Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4
Abstract
1. Introduction
2. Experiment
2.1. Preparation of ZnO Nanowires (ZnO NWs)
2.2. Preparation of g-C3N4
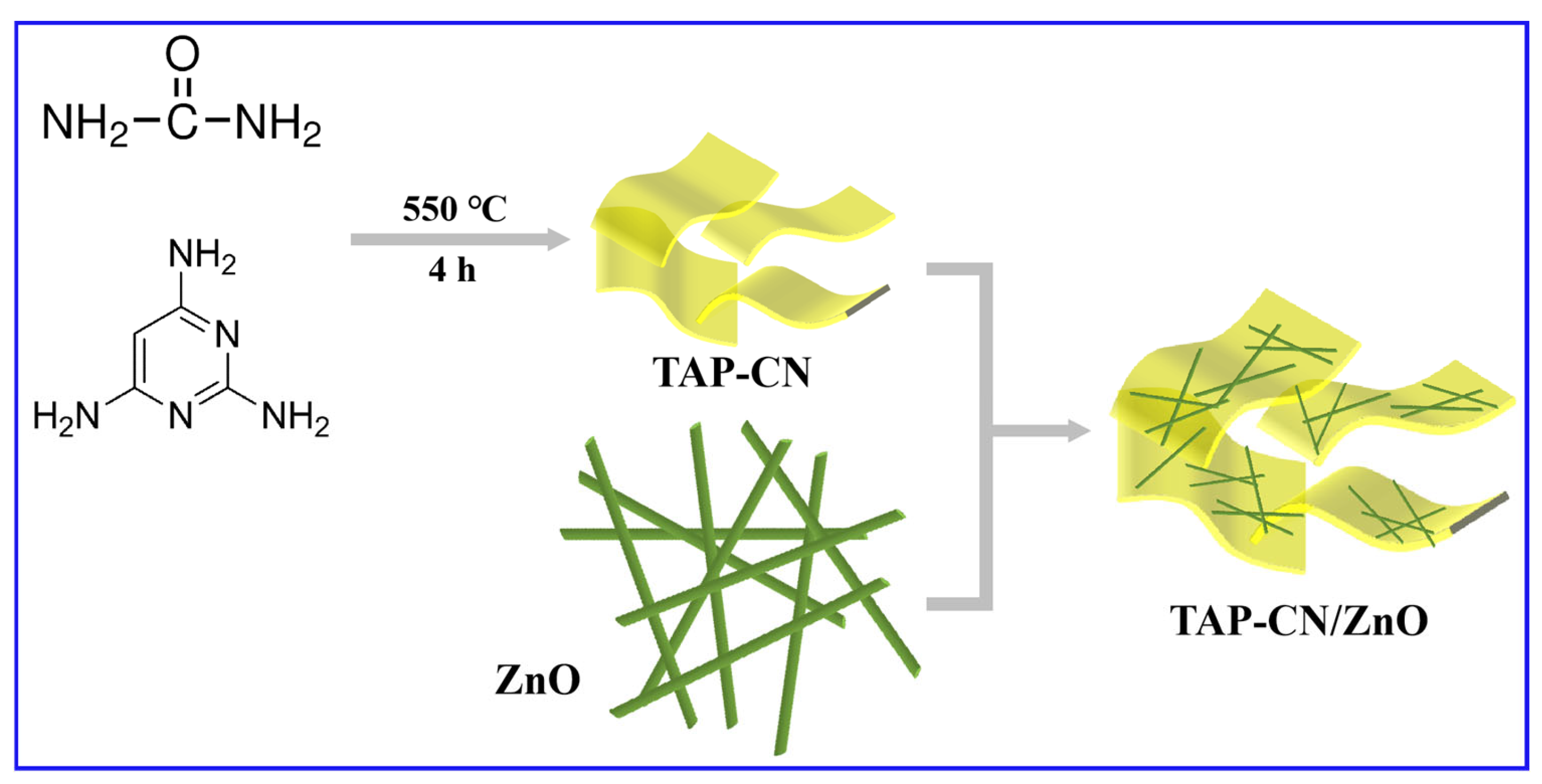
2.3. Preparation of 2,4,6-Triaminopyrimidine-g-C3N4 (TAP-CN) and TAP-CN/ZnO NWs
3. Results and Discussion
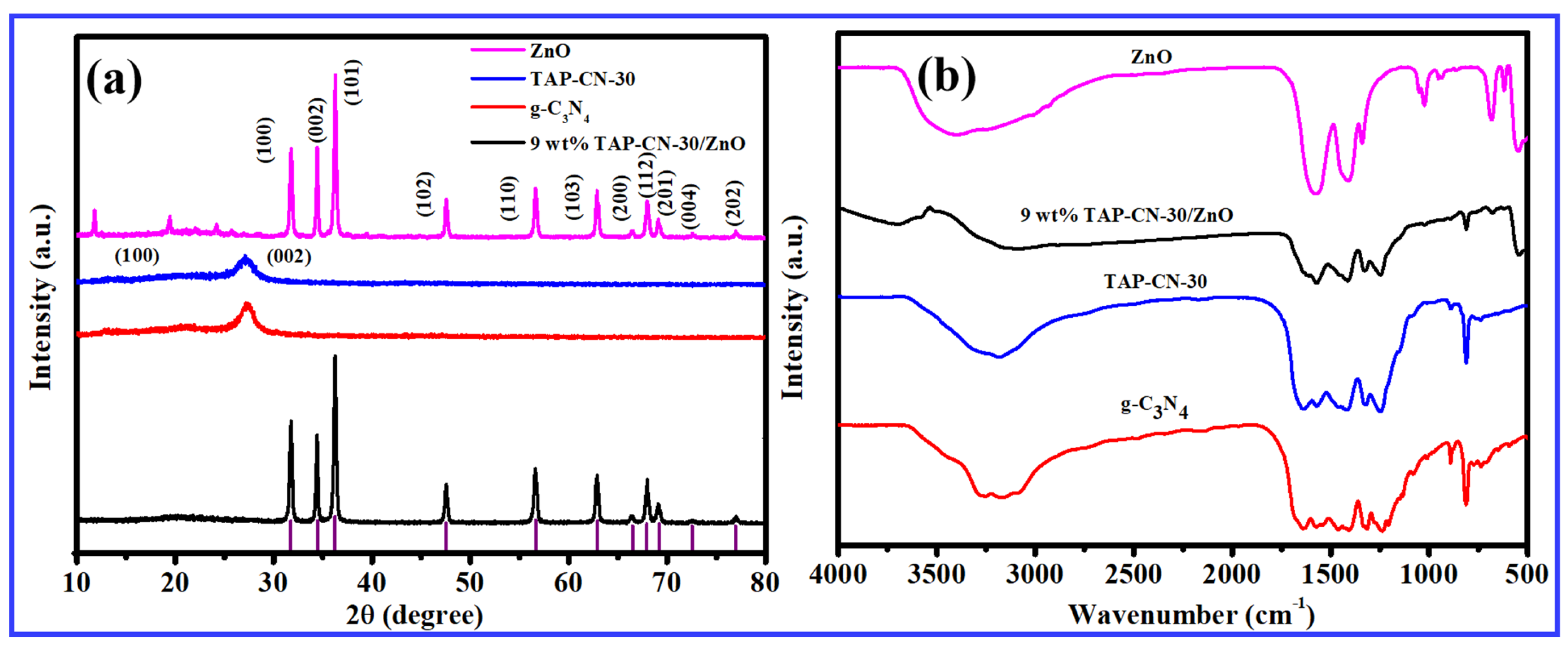
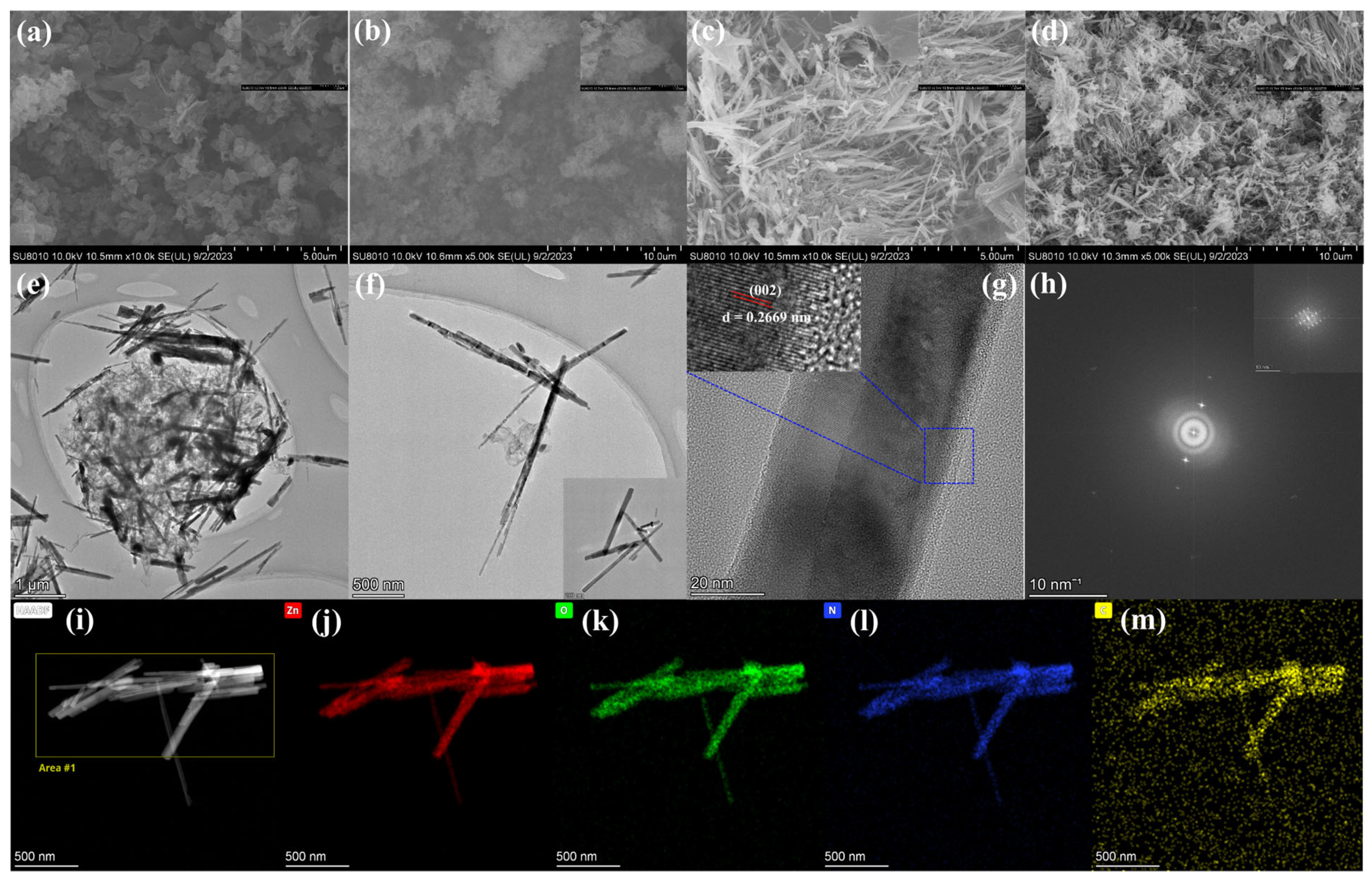
3.1. Morphological and Structural Characterization
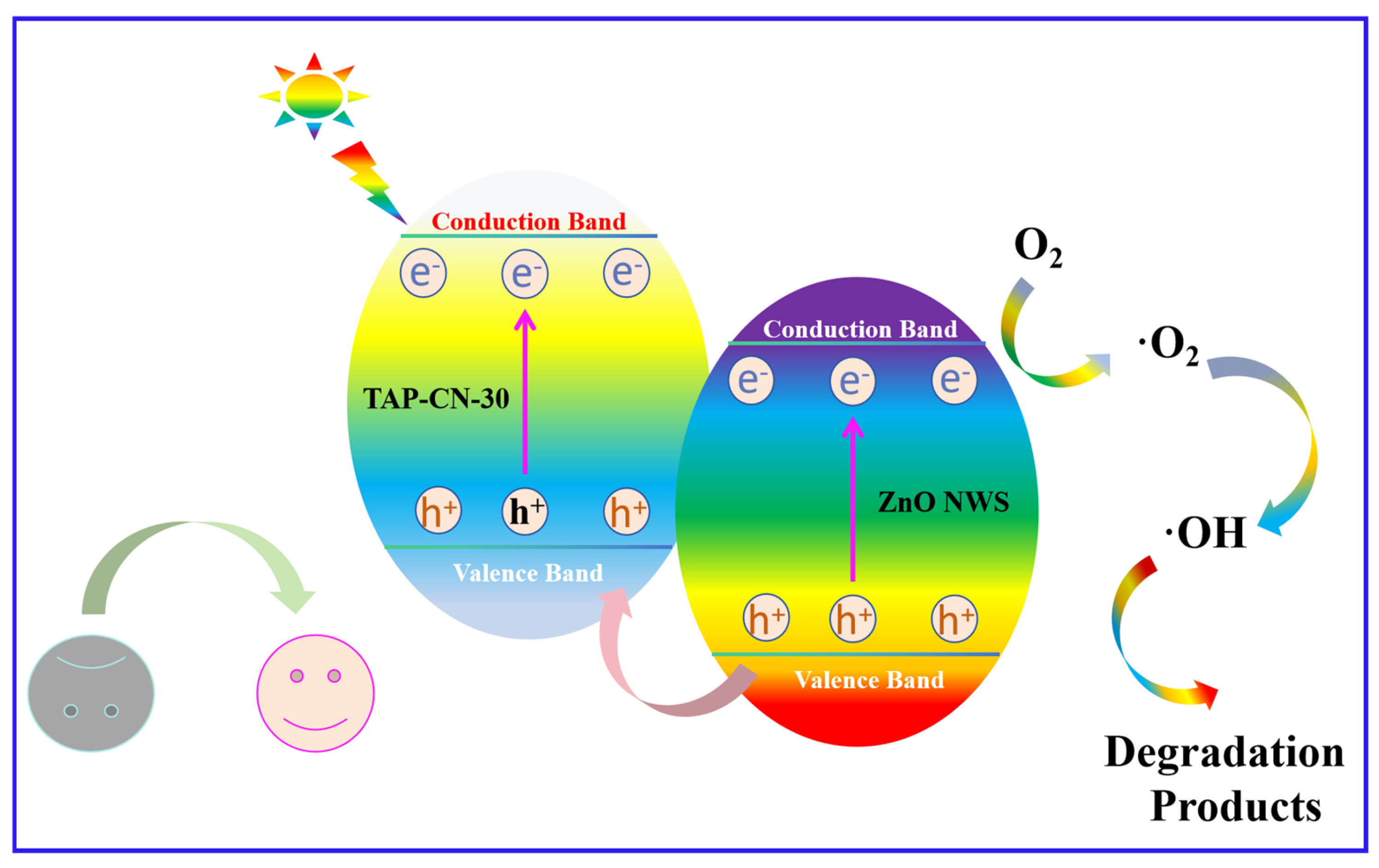
3.2. Evaluation of Photocatalytic Degradation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, R.; Jing, H.; Xiao, J.; Xie, H.; Hong, F.; Ta, N.; Zhang, X.; Zhu, J.; Li, C. Blocking the reverse reactions of overall water splitting on a Rh/GaN-ZnO photocatalyst modified with Al2O3. Nat. Catal. 2023, 6, 80–88. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Ng, Y.H.; Voiry, D.; et al. 2D transition metal dichalcogenides for photocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202218016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Photoredox-catalyzed plastic waste conversion: Nonselective degradation versus selective synthesis. ACS Catal. 2023, 13, 3575–3590. [Google Scholar] [CrossRef]
