Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Activity
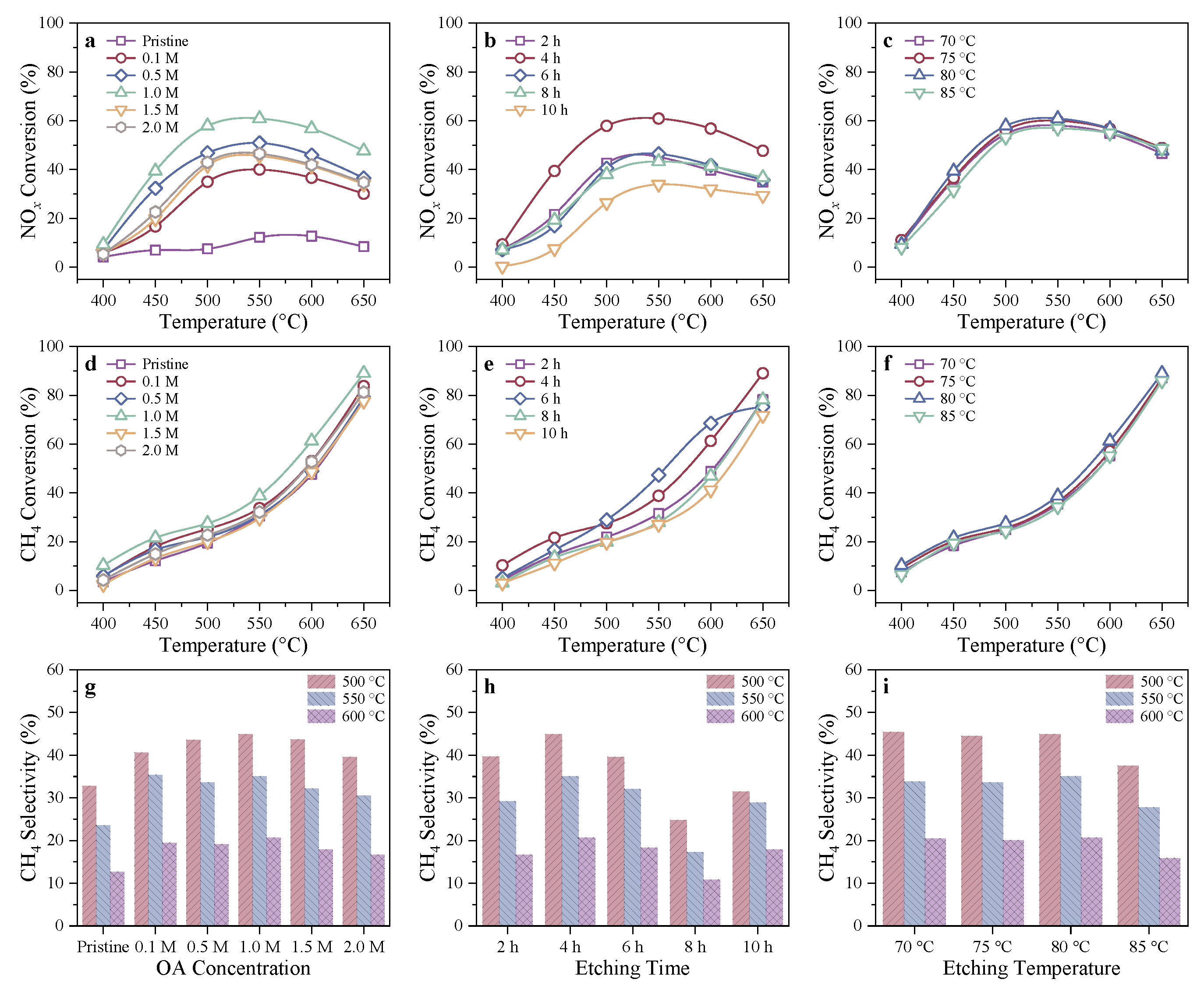
2.1.1. CH4-SCR Activity of In/H-SSZ-39(OA) Catalysts
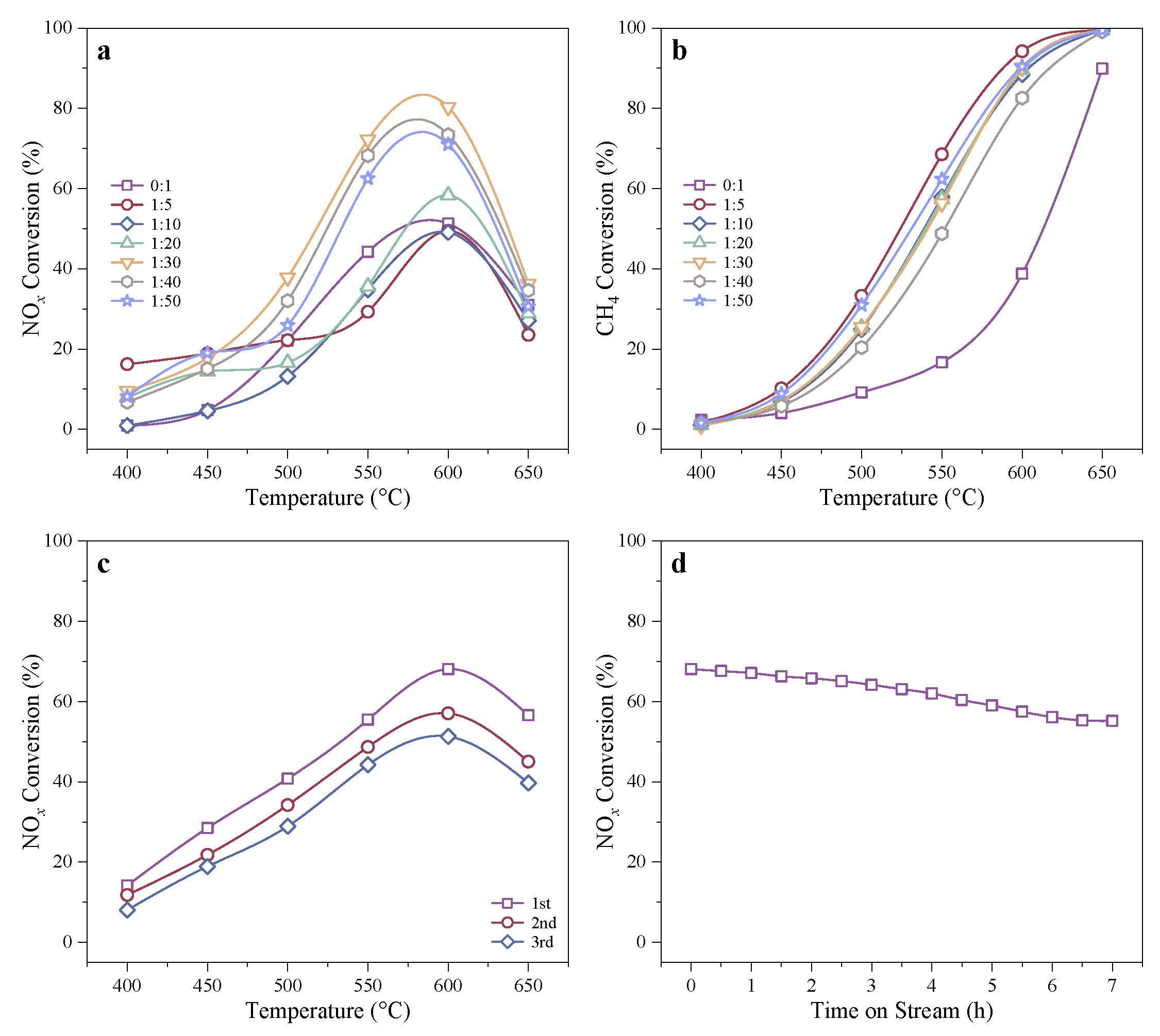
2.1.2. CH4-SCR Activity of In-Co3O4/H-SSZ-39(OA) Catalysts
2.2. Catalyst Characterization
2.2.1. Microscopy
2.2.2. Crystalline Properties
2.2.3. Textural Properties
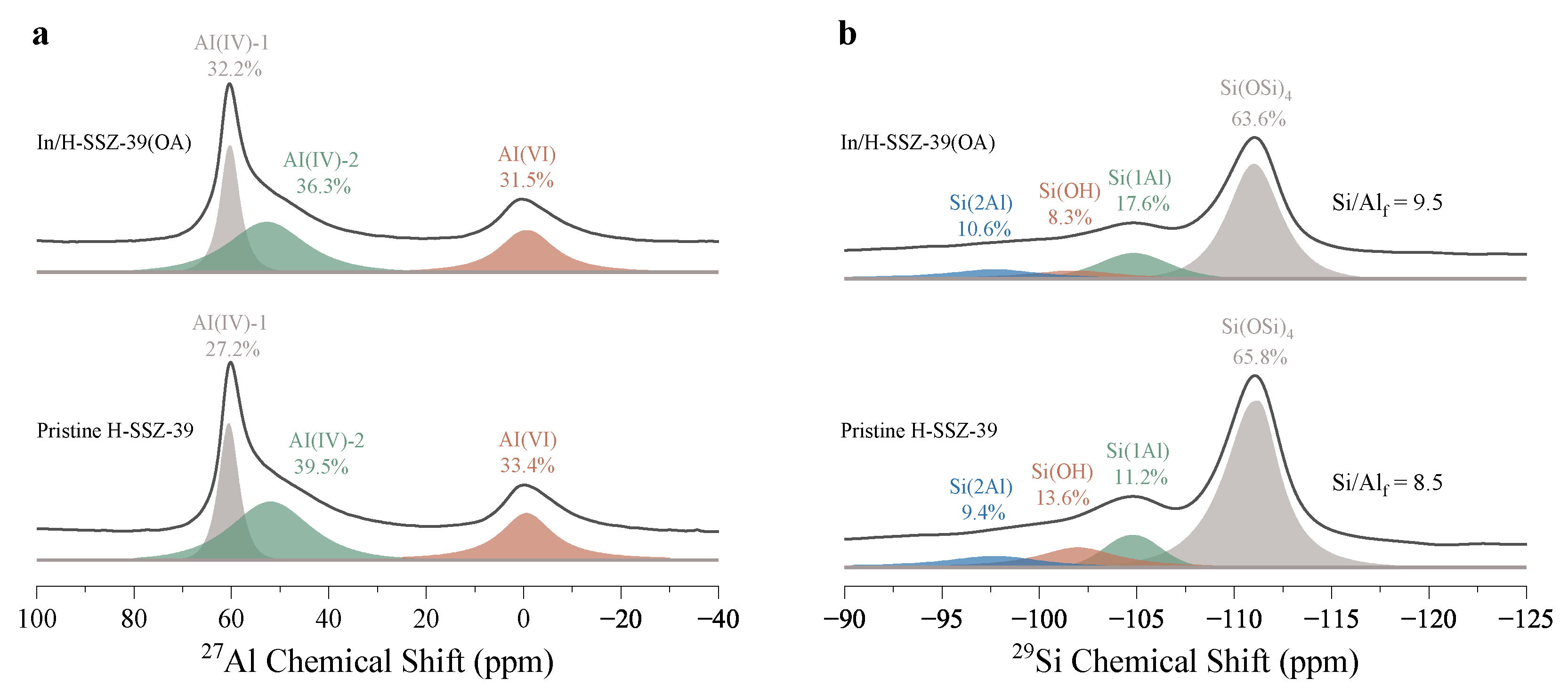
2.2.4. Coordination Environment of T-Atoms
2.2.5. Surface Chemical State
2.2.6. Surface Acidity
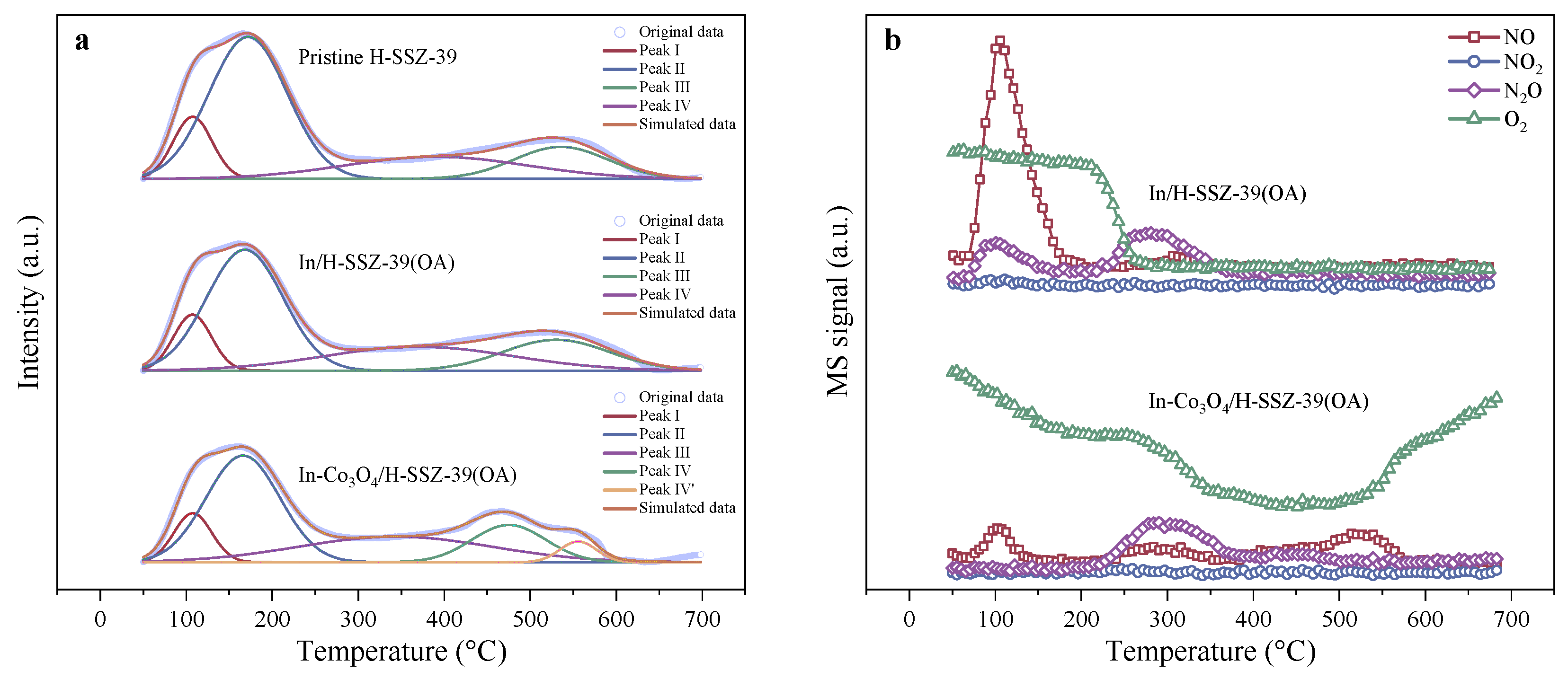
2.2.7. NxOy Intermediates during Reaction
3. Materials and Methods
3.1. Acid Etching
3.2. Indium Exchange
3.3. Catalyst Characterizations
3.4. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Wang, X.; Qin, M.; Li, Q. Selective catalytic reduction of NOx with CH4 over zeolite catalysts: Research progress, challenges and perspectives. J. Environ. Chem. Eng. 2022, 10, 107270. [Google Scholar] [CrossRef]
- Li, Y.; Armor, J. Catalytic reduction of nitrogen oxides with methane in the presence of excess oxygen. Appl. Catal. B 1992, 1, L31–L40. [Google Scholar] [CrossRef]
- Ramallo-López, J.; Requejo, F.; Gutierrez, L.; Miró, E. EXAFS, TDPAC and TPR characterization of PtInFerrierite: The role of surface species in the SCR of NOx with CH4. Appl. Catal. B 2001, 29, 35–46. [Google Scholar] [CrossRef]
