Nitrogen-Tungsten Oxide Nanostructures on Nickel Foam as High Efficient Electrocatalysts for Benzyl Alcohol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
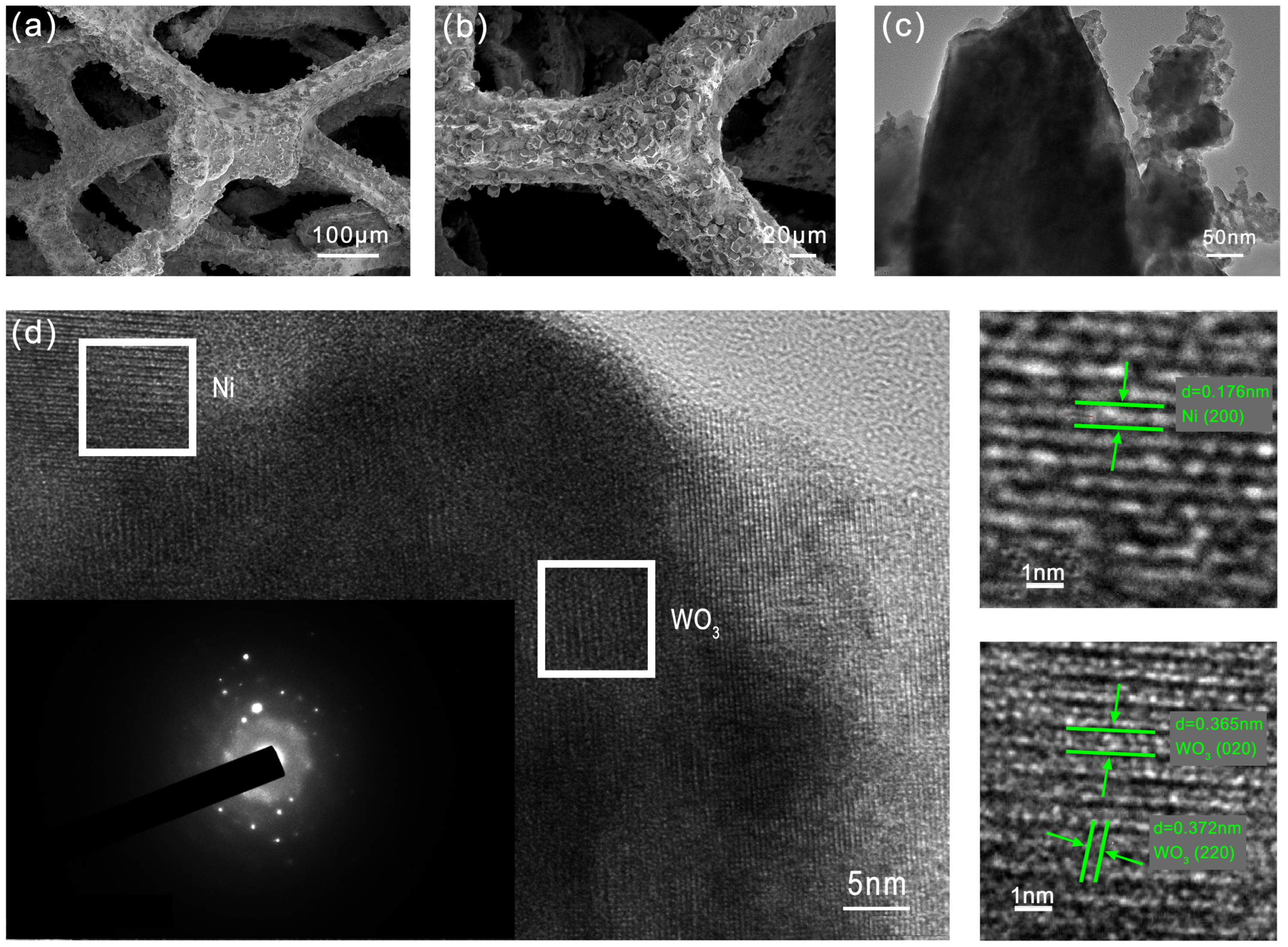
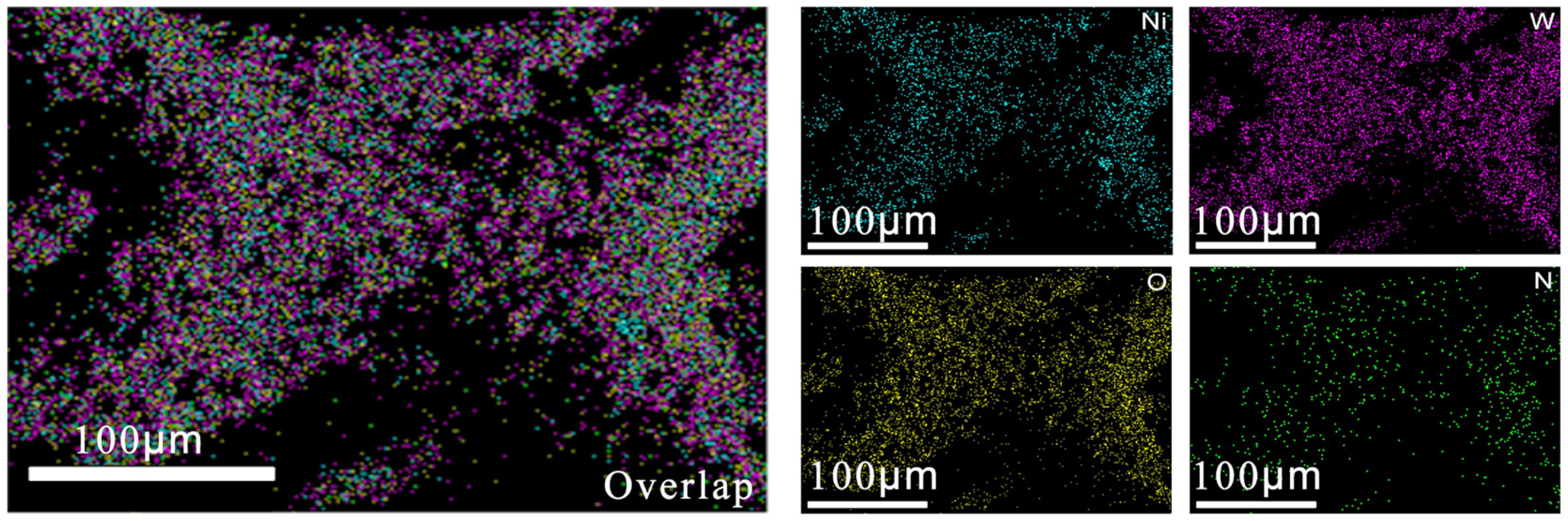
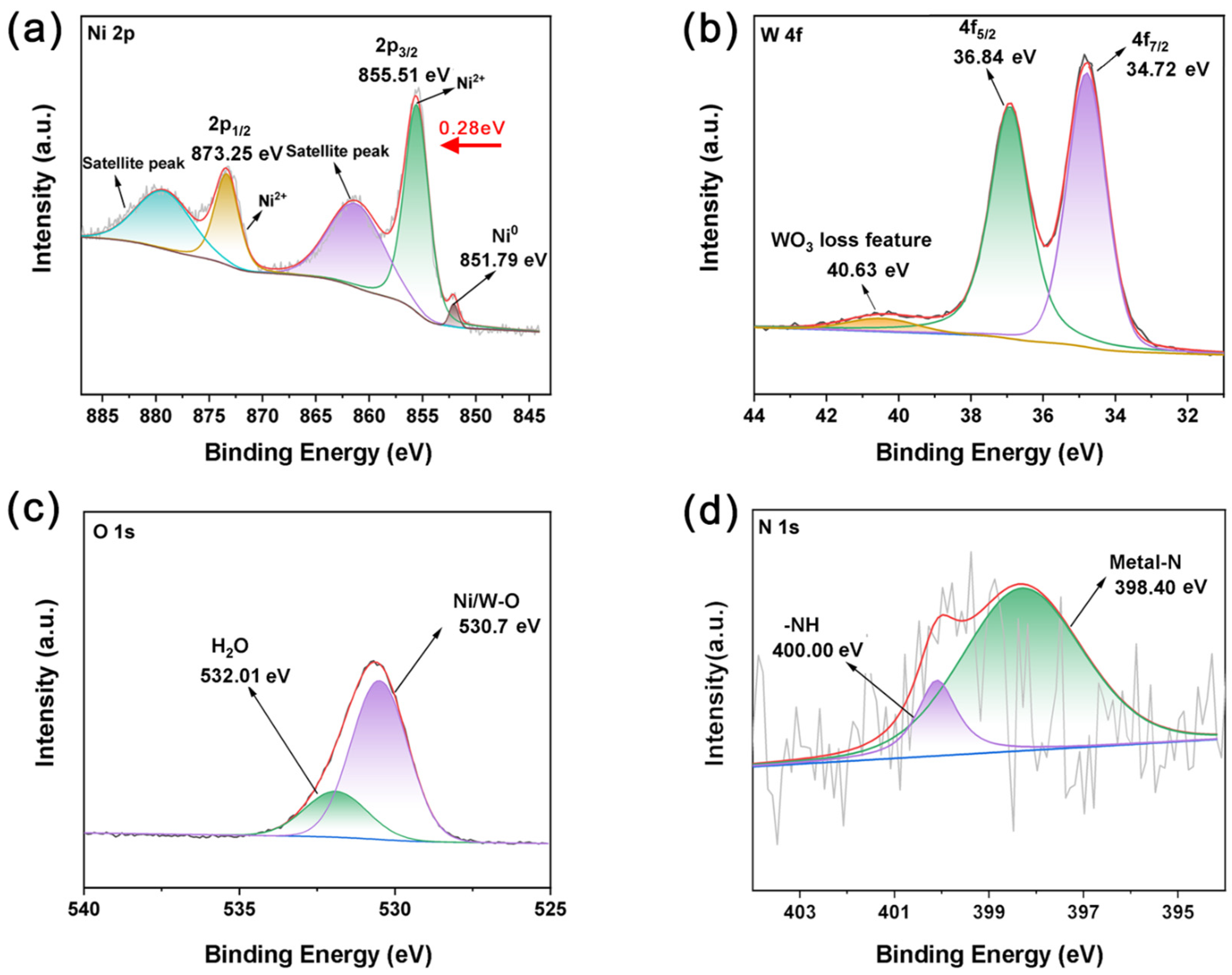
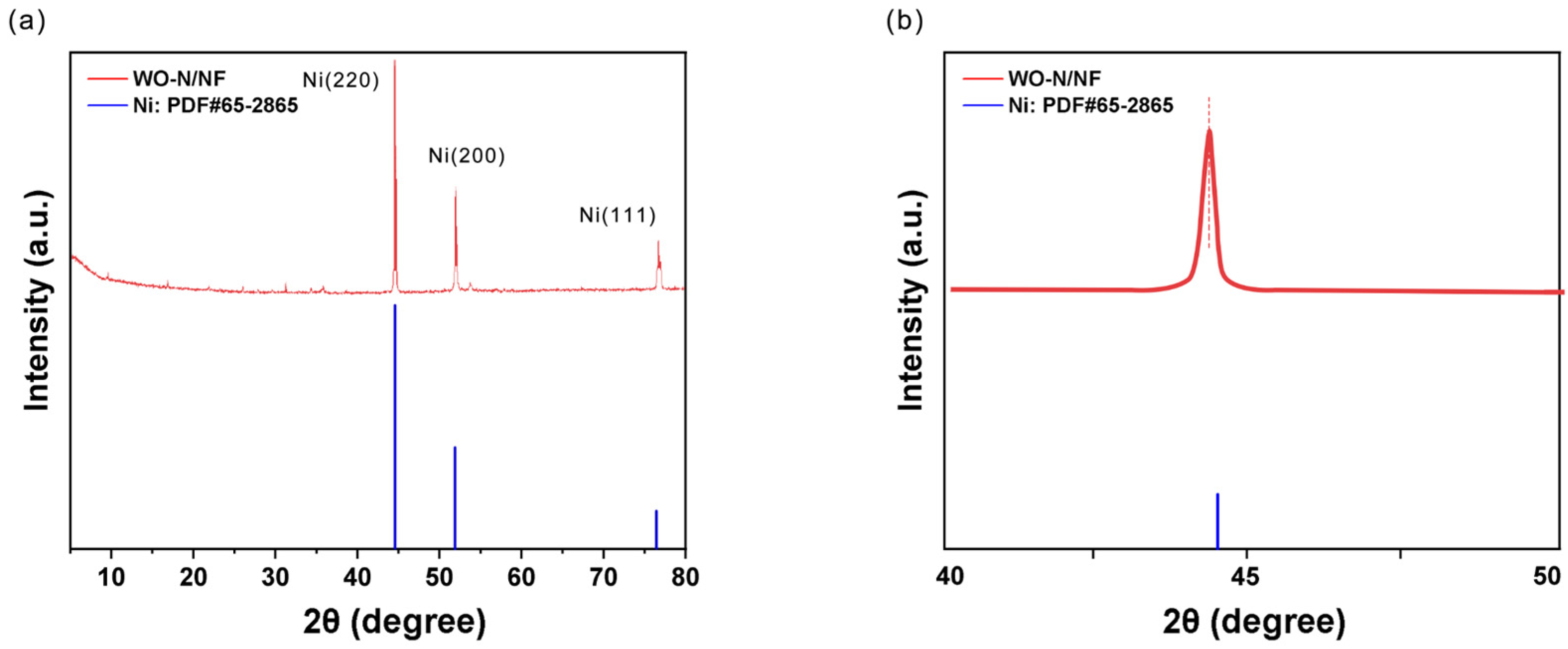
2.1. Characterization of WO-N/NF
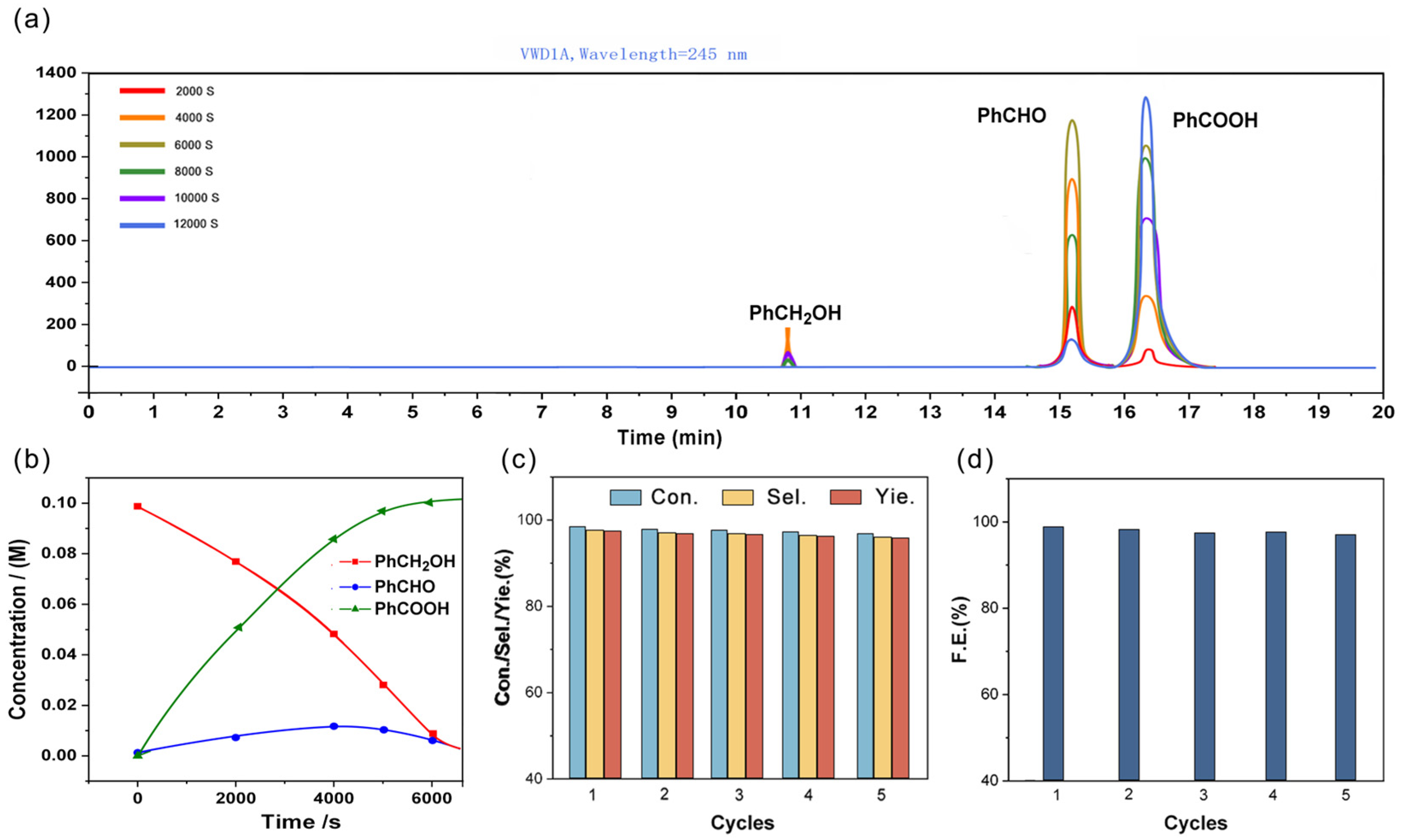
2.2. Electrocatalytic Performance
3. Experimental Section
3.1. Chemicals
3.2. Characterization
3.3. Synthesis of WO-N/NF
3.4. Electrochemical Measurement
3.5. Benzyl Alcohol Electrocatalytic Oxidation Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries Hydroxymethylfurfural, J.G. A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Hauer, A. Energy Storage Solutions for Future Energy Systems. In Advances in Energy Storage; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 743114. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; An, Z.; Kong, Y.; Huang, L.; Duan, D.; Long, R.; Yang, P.; Jiang, Y.-Y.; Liu, J.; et al. Nitrogen-doped carbon for selective pseudo-metal-free hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran: Importance of trace iron impurity. J. Catal. 2023, 417, 396–407. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, L. Electrocatalytic Hydrogenation and Oxidation in Aqueous Conditions. Chin. J. Chem. 2020, 38, 996–1004. [Google Scholar] [CrossRef]
- Li, C.-Q.; Yi, S.-S.; Liu, Y.; Niu, Z.-L.; Yue, X.-Z.; Liu, Z.-Y. In-situ constructing S-scheme/Schottky junction and oxygen vacancy on SrTiO3 to steer charge transfer for boosted photocatalytic H2 evolution. Chem. Eng. J. 2021, 417, 129231. [Google Scholar] [CrossRef]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Tan, C.; Geng, S.; Tan, Z.; Wang, G.; Pu, L.; Guo, X. Integrated energy system–Hydrogen natural gas hybrid energy storage system optimization model based on cooperative game under carbon neutrality. J. Energy Storage 2021, 38, 102539. