Anti-Inflammatory Effect of Xanthones from Hypericum beanii on Macrophage RAW 264.7 Cells through Reduced NO Production and TNF-α, IL-1β, IL-6, and COX-2 Expression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Compounds 1–24
2.2. Cell Viability
2.3. Evaluation of Anti-Inflammatory Activity of the Isolated Compounds
2.4. Effect of Compounds 15, 19, and 22 on NO Production and the mRNA Level of iNOS
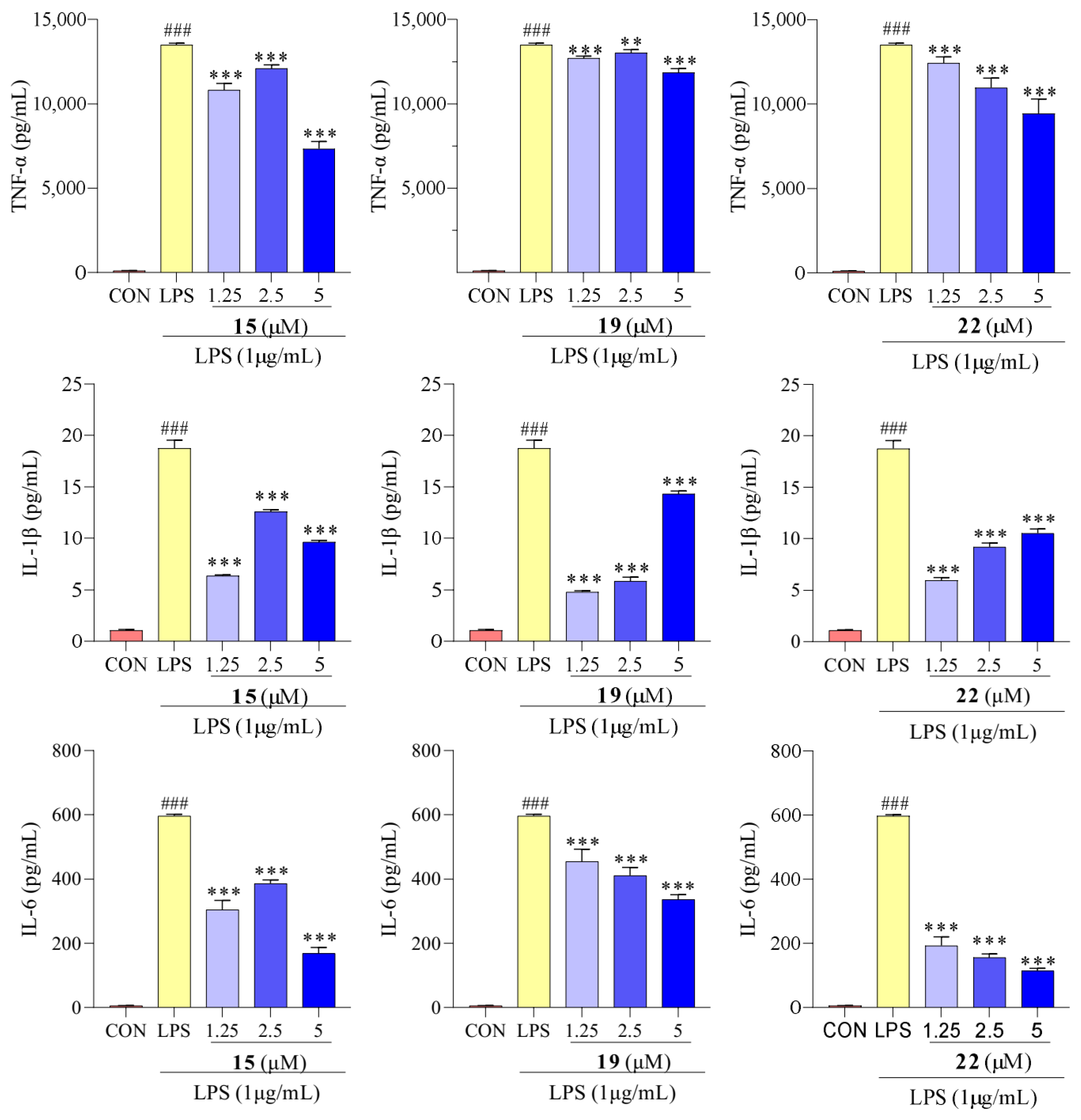
2.5. Effect of Compounds 15, 19, and 22 on TNF-α, IL-1β, and IL-6
2.6. Effect of Compounds 15, 19, and 22 on COX-2
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. CCK-8 Assay
3.6. Anti-Inflammatory Activity
3.7. Determination of NO, TNF-α, IL-1β, and IL-6 Levels
3.8. qPCR Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, Y.L.; Deng, Y.F.; Bu, P.F.; Guo, Y.; Shi, Z.Y.; Cao, Y.Y.; Zhang, Y.T.; Hu, H.; Qi, Z.X.; Hu, C.X.; et al. Discovery of bioactive polycyclic polyprenylated acylphloroglucinols from Hypericum wilsonii. Bioorgan. Chem. 2021, 115, 105246. [Google Scholar] [CrossRef] [PubMed]
- Editorial Board of Zhong Hua Ben Cao; State Administration of Traditional Chinese Medicine. Zhong Hua Ben Cao (China Herbal); Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 3, pp. 586–608. [Google Scholar]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Hypericum L. In Flora of China; Science Press: Beijing, China, 1990; Volume 50, p. 2. Available online: https://www.iplant.cn/info/Hypericum?t=foc (accessed on 1 August 2024).
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Hypericum beanii N. Robson. In Flora of China; Science Press: Beijing, China, 1990; Volume 50, pp. 36–37. Available online: https://www.iplant.cn/info/Hypericum%20beanii?t=foc (accessed on 1 August 2024).
- Yunnan Institute of Materia Medica. Yunnan Natural Medicine Atlas; Yunnan Science and Technology Press: Yunnan, China, 2003; p. 422. [Google Scholar]
- Chen, X.Q.; Li, Y.; Li, K.Z.; Peng, L.Y.; He, J.; Wang, K.; Pan, Z.H.; Cheng, X.; Li, M.M.; Zhao, Q.S.; et al. Spirocyclic acylphloroglucinol derivatives from Hypericum beanii. Chem. Pharm. Bull. 2011, 59, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.Y.; Liu, X.Y.; Liu, X.W.; Li, X.X.; Zhu, T.T.; Ji, T.F.; Liu, B. Four new polyprenylated acylphloroglucinol derivatives from Hypericum beanii. J. Asian Nat. Prod. Res. 2022, 24, 1008–1017. [Google Scholar] [CrossRef]
