In-Column Dehydration Benzyl Alcohols and Their Chromatographic Behavior on Pyridinium-Based Ionic Liquids as Gas Stationary Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Behavior of Ionic Liquid-Based Stationary Phases
2.2. Dehydration of Aromatic Alcohols on the Ionic Liquid-Based Stationary Phases
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koel, M. Ionic liquids in chemical analysis. Crit. Rev. Anal. Chem. 2005, 35, 177–192. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Maciel Filho, R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.A.; Baker, S.N.; Pandey, S.; Bright, F.V. An analytical view of ionic liquids. Analyst 2005, 130, 800–808. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Poole, S.K. Ionic liquid stationary phases for gas chromatography: Gas Chromatography. J. Sep. Sci. 2011, 34, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Anderson, J.L. Retention characteristics of organic compounds on molten salt and ionic liquid-based gas chromatography stationary phases. J. Chromatogr. A 2009, 1216, 1658–1712. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Pandey, S.; Pandey, S. Applications of ionic liquids in biphasic separation: Aqueous biphasic systems and liquid–liquid equilibria. J. Chromatogr. A 2019, 1559, 44–61. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Gas chromatography on wall-coated open-tubular columns with ionic liquid stationary phases. J. Chromatogr. A 2014, 1357, 87–109. [Google Scholar] [CrossRef]
- Shashkov, M.V.; Sidelnikov, V.N.; Bratchikova, A.A. New stationary ionic liquid phases with quinolinium cations for capillary gas chromatography. Anal. Lett. 2020, 53, 84–101. [Google Scholar] [CrossRef]
- Shashkov, M.V.; Sidelnikov, V.N.; Bratchikova, A.A.; Nikolaeva, O.A. New dicationic quinolinium ionic liquids for capillary gas chromatography. Russ. J. Phys. Chem. 2020, 94, 1494–1502. [Google Scholar] [CrossRef]
- Shashkov, M.V.; Sidelnikov, V.N. Properties of columns with several pyridinium and imidazolium ionic liquid stationary phases. J. Chromatogr. A 2013, 1309, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Escobar-Arnanz, J.; Sanz, M.L.; Ramos, L. Evaluation of ionic liquid gas chromatography stationary phases for the separation of polychlorinated biphenyls. J. Chromatogr. A 2018, 1559, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Antle, P.; Zeigler, C.; Robbat, A. Retention behavior of alkylated polycyclic aromatic sulfur heterocycles on immobilized ionic liquid stationary phases. J. Chromatogr. A 2014, 1361, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K. Assessment of ionic liquid stationary phases for the GC analysis of fatty acid methyl esters. Anal. Bioanal. Chem. 2014, 406, 4931–4939. [Google Scholar] [CrossRef] [PubMed]
- Ragonese, C.; Tranchida, P.Q.; Dugo, P.; Dugo, G.; Sidisky, L.M.; Robillard, M.V.; Mondello, L. Evaluation of use of a dicationic liquid stationary phase in the fast and conventional gas chromatographic analysis of health-hazardous C18 cis/trans fatty acids. Anal. Chem. 2009, 81, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, M.V.; Sidelnikov, V.N. Mass spectral evaluation of column bleeding for imidazolium-based ionic liquids as GC liquid phases. Anal. Bioanal. Chem. 2012, 403, 2673–2682. [Google Scholar] [CrossRef]
- Odugbesi, G.A.; Nan, H.; Soltani, M.; Davis, J.H., Jr.; Anderson, J.L. Ultra-high thermal stability perarylated ionic liquids as gas chromatographic stationary phases for the selective separation of polyaromatic hydrocarbons and polychlorinated biphenyls. J. Chromatogr. A 2019, 1604, 460466. [Google Scholar] [CrossRef]
