.1 M) and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford the crude product, which was purified by silica gel column chromatography eluting hexane/EtOAc 99/1 to provide 2-ethynylaniline (734.0 mg, 62% over 2 steps).
- 4-Chloro-2-(phenylethynyl)aniline (1c) [46]. (415.1 mg, 95%). White solid. 1H-NMR (400 MHz, CDCl3) δ: 7.50–7.49 (m, 2H), 7.35–7.32 (m, 4H), 7.06 (dd, J = 8.6, 1.7 Hz, 1H), 6.60 (d, J = 8.6 Hz, 1H), 4.24 (br, 2H) ppm.
- 4-Fluoro-2-(phenylethynyl)aniline (1d) [46]. (1.565 g 85%). Brown solid. 1H-NMR (400 MHz, CDCl3) δ: 7.52–7.51 (m, 2H), 7.36–7.34 (m, 3H), 7.06 (d, J = 8.9 Hz, 1H), 6.85 (t, J = 8.9 Hz, 1H), 6.61 (dd, J = 8.7, 4.7 Hz, 1H), 4.14 (br, 2H) ppm.
- 2-(phenylethynyl)-4-(trifluoromethyl)aniline (1e) [46]. (743 mg, 72%). Brown solid. 1H NMR (400 MHz, CDCl3) δ: 7.63–7.62 (m, 1H), 7.53–7.51 (m, 2H), 7.36–7.33 (m, 4H), 6.73 (d, J = 8.5 Hz, 1H), 4.56 (br, 2H) ppm.
- 2-(oct-1-yn-1-yl)aniline (1f) [47]. (1.858 g, ≥ 99%). Oil. 1H-NMR (400 MHz, CDCl3) δ: 7.22 (d, J = 7.4 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 6.64–6.60 (m, 2H), 4.11 (br, 2H), 2.43 (t, J = 7.1 Hz, 2H) 1.63–1.55 (m, 2H), 1.50–1.40 (m, 2H), 1.37–1.26 (m, 4H), 0.89 (t, J = 6.9 Hz, 3H) ppm.
- 2-((Trimethylsilyl)ethynyl)aniline (1g) [46]. (3.50 g, 94%). Oil. 1H-NMR (400 MHz, CDCl3) δ: 7.26 (d, J = 7.7 Hz, 1H), 7.06 (t, J = 7.7 Hz, 1H), 6.63–6.59 (m, 2H), 4.18 (br, 2H), 0.25 (s, 9H) ppm.
- 4-Chloro-2-((trimethylsilyl)ethynyl)aniline (1h) [48]. (1.271 g, 70%). Oil. 1H-NMR (400 MHz, CDCl3) δ: 7.24 (d, J = 2.3 Hz, 1H), 7.02 (dd, J = 8.7, 2.3 Hz, 1H), 6.55 (d, J = 8.7 Hz, 1H), 4.21 (s, 2H), 0.25 (s, 9H) ppm.
- The typical procedure for the cycloisomerization reaction of 2-alkynylanilines 1 to the corresponding 2-substituted indoles 5: p-TsOH·H2O catalysed the cycloisomerization of 1a to the 2-phenyl indole 5a. To a small vial was added 2-phenylaniline 1a (100 mg, 0.52 mmol), p-TsOH·H2O (19 mg, 0.10 mmol), and 1,2-DCE (2 mL). The reaction mixture was stirred at 40 °C for 24 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give crude material, which was purified by silica gel column chromatography eluting with hexane/EtOAc 99/1 to provide the 2-phenyl-1H-indole (5a) [49]. (54 mg, 54%). White solid. 1H-NMR (400 MHz, CDCl3) δ: 8.27 (br, 1H, NH), 7.64–7.61 (m, 3H), 7.42 (t, J = 7.6 Hz, 2H), 7.37 (d, J = 8.1 Hz, 1H), 7.31 (t, J = 7.3 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H), 6.81 (d, J = 1.9 Hz, 1H) ppm.
- 2-(p-tolyl)-1H-indole (5b) [49]. (37 mg, 90% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 8.28 (br, 1H), 7.60 (dt, J = 7.8, 1.0 Hz, 1H), 7.56–7.54 (m, 2H), 7.38 (d, J = 8.1 Hz, 1H), 7.25–7.23 (m, 2H), 7.17 (td, J = 7.6, 1.3 Hz, 1H), 7.10 (td, J = 7.5, 1.2 Hz, 1H), 6.77 (s, 1H), 2.38 (s, 3H) ppm.
- 5-chloro-2-phenyl-1H-indole (5c) [49]. (30 mg, 65% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 8.36 (br, 1H, NH), 7.66–7.63 (m, 2H), 7.58 (d, J = 1.9 Hz, 1H), 7.47–7.43 (m, 2H), 7.34 (tt, J = 7.4, 1.2 Hz, 1H), 7.13 (dt, J = 8.6, 0.7 Hz, 1H), 7.25 (s, 1H), 7.14 (dd, J = 8.6, 2.0 Hz, 1H), 6.76 (dd, J = 2.2, 0.9 Hz, 1H) ppm.
- 5-fluoro-2-phenyl-1H-indole (5d) [49]. (22 mg, 51% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 8.29 (br, 1H, NH), 7.64 (d, J = 8.1 Hz, 2H), 7.44 (t, J = 7.6 Hz, 2H), 7.35–7.24 (m, 3H), 6.93 (td, J = 9.1, 2.3 Hz, 1H), 6.77 (d, J = 0.8 Hz, 1H) ppm.
- 2-phenyl-5-(trifluoromethyl)-1H-indole (5e) [50]. (26 mg, 16% yield). White solid. 1H-NMR (CDCl3, 400 MHz) δ: 8.50 (br, 1H), 7.91 (s, 1H), 7.66–7.64 (m, 2H), 7.47–7.42 (m, 4H), 7.40–7.34 (m, 1H), 6.87 (d, J = 1.3 Hz, 1H) ppm.
- 2-hexyl-1H-indole (5f) [50]. (13 mg, 7% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 7.86 (br, 1H), 7.51 (d, J = 7.2 Hz, 1H), 7.30 (d, J = 7.9 Hz, 1H), 7.14 (t, J = 7.0 Hz, 1H), 7.09 (t, J = 7.3 Hz, 1H), 6.20 (s, 1H), 2.76 (t, J = 7.7 Hz, 2H), 1.75–1.67 (m, 2H), 1.40–1.24 (m, 6H), 0.88 (t, J = 7.1 Hz, 3H) ppm.
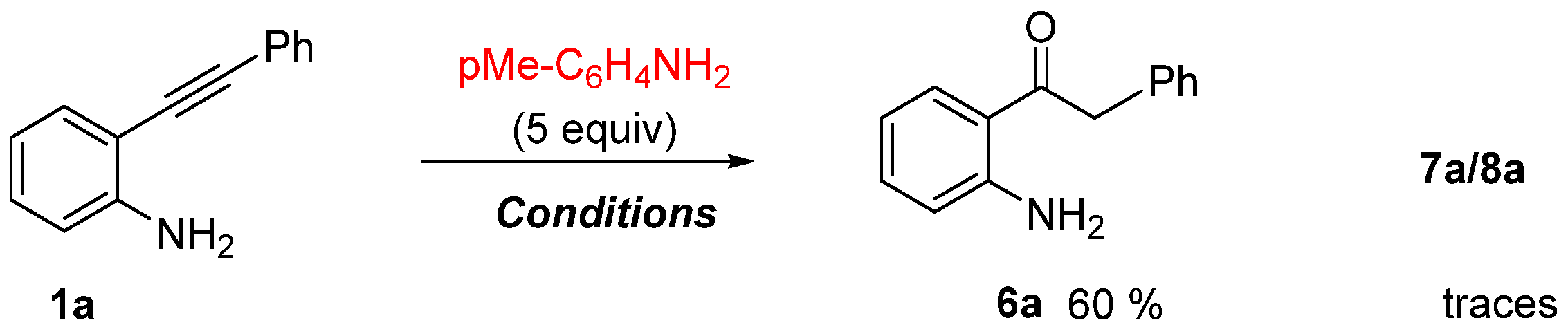
- The typical procedure for the p-TsOH·H2O promoted hydration of 2-alkynylaniline 1: synthesis of 1-(2-aminophenyl)-2-phenylethan-1-one 6a. To a small vial was added 2-phenylaniline 1a (96 mg, 0.49 mmol), p-TsOH·H2O (93 mg, 0.49 mmol), 4-toluidine (268 mg, 2.5 mmol) and ethanol (2 mL). The reaction mixture was stirred at 110 °C for 20 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 95/5 to provide the 1-(2-aminophenyl)-2-phenylethan-1-one (6a) [51]. (62 mg, 60% yield). Oil. 1H-NMR (400 MHz, CDCl3) δ: 7.82 (d, J = 8.0 Hz, 1H), 7.34–7.30 (m, 2H), 7.25–7.24 (m, 4H), 6.64–6.60 (m, 2H), 6.28 (br, 2H), 4.24 (s, 2H) ppm.