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Q.; Wu, J.; Sui, Y.; Tang, P.; Liu, H.; Zhang, W.; Li, H.; Wang, Y.; Cabot, A.; et al. Strain and Shell Thickness Engineering in Pd3Pb@Pt Bifunctional Electrocatalyst for Ethanol Upgrading Coupled with Hydrogen Production. Small 2023, 20, 2306178. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Lavacchi, A.; Vizza, F. Storage of renewable energy in fuels and chemicals through electrochemical reforming of bioalcohols. Curr. Opin. Electrochem. 2020, 21, 140–145. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Z.; Tan, Q.; Zhang, Y. Analysis of electric energy substitution potential and research on calculation method of carbon reduction under “carbon neutrality” background. IOP Conf. Ser. Earth Environ. Sci. 2022, 1087, 012026. [Google Scholar] [CrossRef]
- Ming, L.; Wu, X.-Y.; Wang, S.-S.; Wu, W.; Lu, C.-Z. Facile growth of transition metal hydroxide nanosheets on porous nickel foam for efficient electrooxidation of benzyl alcohol. Green Chem. 2021, 23, 7825–7830. [Google Scholar] [CrossRef]
- Llorente, M.J.; Nguyen, B.H.; Kubiak, C.P.; Moeller, K.D. Paired Electrolysis in the Simultaneous Production of Synthetic Intermediates and Substrates. J. Am. Chem. Soc. 2016, 138, 15110–15113. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Geng, J.; Xu, H.; Zhang, S.; Zhang, H. In situ construction of NiCo2O4 nanosheets on nickel foam for efficient electrocatalytic oxidation of benzyl alcohol. Inorg. Chem. Front. 2023, 10, 2053–2059. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Ahamad, T.; Arunachalam, P.; Ahmad, T.; Khan, A. Bifunctional electro-catalytic performances of CoWO4 nanocubes for water redox reactions (OER/ORR). RSC Adv. 2017, 7, 45615–45623. [Google Scholar] [CrossRef]
