Coupling Electro-Fenton and Electrocoagulation of Aluminum–Air Batteries for Enhanced Tetracycline Degradation: Improving Hydrogen Peroxide and Power Generation
Abstract
1. Introduction
2. Results and Discussion
2.1. Performance of Different Air Cathodes
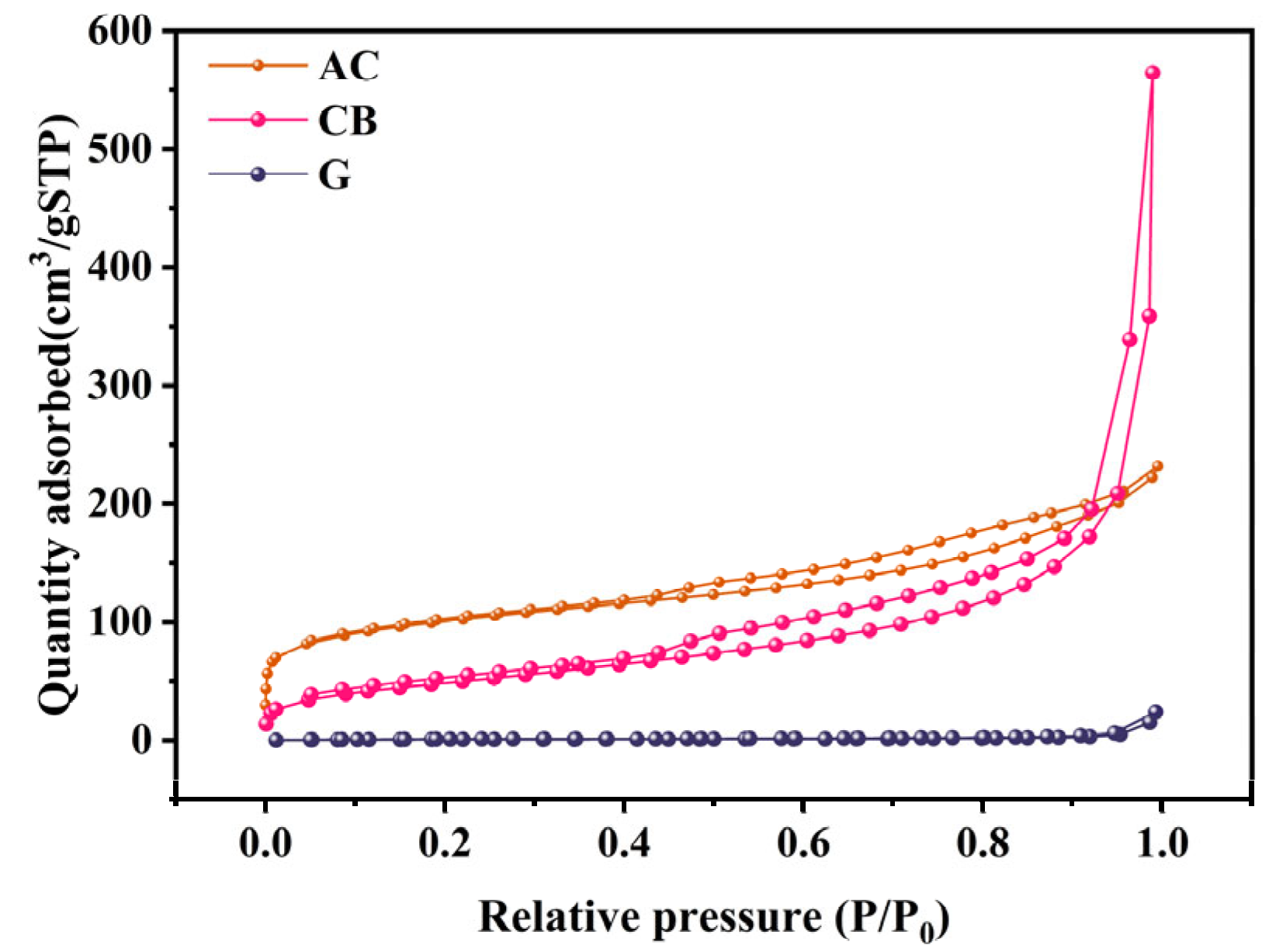