- Xu, W.J.; Tang, P.F.; Lu, W.J.; Zhang, Y.Q.; Wang, X.B.; Zhang, H.; Luo, J.; Kong, L.Y. Hyperberins A and B, type B polycyclic polyprenylated acylphloroglucinols with bicyclo[5.3.1]hendecane core from Hypericum beanii. Org. Lett. 2019, 21, 8558–8562. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Jung, W.K.; Park, S.J.; Kim, Y.T.; Kim, W.S.; Je, J.Y. Gallic Acid-g-Chitosan Modulates Inflammatory Responses in LPS-Stimulated RAW264.7 Cells Via NF-κB, AP-1, and MAPK Pathways. Inflammation 2016, 39, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Taketo, M.M. Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J. Natl. Cancer Inst. 1998, 90, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.L.; Lu, X.Q.; Gan, L.S.; Zhang, Q.W.; Lin, L.G. Xanthones, a promising anti-inflammatory scaffold: Structure, activity, and drug likeness analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef]
- Gnerre, C.; Thull, U.; Gaillard, P.; Carrupt, P.A.; Testa, B.; Fernandes, E.; Silva, F.; Pinto, M.; Pinto, M.I.; Wolfender, J.L.; et al. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biologicalassay and 3D-QSAR. Helv. Chim. Acta 2001, 84, 552–570. [Google Scholar] [CrossRef]
- Habib, A.M.; Reddy, K.S.; McCloud, T.G.; Chang, C.J.; Cassady, J.M. New xanthones from Psorospermum febrifugum. J. Nat. Prod. 1987, 50, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, H.R.; Napagoda, M.T.; Tennakoon, S.B. Xanthones from roots of Calophyllum thwaitesii and their bioactivity. Nat. Prod. Res. 2009, 23, 539–545. [Google Scholar] [CrossRef]
- Cardona, M.L.; Pedro, J.R.; Seoane, E.; Vidal, R. Xanthone constituents of Hypericum canariensis. J. Nat. Prod. 1985, 48, 467–469. [Google Scholar] [CrossRef]
- Frédérich, M.; Kikuchi, H.; Tane, P.; Tchinda, A.T.; Lonfouo, A.H.N.; Kowa, T.K.; Wabo, H.K.; Oshima, Y. Phenolic Compounds and terpenoids from Hypericum lanceolatum. Res. Nat. Prod. 2012, 6, 94–100. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, M.I.; Liou, S.J.; Lee, T.H.; Wang, J.P. Synthesis and anti-inflammatory effects of xanthone derivatives. J. Pharm. Pharmacol. 1996, 48, 532–538. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhong, F.F.; Zhao, Y.H.; Yang, G.Z.; Chen, Y. Study on the antioxidant constituents from the barks of Garcinia xanthochymus. Nat. Prod. Res. Dev. 2008, 20, 836–838. [Google Scholar] [CrossRef]