- Bustamante, F.; Córdoba, F.; Yates, M.; Montes de Correa, C. The promotion of cobalt mordenite by palladium for the lean CH4-SCR of NOx in moist streams. Appl. Catal. A 2002, 234, 127–136. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Beltramone, A.R.; Requejo, F.G. In-containing BEA zeolite for selective catalytic reduction of NOx: Part I: Synthesis, characterization and catalytic activity. J. Mol. Catal. A Chem. 2007, 267, 194–201. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Ru-In/H-SSZ-13 for the selective reduction of nitric oxide by methane: Insights from temperature-programmed desorption studies. Appl. Catal. B 2018, 236, 404–412. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Bimetallic Cr-In/H-SSZ-13 for selective catalytic reduction of nitric oxide by methane. Chin. J. Catal. 2018, 39, 1004–1011. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, L.; Wang, Y.; Zhang, J.; Zhu, R.; Li, C.; Hong, M. Amino-acid modulated hierarchical In/H-Beta zeolites for selective catalytic reduction of NO with CH4 in the presence of H2O and SO2. Nanoscale 2022, 14, 5915–5928. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, G.; Li, Y.; Liu, Y.; Song, C. Selective catalytic reduction of NOX with CH4 over In/SSZ-13 zeolites: The enhancement of high-temperature catalytic activity by Ce modification. J. Environ. Chem. Eng. 2024, 12, 111830. [Google Scholar] [CrossRef]
- Wang, X.; Ge, W.; Liu, Y.; Miao, B.; Qin, M.; Ji, C.; Li, Q. Boosting the activity of In-H-SSZ-13 via tuning acid sites for synergistic abatement of NOx and CH4. Fuel 2024, 359, 130466. [Google Scholar] [CrossRef]
- Jabłońska, M. Review of the application of Cu-containing SSZ-13 in NH3-SCR-DeNOx and NH3-SCO. RSC Adv. 2022, 12, 25240–25261. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B 2020, 264, 118511. [Google Scholar] [CrossRef]
