Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil
Abstract
:1. Introduction
2. Results and Discussion
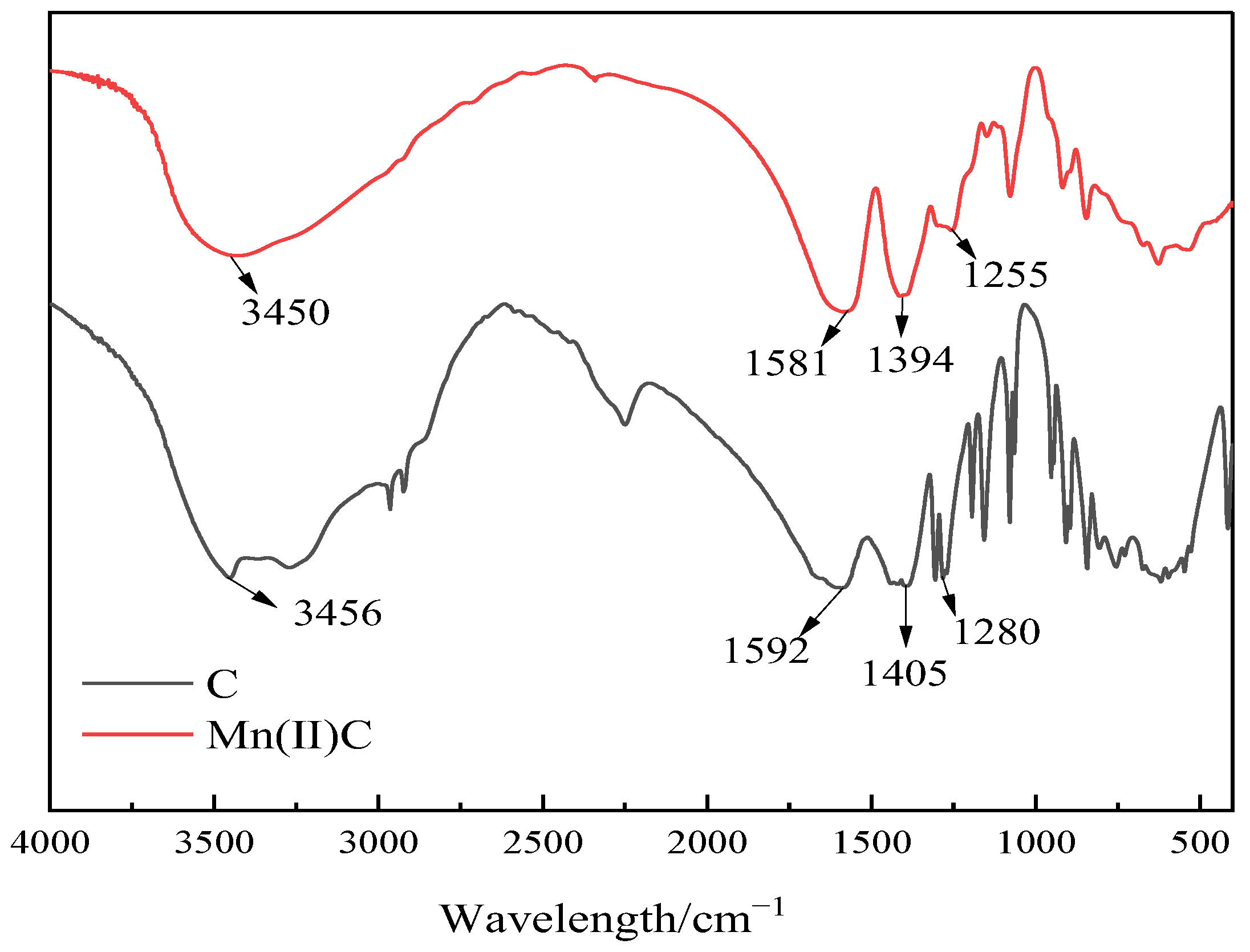
2.1. Infrared Analysis
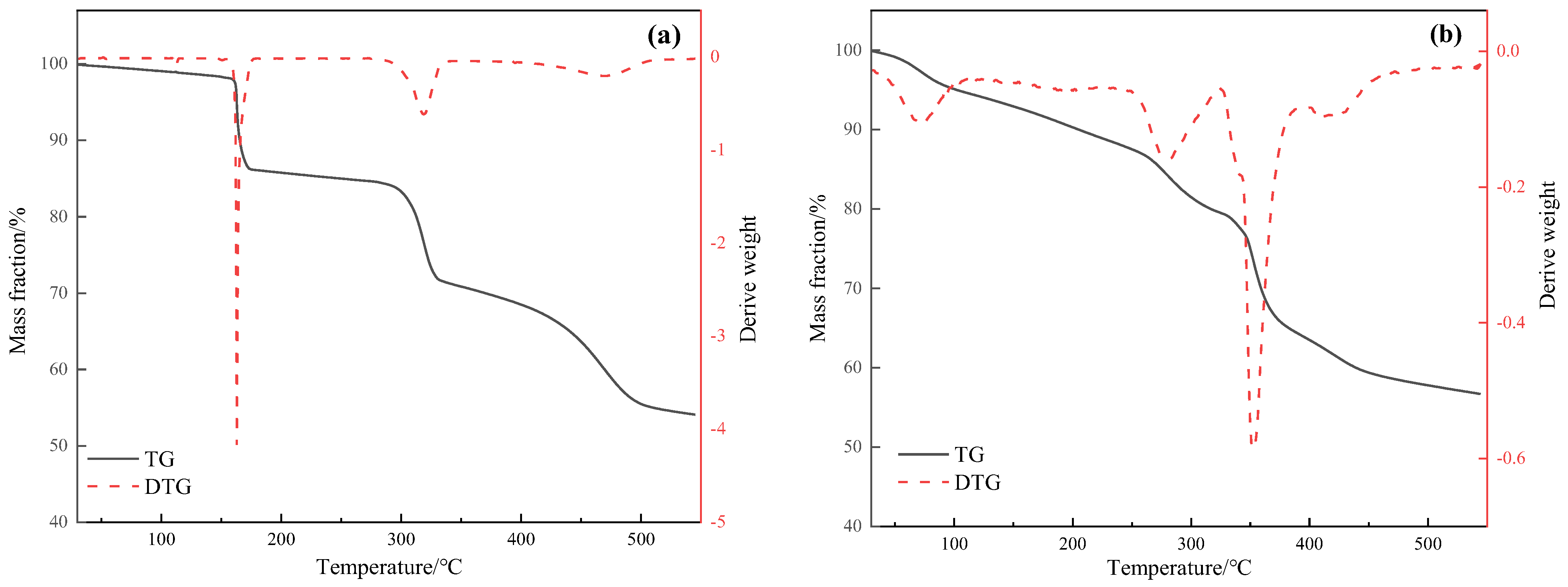
2.2. Thermogravimetric Analysis
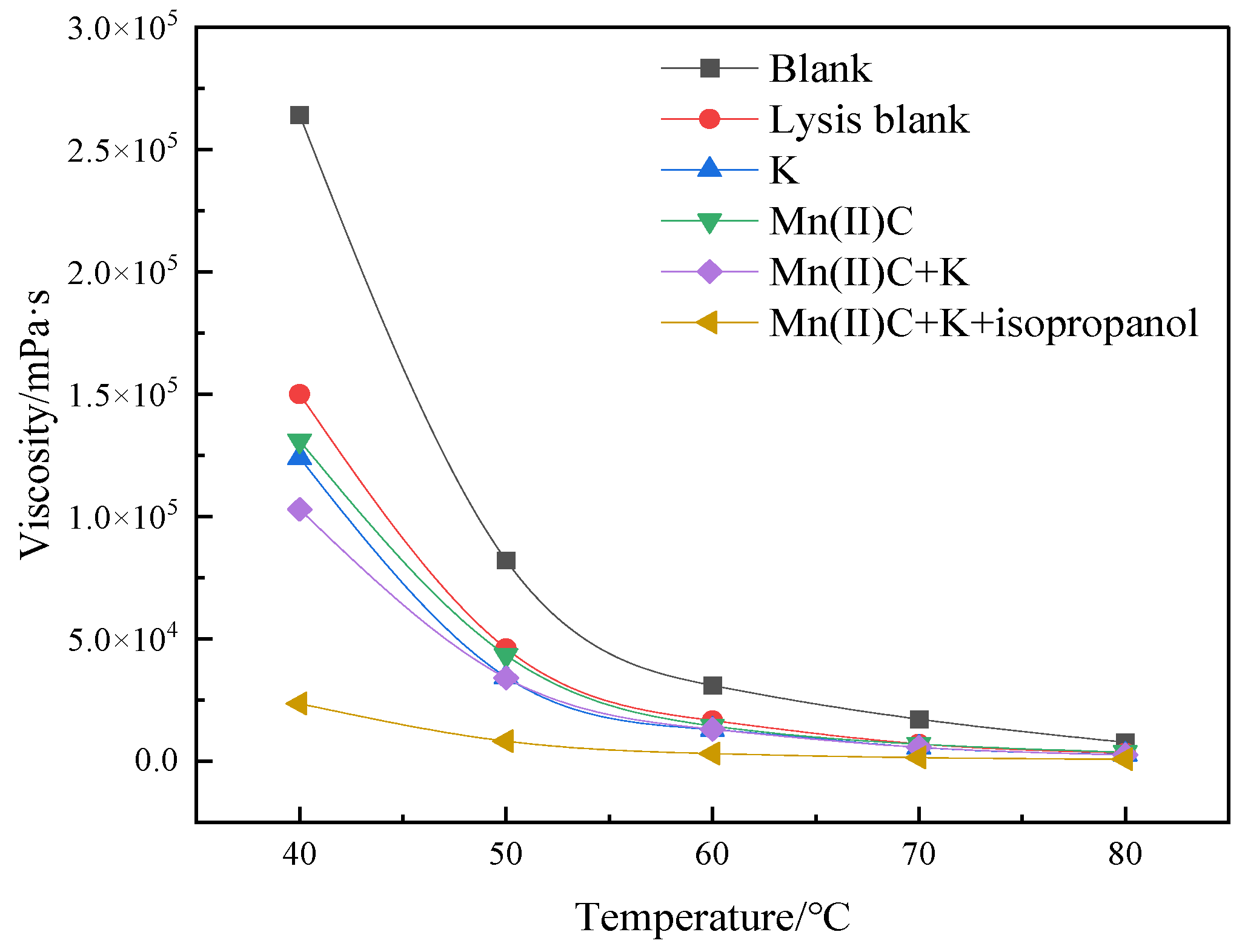
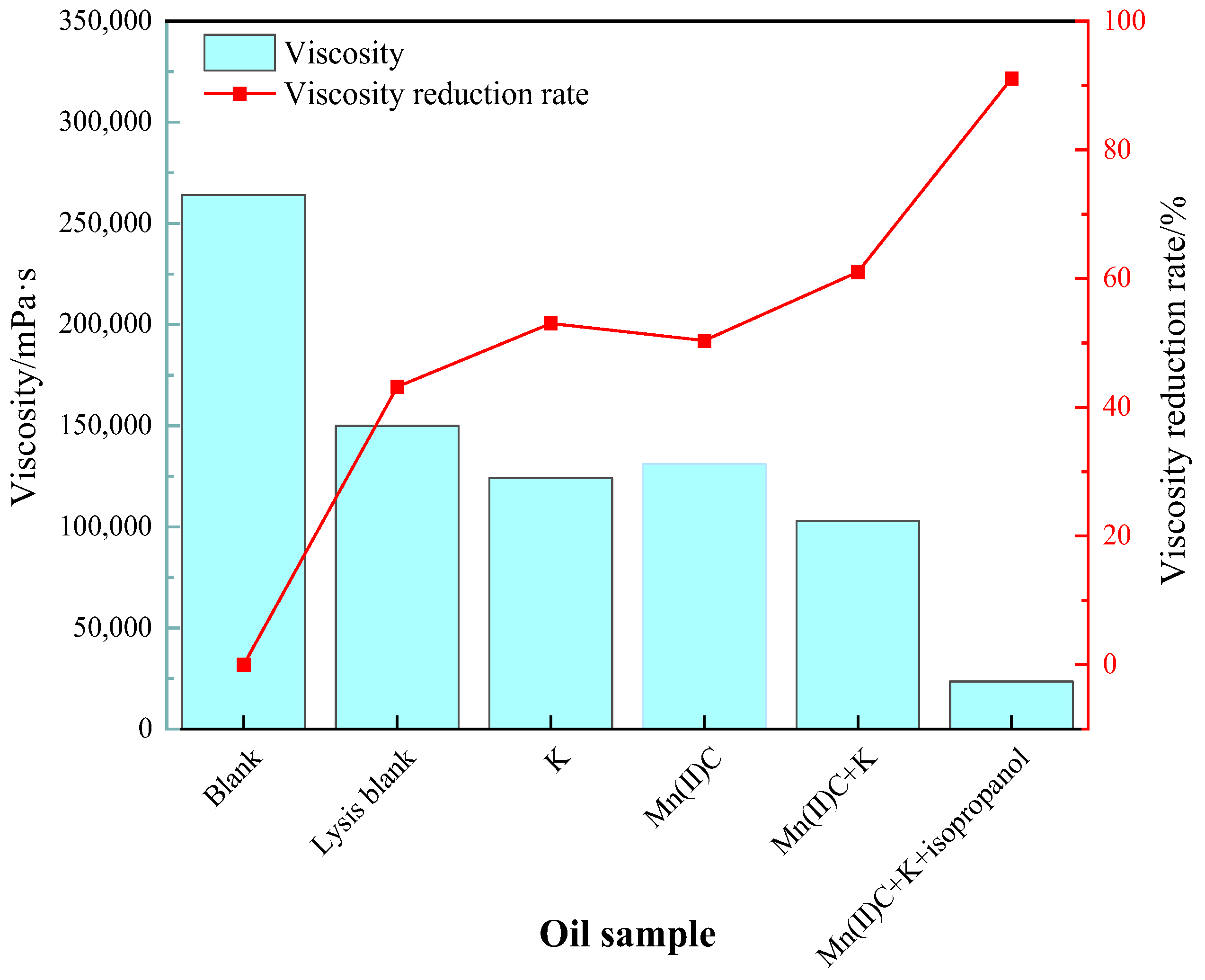
2.3. Changes in Viscosity