- Tala, M.F.; Talontsi, F.M.; Zeng, G.Z.; Wabo, H.K.; Tan, N.H.; Spiteller, M.; Tane, P. Antimicrobial and cytotoxic constituents from native Cameroonian medicinal plant Hypericum riparium. Fitoterapia 2015, 102, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Poobrasert, O.; Constant, H.L.; Beecher, C.W.; Farnsworth, N.R.; Kinghorn, A.D.; Pezzuto, J.M.; Cordell, G.A.; Santisuk, T.; Reutrakul, V. Xanthones from the twigs of Mammea siamensis. Phytochemistry 1998, 47, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Huang, S.T. Xanthones from Swertia nervosa and their inhibitory effects on nitric oxide production. Chem. Nat. Compd. 2020, 56, 732–735. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, N.F.; Xie, Z.; Zhao, Y.M.; Liang, C.H.; Deng, Y.Y.; Wang, R.; Liu, Y.P.; Fu, Y.H. Chemical constituents from stems and leaves of Cratoxylum cochinchinense and their inhibitory effects on proliferation of synoviocytes in vitro. China J. Chin. Mater. Medica 2023, 48, 5014–5023. [Google Scholar] [CrossRef]
- Marston, A.; Hamburger, M.; Sordat-Diserens, I.; Msonthi, J.D.; Hostettmann, K. Xanthones from Polygala nyikensis. Phytochemistry 1993, 33, 809–812. [Google Scholar] [CrossRef]
- Deng, J.T.; Hao, J.; Ma, Y.R.; Zhou, T.X.; Huang, H.Q. Study on chemical components of Hypericum wilsonii N. Robson. J. Yunnan Univ. Nat. Sci. Ed. 2021, 43, 369–376. [Google Scholar] [CrossRef]
- Cardona, M.L.; Fernández, M.I.; Pedro, J.R.; Seoane, E.; Vidal, R. Additional new xanthones and xanthonolignoids from Hypericum canariensis. J. Nat. Prod. 2004, 49, 95–100. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; ElSohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. Natural products inhibiting Candida albicans secreted aspartic proteases from Tovomita krukovii. Planta. Med. 2002, 68, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Li, Z.L.; Hua, H.M.; Li, Z.G.; Liu, M.S. Studies on flavonoids from stems and leaves of Calophyllum inophyllum. China J. Chin. Mater. Medica 2007, 32, 692–694. Available online: https://pubmed.ncbi.nlm.nih.gov/17608221/ (accessed on 1 August 2024).