- Gui, R.; Yan, Q.; Xue, T.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. The promoting/inhibiting effect of water vapor on the selective catalytic reduction of NOx. J. Hazard. Mater. 2022, 439, 129665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, G.; He, J.; Wen, Z.; Li, Z.; Gu, T.; Ding, R.; Zhu, Y.; Zhu, R. Effect of preparation and reaction conditions on the performance of In/H-Beta for selective catalytic reduction of NOx with CH4. Chemosphere 2020, 252, 126458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Zhu, R.; Zhang, J.; Ding, R.; Wen, Z.; Zhu, Y.; Zhang, G.; Chen, B. Mechanism of the selective catalytic reduction of NOx with CH4 on In/H-beta. Catal. Sci. Technol. 2021, 11, 5050–5061. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, Y.; Shan, Y.; Du, J.; Liu, Z.; Gao, M.; Shi, X.; He, G.; Xue, S.; Han, X.; et al. Si/Al Ratio Determines the SCR Performance of Cu-SSZ-13 Catalysts in the Presence of NO2. Environ. Sci. Technol. 2022, 56, 17946–17954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, Y.; Lafon, O.; Trébosc, J.; Duk Kim, K.; Stampfl, C.; Baiker, A.; Amoureux, J.P.; Huang, J. Brønsted acid sites based on penta-coordinated aluminum species. Nat. Commun. 2016, 7, 13820. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Malta, G.; Kubota, H.; Toyao, T.; Maeno, Z.; Shimizu, K.i. Mechanism of NH3–Selective Catalytic Reduction (SCR) of NO/NO2 (Fast SCR) over Cu-CHA Zeolites Studied by In Situ/Operando Infrared Spectroscopy and Density Functional Theory. J. Phys. Chem. C 2021, 125, 21975–21987. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, N.; Chen, Y.; Zhao, J.; Zhu, R.; Hong, M. Recent advances in synthetic strategies and physicochemical modifications of SSZ-13 zeolites: A review. Mater. Today Catal. 2024, 4, 100037. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, Z.; Zhu, R.; Li, Z.; Ding, R.; Zhu, Y.; Gu, T.; Yang, R.; Zhu, Z. In/H-Beta modified by Co3O4 and its superior performance in the presence of H2O and SO2 for selective catalytic reduction of NOx with CH4. Chem. Eng. J. Adv. 2020, 3, 100029. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Ding, R.; Zhu, R.; Li, H.; Li, Z.; Zhang, J.; Zhu, Y.; Li, H. Insights into the superior resistance of In-Co3O4-Ga2O3/H-Beta to SO2 and H2O in the selective catalytic reduction of NOx by CH4. J. Colloid Interface Sci. 2022, 626, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, J.; Top, H.; Vollink, F.; Hoving, K.; van den Brink, R. Selective catalytic reduction of NOx in real exhaust gas of gas engines using unburned gas: Catalyst deactivation and advances toward long-term stability. Chem. Eng. J. 2006, 120, 17–23. [Google Scholar] [CrossRef]
- Li, Z.; Flytzani-Stephanopoulos, M. Effects of water vapor and sulfur dioxide on the performance of Ce–Ag-ZSM-5 for the SCR of NO with CH4. Appl. Catal. B 1999, 22, 35–47. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Wang, Y.; Li, Z.; Nkinahamira, F.; Zhu, R.; Zhang, J.; Sun, S.; Zhu, Y.; Li, H.; et al. The poisoning mechanism of H2O/SO2 to In/H-Beta for selective catalytic reduction of NOx with methane. Appl. Catal. A 2023, 649, 118973. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, G.; Zhang, Y.; Chang, C.; Jiang, M.; Ruan, L.; Xiao, M.; Yan, Z.; Yu, Y.; He, H. Designing a Ce/In-CHA OXZEO catalyst for high-efficient selective catalytic reduction of nitrogen oxide with methane. Appl. Catal. B 2024, 348, 123820. [Google Scholar] [CrossRef]
- Kikuchi, E.; Ogura, M.; Terasaki, I.; Goto, Y. Selective Reduction of Nitric Oxide with Methane on Gallium and Indium Containing H-ZSM-5 Catalysts: Formation of Active Sites by Solid-State Ion Exchange. J. Catal. 1996, 161, 465–470. [Google Scholar] [CrossRef]
- Jing, G.; Li, J.; Yang, D.; Hao, J. Promotional mechanism of tungstation on selective catalytic reduction of NOx by methane over In/WO3/ZrO2. Appl. Catal. B 2009, 91, 123–134. [Google Scholar] [CrossRef]
- Chen, K.; Horstmeier, S.; Nguyen, V.T.; Wang, B.; Crossley, S.P.; Pham, T.; Gan, Z.; Hung, I.; White, J.L. Structure and Catalytic Characterization of a Second Framework Al(IV) Site in Zeolite Catalysts Revealed by NMR at 35.2 T. J. Am. Chem. Soc. 2020, 142, 7514–7523. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gan, Z.; Horstmeier, S.; White, J.L. Distribution of Aluminum Species in Zeolite Catalysts: 27Al NMR of Framework, Partially-Coordinated Framework, and Non-Framework Moieties. J. Am. Chem. Soc. 2021, 143, 6669–6680. [Google Scholar] [CrossRef]
- Fan, B.; Zhu, D.; Wang, L.; Xu, S.; Wei, Y.; Liu, Z. Dynamic evolution of Al species in the hydrothermal dealumination process of CHA zeolites. Inorg. Chem. Front. 2022, 9, 3609–3618. [Google Scholar] [CrossRef]