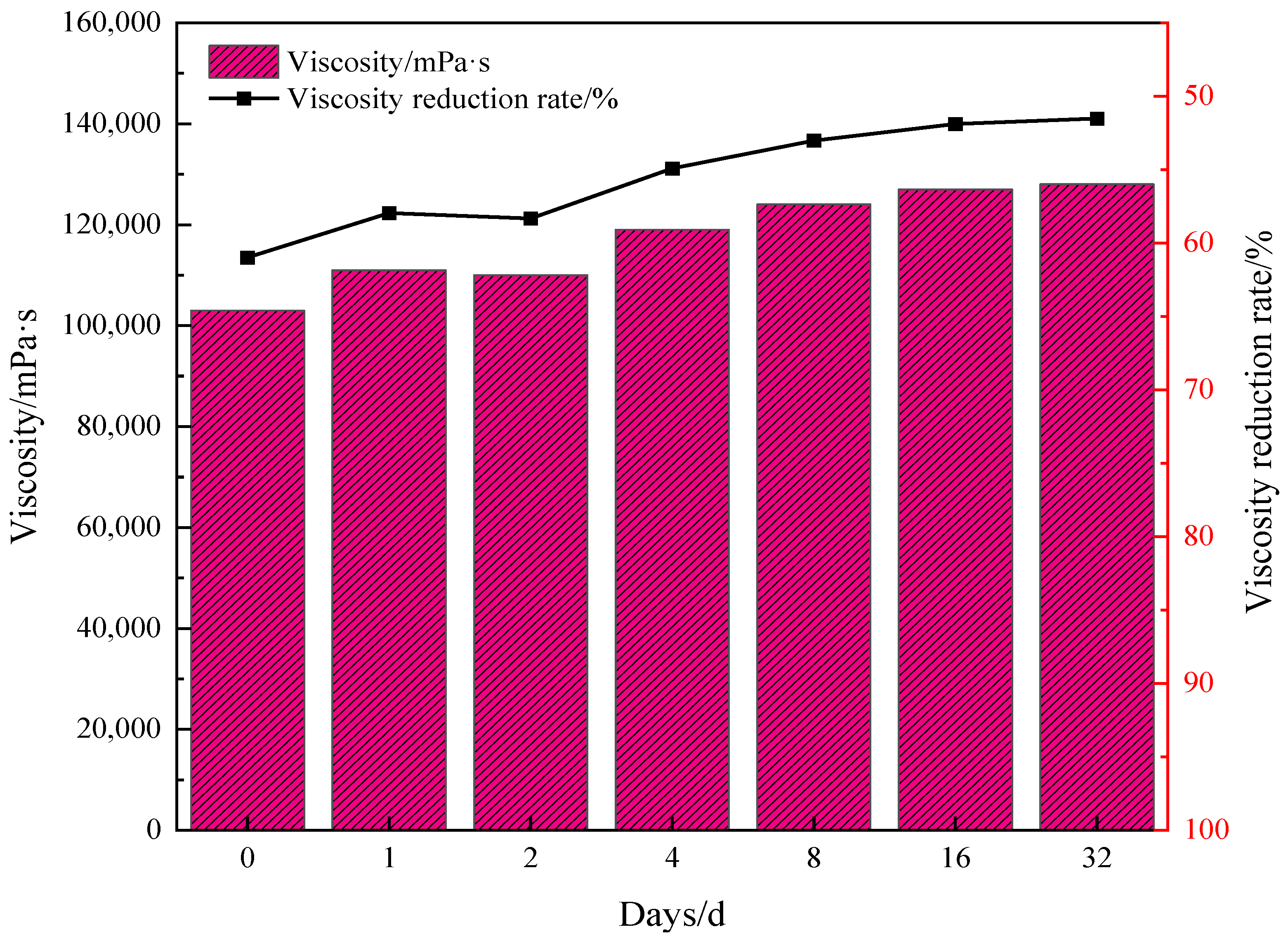
2.4. Assessment of the Stability and Universality of Catalysts in Reducing Viscosity
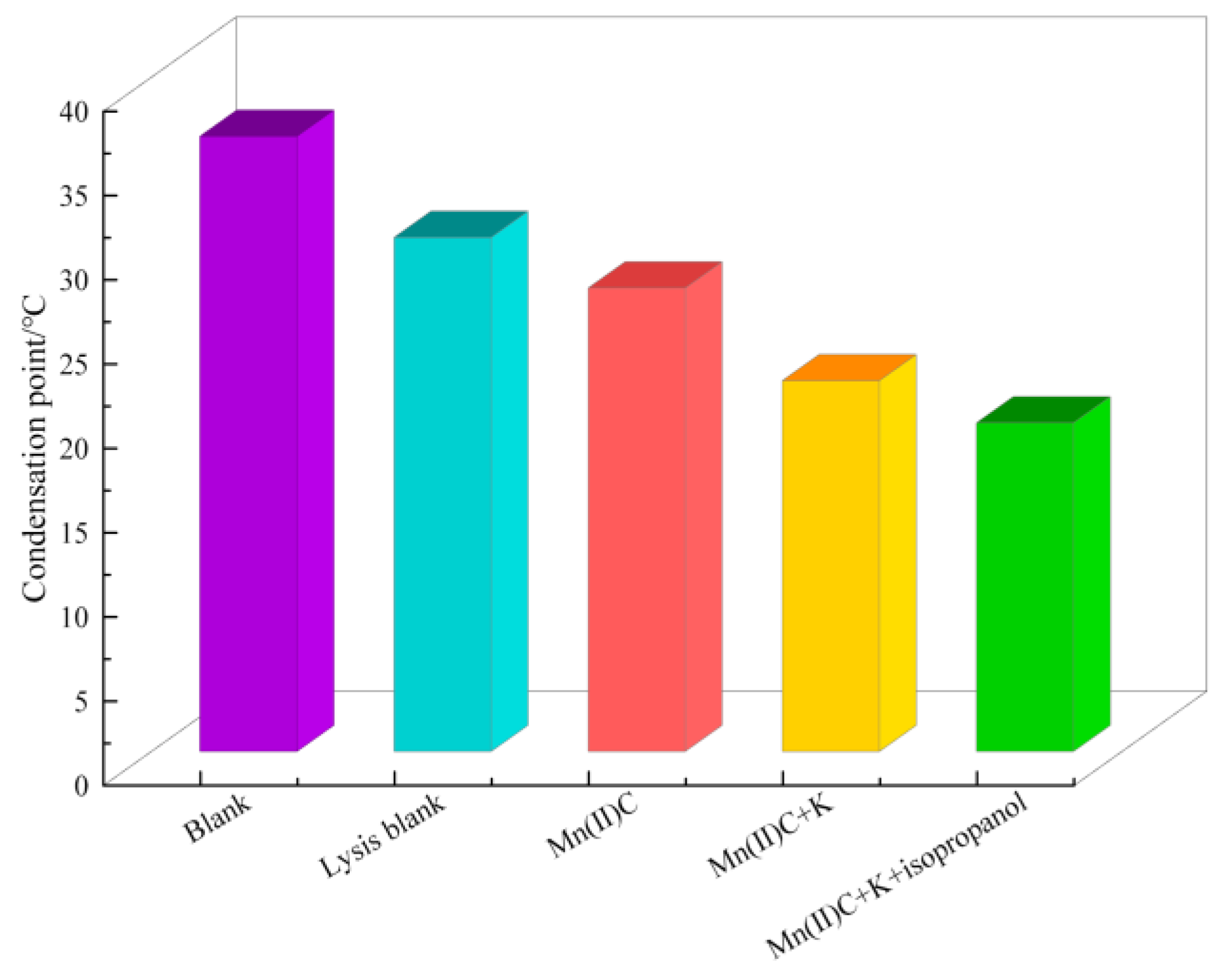
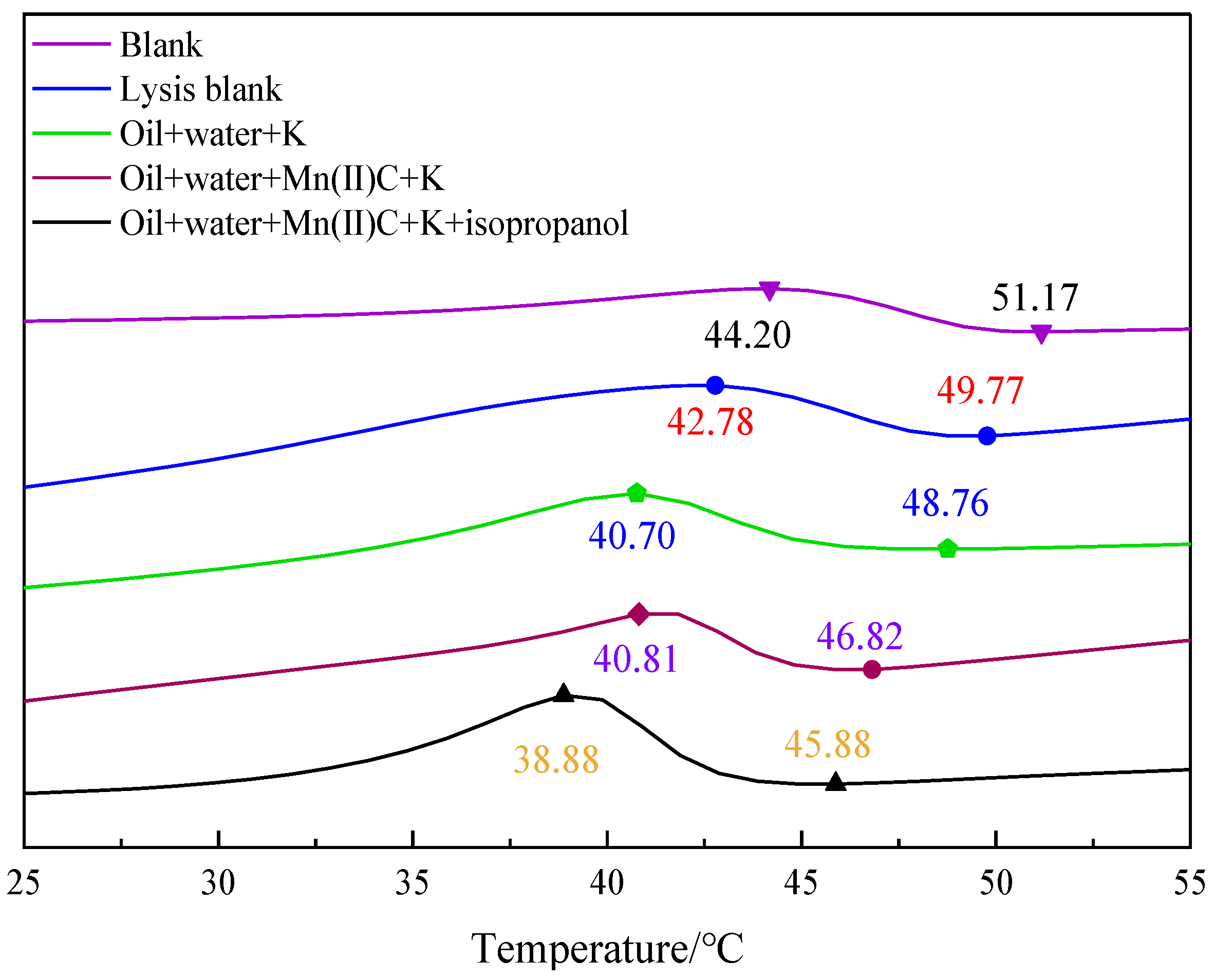
2.5. Variations in Heavy Oil’s Pour Point Both before and after the Reaction
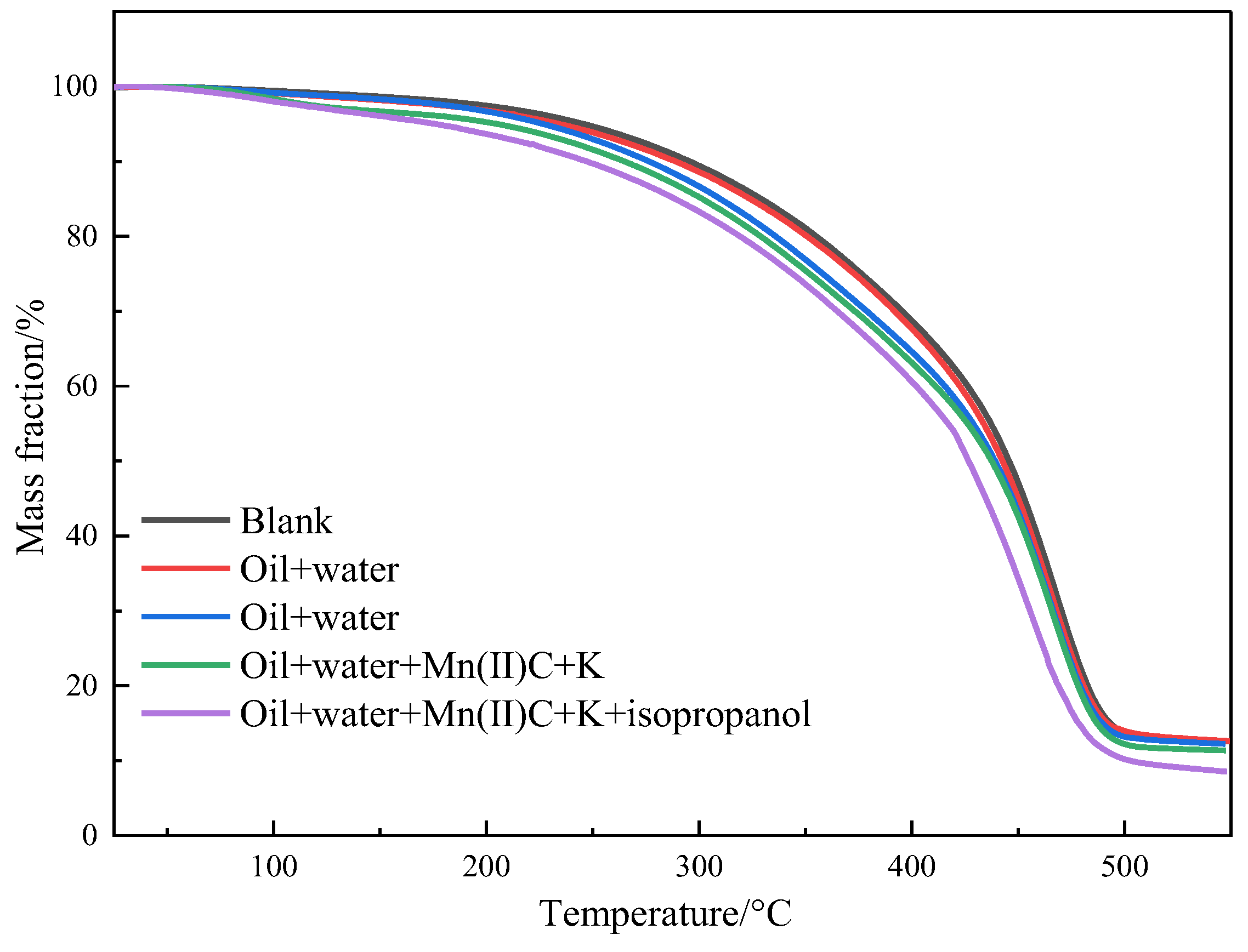
2.6. Thermogravimetric Analysis of Heavy Oil before and after Reaction