- Su, C.Y.; Lin, C.K.; Yang, T.K.; Lin, H.-C.; Pan, C.T. Oxygen partial pressure effect on the preparation of nanocrystalline tungsten oxide powders by a plasma arc gas condensation technique. Int. J. Refract. Met. Hard Mater. 2008, 26, 423–428. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Xiong, S.; Wu, X.; Shan, Y.; Chu, P.K. Synergistic WO3·2H2O Nanoplates/WS2 Hybrid Catalysts for High-Efficiency Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 13966–13972. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Si, R.; Michorczyk, P.; Martínez-Arias, A.; Xu, W.; Hanson, J.C.; Rodriguez, J.A.; Fernández-García, M. Tungsten as an interface agent leading to highly active and stable copper–ceria water gas shift catalyst. Appl. Catal. B Environ. 2013, 132-133, 423–432. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Liu, X.; Fu, H.-G.; Yu, H.-T. CoWO4 nanopaticles wrapped by RGO as high capacity anode material for lithium ion batteries. Rare Met. 2017, 36, 411–417. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Liu, Y.; Zheng, Z.; Liu, B.; Chen, M.; Guan, G.; Yan, K. Recent Advances on Transition-Metal-Based Layered Double Hydroxides Nanosheets for Electrocatalytic Energy Conversion. Adv. Sci. 2023, 10, 2207519. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Wu, L.; Qin, H.; Ji, Z.; Zhou, H.; Shen, X.; Zhu, G.; Yuan, A. Nitrogen-Doped Carbon Dots Modified Fe–Co Sulfide Nanosheets as High-Efficiency Electrocatalysts toward Oxygen Evolution Reaction. Small 2023, 20, 2305965. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Meibom, J.L.; Hassan, N.U.; Chang, M.; Chu, Y.-C.; Krammer, A.; Sun, S.; Zheng, Y.; Bai, L.; Ma, W.; et al. Synergistic interactions between PtRu catalyst and nitrogen-doped carbon support boost hydrogen oxidation. Nat. Catal. 2023, 6, 773–783. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Huang, F.; Qu, L.; Li, J.; Owusu, K.A.; Liu, Z.; Lin, Z.; Xiang, B.; Liu, X.; et al. Copper–Nickel Nitride Nanosheets as Efficient Bifunctional Catalysts for Hydrazine-Assisted Electrolytic Hydrogen Production. Adv. Energy Mater. 2019, 9, 1900390. [Google Scholar] [CrossRef]