- Xing, Y.; Li, G.; Lin, Z.; Xu, Z.; Huang, H.; Zhu, Y.; Tsang, S.C.E.; Li, M.M.J. In situ hierarchical pore engineering in small pore zeolite via methanol-mediated NH4F etching. J. Mater. Chem. A 2023, 11, 14058–14066. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, J.; Yang, L.; Wu, X.; Gao, Y.; Ran, R.; Weng, D. Flexible Al Coordination with H2O Explaining the Deviation of Strong Acid Amount from the Framework Al Content in Al-Rich SSZ-13. J. Phys. Chem. C 2023, 127, 16598–16606. [Google Scholar] [CrossRef]
- Schmidt, C.; Sowade, T.; Löffler, E.; Birkner, A.; Grünert, W. Preparation and Structure of In-ZSM-5 Catalysts for the Selective Reduction of NO by Hydrocarbons. J. Phys. Chem. B 2002, 106, 4085–4097. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Moroz, I.B.; Prosvirin, I.P.; Toktarev, A.V.; Wang, W.; Stepanov, A.G. Methane Activation on In-Modified ZSM-5: The State of Indium in the Zeolite and Pathways of Methane Transformation to Surface Species. J. Phys. Chem. C 2014, 118, 8034–8043. [Google Scholar] [CrossRef]
- Maunula, T.; Ahola, J.; Hamada, H. Reaction mechanism and kinetics of NOx reduction by methane on In/ZSM-5 under lean conditions. Appl. Catal. B 2006, 64, 13–24. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F.; Peden, C.H.F.; Li, J.; Kamasamudram, K.; Epling, W.S. Selective Catalytic Reduction of NOx with NH3 over a Cu-SSZ-13 Catalyst Prepared by a Solid-State Ion-Exchange Method. ChemCatChem 2014, 6, 1579–1583. [Google Scholar] [CrossRef]