2.7. DSC Analysis
2.8. Elements and Four Components
2.9. GC Analysis of Saturated Hydrocarbon Components
2.10. Wax Crystal Morphology
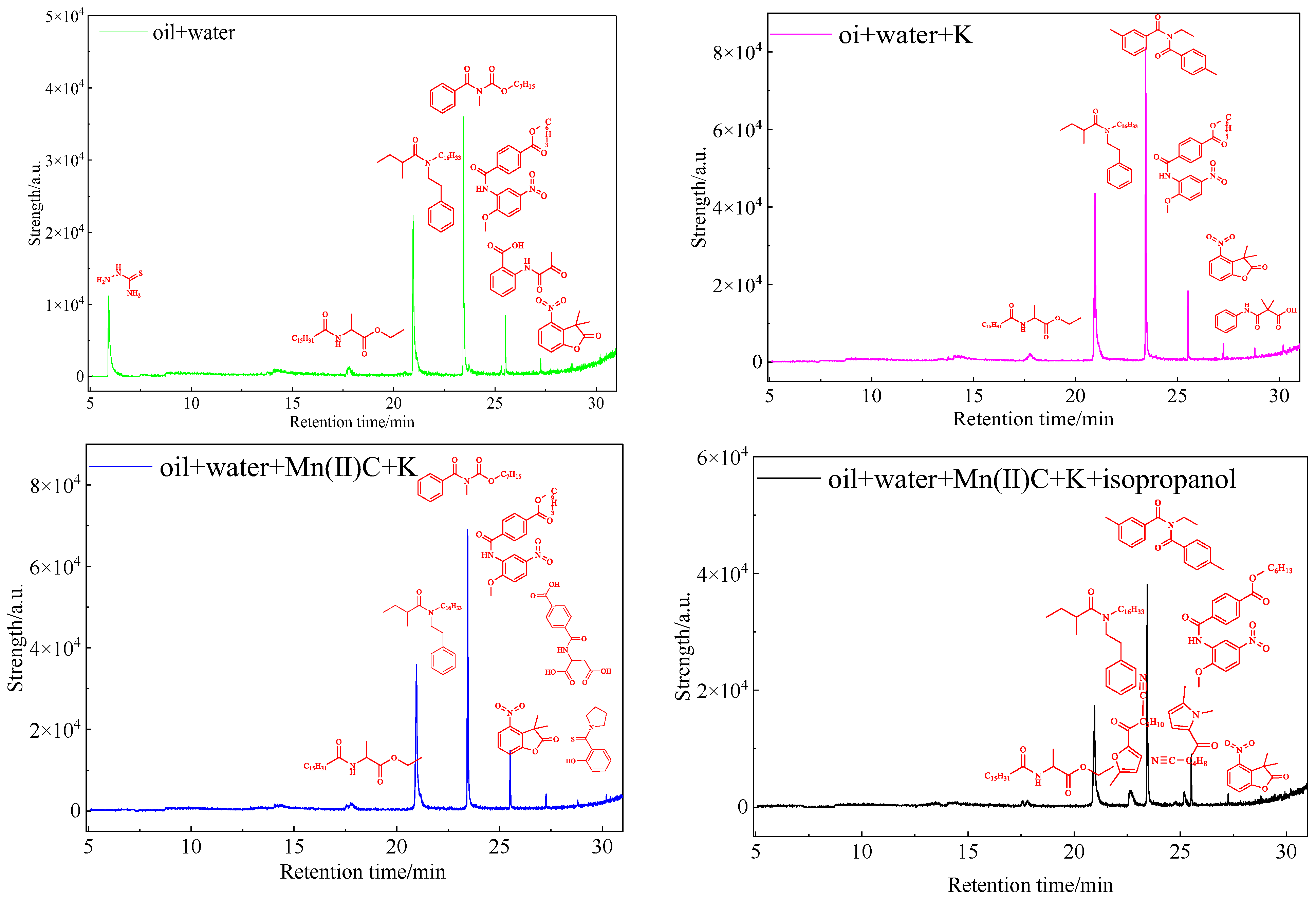
2.11. GC-MS of Heavy Oil Aqueous Phase
3. Mechanism
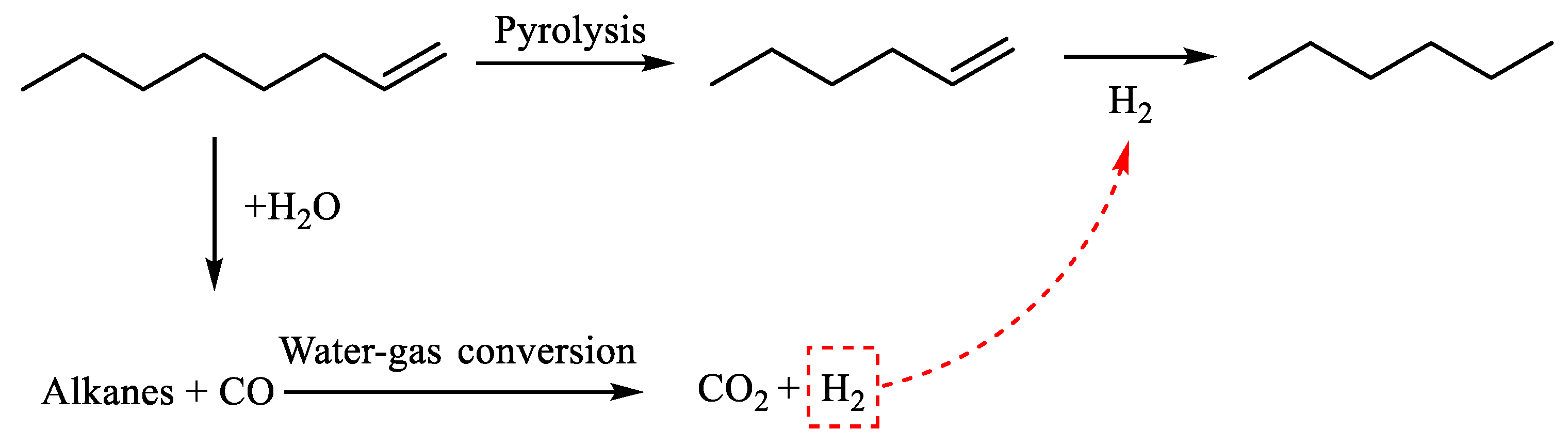
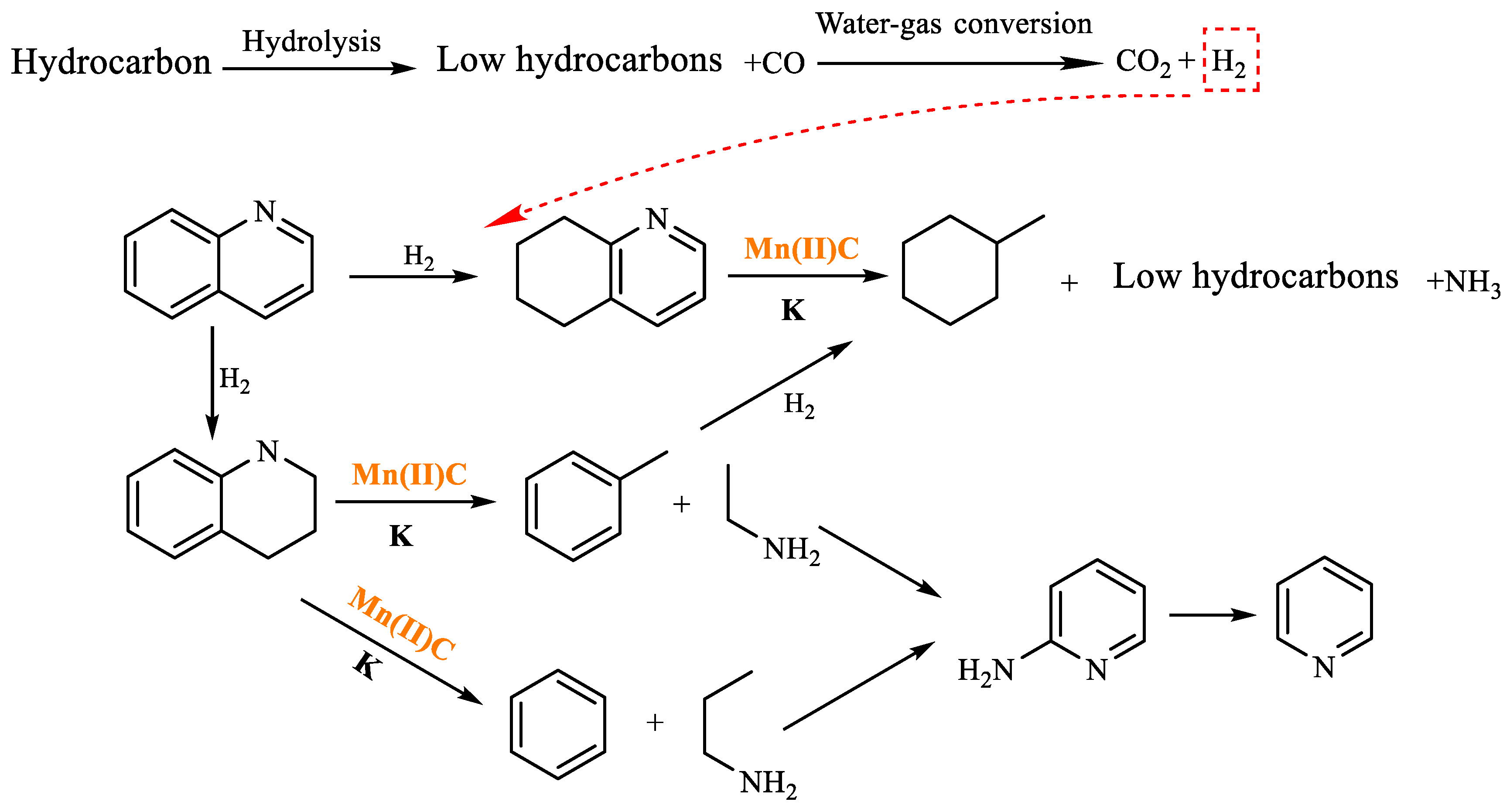
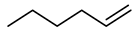
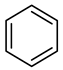
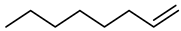
3.1. Catalytic Aquathermolysis of Model Compounds
3.2. Catalytic Mechanism
- (1)
- The abundance of glial asphaltene in heavy oil leads to a pronounced van der Waals force between the layers, causing the units to stick together. This phenomenon is visually observed as high viscosity and limited fluidity. The introduction of external catalysts has a significant impact on the active site, leading to both partial and permanent depolymerization as well as partial and loose binding. Consequently, certain unstable units undergo depolymerization and separation, leading to a substantial decrease in the viscosity of heavy oil.