- Li, J.-K.; Wang, A.; Dong, X.-Y.; Huang, S.; Meng, Y.; Song, J.-L. Construction of 2D C,N-co-doped ZnO/Co3O4 over Ni(OH)2 mesoporous ultrathin nanosheets on Ni foam as high-performance electrocatalysts for benzyl-alcohol oxidation and accelerating hydrogen evolution. New J. Chem. 2023, 47, 5970–5976. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhou, L.; Duan, W.; Song, Y.; Zhang, F.; Zhen, Y.; Zhang, J.; Bao, W.; Lu, Y.; et al. Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation via coupling electrocatalytic oxidation 5-hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Ding, L.; Chen, Z.; Ong, A.; Lu, W.; Herng, T.S.; Li, X.; Ding, J. NiFe (sulfur)oxyhydroxide porous nanoclusters/Ni foam composite electrode drives a large-current-density oxygen evolution reaction with an ultra-low overpotential. J. Mater. Chem. A 2019, 7, 18816–18822. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Heterointerface engineered NiFe(OH)x/Ni3S2 electrocatalysts to overcome the scaling relationship for ultrahigh-current-density water oxidation. Sci. China Mater. 2023, 66, 634–640. [Google Scholar] [CrossRef]
- Ge, Z.; Fu, B.; Zhao, J.; Li, X.; Ma, B.; Chen, Y. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides. J. Mater. Sci. 2020, 55, 14081–14104. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Liu, X. MoS2/NiS Yolk–Shell Microsphere-Based Electrodes for Overall Water Splitting and Asymmetric Supercapacitor. Small 2019, 15, 1803639. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Geng, H.; Wang, J.; Luo, Y.; Li, B.; Zong, Y.; Yang, J.; Guo, Y.; Zheng, Y.; Wang, X.; et al. Hexagonal-Phase Cobalt Monophosphosulfide for Highly Efficient Overall Water Splitting. ACS Nano 2017, 11, 11031–11040. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, H.; Liang, P.; Liu, K.; Cai, M.; Huang, Z.; Wang, C.-A.; Zhong, M. A new binder-free and conductive-additive-free TiO2/WO3-W integrative anode material produced by laser ablation. J. Power Sources 2018, 378, 362–368. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.; Zhao, H.; Liu, Y.; Li, S.; Wang, L. The rational doping of P and W in multi-stage catalysts to trigger Pt-like electrocatalytic performance. J. Mater. Chem. A 2020, 8, 25165–25172. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Bao, K.; Cao, J.; Feng, J.; Qi, J. W doping dominated NiO/NiS2 interfaced nanosheets for highly efficient overall water splitting. J. Colloid Interface Sci. 2020, 562, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Niu, S.; Liang, X.; Wang, G.; Chen, M. Phosphorus incorporation activates the basal plane of tungsten disulfide for efficient hydrogen evolution catalysis. Nano Res. 2022, 15, 2855–2861. [Google Scholar] [CrossRef]
- Cao, B.; Veith, G.M.; Diaz, R.E.; Liu, J.; Stach, E.A.; Adzic, R.R.; Khalifah, P.G. Cobalt Molybdenum Oxynitrides: Synthesis, Structural Characterization, and Catalytic Activity for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 10753–10757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Han, D.; Song, X.; Shi, L.; Song, Y.; Niu, S.; Xie, Y.; Cai, J.; Wu, S.; et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Ji, S.; Tang, Y.; Chen, W.; Li, A.; Zhao, J.; Xiong, Y.; Wu, Y.; Gong, Y.; et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host–guest strategy. Nat. Chem. 2020, 12, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.; Liu, Y.; Zheng, J.; Li, X. A highly efficient and stable biphasic nanocrystalline Ni–Mo–N catalyst for hydrogen evolution in both acidic and alkaline electrolytes. Nano Energy 2016, 22, 111–119. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Ke, G.; Liu, B.; Dong, F.; Yang, L.; He, H.; Zhou, Y. WO3 homojunction photoanode: Integrating the advantages of WO3 different facets for efficient water oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, X.; Hu, H.; Pan, Y.; Zhao, W.; Liu, J.; Zhao, X.; Wang, J.; Yang, Z.; Zhao, Q.; et al. Robust NiCoP/CoP Heterostructures for Highly Efficient Hydrogen Evolution Electrocatalysis in Alkaline Solution. ACS Appl. Mater. Interfaces 2019, 11, 15528–15536. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Sun, Y.; Zhang, J.; Ai, F.; Xi, S.; Wang, J. High-entropy alloy nanocrystal assembled by nanosheets with d–d electron interaction for hydrogen evolution reaction. Energy Environ. Sci. 2023, 16, 4009–4019. [Google Scholar] [CrossRef]
- Zhao, Y.; Björk, E.M.; Yan, Y.; Schaaf, P.; Wang, D. Recent progress in transition metal based catalysts and mechanism analysis for alcohol electrooxidation reactions. Green Chem. 2024, 26, 4987–5003. [Google Scholar] [CrossRef]
- He, G.; Han, X.; Moss, B.; Weng, Z.; Gadipelli, S.; Lai, F.; Kafizas, A.G.; Brett, D.J.L.; Guo, Z.X.; Wang, H.; et al. Solid solution nitride/carbon nanotube hybrids enhance electrocatalysis of oxygen in zinc-air batteries. Energy Storage Mater. 2018, 15, 380–387. [Google Scholar] [CrossRef]
- Wang, H.; Carter, E.A. Metal-metal bonding in Engel-Brewer intermetallics: “anomalous” charge transfer in zirconium-platinum (ZrPt3). J. Am. Chem. Soc. 1993, 115, 2357–2362. [Google Scholar] [CrossRef]
- Wang, Z.; Goddard, W.A.; Xiao, H. Potential-dependent transition of reaction mechanisms for oxygen evolution on layered double hydroxides. Nat. Commun. 2023, 14, 4228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, X.-J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Guo, T.; Li, L.; Wang, Z. Recent Development and Future Perspectives of Amorphous Transition Metal-Based Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2022, 12, 2200827. [Google Scholar] [CrossRef]
- Chai, K.; Yang, X.; Shen, R.; Chen, J.; Su, W.; Su, A. A high activity mesoporous Pt@KIT-6 nanocomposite for selective hydrogenation of halogenated nitroarenes in a continuous-flow microreactor. Nanoscale Adv. 2023, 5, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhang, M.; Liu, B.; Yang, Z.; Li, R.; Yan, K. Efficient electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using the facilely synthesized 3D porous WO3/Ni electrode. Mol. Catal. 