- 1-(2-aminophenyl)-2-(p-tolyl)ethan-1-one (6b) [22] (38 mg, 26% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 7.83 (d, J = 8.2 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 7.14–7.12 (m, 4H), 6.65–6.61 (m, 2H), 6.27 (br, 2H), 4.21 (s, 2H), 2.32 (s, 3H) ppm.
- 1-(2-amino-5-chlorophenyl)-2-phenylethan-1-one (6c) [52]. (25 mg, 27% yield). White solid. 1H-NMR (400 MHz, CDCl3) δ: 7.79 (d, J = 2.3 Hz, 1H), 7.36–7.32 (m, 2H), 7.28–7.26 (m, 1H), 7.24–7.22 (m, 2H), 7.19 (dd, J = 8.8, 2.4 Hz, 1H), 6.58 (d, J = 8.9 Hz, 1H), 6.28 (br, 2H), 4.21 (s, 2H) ppm.
- 1-(2-amino-5-fluorophenyl)-2-phenylethan-1-one (6d). (30 mg, 17% yield). Oil. 1H-NMR (400 MHz, CDCl3) δ: 7.49 (dd, J = 9.9, 2.9 Hz, 1H), 7.34–7.31 (m, 2H), 7.27–7.22 (m, 3H), 7.03 (ddd, J = 9.0, 7.7, 2.9 Hz, 1H), 6.64 (dd, J = 9.1, 4.6 Hz, 1H), 5.75 (br, 2H), 4.19 (s, 2 H) ppm; 19F{1H} NMR (376 MHz, CDCl3) δ: −127.64 (s, 1F) ppm; 13C {1H} NMR (150 MHz, CDCl3) δ: 199.1 (d, J = 2.8 Hz, Cq), 153.6 (d, J = 235.3 Hz, Cq), 146.8 (Cq), 134.8 (Cq), 129.4 (2CH), 128.7 (2CH), 126.9 (CH), 122.6 (d, J = 23.5 Hz, CH), 118.9 (d, J = 7.0 Hz, CH), 117.2 (d, J = 5.3 Hz, Cq), 116.1 (d, J = 22.2 Hz, CH), 46.2 (CH2) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C14H12FKNO: [M + K]+ 268.0540, Found: 268.0538.
- 1-(2-amino-5-trifluoromethyl)phenyl)-2-phenylethan-1-one (6e). (51 mg, 50% yield). Oil. 1H-NMR (400 MHz, CDCl3) δ: 8.10 (s, 1H), 7.43 (dd, J = 8.7, 1.9 Hz, 1 H), 7.36–7.32 (m, 2H), 7.28–7.23 (m, 3H), 6.68 (d, J = 8.7 Hz, 1H), 6.61 (br, 2H), 4.27 (s, 2H) ppm; 19F{1H} NMR (376 MHz, CDCl3) δ: −127.64 (s, 3F) ppm; 13C {1H} NMR (150 MHz, CDCl3) δ: 119.5 (Cq), 152.9 (Cq), 134.6 (Cq), 130.7 (q, J = 3.2 Hz, CH), 129.5 (2CH), 129.2 (q, J = 4.1 Hz, CH), 128.8 (2CH), 127.1 (CH), 124.3 (q, J = 270.4 Hz, CF3), 117.68 (CH), 117.67 (q, J = 33.3 Hz, Cq), 116.2 (Cq), 46.1 (CH2) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C15H12F3KNO: [M + K]+ 318.0508, Found: 318.0510.
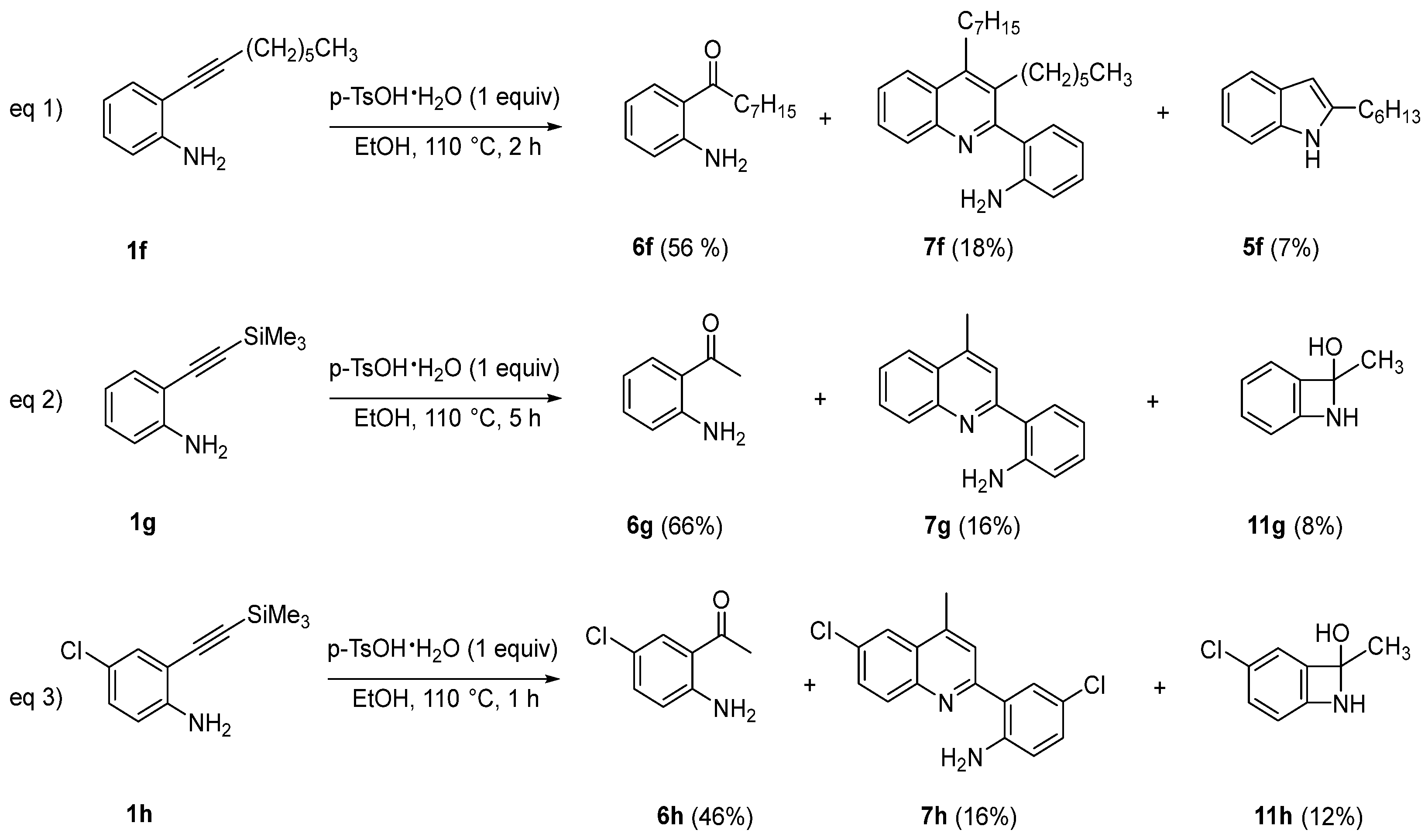
- 1-(2-aminophenyl)octan-1-one (6f) [53]. (98 mg, 56% yield). Oil. 1H NMR (400 MHz, CDCl3) δ: 7.72 (d, J = 8.3 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 6.63–6.60 (m, 2H), 6.26 (br, 2H), 2.90 (t, J = 7.5 Hz, 2H), 1.72–1.67 (m, 2H), 1.35–1.29 (m, 8H), 0.88 (t, J = 6.6 Hz, 3H) ppm.