- Qiao, S.; Di, M.; Jiang, J.X.; Han, B.H. Conjugated Porous Polymers for Photocatalysis: The Road From Catalytic Mechanism, Molecular Structure to Advanced Applications. EnergyChem 2022, 4, 100094. [Google Scholar] [CrossRef]
- Zare, A.; Saadati, A.; Sheibani, S. Modification of a Z-scheme ZnO-CuO nanocomposite by Ag loading as a highly efficient visible light photocatalyst. Mater. Res. Bull. 2023, 158, 112048. [Google Scholar] [CrossRef]
- Guaraldo, T.T.; Vakili, R.; Wenk, J.; Mattia, D. Highly efficient ZnO photocatalytic foam reactors for micropollutant degradation. Chem. Eng. J. 2023, 455, 140784. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, J.; Gao, X.; Liu, W.; Che, H.; Liu, B.; Ao, Y. Two birds with one stone: Cobalt-doping induces to enhanced piezoelectric property and persulfate activation ability of ZnO nanorods for efficient water purification. Nano Energy 2023, 107, 108173. [Google Scholar] [CrossRef]
- Wu, J.; Ke, K.; Qin, N.; Lin, E.; Kang, Z.; Bao, D. Magnetically retrievable Fe3O4@ SiO2@ ZnO piezo-photocatalyst: Synthesis and multiple catalytic properties. J. Colloid Interface Sci. 2023, 636, 167–175. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.-V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Liew, R.K.; Nguyen, D.T.C.; Tran, T.V. Recent advances on botanical biosynthesis of nanoparticles for catalytic, water treatment and agricultural applications: A review. Sci. Total Environ. 2022, 827, 154160. [Google Scholar] [CrossRef]