2021, 504, 111459. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Zhong, X.; Li, S.; Liu, T.; Zhuang, G.; Li, X.; Deng, S.; Mei, D.; Wang, J.-G. Hierarchical Porous NC@CuCo Nitride Nanosheet Networks: Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting and Selective Electrooxidation of Benzyl Alcohol. Adv. Funct. Mater. 2017, 27, 1704169. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, D.; Kong, X.; Zhang, F.; Lei, X. Multi-vacancy Co3O4 on nickel foam synthesized via a one-step hydrothermal method for high-efficiency electrocatalytic benzyl alcohol oxidation. J. Mater. Sci. 2021, 56, 6689–6703. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Han, X.; Huang, H.; Wei, Q.; Guo, W.; Wang, Z.; Qiu, J. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm−2. Energy Environ. Sci. 2020, 13, 4990–4999. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Y.; Wang, H.; Li, J.; Zhu, Q.; Wang, Y.; Ma, N.; Hu, G.; He, B.; Knop-Gericke, A.; et al. Engineering Interface with One-Dimensional Co3O4 Nanostructure in Catalytic Membrane Electrode: Toward an Advanced Electrocatalyst for Alcohol Oxidation. ACS Nano 2017, 11, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, X.; Yuan, B.; Li, S.; Gu, Y.; Zhang, Q.; Zhuang, G.; Li, X.; Deng, S.; Wang, J.-G. Defect engineering of nickel hydroxide nanosheets by Ostwald ripening for enhanced selective electrocatalytic alcohol oxidation. Green Chem. 2019, 21, 578–588. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Liu, X.; Sun, Y. Efficient H2 Evolution Coupled with Oxidative Refining of Alcohols via A Hierarchically Porous Nickel Bifunctional Electrocatalyst. ACS Catal. 2017, 7, 4564–4570. [Google Scholar] [CrossRef]