- 1-(2-aminophenyl)ethan-1-one (6g) [Commercial Product]. (96 mg, 66% yield); 1H NMR (400 MHz, CDCl3) δ: 7.70 (dt, J = 8.0, 1.5 Hz, 1H), 7.29–7.24 (m, 1H), 6.69–6.64 (m, 2H), 6.23 (br, 2H), 2.56 (s, 3H) ppm.
- 1-(2-amino-5-chlorophenyl)ethan-1-one (6h) [Commercial Product]. 1H NMR (400 MHz, CDCl3) δ: 7.65 (d, J = 2.7 Hz, 1H), 7.19 (m, 1H), 6.59 (d, J = 8.8 Hz, 1H), 6.27 (s, 2H), 2.54 (m, 3H) ppm.
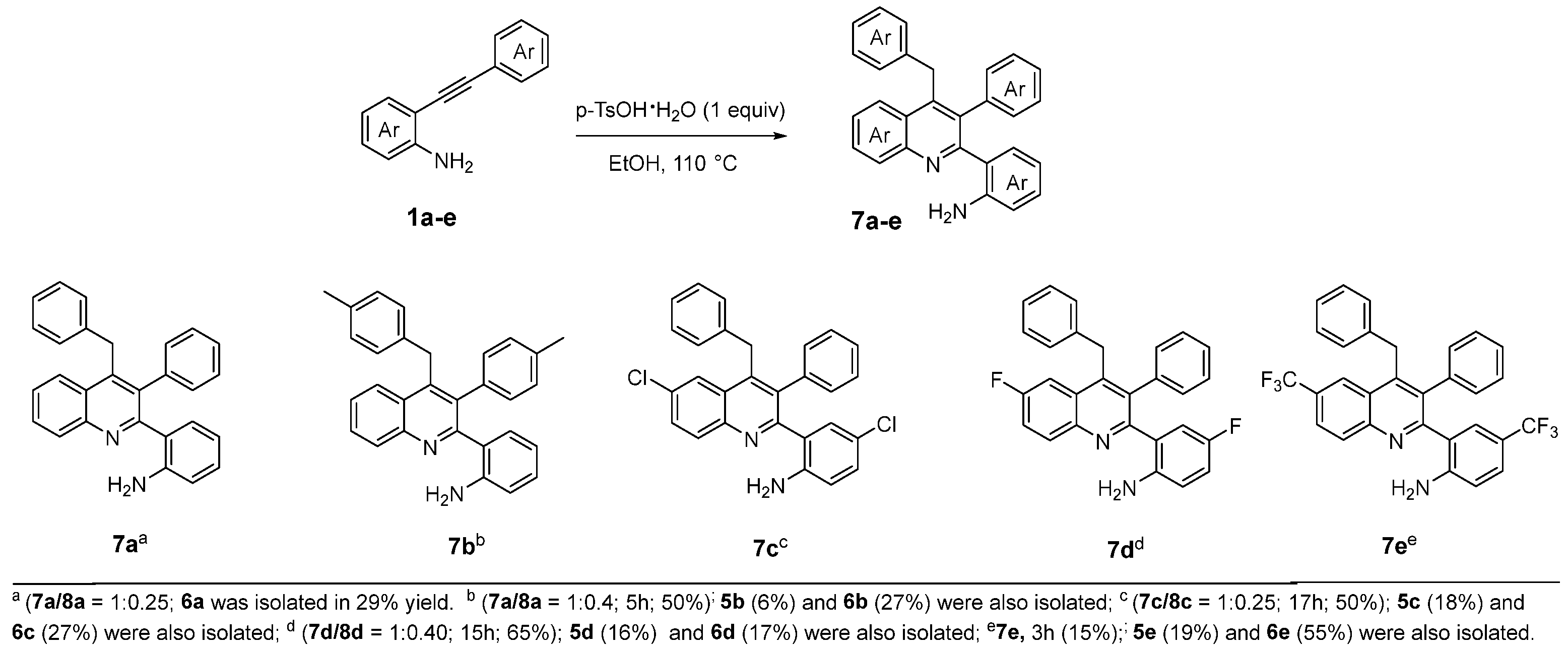
- The typical procedure for the regioselective p-TsOH·H2O promoted dimerization reaction of 2-alhynylaniline 1 to quinolines 7. To a small vial was added 2-phenylaniline 1a (150 mg, 0.78 mmol), p-TsOH·H2O (148 mg, 0.78 mmol),) and ethanol (2 mL). The reaction mixture was stirred at 110 °C for 20 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 90/10 to provide the 2-(4-benzyl-3-phenylquinolin-2-yl)aniline (7a) [40] and 2-(4-Benzyl-2-phenylquinolin-3-yl)aniline (8a) [41] ratio 1:0.25 calculated by 1H NMR (104 mg, 70% yield). Oil. 1H-NMR (400 MHz, CDCl3) δ: 8.23 (d, J = 8.4 Hz, 1H, 8a), 8.16 (d, J = 8.4 Hz, 1H, 7a), 8.01 (d, J = 8.4 Hz, 1H, 8a), 7.93 (d, J = 8.4 Hz, 1H, 7a), 7.72–7.66 (m, 1H, 7a + 1H, 8a), 7.51–7.45 (m, 1H, 7a + 3H, 8a), 7.22–7.08 (m, 8H, 7a + 6H, 8a), 7.03–6.92 (m, 3H, 7a + 3H, 8a), 6.80 (dt, J = 7.6, 1.3 Hz, 1H, 8a), 6.71 (dt, J = 7.7 Hz, 1.4 Hz, 1H, 7a), 6.67 (dt, J = 8.1 Hz, 1.1 Hz, 1H, 7a), 6.60–6.55 (m, 2H, 8a), 6.41 (tt, J = 7.5 Hz, 1.1 Hz, 1H, 7a), 4.45 (d, J = 15.6 Hz, 1H, AB system 8a), 4.39 (s, 2H, 7a), 4.34 (br, 2H, 7a), 4.29 (d, J = 15.6 Hz, 1H, AB system 8a), 3.29 (br, 2H, 8a) ppm.