- Gu, Q.; David, F.; Lynen, F.; Vanormelingen, P.; Vyverman, W.; Rumpel, K.; Sandra, P. Evaluation of ionic liquid stationary phases for one dimensional gas chromatography–mass spectrometry and comprehensive two dimensional gas chromatographic analyses of fatty acids in marine biota. J. Chromatogr. A 2011, 1218, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.; He, L.; Liu, Y.S. Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal. Chem. 1999, 71, 3873–3876. [Google Scholar] [CrossRef] [PubMed]
- Mutelet, F.; Carre, P.; Skrzypczak, A. Study of interaction between organic compounds and mono or dicationic oxygenated ionic liquids using gas chromatography. Fluid Phase Equilibria 2015, 387, 59–72. [Google Scholar] [CrossRef]
- Poole, C.F.; Atapattu, S.N. Determination of physicochemical properties of ionic liquids by gas chromatography. J. Chromatogr. A 2021, 1644, 461964. [Google Scholar] [CrossRef] [PubMed]
- Sholokhova, A.; Matyushin, D.; Shashkov, M. Quantitative structure-retention relationships for pyridinium-based ionic liquids used as gas chromatographic stationary phases: Convenient software and assessment of reliability of the results. J. Chromatogr. A 2024, 1730, 465144. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, M.V.; Sidelnikov, V.N.; Zaikin, P.A. Selectivity of stationary phases based on pyridinium ionic liquids for capillary gas chromatography. Russ. J. Phys. Chem. 2014, 88, 717–721. [Google Scholar] [CrossRef]
- Filippi, J.J.; Cocolo, N.; Meierhenrich, U.J. Peak-bridges due to in-column analyte transformations as a new tool for establishing molecular connectivities by comprehensive two-dimensional gas chromatography–mass spectrometry. J. Chromatogr. A 2015, 1383, 134–143. [Google Scholar] [CrossRef]
- Poole, C.F.; Poole, S.K. Separation characteristics of wall-coated open-tubular columns for gas chromatography. J. Chromatogr. A 2008, 1184, 254–280. [Google Scholar] [CrossRef]
- Berezkin, V.G.; Korolev, A.A. Investigation of the role of adsorption at the stationary phase interface in capillary columns prepared with cross-linked phases. Chromatographia 1985, 20, 482–486. [Google Scholar] [CrossRef]
- Farkaš, P.; Soják, L.; Kováč, M.; Janák, J. Interface adsorption and reproducibility of retention indices in glass capillary columns with dimethylpolysiloxane stationary phases cross-linked by γ-irradiation. J. Chromatogr. A 1989, 471, 251–261. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y. Efficient and selective dehydration of fructose to 5-hydroxymethylfurfural catalyzed by brønsted-acidic ionic liquids. ChemSusChem Chem. Sustain. Energy Mater. 2010, 3, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Sharma, N.; Kumar, V.; Sinha, A.K. Neutral ionic liquid [hmim] Br as a green reagent and solvent for the mild and efficient dehydration of benzyl alcohols into (E)-arylalkenes under microwave irradiation. EurJOC 2008, 33, 5577–5582. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, B.; Dwivedi, A.R.; Kumar, V. Regioselective alkylation of 1, 2, 4-triazole using ionic liquids under microwave conditions. Green Process. Synth. 2016, 5, 233–237. [Google Scholar] [CrossRef]
- Shashkov, M.V.; Sidel’nikov, V.N. Single cation ionic liquids as high polarity thermostable stationary liquid phases for capillary chromatography. Russ. J. Phys. Chem. 2012, 86, 138–141. [Google Scholar] [CrossRef]
- Available online: https://figshare.com/articles/dataset/Supplementary_data_for_the_article_Pyridinium-based_ionic_liquids_as_gas_chromatographic_stationary_phases_chromatographic_behavior_a_data_set_for_quantitative_structure-retention_relationships_and_an_application_for_non-target_analysis_/16885009/4 (accessed on 24 June 2024).