- (2)
- C-S, C-O, and C-N bonds separate as a result of interactions between the external catalyst and the heteroatoms in the recombination component, which breaks the hydrogen bonds between some high carbon hydrocarbon molecules.
- (3)
- Reservoir minerals have a negatively charged surface as a result of lattice substitution, which allows them to absorb cations. This characteristic allows minerals in reservoirs to function as efficient catalysts and transporters. The transition metals found in the catalyst from another source can easily substitute sodium/calcium ions in the clay, thereby becoming the active sites in the process. Transition metals possess several vacant orbitals, allowing them to readily engage with electron-rich compounds found in heavy oil. This interaction significantly enhances the catalytic efficiency of hydrothermal cracking [37].
- (4)
- At elevated temperatures, clay minerals exhibit strong acidic properties. The catalytic mechanism by which the mineral matrix produces oil and gas involves the formation of carbonium ions. Specifically, the acid centers on the surface of the mineral matrix facilitate the conversion of kerogen into carbonium ions. The catalytic action is accomplished by decomposing and transferring carbonium ions [38,39]. Non-clay minerals such as quartz and calcite have the ability to absorb free cations and create L-acid, which promotes the transformation of kerogen [40,41]. The existence of Lewis acid on mineral surfaces enhances the electron donation by high-carbon hydrocarbon molecules, resulting in the formation of free radicals. These free radicals then undergo rearrangement and encourage the breaking of C-C bonds, resulting in the formation of short-chain alkanes. Clay minerals function as a Brønsted acid by supplying a proton (H+) to adsorbed organic molecules. The proton (H+) is generated through the dissociation of water molecules that are adsorbed and present in the interlayer, along with exchangeable cations. This process mostly involves the formation of transition-state carbonium ions [42,43].
- (5)
- Water molecules adhere to the surface of clay particles by adsorption. This phenomenon arises due to the high electron affinity of L-acid, which enables it to form a covalent bond by sharing a pair of electrons with the hydroxyl group in water. Consequently, the hydroxyl group gets strongly bonded to the surface of L-acid, while the remaining H+ ion is readily released. This process converts L-acid into B-acid. When clay minerals lose water molecules, as a result of proton deficiency, B-acid sites undergo a progressive transformation into L-acid sites [44,45,46]. The presence of clay minerals in this reaction system increases the reactivity of water/steam, reduces the energy needed for the reaction to take place, accelerates the disruption of hydrogen bonds in high-carbon hydrocarbon compounds, and improves the ability to decrease the viscosity of heavy oil.
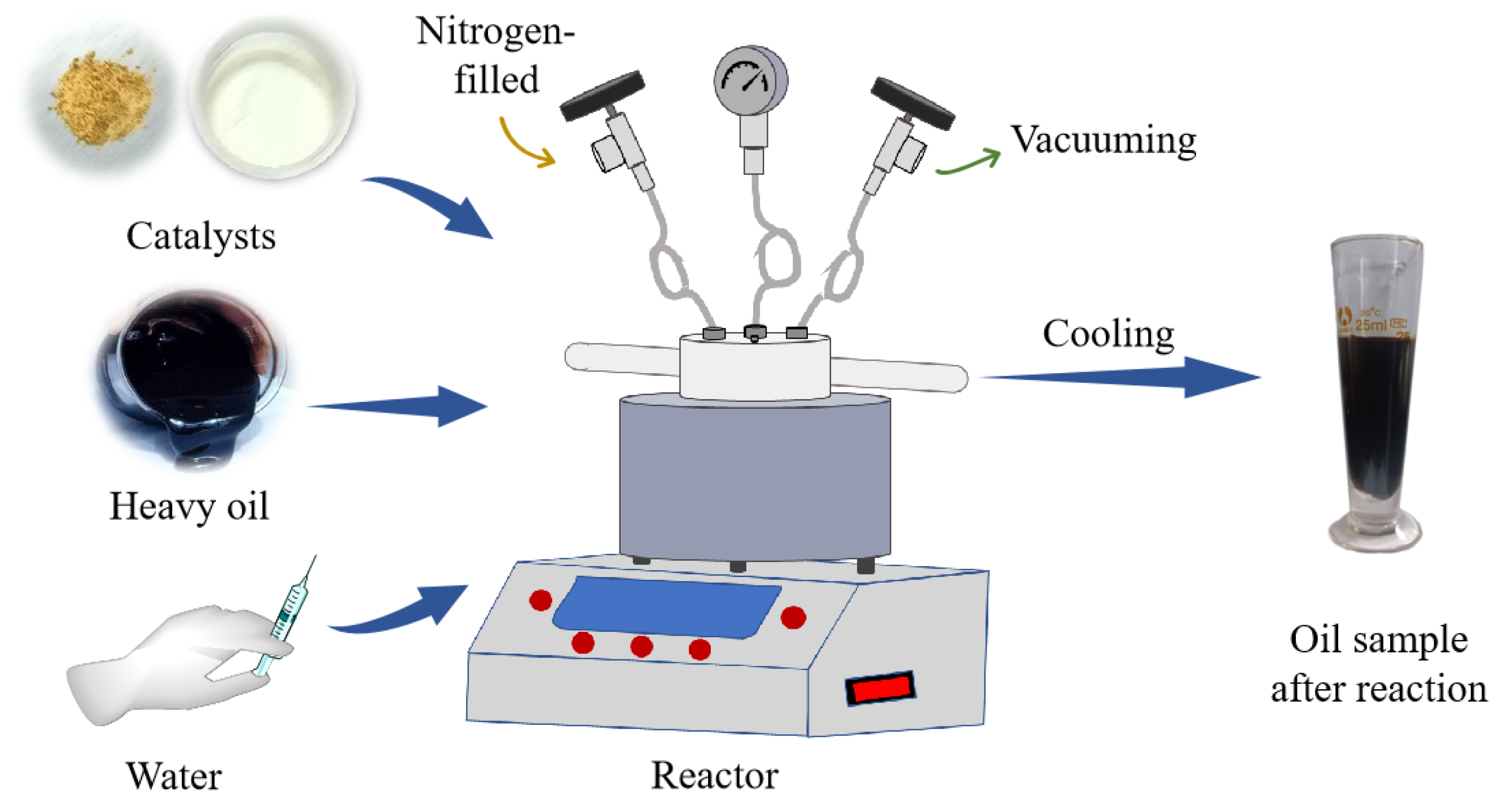
4. Materials and Methods
4.1. Materials
4.2. Preparation of Water-Soluble Exogenous Catalysts
4.3. Water Thermal Cracking
4.4. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chengzao, J.; Zheng, M.; Zhang, Y. Unconventional hydrocarbon resources in China and the prospect of exploration and development. Pet. Explor. Dev. 2012, 39, 139–146. [Google Scholar]
- Lv, S.; Peng, S.; Zhang, R.; Guo, Z.; Du, W.; Zhang, J.; Chen, G. Viscosity reduction of heavy oil by ultrasonic. Pet. Chem. 2020, 60, 998–1002. [Google Scholar]
- Yan, Y.; Prado, G.; Klerk, A. Storage Stability of Products from Visbreaking of Oilsands Bitumen. Energy Fuels 2020, 34, 9585–9598. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.; Li, Q.; Huang, H.; Ni, W.; Wang, Q.; Xin, X.; Zhao, B.; Chen, G. Preparation of Multifunctional Surfactants Derived from Sodium Dodecylbenzene Sulfonate and Their Use in Oil-Field Chemistry. Molecules 2023, 28, 3640. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Laboratory experiments and field test of a difunctional catalyst for catalytic aquathermolysis of heavy oil. Energy Fuel 2012, 26, 1152–1159. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Yu, T.; Li, Y.; Struhárová, A.; Matejdes, M.; Slaný, M.; Chen, G. The Effect of Sodium Bentonite in the Thermo-Catalytic Reduction of Viscosity of Heavy Oils. Molecules 2023, 28, 2651. [Google Scholar] [CrossRef] [PubMed]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Huang, S.; Cao, M.; Huang, Q.; Liu, B.; Jiang, J. Study on reaction equations of heavy oil aquathermolysis with superheated steam. Int. J. Environ. Sci. Technol. 2019, 16, 5023–5032. [Google Scholar] [CrossRef]
- Maity, S.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuel 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, Y.; Fan, H.; Jiang, S. Liaohe extra-heavy crude oil underground aquathermolytic treatments using catalyst and hydrogen donors under steam injection conditions. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 20–21 October 2003; p. SPE-84863-MS. [Google Scholar]
- Ma, L.; Slaný, M.; Guo, R.; Du, W.; Li, Y.; Chen, G. Study on synergistic catalysis of ex-situ catalyst and in-situ clay in aquathermolysis of water-heavy oil-ethanol at low temperature. Chem. Eng. J. 2023, 453, 139872. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Li, W.; Dou, M.; Ma, L.; Wang, Q.; Zhao, B.; Chen, G. Enhanced sorption for the oil spills by SDS-modified rice straw. Gels 2023, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yuan, W.; Wu, Y.; Zhang, J.; Song, H.; Jeje, A.; Song, S.; Qu, C. Catalytic aquathermolysis of heavy oil by coordination complex at relatively low temperature. Pet. Chem. 2017, 57, 881–884. [Google Scholar] [CrossRef]