- 2-(4-(4-methylbenzyl)-3-(p-tolyl)quinolin-2-yl)aniline (7b) and 2-(4-(4-methylbenzyl)-2-(p-tolyl)quinolin-3-yl)aniline (8b) [45] ratio 1:0.40 calculated by 1H NMR (65 mg, 50% yield). Oil. 1H-NMR (400 MHz, CDCl3) δ 8.21 (d, J = 8.4 Hz, 1H, 8b), 8.14 (d, J = 8.4 Hz, 1H, 7b), 7.97 (d, J = 8.4 Hz, 1H, 8b), 7.90 (d, J = 8.5 Hz, 1H, 7b), 7.70 (t, J = 7.1 Hz, 1H, 8b), 7.65 (dd, J = 8.2, 7.1 Hz, 1H, 7b), 7.48 (t, J = 7.2 Hz, 1H, 8b), 7.43 (t, J = 7.1 Hz, 1H, 7b), 7.38 (d, J = 7.9 Hz, 2H 8b), 7.04–6.88 (m, 7H, 7b + 5H, 8b), 6.83–6.81 (m, 3H, 8b), 6.74 (d, J = 7.7 Hz, 1H, 7b), 6.66 (d, J = 8.0 Hz, 1H, 7b), 6.62–6.55 (m, 2H, 8b), 6.43 (t, J = 7.5 Hz, 1H, 7b), 4.39 (d, J = 15.57 Hz, 1H, 8b, AB system), 4.34 (s, 2H, 7b), 4.33 (br, 2H, 7b), 4.22 (d, J = 15.57 Hz, 1H 8b, AB system), 3.29 (br, 2H, 8b), 2.27 (s, 3H, 7b), 2.26 (s, 3H, 8b), 2.23 (s, 3H, 8b), 2.23 (s, 3H, 7b) ppm; 13C {1H} NMR (150 MHz, CDCl3) δ: 159.59 (8b), 158.78 (7b), 147.86 (8b), 146.94 (7b), 145.90 (8b), 144.85 (7b), 144.72 (7b), 144.10 (8b), 138.10 (8b), 137.60 (8b), 137.14 (7b), 136.63 (8b), 135.95 (7b), 135.44 (7b), 135.40 (8b), 135.17 (7b), 131.40 (8b), 131.18 (7b), 131.10 (8b), 130.27 (8b), 129.90 (7b), 129.73 (7b), 129.19 (8b), 129.16 (7b), 129.14 (8b), 129.01 (7b), 128.99 (8b), 128.75 (8b), 128.49 (7b), 128.45 (7b), 128.30 (8b), 128.19 (8b), 128.07 (7b), 127.97 (7b), 126.81 (8b), 126.80 (7b), 126.70 (7b), 126.48 (8b), 126.31 (8b), 125.30 (7b), 125.18 (7b), 124.02 (8b), 118.37 (8b), 117.60 (7b), 116.28 (7b), 115.35 (8b), 35.22 (7b), 34.71 (8b), 21.22 (8b), 21.16 (7b), 20.95 (7b), 20.93 (8b) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C30H26N2Na: [M + Na]+ 437.1994, Found: 437.1998.
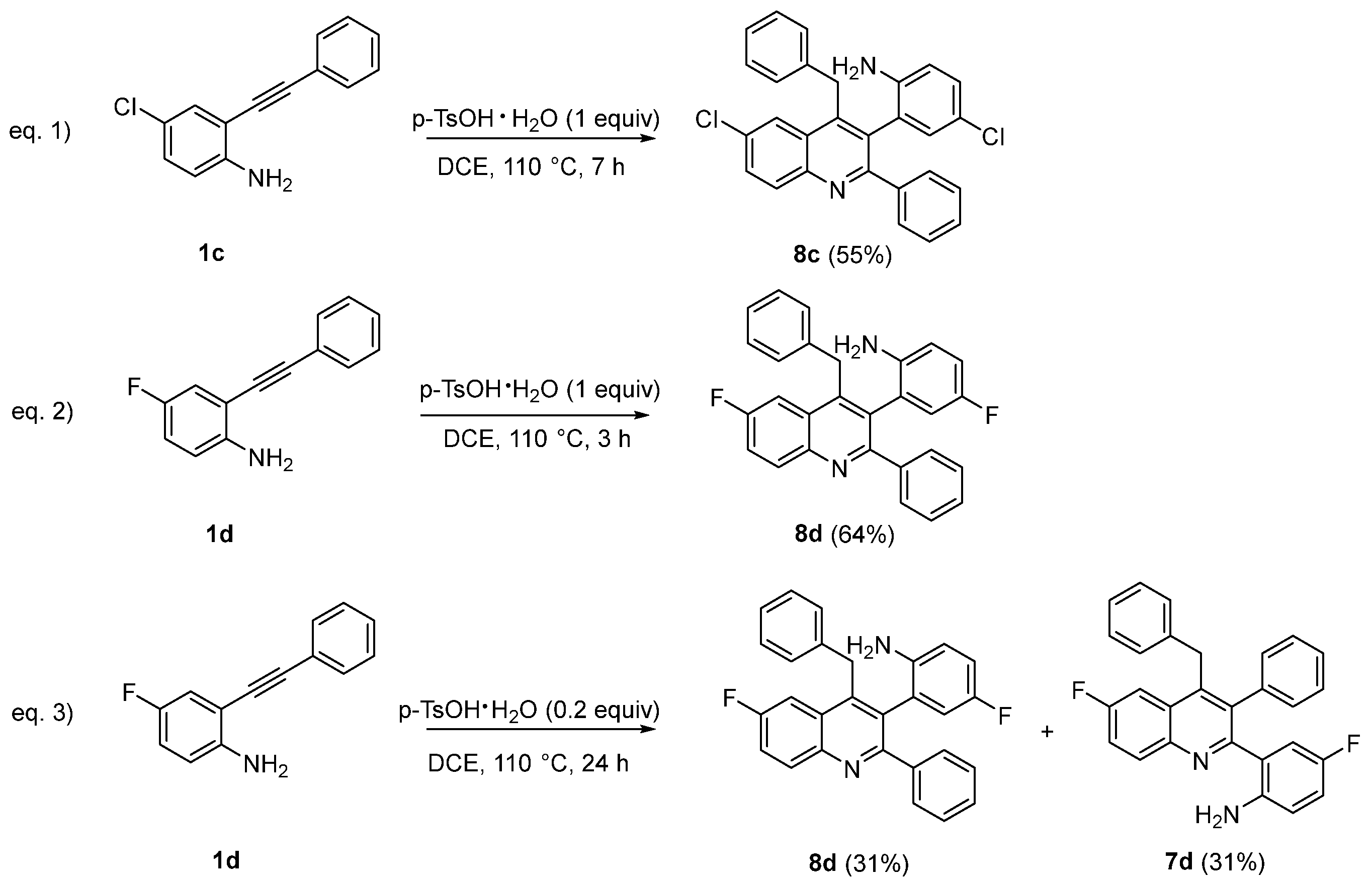
- 2-(4-Benzyl-6-chloro-3-phenylquinolin-2-yl)-4-chloroaniline (7c) and 2-(4-Benzyl-6-chloro-2-phenylquinolin-3-yl)-4-chloroaniline (8c) ratio 1:0.25 calculated by 1H NMR (43 mg, 50% yield). Oil. Eluent: hexane/ethyl acetate (90:10). 1H-NMR (400 MHz, CDCl3) δ: 8.16 (dd, J = 8.9, 0.6 Hz, 1H, 8c), 8.07 (dd, J = 9.0, 0.6 Hz, 1H, 7c), 8.04 (d, J = 2.2 Hz, 1H, 8c), 7.92 (d, J = 2.1 Hz, 1H, 7c), 7.68 (ddd, J = 9.0, 2.4, 1.1 Hz, 1H, 8c), 7.64 (ddd, J = 8.8, 2.2, 1.1 Hz, 1H, 7c), 7.44 (dd, J = 7.8, 2.0 Hz, 2H, 8c), 7.25–7.15 (m, 8H, 7c, 6H, 8c), 6.96 (d, J = 7.6 Hz, 2H, 7c), 7.01 (dd, J = 8.6, 2.2 Hz, 1H, 8c), 6.82 (d, J1 = 8.9 Hz, 1H, 7c), 6.89 (d, J = 6.2 Hz, 2H, 8c), 6.72 (d, J = 2.2 Hz, 1H, 8c), 6.70 (d, J = 2.3 Hz, 1H, 7c), 6.59 (d, J = 8.6 Hz, 1H, 7c), 6.49 (d, J = 8.4 Hz, 1H, 8c), 4.40 (d, J = 15.8 Hz, 1H, 8c, AB system), 4.34 (s, 2H, 7c), 4.20 (d, J = 15.8 Hz, 1H, 8c, AB system), 4.31 (br, 2H, 7c), 3.25 (br, 2H, 8c) ppm.