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Al-Shammari, T.K.; Yokoi, T. OSDA-free chabazite (CHA) zeolite synthesized in the presence of fluoride for selective methanol-to-olefins. Microporous Mesoporous Mater. 2019, 274, 277–285. [Google Scholar] [CrossRef]
- Meng, X.; Lian, Z.; Wang, X.; Shi, L.; Liu, N. Effect of dealumination of HZSM-5 by acid treatment on catalytic properties in non-hydrocracking of diesel. Fuel 2020, 270, 117426. [Google Scholar] [CrossRef]
- Resini, C.; Montanari, T.; Nappi, L.; Bagnasco, G.; Turco, M.; Busca, G.; Bregani, F.; Notaro, M.; Rocchini, G. Selective catalytic reduction of NOx by methane over Co-H-MFI and Co-H-FER zeolite catalysts: Characterisation and catalytic activity. J. Catal. 2003, 214, 179–190. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.; He, C.; Zhang, X.; Hao, Z.; Xu, Z. Cobalt zeolites: Preparation, characterization and catalytic properties for N2O decomposition. Asia-Pac. J. Chem. Eng. 2012, 7, 502–509. [Google Scholar] [CrossRef]
- van Meerten, S.; Franssen, W.; Kentgens, A. ssNake: A cross-platform open-source NMR data processing and fitting application. J. Magn. Reson. 2019, 301, 56–66. [Google Scholar] [CrossRef] [PubMed]
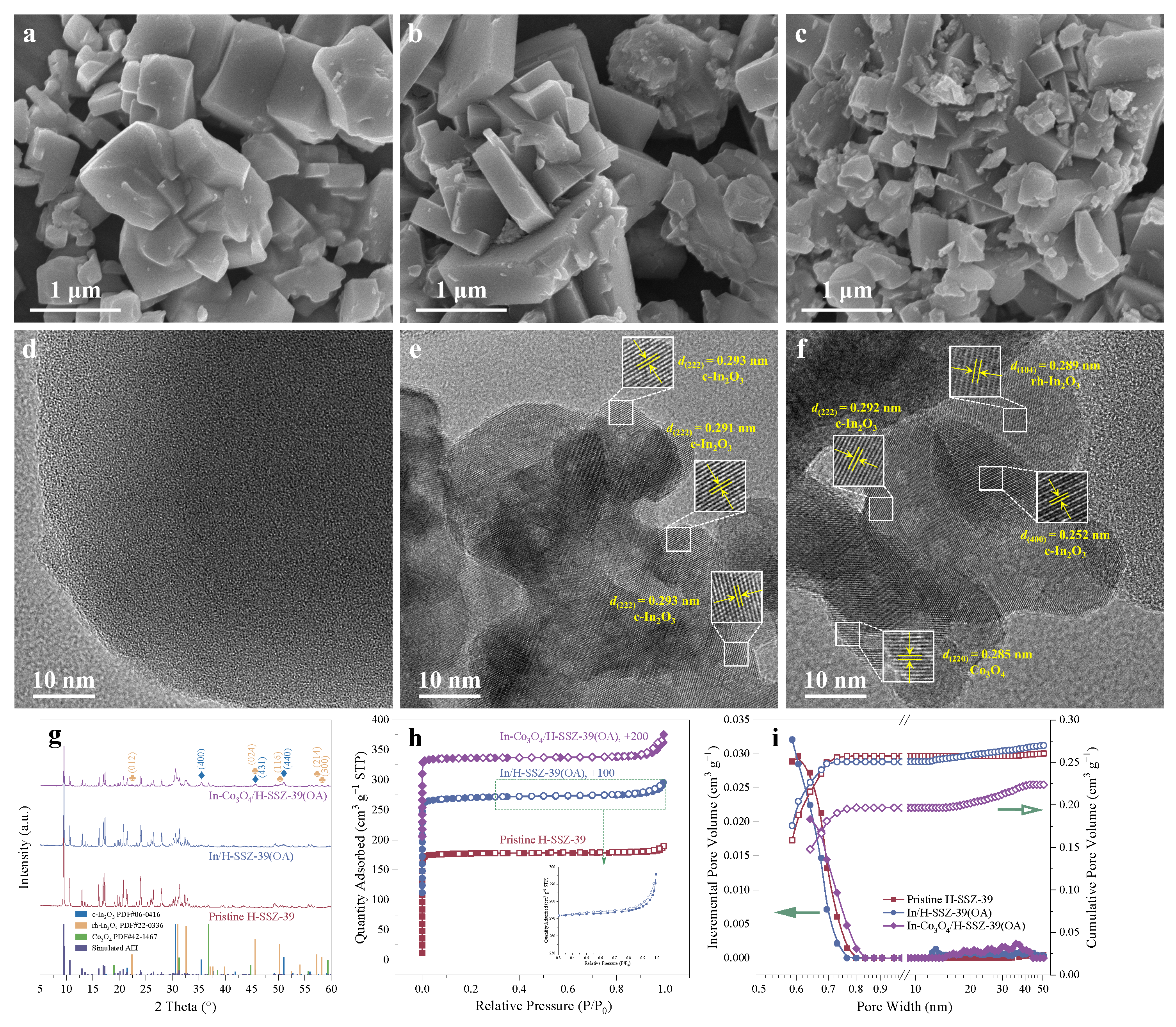

| Sample | Relative Crystallinity a (%) | SBET b (m2 g−1) | Vtot c (cm3 g−1) | Vmicro d (cm3 g−1) | Vmeso e (cm3 g−1) | Si/Al Bulk f | In Content f (wt%) | Co Content f (wt%) |
|---|---|---|---|---|---|---|---|---|
| Pristine H-SSZ-39 | 100.0 | 746.7 | 0.293 | 0.278 | 0.015 | 4.72 | / | / |