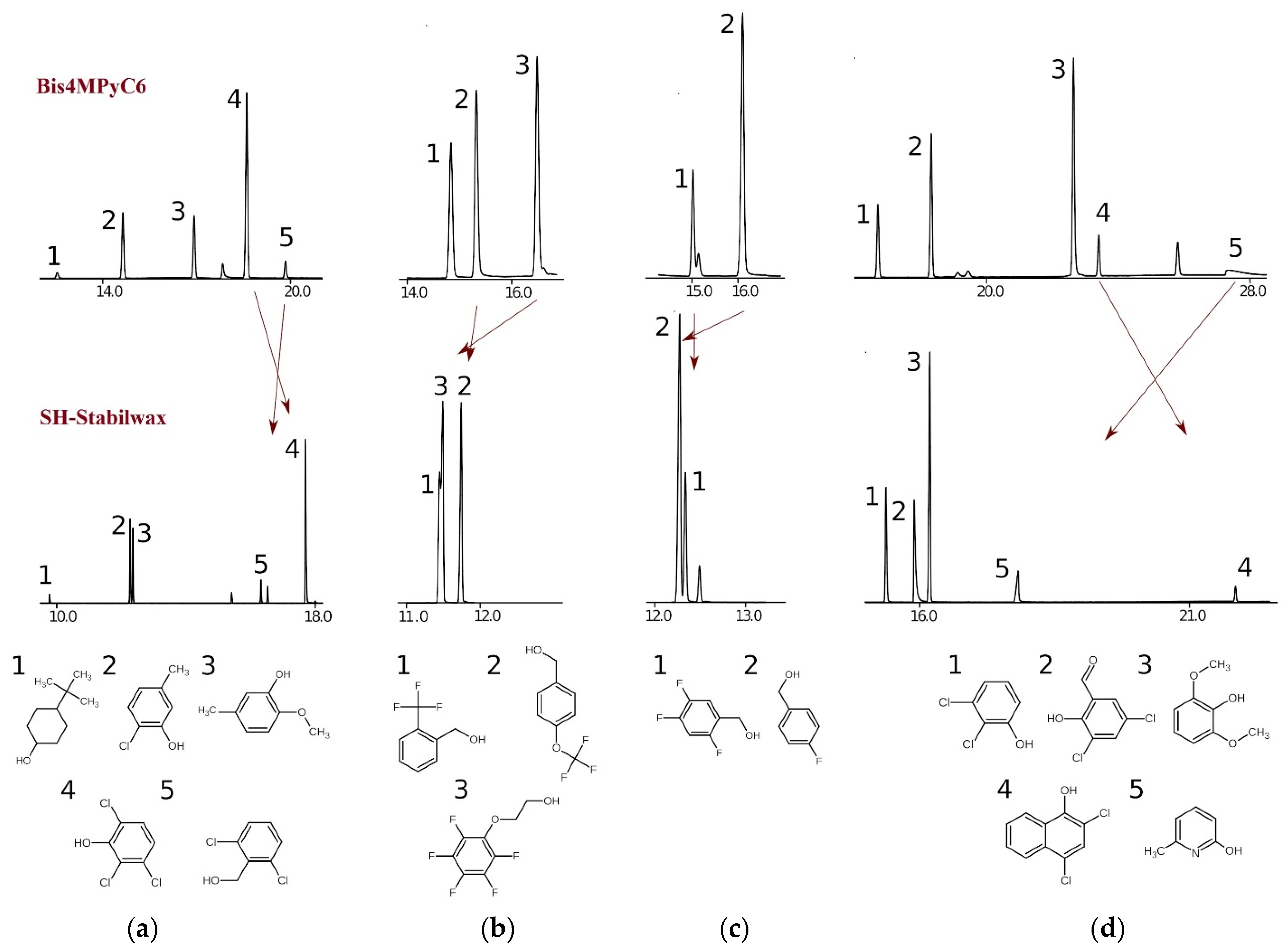
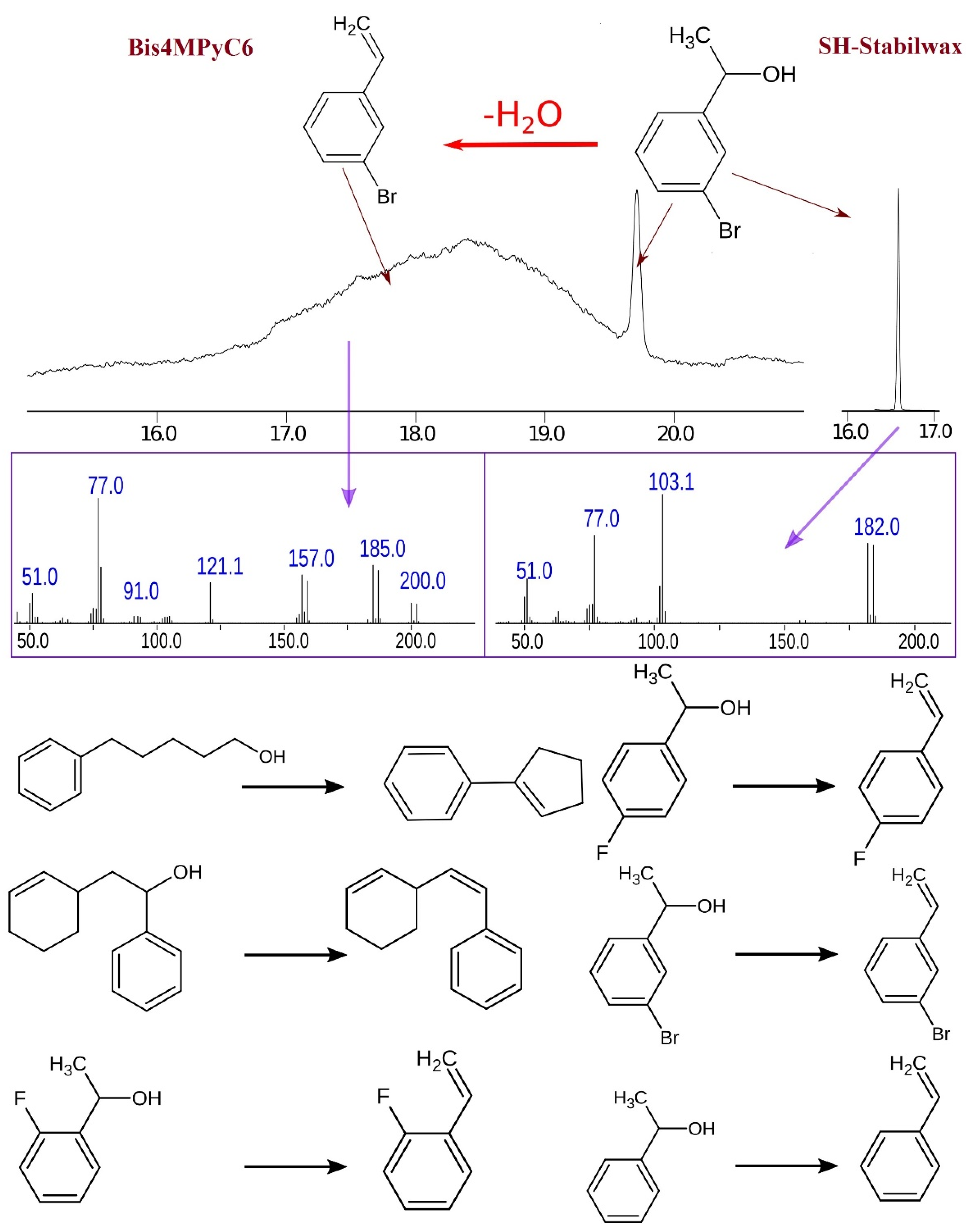
| Stationary Phase | SH-Stabilwax | Bis4MPyC6 |
|---|---|---|
| Importance of descriptor | BCUT2D_MWHI | fr_aryl_methyl |
| PEOE_VSA11 | BCUT2D_MWHI | |
| FractionCSP3 | VSA_EState3 | |
| EState_VSA2 | NumRotatableBonds | |
| fr_aryl_methyl | Chi0n | |
| MaxAbsPartialCharge | Estate_VSA2 | |
| fr_benzene | fr_benzene | |
| Chi0n | PEOE_VSA11 | |
| VSA_EState3 | MaxAbsPartialCharge | |
| NumRotatableBonds | FractionCSP3 |
| Compound | Bis4MPyC6 | SH-Stabilwax | ||
|---|---|---|---|---|
| Rt, min | As | Rt, min | As | |
| 4-tert-Butylcyclohexanol | 12.55 | 1.11 | 9.78 | 1.16 |
| 2-Chloro-5-methylphenol | 14.64 | 0.96 | 12.27 | 1.10 |
| 2-Methoxy-5-methylphenol | 16.92 | 1.06 | 12.36 | 1.03 |
| 2,3,6-Trichlorophenol | 18.60 | 0.92 | 17.69 | 1.30 |
| (2,6-dichlorophenyl)methanol | 19.84 | 1.07 | 16.32 | 1.00 |
| 2-(Trifluoromethylphenyl)methanol | 14.84 | 1.04 | 11.45 | 0.83 |