- 2-(4-(4-Fluorobenzyl)-3-(4-fluorophenyl)quinolin-2-yl)aniline (7d) and 2-(4-Benzyl-6-fluoro-2-phenylquinolin-3-yl)-4-fluoroaniline (8d) ratio 1:0.40 calculated by 1H NMR. (105 mg, 65% yield). Oil. Eluent: hexane/ethyl acetate (90:10). 1H NMR (400 MHz, CDCl3) δ: 8.22 (dd, J = 9.2, 5.6 Hz, 1H, 8d), 8.14 (dd, J = 9.2, 5.6 Hz, 1H, 7d), 7.61 (dd, J = 10.2, 2.8 Hz, 1H, 8d), 7.53 (dd, J = 10.3, 2.7 Hz, 1H, 7d), 7.49 (ddd, J = 9.2, 8.0, 2.8 Hz, 1H, 8d), 7.48–7.43 (m, 3H 7d + 2H 8d), 7.24–7.08 (m, 6H 7d, 6H 8d), 6.99 (d, J = 1.7 Hz, 1H, 7d), 6.97 (d, J = 1.0 Hz, 1H, 7d), 6.90 (d, J = 1.8 Hz, 1H, 8d), 6.83 (d, J = 1.1 Hz, 1H, 8d), 6.77 (td, J = 8.5, 2.9 Hz, 1H, 8d), 6.68 (td, J = 8.5, 2.9 Hz, 1H, 7d), 6.59 (dd, J = 8.78, 4.82 Hz, 1H, 7d), 6.51 (dd, J = 8.8, 1.4 Hz, 1H, 8d), 6.50 (d, J = 8.8 Hz, 1H, 8d), 6.47 (dd, J = 9.53, 2.98 Hz, 1H, 7d), 4.36 (d, J = 15.72 Hz, 1H, AB system, 8d), 4.33 (s, 2H, 7d), 4.23 (d, J = 15.72 Hz, 1H, AB system, 8d), 4.14 (br, 2H, 7d), 3.16 (br, 2H, 8d) ppm; 19F{1H} NMR (376 MHz, CDCl3) δ: −111.32 (s, 1F, 7d), −111.63 (s, 1F, 8d), −126.39 (s, 1F, 8d) −127.68 (s, 1F, 7d) ppm; 13C {1H} NMR (150 MHz, CDCl3) δ: 160.9 (d, J = 248.4 Hz, Cq, 7d), 160.8 (d, J = 248.3 Hz, Cq, 8d), 158.8 (d, J = 2.8 Hz, Cq, 8d), 156.8 (dd, J = 2.7, 2.03 Hz, Cq, 7d), 155.8 (d, J = 237.4 Hz, Cq, 8d), 155.5 (d, J = 235.9 Hz, Cq, 7d), 145.5 (d, J = 5.6 Hz, Cq, 8d), 145.1 (Cq, 8d), 144.3 (d, J = 5.7 Hz, Cq, 7d), 144.2 (Cq, 7d), 141.0 (d, J = 2.1 Hz, 1 Cq, 7d), 140.4 (d, J = 2.0 Hz, Cq, 8d), 140.3 (Cq, 8d), 139.3 (Cq, 7d), 138.8 (Cq, 8d), 137.4 (Cq, 7d), 136.5 (d, J = 0.5 Hz, Cq, 7d), 132.9 (d, J = 9.3 Hz, CH, 8d), 132.3 (d, J = 9.3 Hz, CH, 7d), 130.9 (d, J = 1.0 Hz, Cq, 8d), 129.8 (2CH, 7d), 129.1 (2CH, 8d), 128.7 (2CH, 7d), 128.6 (2CH, 8d), 128.2 (CH, 8d), 128.1 (2CH, 8d), 128.04 (2CH, 7d), 128.01 (2CH, 7d), 127.9 (d, J = 9.5 Hz, Cq, 7d), 127.8 (2CH, 8d), 127.71 (d J = 8.9 Hz, Cq, 8d), 127.70 (d J = 12.7 Hz, CH, 7d), 126.8 (d, J = 7.13 Hz, Cq, 7d), 126.4 (CH, 8d), 126.3 (CH, 7d), 124.6 (d, J = 7.4 Hz, Cq, 8d), 119.9 (d, J = 25.8 Hz, CH, 8d), 119.6 (d, J = 25.9 Hz, CH, 7d), 117.4 (d, J = 22.5 Hz, CH, 8d), 117.3 (d, J = 12.1 Hz, CH, 7d), 117.1 (d, J = 3.7 Hz, CH, 7d), 116.5 (d, J = 7.8 Hz, CH, 8d), 115.8 (d, J = 22.4 Hz, CH, 8d), 115.5 (d, J = 22.4 Hz, CH, 7d), 108.9 (d, J = 22.8 Hz, CH, 7d), 108.7 (d, J = 22.9 Hz, CH, 8d), 35.71 (CH2, 7d), 35.2 (CH2, 8d) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C28H20F2KN2: [M + K]+ 461.1232, Found: 461.1231.
- 2-(4-(4-(Trifluoromethyl)benzyl)-3-(4-(trifluoromethyl)phenyl)quinolin-2-yl)aniline (7e) (21 mg, 20% yield). Oil. Eluent: hexane/ethyl acetate (90:10). 1H NMR (400 MHz, CDCl3) δ: 8.30 (s, 1H), 8.24 (d, J = 9.1 Hz, 1H), 7.89 (d, J = 8.2 Hz, 1H), 7.34–7.18 (m, 7H), 7.06–7.04 (m, 2 H), 6.97–6.95 (m, 3 H), 6.72 (d, J = 8.7 Hz, 1H), 4.79 (br, 2H), 4.44 (s, 2H) ppm; 19F {1H} NMR (376 MHz, CDCl3) δ: −61.58 (s, 3F), −62.46 (s, 3F) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C30H20F6KN2: [M + K]+ 561.1168, Found: 561.1170.
- 2-(3-hexyl-4-octylquinolin-2-yl)aniline (7f) (32 mg, 18% yield). Oil. Eluent: hexane/ethyl acetate (90:10). 1H NMR (400 MHz, CDCl3): δ 8.00 (d, J = 8.3 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 1H), 7.23 (t, J = 8.6 Hz, 1H), 6.99 (d, J = 7.3 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 6.80 (d, J = 8.1 Hz, 1H), 3.38 (br, 2H), 2.93 (td, J = 12.1, 4.89 Hz, 1H), 2.75–2.60 (m, 3H), 1.69–1.43 (m, 3H), 1.29–1.16 (m, 15H), 0.84 (t, J = 6.8 Hz, 3H), 0.81 (t, J = 7.1 Hz, 3H) ppm; 13C {1H} NMR (150 MHz, CDCl3): δ 162.5 (Cq),148.0 (Cq), 147.7 (Cq), 143.8 (Cq), 130.8 (CH), 130.3 (Cq), 129.7 (CH), 128.9 (CH), 128.8 (CH), 126.1 (Cq), 125.6 (CH), 124.1 (CH), 123.7 (Cq), 118.4 (CH), 115.2 (CH), 37.1 (CH2), 31.6 (CH2), 31.5 (CH2), 30.5 (CH2), 30.0 (CH2), 29.5 (CH2), 29.4 (CH2), 29.3 (CH2), 28.7 (CH2), 22.6 (CH2), 22.5 (CH2), 14.1 (CH3), 14.0 (CH3) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C28H38N2Na: [M + Na]+ 425.2933, Found: 425.2930.
- 2-(4-Methylquinolin-2-yl)aniline (7g) [45]. Eluent: petroleum ether/ethyl acetate (20:1). (25.6 mg, 73%). Yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 8.3 Hz, 1H), 7.64–7.62 (m, 2H), 7.47 (t, J = 7.6 Hz, 1H), 7.22–7.15 (m, 1H), 6.80–6.75 (m, 1H), 6.61–6.57 (m, 2H), 6.18 (br, 2H), 2.67 (s, 3H) ppm.
- 4-chloro-2-(6-chloro-4-methylquinolin-2-yl)aniline (7h) [44]. Eluent: petroleum ether/ethyl acetate (20:1). (28 mg, 16%). Yellow solid. 1H NMR (400 MHz, CDCl3) δ: 7.93 (d, J = 7.2 Hz, 1H), 7.92 (s, 1H), 7.62–7.59 (m, 3H), 7.13 (dd, J = 8.6, 2.4 Hz, 1H), 6.70 (d, J = 8.6 Hz, 1H), 6.15 (br, 2H), 2.68 (d, J = 0.9 Hz, 3H) ppm.