| In/H-SSZ-39(OA) | 72.0 | 699.5 | 0.303 | 0.242 | 0.060 | 6.79 | 4.40 | / |
| In-Co3O4/H-SSZ-39(OA) | 33.4 | 566.6 | 0.272 | 0.202 | 0.070 | 6.86 | 7.17 | 1.14 |
| Sample | Peak I | Peak II | Peak III | Peak IV | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Q (mmol g−1) | T (°C) | Q (mmol g−1) | T (°C) | Q (mmol g−1) | T (°C) | Q (mmol g−1) | |
| Pristine H-SSZ-39 | 107.4 | 0.160 | 171.8 | 0.737 | 390.4 | 0.199 | 535.7 | 0.269 |
| In/H-SSZ-39(OA) | 107.2 | 0.141 | 168.1 | 0.615 | 370.3 | 0.301 | 530.1 | 0.219 |
| In-Co3O4/H-SSZ-39(OA) | 107.6 | 0.127 | 165.9 | 0.541 | 343.0 | 0.340 | 475.1 + 555.6 | 0.190 + 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhang, W.; Zhu, R.; Chen, Y.; Zhao, M.; Hong, M. Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification. Molecules 2024, 29, 3747. https://doi.org/10.3390/molecules29163747
Chen G, Zhang W, Zhu R, Chen Y, Zhao M, Hong M. Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification. Molecules. 2024; 29(16):3747. https://doi.org/10.3390/molecules29163747
Chicago/Turabian StyleChen, Guanyu, Weixin Zhang, Rongshu Zhu, Yanpeng Chen, Minghu Zhao, and Mei Hong. 2024. "Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification" Molecules 29, no. 16: 3747. https://doi.org/10.3390/molecules29163747
APA StyleChen, G., Zhang, W., Zhu, R., Chen, Y., Zhao, M., & Hong, M. (2024). Engineering In-Co3O4/H-SSZ-39(OA) Catalyst for CH4-SCR of NOx: Mild Oxalic Acid (OA) Leaching and Co3O4 Modification. Molecules, 29(16), 3747. https://doi.org/10.3390/molecules29163747