- Jiang, D.J.; Hu, G.Y.; Jiang, J.L.; Xiang, H.L.; Deng, H.W.; Li, Y.J. Relationship between protective effect of xanthone on endothelial cells and endogenous nitric oxide synthase inhibitors. Bioorganic Med. Chem. 2003, 11, 5171–5177. [Google Scholar] [CrossRef]
- Gonda, R.; Takeda, T.; Akiyama, T. Studies on the constituents of Anaxagorea luzonensis A. GRAY II. Nat. Med. 2001, 55, 316. [Google Scholar]
- Ghosal, S.; Chaudhuri, R.K.; Nath, A. Chemical constituents of gentianaceae IV: New xanthones of Canscora decussata. J. Pharm. Sci. 1973, 62, 137–139. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Hamburger, M.; Msonthi, J.D.; Hostettmann, K. Xanthones from Chironia krebsii. Phytochemistry 1991, 30, 3625–3629. [Google Scholar] [CrossRef]
- Wang, H.; Ye, G.; Ma, C.H.; Tang, Y.H.; Fan, M.S.; Li, Z.X.; Huang, C.G. Identification and determination of four metabolites of mangiferin in rat urine. J. Pharm. Biomed. Anal. 2007, 45, 793–798. [Google Scholar] [CrossRef]
- Ngouela, S.; Zelefack, F.; Lenta, B.N.; Ngouamegne, E.T.; Tchamo, D.N.; Tsamo, E.; Connolly, J.D. Xanthones and other constituents of Allanblackia monticola (Guttiferae). Nat. Prod. Res. 2005, 19, 685–688. [Google Scholar] [CrossRef]
- Hu, L.H.; Yip, S.C.; Sim, K.Y. Xanthones from Hypericum ascyron. Phytochemistry 1999, 52, 1371–1373. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef]
- Khatua, S.; Simal-Gandara, J.; Acharya, K. Understanding immune-modulatory efficacy in vitro. Chem. Biol. Interact. 2022, 352, 109776. [Google Scholar] [CrossRef]
- Ma, W.; Ren, F.C.; Yan, X.W.; Wang, X.R.; Wu, T.N.; Li, N. Cytotoxic and anti-inflammatory constituents from roots of Hypericum beanii and the antitumor potential under the view of cancer-related inflammation. Fitoterapia 2024, 172, 105745. [Google Scholar] [CrossRef]
- DeRijk, R.; Michelson, D.; Karp, B.; Petrides, J.; Galliven, E.; Deuster, P.; Paciotti, G.; Gold, P.W.; Sternberg, E.M. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) production in humans: High sensitivity of TNF-α and resistance of IL-6. J. Clin. Endocrinol. Metab. 1997, 82, 2182–2191. [Google Scholar] [CrossRef]
- Cho, B.O.; Ryu, H.W.; So, Y.; Chang, W.L.; Chang, H.J.; Hong, S.Y.; Yong, W.J.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of mangostenone F in lipopolysaccharide-stimulated RAW264.7 macrophages by suppressing NF-κB and MAPK activation. Biomol. Ther. 2014, 22, 288–294. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Sun, W.; Chen, X.; Wang, Y.; Sun, Y.; Lin, L. 1, 3, 6, 7-Tetrahydroxy-8-prenylxanthone ameliorates inflammatory responses resulting from the paracrine interaction of adipocytes and macrophages. Br. J. Pharmacol. 2018, 175, 1590–1606. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, D.S.; Kim, Y.C. Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246. [Google Scholar] [CrossRef]
- Toshihiro, N.; Takahashi-Yanaga, F.; Arioka, M.; Mori, Y.; Sasaguri, T. Inhibition of GSK-3 reduces prostaglandin E2 production by decreasing the expression levels of COX-2 and mPGES-1 in monocyte/macrophage lineage cells. Biochem. Pharmacol. 2016, 116, 120–129. [Google Scholar] [CrossRef]
- Koopklang, K.; Choodej, S.; Hantanong, S.; Intayot, R.; Jungsuttiwong, S.; Insumran, Y.; Ngamrojanavanich, N.; Pudhom, K. Anti-inflammatory properties of oxygenated isocoumarins and xanthone from thai mangrove-associated endophytic fungus Setosphaeria rostrata. Molecules 2024, 29, 603. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Ma, J.C.; Deng, P.; Ren, F.C.; Li, N. Chemical constituents of thesium chinense turcz and their in vitro antioxidant, anti-inflammatory and cytotoxic activities. Molecules 2023, 28, 2685. [Google Scholar] [CrossRef] [PubMed]

 ) of compound 1.