- Mironova, E.Y.; Lytkina, A.; Ermilova, M.; Efimov, M.; Zemtsov, L.; Orekhova, N.; Karpacheva, G.; Bondarenko, G.; Muraviev, D.; Yaroslavtsev, A. Ethanol and methanol steam reforming on transition metal catalysts supported on detonation synthesis nanodiamonds for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 3557–3565. [Google Scholar] [CrossRef]
- Tajik, A.; Farhadian, A.; Khelkhal, M.A.; Rezaeisadat, M.; Petrov, S.M.; Eskin, A.A.; Vakhin, A.V.; Babapour Golafshani, M.; Lapuk, S.E.; Buzurov, A.E.; et al. Sunflower oil as renewable biomass source to develop highly effective oil-soluble catalysts for in-situ combustion of heavy oil. Chem. Eng. J. 2023, 453, 139813. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, Y.; Qi, G.; Yu, T.; Xin, X.; Zhao, B.; Chen, G. Oil-Soluble Exogenous Catalysts and Reservoir Minerals Synergistically Catalyze the Aquathermolysis of Heavy Oil. Molecules 2023, 28, 6766. [Google Scholar] [CrossRef] [PubMed]
- Boytsova, A.; Kondrasheva, N.; Ancheyta, J. Pyrolysis kinetics of heavy oil asphaltenes under steam atmosphere at different pressures. Energy Fuels 2018, 32, 1132–1138. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.; Zhao, J.; Austin, D.E.; Zhang, Z. Matrix-assisted nanoelectrospray mass spectrometry for soft ionization of metal (i)–protein complexes. Analyst 2020, 145, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Hu, X.; Wang, W.; Du, C. Non-covalent loading of ionic liquid-functionalized nanoparticles for bovine serum albumin: Experiments and theoretical analysis. RSC Adv. 2019, 9, 19114–19120. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro-and nano-metal particles during aqueous and non-aqueous thermal applications. J. Pet. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Fan, H.F.; Liu, Y.J.; Zhong, L.G. Studies on the Synergetic Effects of Mineral and Steam on the Composition Changes of Heavy Oils. Energy Fuel 2001, 15, 1475–1479. [Google Scholar] [CrossRef]
- Ambalae, A.; Mahinpey, N.; Freitag, N. Thermogravimetric studies on pyrolysis and combustion behavior of a heavy oil and its asphaltenes. Energy Fuels 2006, 20, 560–565. [Google Scholar] [CrossRef]
- Dong, S.; Li, H.; Bloede, I.K.; Al Abdulghani, A.J.; Lebrón-Rodríguez, E.A.; Huber, G.W.; Hermans, I. Catalytic conversion of model compounds of plastic pyrolysis oil over ZSM-5. Appl. Catal. B Environ. 2023, 324, 122219. [Google Scholar] [CrossRef]
- Yoosuk, B.; Tumnantong, D.; Prasassarakich, P. Amorphous unsupported Ni–Mo sulfide prepared by one step hydrothermal method for phenol hydrodeoxygenation. Fuel 2012, 91, 246–252. [Google Scholar] [CrossRef]
- Yang, Y.; Gilbert, A.; Xu, C.C. Hydrodeoxygenation of bio-crude in supercritical hexane with sulfided CoMo and CoMoP catalysts supported on MgO: A model compound study using phenol. Appl. Catal. A Gen. 2009, 360, 242–249. [Google Scholar] [CrossRef]
- Canıaz, R.O.; Arca, S.; Yaşar, M.; Erkey, C. Refinery bitumen and domestic unconventional heavy oil upgrading in supercritical water. J. Supercrit. Fluids 2019, 152, 104569. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, B.; Wang, Y.; Qu, H.; Ding, Z.; Li, Y.; Xiao, Q. Experimental study of slickwater volume effect on methane desorption on Longmaxi shale. J. Nat. Gas Sci. Eng. 2021, 91, 103950. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B. Chemistry of organosulphur compound types occurring in heavy oil sands: 3. Reaction of thiophene and tetrahydrothiophene with vanadyl and nickel salts. Fuel 1984, 63, 1649–1654. [Google Scholar] [CrossRef]
- Laine, R.M. Modeling heterogeneous catalysts with homogeneous catalysts: Modeling the hydrodenitrogenation reaction. J. Mol. Catal. 1983, 21, 119–132. [Google Scholar] [CrossRef]
- Perot, G. The reactions involved in hydrodenitrogenation. Catal. Today 1991, 10, 447–472. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Y.; Yang, H.; Qu, H.; Li, X.; Zeng, S.; Ding, Z. Interaction between slick water and gas shale and its impact on methane desorption: A case study of Longmaxi member shale formation in the northeast of Chongqing, China. Pet. Sci. Technol. 2022, 42, 1–19. [Google Scholar] [CrossRef]
- Muraza, O. Hydrous pyrolysis of heavy oil using solid acid minerals for viscosity reduction. J. Anal. Appl. Pyrol. 2015, 114, 1–10. [Google Scholar] [CrossRef]
- Zeshi, Z.; Xiaowa, N.; Chunshan, S.; Xinwen, G. Theoretical Study on Hydrodeoxygenation of o-Cresol Over Pd-Doped Fe Catalyst. Acta Pet. Sin. Pet. Process. Sect. 2021, 37, 728. [Google Scholar]
- Tirado, A.; Félix, G.; Al-Muntaser, A.A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Experimental Considerations for Proper Development of Aquathermolysis Tests in Batch Reactor Systems. Ind. Eng. Chem. Res. 2023, 62, 11491–11503. [Google Scholar] [CrossRef]
- Yan, J.; Li, Y.; Xie, X.; Slaný, M.; Dong, S.; Wu, Y.; Chen, G. Research of a novel fracturing-production integral fluid based on cationic surfactant. J. Mol. Liq. 2023, 369, 120858. [Google Scholar] [CrossRef]
- Cornejo, J.; Celis, R.; Pavlovic, I.; Ulibarri, M. Interactions of pesticides with clays and layered double hydroxides: A review. Clay Miner. 2008, 43, 155–175. [Google Scholar] [CrossRef]
- Karpiński, B.; Szkodo, M. Clay minerals–mineralogy and phenomenon of clay swelling in oil & gas industry. Adv. Mater. Sci. 2015, 15, 37–55. [Google Scholar]
- Zhao, K.; Wang, X.; Pan, H.; Li, Q.; Yang, J.; Li, X.; Zhang, Z. Preparation of molybdenum-doped akaganeite nano-rods and their catalytic effect on the viscosity reduction of extra heavy crude oil. Appl. Surf. Sci. 2018, 427, 1080–1089. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Shawabkeh, R.A.; Al-Harahsheh, A.M.; Shawaqfeh, A.T. The chemistry of minerals obtained from the combustion of Jordanian oil shale. Energy 2005, 30, 611–619. [Google Scholar] [CrossRef]