- Cui, X.; Chen, M.; Xiong, R.; Sun, J.; Liu, X.; Geng, B. Ultrastable and efficient H2 production via membrane-free hybrid water electrolysis over a bifunctional catalyst of hierarchical Mo–Ni alloy nanoparticles. J. Mater. Chem. A 2019, 7, 16501–16507. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, R.; Anandhababu, G.; Xie, J.; Lv, J.; Zhao, X.; Wang, X.; Wu, M.; Li, Q.; Wang, Y. Cobalt/Iron(Oxides) Heterostructures for Efficient Oxygen Evolution and Benzyl Alcohol Oxidation Reactions. ACS Energy Lett. 2018, 3, 1854–1860. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, M.; Su, W.; Guo, D.; Chen, X.; Sun, G.; Zhang, W. Ultrathin Two-Dimensional Bimetal–Organic Framework Nanosheets as High-Performance Electrocatalysts for Benzyl Alcohol Oxidation. Inorg. Chem. 2022, 61, 7308–7317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ci, S.; Peng, X.; Huang, J.; Cai, P.; Ding, Y.; Wen, Z. Tri-profit electrolysis for energy-efficient production of benzoic acid and H2. J. Energy Chem. 2021, 54, 30–35. [Google Scholar] [CrossRef]
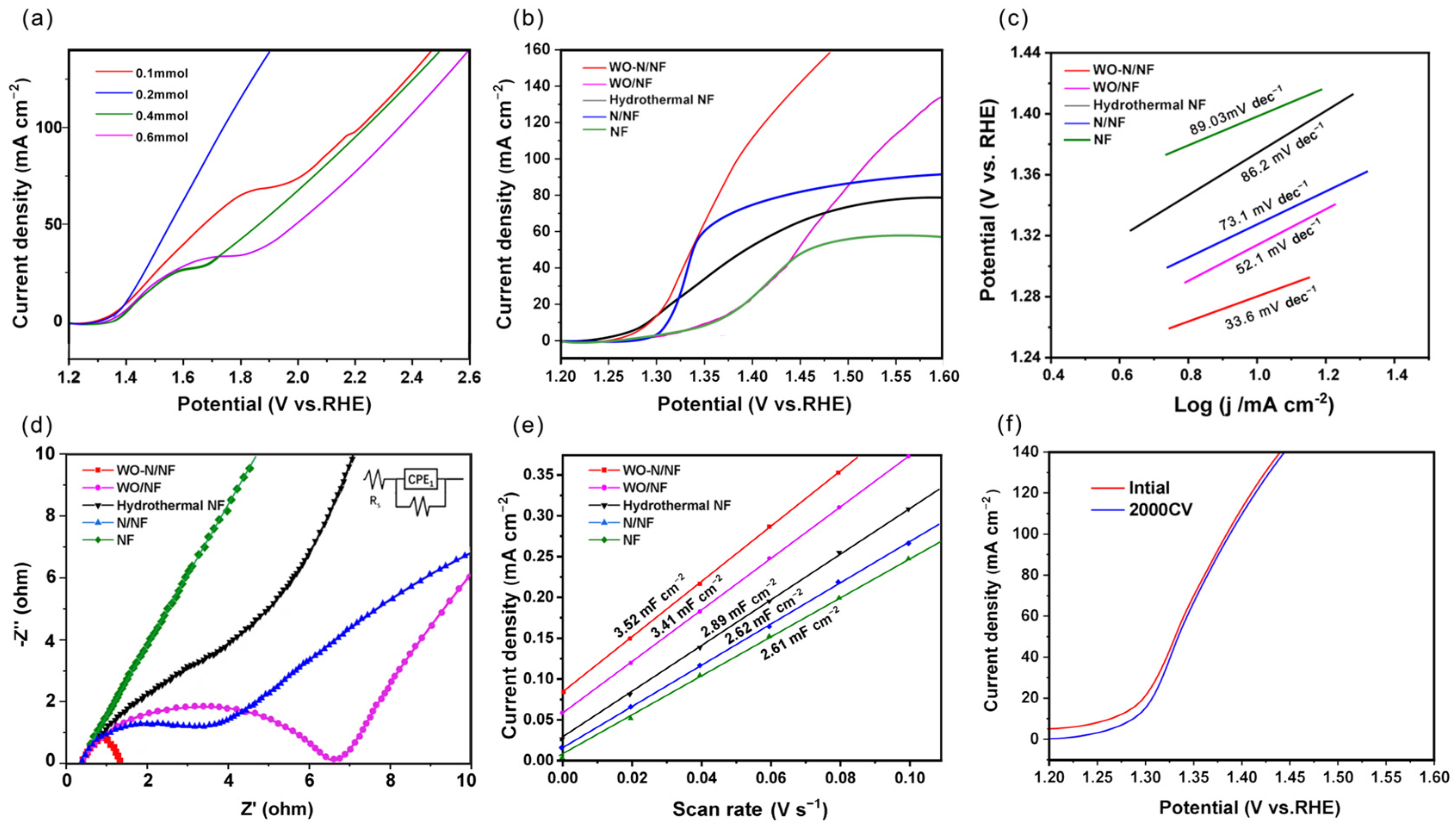
| Electrode | Rs/(Ω) | Rct/(Ω) |
|---|---|---|
| NF | 0.30 | 590.02 |
| WO-N/NF | 0.32 | 1.36 |
| WO/NF | 0.32 | 6.84 |
| N/NF | 0.33 | 88.52 |
| Hydrothermal NF | 0.33 | 97.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, X.; Zhang, Y.; Zhu, Z.; Chen, H.; Chai, K.; Xu, W. Nitrogen-Tungsten Oxide Nanostructures on Nickel Foam as High Efficient Electrocatalysts for Benzyl Alcohol Oxidation. Molecules 2024, 29, 3734. https://doi.org/10.3390/molecules29163734
Zhu Y, Chen X, Zhang Y, Zhu Z, Chen H, Chai K, Xu W. Nitrogen-Tungsten Oxide Nanostructures on Nickel Foam as High Efficient Electrocatalysts for Benzyl Alcohol Oxidation. Molecules. 2024; 29(16):3734. https://doi.org/10.3390/molecules29163734
Chicago/Turabian StyleZhu, Yizhen, Xiangyu Chen, Yuanyao Zhang, Zhifei Zhu, Handan Chen, Kejie Chai, and Weiming Xu. 2024. "Nitrogen-Tungsten Oxide Nanostructures on Nickel Foam as High Efficient Electrocatalysts for Benzyl Alcohol Oxidation" Molecules 29, no. 16: 3734. https://doi.org/10.3390/molecules29163734