) of compound 1.





| NO | 1 | |
|---|---|---|
| δC | δH | |
| 1 | 161.6 | |
| 2 | 94.8 | 6.33s |
| 3 | 163.6 | |
| 4 | 95.6 | 6.37s |
| 4a | 159.0 | |
| 4b | 143.7 | |
| 5 | 99.5 | 7.01s |
| 6 | 153.4 | |
| 7 | 149.0 | |
| 8 | 108.8 | 7.34s |
| 8a | 115.4 | |
| 8b | 104.7 | |
| 9 | 172.7 | |
| 1-OCH3 | 55.8 | 3.80 (s) |
| 6-OCH3 | 56.1 | 3.88 (s) |
| Gene | Name | Primers Sequence |
|---|---|---|
| GAPDH | Forward Primer Reverse Primer | AGGTCGTGTGAACGGATTTG GGGGTCGTTGATGGCAACA |
| iNOS | Forward Primer Reverse Primer | GGAGTGACGGCAAACATGACT TCGATGCACAACTGGGTGAAC |
| TNF-α | Forward Primer Reverse Primer | CGCTCTTCTGTCTACTGAACTTCGG GTGGTTTGTGAGTGTGAGGGTCTG |
| IL-1β | Forward Primer Reverse Primer | CACTACAGGCTCCGAGATGAACAAC TGTCGTTGCTTGGTTCTCCTTGTAC |
| IL-6 | Forward Primer Reverse Primer | CTTCTTGGGACTGATGCTGGTGAC TCTGTTGGGAGTGGTATCCTCTGTG |
| COX-2 | Forward Primer Reverse Primer | TCCAACACACTCTATCACTGGC AGAAGCGTTTGCGGTACTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Ren, F.-C.; Wang, X.-R.; Li, N. Anti-Inflammatory Effect of Xanthones from Hypericum beanii on Macrophage RAW 264.7 Cells through Reduced NO Production and TNF-α, IL-1β, IL-6, and COX-2 Expression. Molecules 2024, 29, 3705. https://doi.org/10.3390/molecules29153705
Ma W, Ren F-C, Wang X-R, Li N. Anti-Inflammatory Effect of Xanthones from Hypericum beanii on Macrophage RAW 264.7 Cells through Reduced NO Production and TNF-α, IL-1β, IL-6, and COX-2 Expression. Molecules. 2024; 29(15):3705. https://doi.org/10.3390/molecules29153705
Chicago/Turabian StyleMa, Wei, Fu-Cai Ren, Xue-Ru Wang, and Ning Li. 2024. "Anti-Inflammatory Effect of Xanthones from Hypericum beanii on Macrophage RAW 264.7 Cells through Reduced NO Production and TNF-α, IL-1β, IL-6, and COX-2 Expression" Molecules 29, no. 15: 3705. https://doi.org/10.3390/molecules29153705