- Kazakov, M.; Klimov, O.; Dik, P.; Shaverina, A.; Pereyma, V.Y.; Noskov, A. Hydroconversion of oil shale on natural mineral matrices. Pet. Chem. 2017, 57, 1169–1172. [Google Scholar] [CrossRef]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic study and thermal decomposition behavior of lignite coal. Int. J. Chem. Eng. 2015, 2015, 481739. [Google Scholar] [CrossRef]
- Johns, W.D. Clay mineral catalysis and petroleum generation. Annu. Rev. Earth Planet. Sci. 1979, 7, 183–198. [Google Scholar] [CrossRef]
- Brown, D.; Rhodes, C. Brønsted and Lewis acid catalysis with ion-exchanged clays. Catal. Lett. 1997, 45, 35–40. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.; Nagendrappa, G.; Prakash, B.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- Félix, G.; Tirado, A.; Al-Muntaser, A.; Kwofie, M.; Varfolomeev, M.A.; Yuan, C.; Ancheyta, J. SARA-based kinetic model for non-catalytic aquathermolysis of heavy crude oil. J. Pet. Sci. Eng. 2022, 216, 110845. [Google Scholar] [CrossRef]
- ASTM D97; Standard Test Method for Pour Point of Petroleum Products. American Society Testing and Materials: Conshohoken, PA, USA, 2022.
- SY/T 5119; Analysis of Soluble Organics and Crude Oil Groups in Rocks. National Energy Administration: Beijing, China, 2016.
- NB/SH/T 0509; Method for Determination of Four Components of Petroleum Asphalt. National Energy Administration: Beijing, China, 2010.
- SY/T 0545; Recommended Practice for Thread Grease for Casing, Tubing and Pipe Lines. National Energy Administration: Beijing, China, 2012.









| Oil Sample | C/% | H/% | N/% | S/% | C/H |
|---|---|---|---|---|---|
| Blank | 86.22 | 10.10 | 2.25 | 0.45 | 8.54 |
| Lysis blank | 85.08 | 10.06 | 2.17 | 0.39 | 8.46 |
| Oil + water + K | 84.11 | 10.01 | 2.15 | 0.35 | 8.40 |
| Oil + water + Mn(Ⅱ)C + K | 84.21 | 10.09 | 1.91 | 0.33 | 8.35 |
| Oil + water + Mn(Ⅱ)C + K + isopropanol | 84.13 | 10.10 | 1.58 | 0.30 | 8.33 |
| Compound Structural Formula α-Octene + Water | Compound Structural Formula α-Octene + Water + K | Compound Structural Formula α-Octene + Water + Mn(Ⅱ)C + K | Compound Structural Formula α-Octene + Water+ Mn(Ⅱ)C + K+ Isopropanol |
|---|---|---|---|
 | |||
 | |||
 | |||
 | |||
| Compound Structural Formula Phenol + Water | Compound Structural Formula Phenol + Water + K | Compound Structural Formula Phenol + Water + Mn(Ⅱ)C + K | Compound Structural Formula Phenol + Water + Mn(Ⅱ)C + K + Isopropanol |
|---|---|---|---|
 | |||
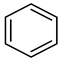 | |||
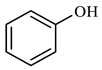 | |||
| Compound Structural Formula Thiophene + Water | Compound Structural Formula Thiophene + Water + K | Compound Structural Formula Thiophene + Water + Mn(Ⅱ)C +K | Compound Structural Formula Thiophene + Water + Mn(Ⅱ)C + K+ Isopropanol |
|---|---|---|---|
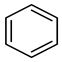 | |||
 | |||
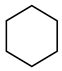 | |||
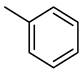 | |||
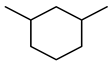 | |||
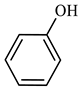 | |||
| Compound Structural Formula Quinoline + Water | Compound Structural Formula Quinoline + Water + K | Compound Structural Formula Quinoline + Water + Mn(II)C + K | Compound Structural Formula Quinoline + Water + Mn(Ⅱ)C + K + Isopropanol |
|---|---|---|---|
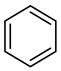 | |||
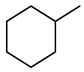 | |||
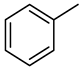 | |||
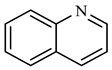 | |||
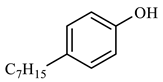
| Compound Structural Formula Nonylphenol + Water | Compound Structural Formula Nonylphenol + Water + K | Compound Structural Formula Nonylphenol + Water + Mn(Ⅱ)C + K | Compound Structural Formula Nonylphenol + Water + Mn(Ⅱ)C + K + Isopropanol |
|---|---|---|---|
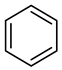 | |||
 | |||
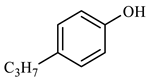 | |||
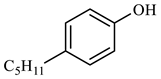 | |||
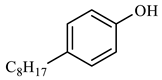 | |||
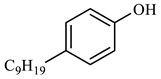 | |||
 | |||
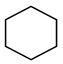
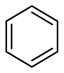
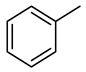
| Compound Structural Formula Benzothiophene + Water | Compound Structural Formula Benzothiophene + Water + K | Compound Structural Formula Benzothiophene + Water + Mn(Ⅱ)C + K | Compound Structural Formula Benzothiophene + Water + Mn(Ⅱ)C + K + Isopropanol |
|---|---|---|---|
 | |||
 | |||
 | |||
 | |||
| Heavy Oil | Pour Point (°C) | Water Content (%) | Saturates (%) | Aromatics (%) | Resins (%) | Asphaltenes (%) |
|---|---|---|---|---|---|---|
| Oil sample 1 | 38.0 | 15.5 | 25.26 | 33.98 | 25.55 | 15.21 |
| Oil sample 2 | 20.0 | 17.0 | 31.16 | 28.73 | 16.67 | 23.44 |
| Oil sample 3 | 19.6 | 12.5 | 24.76 | 31.28 | 18.57 | 25.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, S.; Chen, X.; Ni, J.; Du, J.; Li, Y.; Xin, X.; Zhao, B.; Chen, G. Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil. Molecules 2024, 29, 3761. https://doi.org/10.3390/molecules29163761
Wang Q, Zhang S, Chen X, Ni J, Du J, Li Y, Xin X, Zhao B, Chen G. Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil. Molecules. 2024; 29(16):3761. https://doi.org/10.3390/molecules29163761
Chicago/Turabian StyleWang, Qian, Shu Zhang, Xiang Chen, Jianjun Ni, Jialu Du, Yongfei Li, Xin Xin, Bin Zhao, and Gang Chen. 2024. "Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil" Molecules 29, no. 16: 3761. https://doi.org/10.3390/molecules29163761