- The typical procedure for the regioselective p-TsOH·H2O promoted the dimerization reaction of 2-alhynylaniline 1 to quinolines 8. To a small vial was added 2-phenylaniline 1a (101 mg, 0.52 mmol), p-TsOH·H2O (99 mg, 0.52 mmol), and DCE (2 mL). The reaction mixture was stirred at 110 °C for 4 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 90/10 to provide 2-(4-Benzyl-2-phenylquinolin-3-yl)aniline (8a) (48 mg, 48% yield). Unseparable mixture 8a:7a Ratio 9:2 calculated by 1H NMR.
- 2-(4-Benzyl-6-chloro-2-phenylquinolin-3-yl)-4-chloroaniline (8c) (45 mg, 55% yield). Oil. hexane/EtOAc 90/10. Unseparable mixture 8c:7c Ratio 9:1 calculated by 1H NMR.
- 2-(4-Benzyl-6-fluoro-2-phenylquinolin-3-yl)-4-fluoroaniline (8d) (64 mg, 64% yield) hexane/EtOAc 90/10. Oil. 1H NMR (400 MHz, CDCl3) δ: 8.25 (dd, J = 9.2, 5.6 Hz, 1H), 7.60 (dd, J = 10.2, 2.8 Hz, 1H), 7.49 (ddd, J = 9.2, 8.0, 2.8 Hz, 1H), 7.44–7.43 (m, 2H), 7.24–7.20 (m, 3H), 7.18–7.12 (m, 3H), 6.90 (d, J = 1.8 Hz, 1H), 6.83 (d, J = 1.1 Hz, 1H), 6.77 (td, J = 8.5, 2.9 Hz, 1H), 6.51 (dd, J = 8.8, 1.4 Hz, 1H), 6.50 (d, J = 8.8 Hz, 1H), 4.36 (d, J = 15.72 Hz, 1H, AB system), 4.23 (d, J = 15.72 Hz, 1H, AB system), 3.16 (br, 2H) ppm; 19F{1H} NMR (376 MHz, CDCl3) δ: −111.41 (s, 1F), −126.38 (s, 1F) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ: 160.8 (d, J = 248.3 Hz, Cq), 158.8 (d, J = 2.8 Hz, Cq), 155.8 (d, J = 237.4 Hz, Cq), 145.5 (d, J = 5.6 Hz, Cq), 145.1 (Cq), 140.4 (d, 4J = 2.0 Hz, Cq), 140.3 (Cq), 138.8 (Cq), 132.9 (d, J = 9.3 Hz, CH), 130.9 (d, J = 1.0 Hz, Cq), 129.1 (2CH), 128.6 (2CH), 128.2 (CH), 128.1 (2CH), 127.8 (2CH), 127.7 (d J = 8.9 Hz, Cq), 126.4 (CH), 124.6 (d, J = 7.4 Hz, Cq), 119.9 (d, J = 25.8 Hz, CH), 117.4 (d, J = 22.5 Hz, CH), 116.5 (d, J = 7.8 Hz, CH), 115.8 (d, J = 22.4 Hz, CH), 108.7 (d, J = 22.9 Hz, CH), 35.2 (CH2) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C28H20F2KN2: [M + K]+ 461.1232, Found: 461.1235.
- Synthesis of the 3-phenylquinolin-4(1H)-one 9a [54]. To a small vial was added 2-phenylaniline 1a (72 mg, 0.37 mmol), p-TsOH·H2O (71 mg, 0.37 mmol), and DMF (2 mL). The reaction mixture was stirred at 110 °C for 18 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 70/30 to provide 3-phenylquinolin-4(1H)-one 9a (41 mg, 33% yield). Yellow solid. 1H NMR (400 MHz, DMSO) δ: 12.04 (s, 1H), 8.20 (d, J = 8.3 Hz, 1H), 8.15 (s, 1H), 7.73–7.71 (m, 2H), 7.65 (t, J = 7.6 Hz, 1H), 7.58 (d, J = 8.3 Hz, 1H), 7.40–7.32 (m, 3H), 7.27 (t, J = 7.2 Hz, 1H) ppm.
- Synthesis of the 2,2′-Diphenyl-1H,1′H-3,3′-biindole 10a [25]. To a small vial was added 2-phenylaniline 1a (98 mg, 0.5 mmol), p-TsOH·H2O (20 mg, 0.10 mmol), dimethoxymethane (190 mg, 2.50 mmol), and DCE (2 mL). The reaction mixture was stirred at 40 °C for 4 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 90/10 to provide the 2,2′-Diphenyl-1H,1′H-3,3′-biindole (10a) (73 mg, 76% yield). White solid. 1H NMR (400 MHz, CDCl3) δ: 8.02 (s, 2H), 7.58–7.56 (m, 4H), 7.40 (t, J = 6.9 Hz, 4H), 7.34–7.19 (m, 6H), 7.07 (t, J = 7.2 Hz, 2H) 6.85 (t, J = 7.2 Hz, 2H) ppm.
- 8-methyl-7-azabicyclo [4.2.0]octa-1,3,5-trien-8-ol (11g). (8 mg, 8% yield). Oil. 1H NMR (400 MHz, CDCl3) δ: 7.19 (d, J = 7.7 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 6.80 (t, J = 7.5 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H), 4.49 (br, 1H), 1.86 (s, 3H), 1.57 (br, 1H) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C8H9KNO: [M + K]+ 174.0321, Found: 174.0317.
- 3-chloro-8-methyl-7-azabicyclo [4.2.0]octa-1,3,5-trien-8-ol (11h). (11 mg, 12% yield). Oil. 1H NMR (400 MHz, CDCl3) δ: 7.16 (d, J = 2.3 Hz, 1H), 7.01 (dd, J = 8.6, 2.3 Hz, 1H), 6.58 (d, J = 8.6 Hz, 1H), 4.48 (br, 1H), 1.83 (s, 3H), 1.54 (br, 1H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ: 139.1 (Cq), 129.5 (Cq), 128.4 (CH), 125.6 (CH), 125.3 (Cq), 119.2 (CH), 81.4 (Cq), 27.0 (CH3) ppm; HRMS: m/z (MALDI-TOF) positive ion, calculated for C8H8ClNNaO: [M + Na]+ 192.0195, Found: 192.0195.