2.1.1. Morphology and Porosity
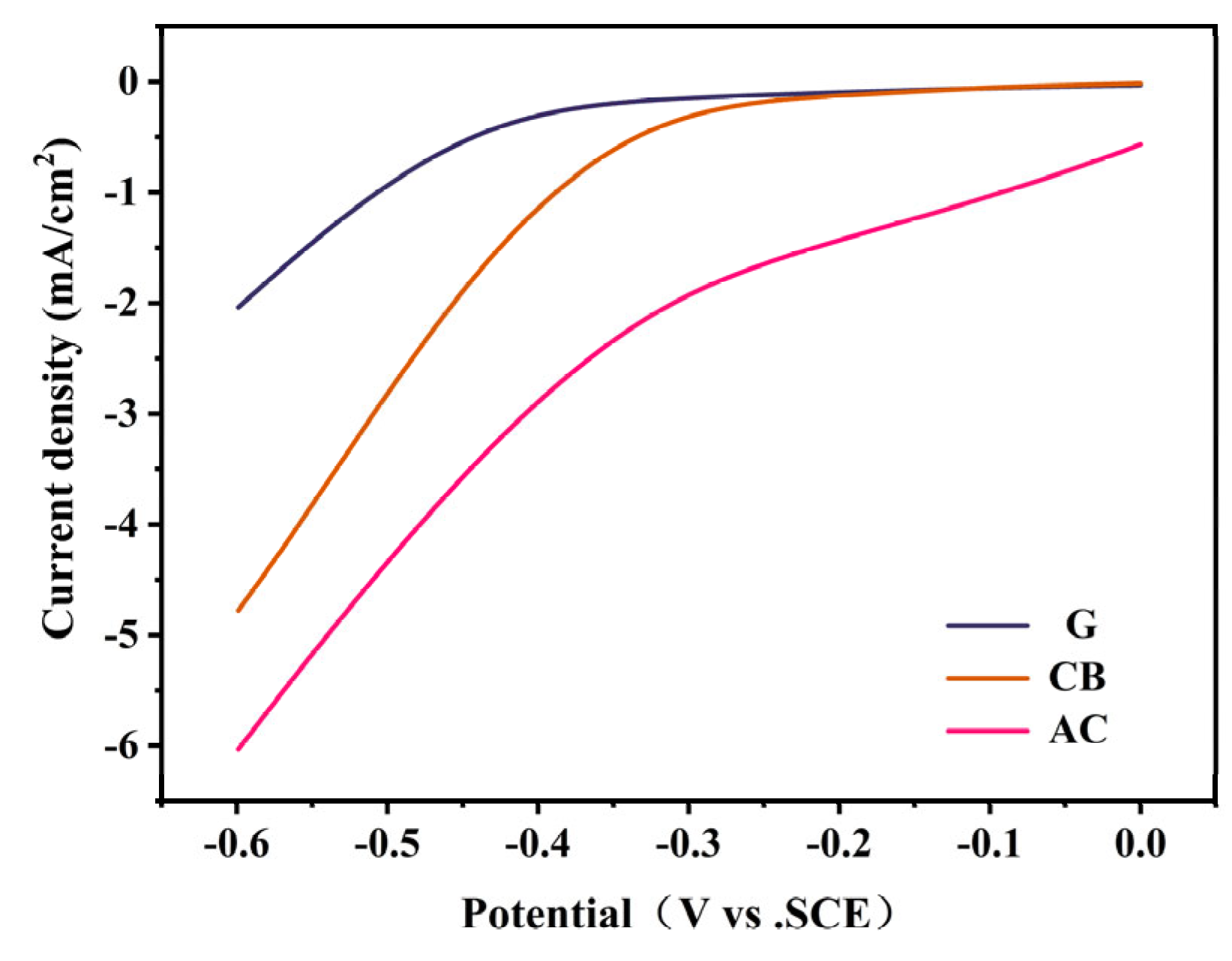
2.1.2. Electrochemical Analysis
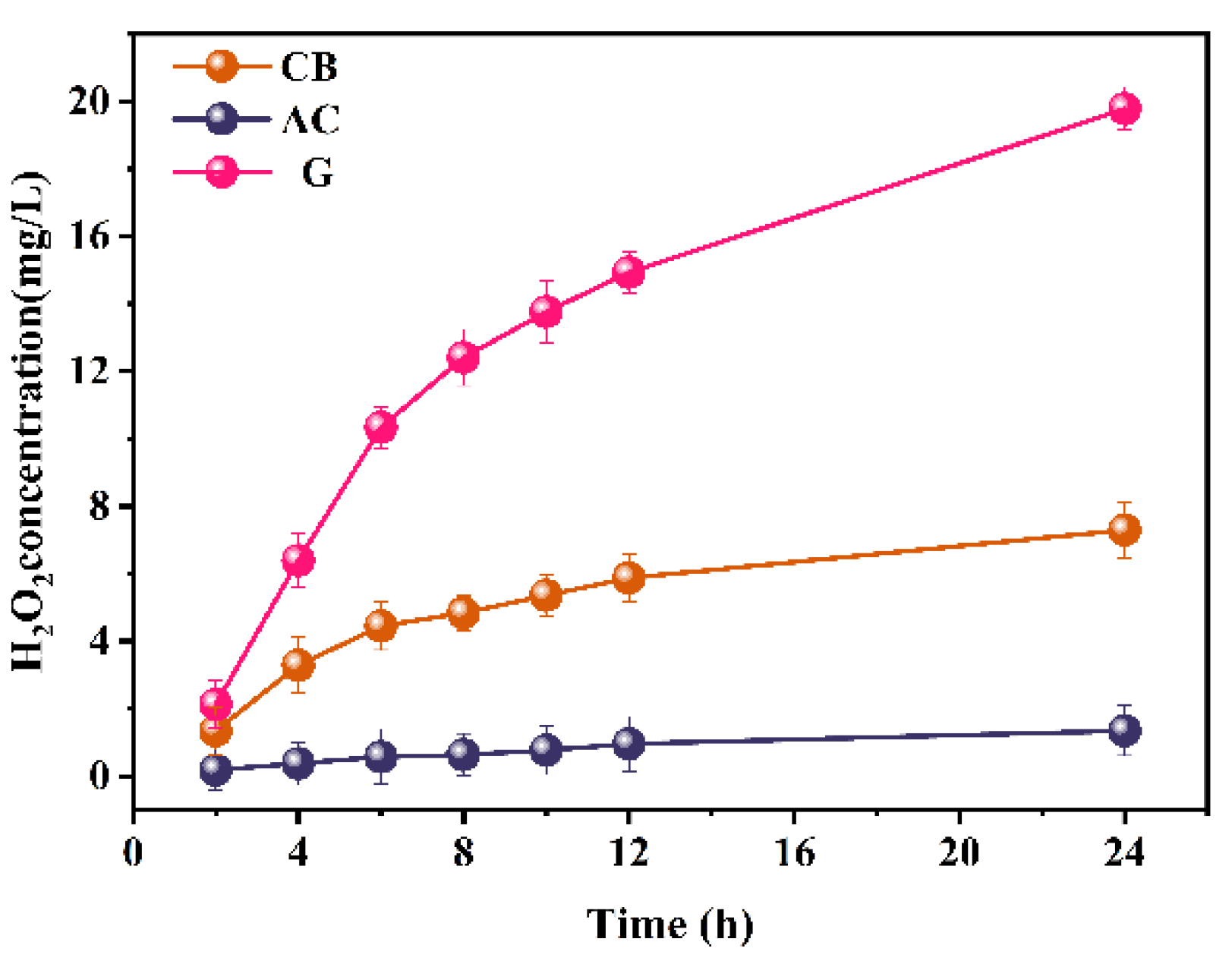