- Rai, R.S.; Bajpai, V.; Khan, M.I.; Elboughdiri, N.; Shanableh, A.; Luque, R. An eco-friendly approach on green synthesis, bio-engineering applications, and future outlook of ZnO nanomaterial: A critical review. Environ. Res. 2023, 221, 114807. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Altuner, E.E.; El Houda Tiri, R.N.; Bekmezci, M.; Gulbagca, F.; Aygun, A.; Xia, C.; Van Le, Q.; Sen, F.; Karimi-Maleh, H. Hydrogen generation from methanolysis of sodium borohydride using waste coffee oil modified zinc oxide nanoparticles and their photocatalytic activities. Int. J. Hydrogen Energy 2023, 48, 6613–6623. [Google Scholar] [CrossRef]
- Haounati, R.; Ighnih, H.; Malekshah, R.E.; Alahiane, S.; Alakhras, F.; Alabbad, E.; Alghamdi, H.; Ouachtak, H.; Addi, A.A.; Jada, A. Exploring ZnO/montmorillonite photocatalysts for the removal of hazardous RhB Dye: A combined study using molecular dynamics simulations and experiments. Mater. Today Commun. 2023, 35, 105915. [Google Scholar] [CrossRef]
- Zheng, Z.; Liang, W.; Lin, R.; Hu, Z.; Wang, Y.; Lu, H.; Zhong, W.; Shen, S.; Pan, Y. Facile synthesis of zinc indium oxide nanofibers distributed with low content of silver for superior antibacterial activity. Small Struct. 