- Control experiment ruling out the formation of 7a from 6a. To a small vial was added 1-(2-aminophenyl)-2-phenylethan-1-one 6a (87 mg, 0.78 mmol), 2-(phenylethynyl)aniline 1a (80 mg, 0.41 mmol), p-TsOH·H2O (148 mg, 0.41 mmol), and ethanol (2 mL). The reaction mixture was stirred at 110 °C for 20 h. Then, the mixture was diluted with a saturated solution of NaHCO3 and extracted with CHCl3. The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give crude material which was purified by silica gel column chromatography eluting with hexane/EtOAc 90/10 to provide 1-(2-aminophenyl)-2-phenylethan-1-one 6a (95 mg; 84 mg recovered + 11 mg, 13% from 1a), 2-phenyl-1H-indole 5a (21 mg from 1a, 24% from 1a) and 2-(4-benzyl-3-phenylquinolin-2-yl)aniline 7a (36 mg, 45% from 1a).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasseri, M.A.; MShahabi, M.; Alavi, S.A.; Allahresani, G.A. A novel, efficient and magnetically recyclable Cu–Ni bimetallic alloy nanoparticle as a highly active bifunctional catalyst for Pd-free Sonogashira and C–N cross-coupling reactions: A combined theoretical and experimental study. RSC Adv. 2023, 13, 22158–22171. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Vavsari, V.F.; Nikbakht, A.; Balalaie, S. Annulation of 2-Alkynylanilines: The Versatile Chemical Compounds. Asian J. Org. Chem. 2022, 11, e202100772. [Google Scholar] [CrossRef]
- Festa, A.A.; Raspertov, P.V.; Voskressensky, L.G. 2-(Alkynyl)anilines and Derivatives—Versatile Reagents for Heterocyclic Synthesis. Adv. Synth. Catal. 2022, 364, 466–486. [Google Scholar] [CrossRef]
- Kamble, O.S.; Khatravath, M.; Dandela, R. Applications of Ethynylanilines as Substrates for Construction of Indoles and Indole-Substituted Derivatives. ChemistrySelect 2021, 6, 7408–7427. [Google Scholar] [CrossRef]
- Kotha, S.; Ansari, S.; Gupta, N.K. Selectivity: A Goal for Synthetic Economy. Synlett 2023, 34, 535–551. [Google Scholar] [CrossRef]
- Mondal, D.; Kalar, P.L.; Kori, S.; Gayen, S.; Das, K. Recent Developments on Synthesis of Indole Derivatives Through Green Approaches and Their Pharmaceutical Applications. Curr. Org. Chem. 2020, 24, 2665–2693. [Google Scholar] [CrossRef]
- Zeng, W.; Han, C.; Mohammed, S.; Li, S.; Song, Y.; Sun, F.; Du, Y. Indole-containing pharmaceuticals: Targets, pharmacological activities, and SAR studies. RSC Med. Chem. 2024, 15, 788–808. [Google Scholar] [CrossRef]
- Vinod, A.; Mouli, H.M.C.; Jana, A.; Peraman, R. Unlocking therapeutic potential: Exploring indole scaffolds and their structural insights as pharmacophores in designing anti-breast cancer agents. Med. Chem. Res. 2024, 33, 1100–1132. [Google Scholar] [CrossRef]
- Barresi, E.; Baglini, E.; Poggetti, V.; Castagnoli, J.; Giorgini, D.; Salerno, S.; Taliani, S.; Da Settimo, F. Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents. Molecules 2024, 29, 2127. [Google Scholar] [CrossRef]
- Shivam; Tiwari, G.; Kumar, M.; Chauhan, A.N.S.; Erande, R.D. Recent advances in cascade reactions and their mechanistic insights: A concise strategy to synthesize complex natural products and organic scaffolds. Org. Biomol. Chem. 2022, 20, 3653–3674. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Morlacci, V.; Palombi, L. Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls. Molecules 2023, 28, 4725. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Gritti, A.; Pirovano, V.; Arcadi, A.; Germani, R.; Tiecco, M.; Abbiati, G. Acidic Deep Eutectic Solvents as Active Media for Sustainable Synthesis of Biindoles Starting from 2,2′-Diaminotolanes and Aldehydes. Eur. J. Org. Chem. 2023, 26, e202300204. [Google Scholar] [CrossRef]
- Arcadi, A.; Chiarini, M.; D’anniballe, G.; Marinelli, F.; Pietropaolo, E. Brønsted Acid Catalyzed Cascade Reactions of 2-[(2-Aminophenyl)ethynyl]phenylamine Derivatives with Aldehydes: A New Approach to the Synthesis of 2,2′-Disubstituted 1H,1′H-3,3′-Biindoles. Org. Lett. 2014, 16, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A. Au-Catalyzed Synthesis and Functionalization of Heterocycles; Bandini, M., Ed.; Topics in Heterocyclic Chemistry; Springer International Publishing: Cham, Switzerland, 2016; Volume 46, pp. 53–85. [Google Scholar]
- Ohno, H. Recent Advances in the Construction of Polycyclic Compounds by Palladium-Catalyzed Atom-Economical Cascade Reactions. Asian J. Org. Chem. 2013, 2, 18–28. [Google Scholar] [CrossRef]
- Ohno, H. Gold-Catalyzed Cascade Reactions of Alkynes for Construction of Polycyclic Compounds. Isr. J. Chem. 2013, 53, 869–882. [Google Scholar] [CrossRef]
- Abbiati, G.; Marinelli, F.; Rossi, E.; Arcadi, A. Synthesis of Indole Derivatives from 2-Alkynylanilines by Means of Gold Catalysis. Isr. J. Chem. 2013, 53, 856–868. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A. Copper catalysis in the construction of indole and benzo[b]furan rings. Org. Biomol. Chem. 2011, 9, 641–652. [Google Scholar] [CrossRef]
- Marsicano, V.; Arcadi, A.; Chiarini, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Synthesis of functionalised 2,3-dihydroquinolin-4(1H)-ones vs. quinoline or N-alkenylindole derivatives through sequential reactions of 2-alkynylanilines with ketones. Org. Biomol. Chem. 2021, 19, 421–438. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Ding, C.; Wang, Y.; Ma, C. N,N-Dimethylformamide as Carbon Synthons for the Synthesis of N-Heterocycles: Pyrrolo/Indolo[1,2-a]quinoxalines and Quinazolin-4-ones. J. Org. Chem. 2021, 86, 16848–16857. [Google Scholar] [CrossRef]
- Lee, S.B.; Jang, Y.; Ahn, J.; Chun, S.; Oh, D.-C.; Hong, S. One-Pot Synthesis of 4-Quinolone via Iron-Catalyzed Oxidative Coupling of Alcohol and Methyl Arene. Org. Lett. 2020, 22, 8382–8386. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Gao, Z.; Davis, L.; Chiang, Y.; Munson, R.; Hendrix, J.A. Regioselective assembly of 3,4-dihydroisoquinoline derivatives. Tetrahedron Lett. 2012, 53, 4429–4432. [Google Scholar] [CrossRef]
- Perea-Buceta, J.E.; Wirtanen, T.; Laukkanen, O.; Mäkelä, M.K.; Nieger, M.; Melchionna, M.; Huittinen, N.; Lopez-Sanchez, J.A.; Helaja, J. Cycloisomerization of 2-Alkynylanilines to Indoles Catalyzed by Carbon-Supported Gold Nanoparticles and Subsequent Homocoupling to 3,3′-Biindoles. Angew. Chem. Int. Ed. 2013, 52, 11835–11839. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Li, G.-D.; Mao, S.-P.; Chai, J.-D. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Asahara, H.; Mukaijo, Y.; Muragishi, K.; Iwai, K.; Ito, A.; Nishiwaki, N. Metal-Free and syn-Selective Hydrohalogenation of Alkynes through a Pseudo-Intramolecular Process. Eur. J. Org. Chem. 2021, 2021, 5747–5755. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, L.; Wang, H.; Zhao, J.; Zhu, Q. p-Toluenesulfonic Acid Promoted Annulation of 2-Alkynylanilines with Activated Ketones: Efficient Synthesis of 4-Alkyl-2,3-Disubstituted Quinolines. Eur. J. Org. Chem. 2010, 2010, 818–822. [Google Scholar] [CrossRef]