| 4-(Trifluoromethoxyphenyl)methanol | 15.32 | 1.06 | 11.74 | 0.93 |
| 2-(Pentafluorophenoxy)ethanol | 16.49 | 1.06 | 11.49 | 1.01 |
| (2,4,5-Trifluorophenyl)methanol | 15.02 | 1.06 | 12.34 | 0.96 |
| (4-Fluorophenyl)methanol | 16.11 | 0.96 | 12.28 | 0.83 |
| 2,3-Dichlorophenol | 16.69 | 1.00 | 15.38 | 1.20 |
| 3,5-Dichloro-2-hydroxybenzaldehyde | 18.32 | 1.04 | 15.90 | 1.67 |
| 2,6-Dimethoxyphenol | 22.64 | 1.13 | 16.20 | 0.98 |
| 2,4-Dichloro-1-naphthol | 23.41 | 0.98 | 21.79 | 1.01 |
| 6-Methylpyridine-2-ol | 27.34 | 4.00 | 17.69 | 0.84 |
| Compounds | Rs | ||
|---|---|---|---|
| Bis4MPyC6 | PEG | ||
| 2-Chloro-5-methylphenol | 2-Methoxy-5-methylphenol | 3.84 | 0.35 |
| 2-(Trifluoromethylphenyl)methanol | 2-(Pentafluorophenoxy)ethanol | 5.07 | 0.25 |
| (2,4,5-Trifluorophenyl)methanol | (4-Fluorophenyl)methanol | 5.28 | 0.50 |
| 3,5-Dichloro-2-hydroxybenzaldehyde | 2,6-Dimethoxyphenol | 1.70 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sholokhova, A.Y.; Borovikova, S.A. In-Column Dehydration Benzyl Alcohols and Their Chromatographic Behavior on Pyridinium-Based Ionic Liquids as Gas Stationary Phases. Molecules 2024, 29, 3721. https://doi.org/10.3390/molecules29163721
Sholokhova AY, Borovikova SA. In-Column Dehydration Benzyl Alcohols and Their Chromatographic Behavior on Pyridinium-Based Ionic Liquids as Gas Stationary Phases. Molecules. 2024; 29(16):3721. https://doi.org/10.3390/molecules29163721
Chicago/Turabian StyleSholokhova, Anastasia Yu., and Svetlana A. Borovikova. 2024. "In-Column Dehydration Benzyl Alcohols and Their Chromatographic Behavior on Pyridinium-Based Ionic Liquids as Gas Stationary Phases" Molecules 29, no. 16: 3721. https://doi.org/10.3390/molecules29163721
APA StyleSholokhova, A. Y., & Borovikova, S. A. (2024). In-Column Dehydration Benzyl Alcohols and Their Chromatographic Behavior on Pyridinium-Based Ionic Liquids as Gas Stationary Phases. Molecules, 29(16), 3721. https://doi.org/10.3390/molecules29163721