2023, 4, 2200291. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.R.; Khiadani, M.; Zhong, Y.; Wang, W.; Su, C.; Shao, Z. Prussian blue-conjugated ZnO nanoparticles for near-infrared light-responsive photocatalysis. Mater. Today Energy 2022, 23, 100895. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Yang, M.; Wang, P.; Li, Y.; Tang, S.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Graphene aerogel-based NiAl-LDH/g-C3N4 with ultratight sheet-sheet heterojunction for excellent visible-light photocatalytic activity of CO2 reduction. Appl. Catal. B Environ. 2022, 306, 121065. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Liu, X.; Du, Y.; Zhao, Y.; Song, X.; Jing, X.; Yu, L.; Sun, M. 2D Benzodithiophene based conjugated polymer/g-C3N4 heterostructures with enhanced photocatalytic activity: Synergistic effect of antibacterial carbazole side chain and main chain copolymerization. Appl. Catal. B Environ. 2022, 312, 121401. [Google Scholar] [CrossRef]
- Yuan, T.; Sun, L.; Wu, Z.; Wang, R.; Cai, X.; Lin, W.; Zheng, M.; Wang, X. Mild and metal-free Birch-type hydrogenation of (hetero) arenes with boron carbonitride in water. Nat. Catal. 2022, 5, 1157–1168. [Google Scholar] [CrossRef]