- Marsicano, V.; Arcadi, A.; Aschi, M.; Michelet, V. Experimental and computational evidence on gold-catalyzed regioselective hydration of phthalimido-protected propargylamines: An entry to β-amino ketones. Org. Biomol. Chem. 2020, 18, 9438–9447. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Bamford, M.J.; Blacker, A.J.; Lynch, J.; Marsden, S.P.; Plucinski, P.; Watson, R.J.; Williams, J.M. Iridium-catalyzed formylation of amines with paraformaldehyde. Tetrahedron Lett. 2010, 51, 5804–5806. [Google Scholar] [CrossRef]
- He, Y.-Y.; Sun, X.-X.; Li, G.-H.; Mei, G.-J.; Shi, F. Substrate-Controlled Regioselective Arylations of 2-Indolylmethanols with Indoles: Synthesis of Bis(indolyl)methane and 3,3′-Bisindole Derivatives. J. Org. Chem. 2017, 82, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, H.; Peng, C.; Ding, K.; Zhu, Q. Sequential Hydration-Condensation-Double Cyclization of Pyridine-Substituted 2-Alkynylanilines: An Efficient Approach to Quinoline-Based Heterocycles. Synthesis 2011, 2011, 1723–1732. [Google Scholar] [CrossRef]
- Politanskaya, L.; Shteingarts, V.; Tretyakov, E.; Potapov, A. The p-toluenesulfonic acid-catalyzed transformation of polyfluorinated 2-alkynylanilines to 2-aminoarylketones and indoles. Tetrahedron Lett. 2015, 56, 5328–5332. [Google Scholar] [CrossRef]
- Jia, R.; Li, B.; Zhang, X.; Fan, X. Selective Synthesis of 2-Indolyl-3-oxoindolines or 2-(2-Aminophenyl)quinolines through Cu(II)- or Bi(III)-Catalyzed Tunable Dimerizations of 2-Alkynylanilines. Org. Lett. 2020, 22, 6810–6815. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Annaka, K.; Fujita, A.; Sato, A.; Konakahara, T. InBr3-Promoted Divergent Approach to Polysubstituted Indoles and Quinolines from 2-Ethynylanilines: Switch from an Intramolecular Cyclization to an Intermolecular Dimerization by a Type of Terminal Substituent Group. J. Org. Chem. 2008, 73, 4160–4165. [Google Scholar] [CrossRef]
- Perumal, P.; Praveen, C.; Jegatheesan, S. Gold(III) Chloride Catalyzed Intermolecular Dimerization of 2-Ethynylanilines: Synthesis of Substituted Quinolines. Synlett 2009, 2009, 2795–2800. [Google Scholar] [CrossRef]
- Shelton, P.A.; Hilliard, C.R.; Swindling, M.; McElwee-White, L. Dimerization of ethynylaniline to a quinoline derivative using a ruthenium/gold heterobimetallic catalyst. ARKIVOC 2010, viii, 160–166. [Google Scholar] [CrossRef]
- Praveen, C.; Perumal, P.T. Revisiting the Gold-Catalyzed Dimerization of 2-Ethynylanilines: A Room-Temperature and Silver-Free Protocol for the Synthesis of Multifunctional Quinolines. Synthesis 2016, 48, 855–864. [Google Scholar] [CrossRef]
- Jia, R.; Li, B.; Liang, R.; Zhang, X.; Fan, X. Tunable Synthesis of Indolo[3,2-c]quinolines or 3-(2-Aminophenyl)quinolines via Aerobic/Anaerobic Dimerization of 2-Alkynylanilines. Org. Lett. 2019, 21, 4996–5001. [Google Scholar] [CrossRef] [PubMed]
- Punjajom, K.; Ruengsangtongkul, S.; Tummatorn, J.; Paiboonsombat, P.; Ruchirawat, S.; Thongsornkleeb, C. Mn(OAc)3-Mediated One-Pot Condensation-Oxidative Annulation of 2-Alkynylanilines and 1,3-Ketoesters: Synthesis of 2-Substituted Quinolines. J. Org. Chem. 2023, 88, 6736–6749. [Google Scholar] [CrossRef]
- Álvarez, R.; Martínez, C.; Madich, Y.; Denis, J.G.; Aurrecoechea, J.M.; de Lera, R. A General Synthesis of Alkenyl-Substituted Benzofurans, Indoles, and Isoquinolones by Cascade Palladium-Catalyzed Heterocyclization/Oxidative Heck Coupling. Chem.-A Eur. J. 2010, 16, 12746–12753. [Google Scholar] [CrossRef]
- Liang, S.; Hammond, L.; Xu, B.; Hammond, G.B. Commercial Supported Gold Nanoparticles Catalyzed Alkyne Hydroamination and Indole Synthesis. Adv. Synth. Catal. 2016, 358, 3313–3318. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Lee, S.; Son, S.H.; Yoo, H.-S.; Jang, Y.H.; Shin, J.-W.; Won, H.-J.; Sim, J.; Kim, N.-J. Ligand-controlled regiodivergent direct arylation of indoles via oxidative boron Heck reaction. Org. Chem. Front. 2022, 9, 5906–5911. [Google Scholar] [CrossRef]
- Shen, M.; Leslie, B.E.; Driver, T.G. Dirhodium(II)-Catalyzed Intramolecular C-H Amination of Aryl Azides. Angew. Chem. Int. Ed. 2008, 47, 5056–5059. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, S.; Ponticelli, F.; Taddei, M. Rapid Approach to 3,5-Disubstituted 1,4-Benzodiazepines via the Photo-Fries Rearrangement of Anilides. J. Org. Chem. 2006, 71, 9217–9220. [Google Scholar] [CrossRef]
- Patterson, S.; Alphey, M.S.; Jones, D.C.; Shanks, E.J.; Street, I.P.; Frearson, J.A.; Wyatt, P.G.; Gilbert, I.H.; Fairlamb, A.H. Dihydroquinazolines as a Novel Class of Trypanosoma brucei Trypanothione Reductase Inhibitors: Discovery, Synthesis, and Characterization of their Binding Mode by Protein Crystallography. J. Med. Chem. 2011, 54, 6514–6530. [Google Scholar] [CrossRef]
- Mamidala, R.; Subramani, M.S.; Samser, S.; Biswal, P.; Venkatasubbaiah, K. Chemoselective Alkylation of Aminoacetophenones with Alcohols by Using a Palladacycle-Phosphine Catalyst. Eur. J. Org. Chem. 2018, 2018, 6286–6296. [Google Scholar] [CrossRef]
- Ji, F.; Li, X.; Wu, W.; Jiang, H. Palladium-Catalyzed Oxidative Carbonylation for the Synthesis of Polycyclic Aromatic Hydrocarbons (PAHs). J. Org. Chem. 2014, 79, 11246–11253. [Google Scholar] [CrossRef] [PubMed]



| Entry a | Solvent | Brønsted Acid/ (Equiv.) | T (°C)/ Time (h) | Products/ Yield (%) b |
|---|---|---|---|---|
| 1 | EtOH | p-TsOH·H2O/1.0 | 110/24 | 6a (29) + 7a/8a (80/20) (70) |
| 2 | EtOH | p-TsOH·H2O/0.2 | 110/24 | 6a (46) + 7a (53) |
| 3 | EtOH | MsOH/1.0 | 110/24 | 6a (16) + 7a/8a (67/33) (53) |
| 4 | EtOH | TfOH/1.0 | 110/24 | 5a (13) + 6a (22) + 7a/8a (44/66) (34) |
| 5 | Toluene | TfOH/1.0 | 110/24 | 5a (7) + 6a (3) + 7a/8a (43/67) (50) |
| 6 | iPrOH | p-TsOH·H2O/1.0 | 110/24 | 5a (5) + 6a (12) + 7a/8a (55/45) (48) |
| 7 | DCE | p-TsOH·H2O/0.2 | 40/24 | 5a (54) + 1a (34%) |
| 8 | DCE | p-TsOH·H2O/1.0 | 40/24 | 6a (62) |
| 9 | DCE | p-TsOH·H2O/0.2 | 60/3 | 5a (32) + 6a (8) + 8a (9) |
| 10 | DCE | p-TsOH·H2O/0.2 | 80/3 | 5a (42) + 6a (13) + 8a (30) |
| 11 | DCE | p-TsOH·H2O/1.0 | 110/4 | 6a (17) + 7a (19)+ 8a (49) |
| 12 | THF | p-TsOH·H2O/1.0 | 60/24 | 6a (15) + 1a (56) |
| 13 | DMF | p-TsOH·H2O/1.0 | 110/17 | 5a (45) + 6a (22) + 9a (30) |
| 14 | DCE/DMM | p-TsOH·H2O/traces | 40/24 | 10a (76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morlacci, V.; Aschi, M.; Chiarini, M.; Momoli, C.; Palombi, L.; Arcadi, A. Product Selectivity Control in the Brønsted Acid-Mediated Reactions with 2-Alkynylanilines. Molecules 2024, 29, 3693. https://doi.org/10.3390/molecules29153693
Morlacci V, Aschi M, Chiarini M, Momoli C, Palombi L, Arcadi A. Product Selectivity Control in the Brønsted Acid-Mediated Reactions with 2-Alkynylanilines. Molecules. 2024; 29(15):3693. https://doi.org/10.3390/molecules29153693
Chicago/Turabian StyleMorlacci, Valerio, Massimiliano Aschi, Marco Chiarini, Caterina Momoli, Laura Palombi, and Antonio Arcadi. 2024. "Product Selectivity Control in the Brønsted Acid-Mediated Reactions with 2-Alkynylanilines" Molecules 29, no. 15: 3693. https://doi.org/10.3390/molecules29153693
APA StyleMorlacci, V., Aschi, M., Chiarini, M., Momoli, C., Palombi, L., & Arcadi, A. (2024). Product Selectivity Control in the Brønsted Acid-Mediated Reactions with 2-Alkynylanilines. Molecules, 29(15), 3693. https://doi.org/10.3390/molecules29153693