- Zhang, C.; Ouyang, Z.; Yang, Y.; Long, X.; Qin, L.; Wang, W.; Zhou, Y.; Qin, D.; Qin, F.; Lai, C. Molecular engineering of donor-acceptor structured g-C3N4 for superior photocatalytic oxytetracycline degradation. Chem. Eng. J. 2022, 448, 137370. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.Z.S.; Ng, S.F.; Ong, W.J. Tailor-engineered 2D cocatalysts: Harnessing electron-hole redox center of 2D g-C3N4 photocatalysts toward solar-to-chemical conversion and environmental purification. Adv. Funct. Mater. 2022, 32, 2111875. [Google Scholar] [CrossRef]
- He, W.; Liu, L.; Ma, T.; Han, H.; Zhu, J.; Liu, Y.; Fang, Z.; Yang, Z.; Guo, K. Controllable morphology CoFe2O4/g-C3N4 pn heterojunction photocatalysts with built-in electric field enhance photocatalytic performance. Appl. Catal. B Environ. 2022, 306, 121107. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Guo, F.; Shi, Y.; Li, L.; Xu, Z.; Yan, X.; Shi, W. Fe-doped g-C3N4 derived from biowaste material with Fe-N bonds for enhanced synergistic effect between photocatalysis and Fenton degradation activity in a broad pH range. J. Alloys Compd. 2022, 900, 163410. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, L.; Zhu, Y.; Zhang, Y.; Liu, J.; Wang, J.; Feng, C.; Qiao, L.; Chen, Y. Conjugated polymers S-scheme homojunction with large internal electric field and matching interface for efficient visible light photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2023, 419, 138199. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Zhu, Y. Deep degradation of pollutants by perylene diimide supramolecular photocatalyst with unique Bi-planar π-π conjugation. Chem. Eng. J. 2022, 438, 135667. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, X.; Chen, P.; Li, D.; Zhang, Q.; Liu, H.; Zhong, J.; Lv, W.; Liu, G. Synchronous construction of a porous intramolecular DA conjugated polymer via electron donors for superior photocatalytic decontamination. J. Hazard. Mater. 2022, 424, 127379. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Gao, X.; Che, H.; Wang, P.; Liu, B.; Ao, Y. Enhanced photocatalytic degradation of bisphenol A by a novel donor-acceptor g-C3N4: π-π interactions boosting the adsorption and electron transfer behaviors. Sep. Purif. Technol. 2022, 300, 121947. [Google Scholar] [CrossRef]
- Swedha, M.; Okla, M.K.; Al-amri, S.S.; Alaraidh, I.A.; Al-ghamdi, A.A.; Mohebaldin, A.; Abdel-Maksoud, M.A.; Aufy, M.; Studenik, C.R.; Thomas, A.M.; et al. Green synthesis of two-electron centre based ZnO/NiCo2S4 QDs-OVs using Punica granatum fruit peel extract for an exceptional visible light photocatalytic degradation of doxycycline and ciprofloxacin. Chemosphere 2022, 304, 135225. [Google Scholar] [CrossRef] [PubMed]
- Abubshait, H.A.; Saad, M.; Iqbal, S.; Abubshait, S.A.; Bahadur, A.; Raheel, M.; Alshammari, F.H.; Alwadai, N.; Alrbyawi, H.; Abourehab, M.A.S.; et al. Co-doped zinc oxide nanoparticles embedded in Polyvinylalcohol Hydrogel as solar light derived photocatalyst disinfection and removal of coloured pollutants. J. Mol. Struct. 2023, 1271, 134100. [Google Scholar] [CrossRef]
- Cong, C.Q.; Dat, N.M.; Hai, N.D.; Nam, N.T.; An, H.; Dat, T.D.; Dat, N.T.; Phong, M.T.; Hieu, N.H. Green synthesis of carbon-doped zinc oxide using Garcinia mangostana peel extract: Characterization, photocatalytic degradation, and hydrogen peroxide production. J. Clean. Prod. 2023, 392, 136269. [Google Scholar]
- Vijayan, M.; Easwaran, G.; Sivakumar, K.; Palanisamy, G.; Bhuvaneswari, K. Energetic two-dimensional g-C3N4 nanosheets combined with ZnO nanoparticles as effectual catalyst for degradation of MB dye under UV-Visible-light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 24340–24353. [Google Scholar] [CrossRef]
- Guo, J.; Sun, Y.; Luo, Q.; Zhang, J.; Fang, L. Construction of Z-scheme Ti/Ga co-doped ZnO heterostructure photocatalyst with graphitic carbon nitride for efficient visible-light-driven dye degradation. Environ. Sci. Pollut. Res. 2023, 30, 43702–43713. [Google Scholar] [CrossRef]

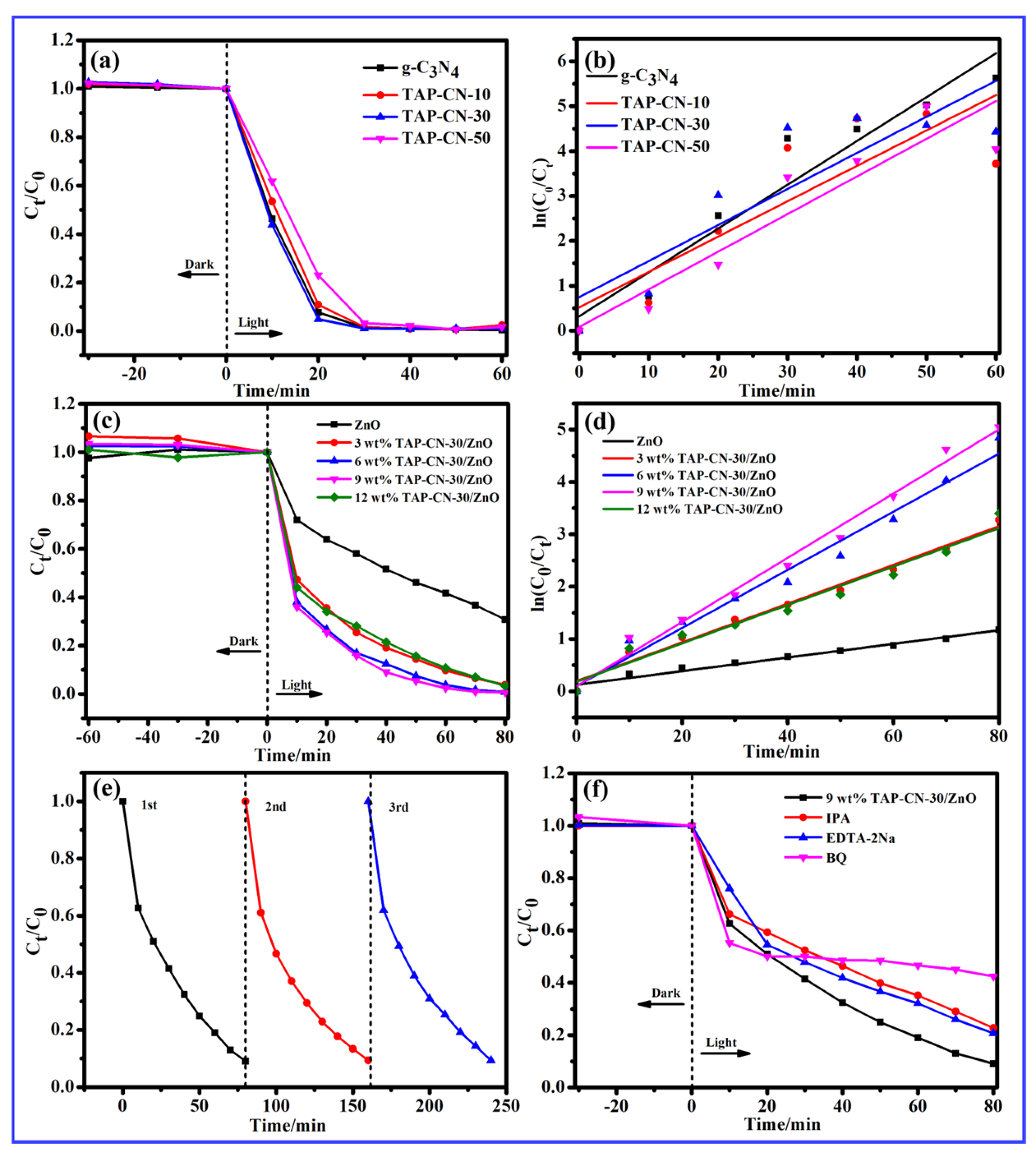

| y = ln (C0/Ct) | R2 | Degradation Rate (%) | |
|---|---|---|---|
| ZnO | y = 0.01303x + 0.12447 | 0.96929 | 69.18% |
| 3 wt% TAP-CN/ZnO | y = 0.03701x + 0.19386 | 0.96102 | 96.23% |
| 6 wt% TAP-CN/ZnO | y = 0.05552x + 0.010009 | 0.98309 | 99.20% |
| 9 wt% TAP-CN/ZnO | y = 0.06134x + 0.09746 | 0.97750 | 99.36% |
| 12 wt% TAP-CN/ZnO | y = 0.03669x + 0.18199 | 0.98738 | 96.67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, J.; Wang, L.; Huang, Y.; Xing, J.; Yang, X. Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4. Molecules 2024, 29, 3716. https://doi.org/10.3390/molecules29163716
Lou J, Wang L, Huang Y, Xing J, Yang X. Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4. Molecules. 2024; 29(16):3716. https://doi.org/10.3390/molecules29163716
Chicago/Turabian StyleLou, Jiahui, Lihong Wang, Yaqiong Huang, Jun Xing, and Xiaojie Yang. 2024. "Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4" Molecules 29, no. 16: 3716. https://doi.org/10.3390/molecules29163716
APA StyleLou, J., Wang, L., Huang, Y., Xing, J., & Yang, X. (2024). Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4. Molecules, 29(16), 3716. https://doi.org/10.3390/molecules29163716