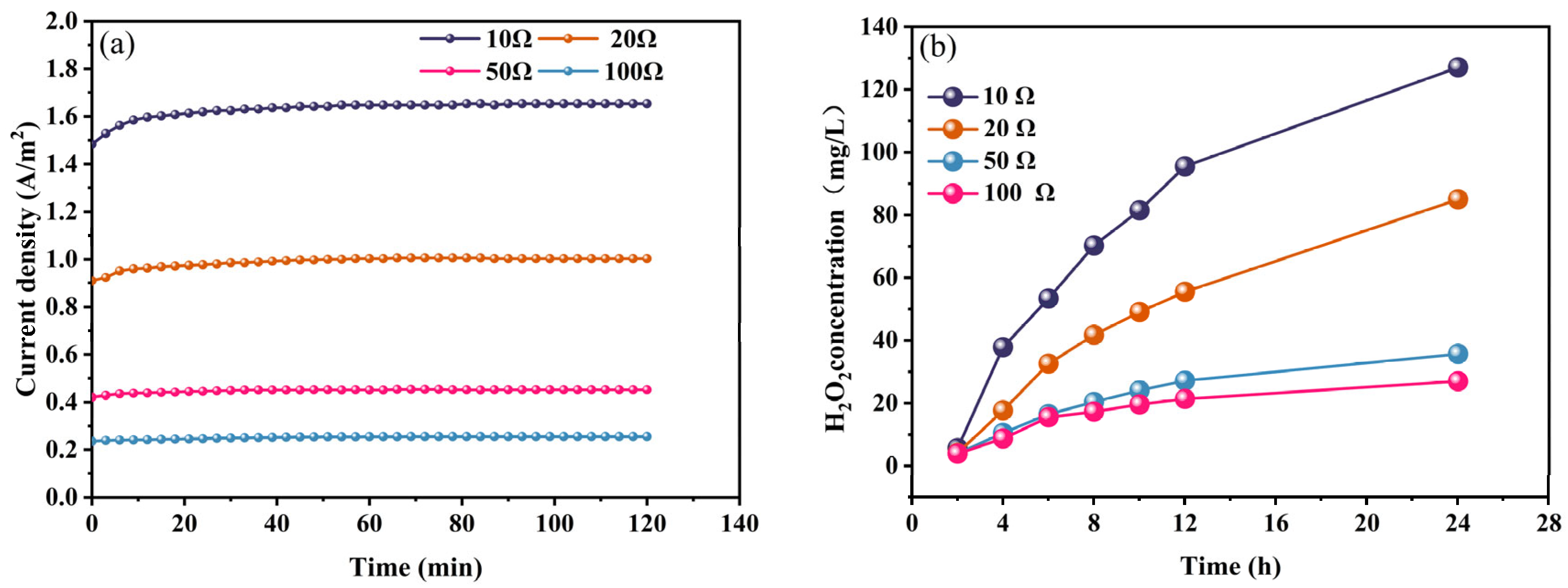
2.1.3. Performance of H2O2 Production
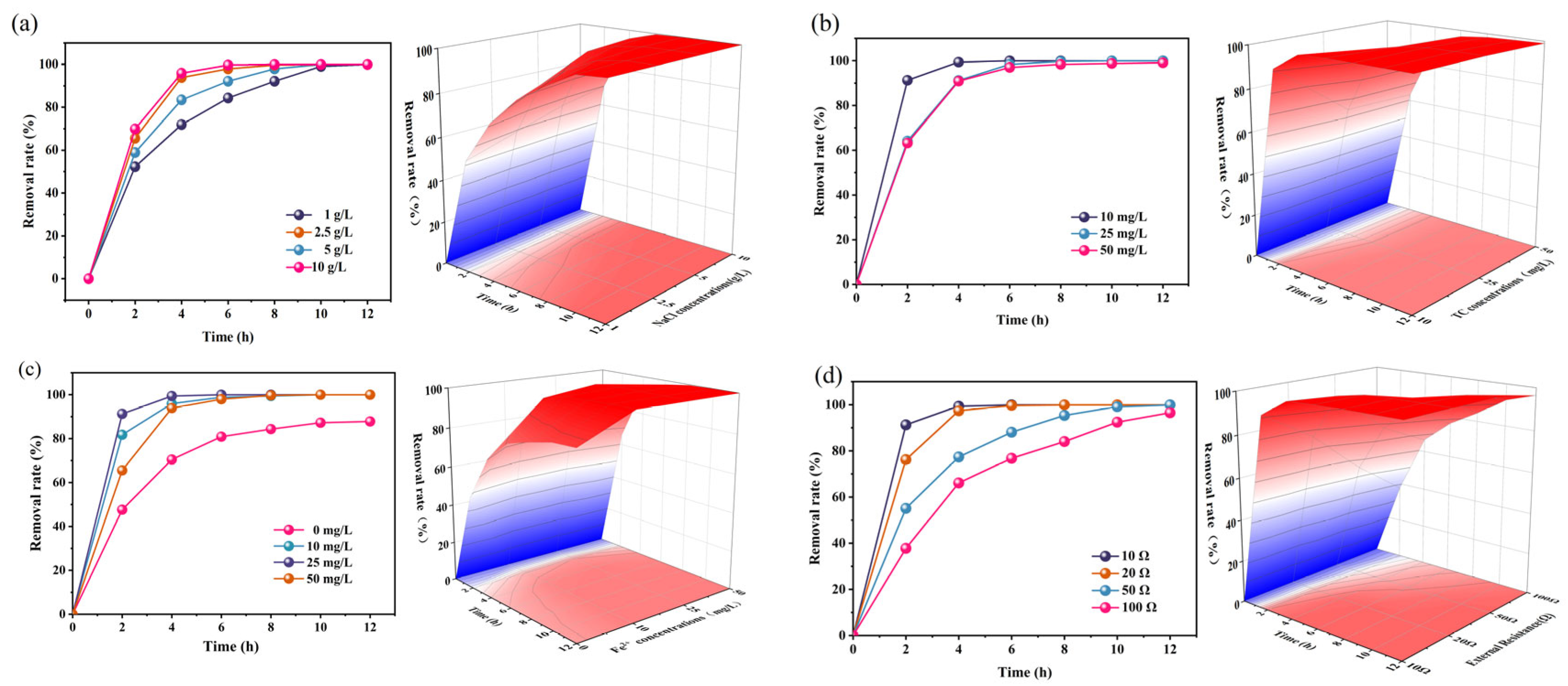
2.2. Removal of TC
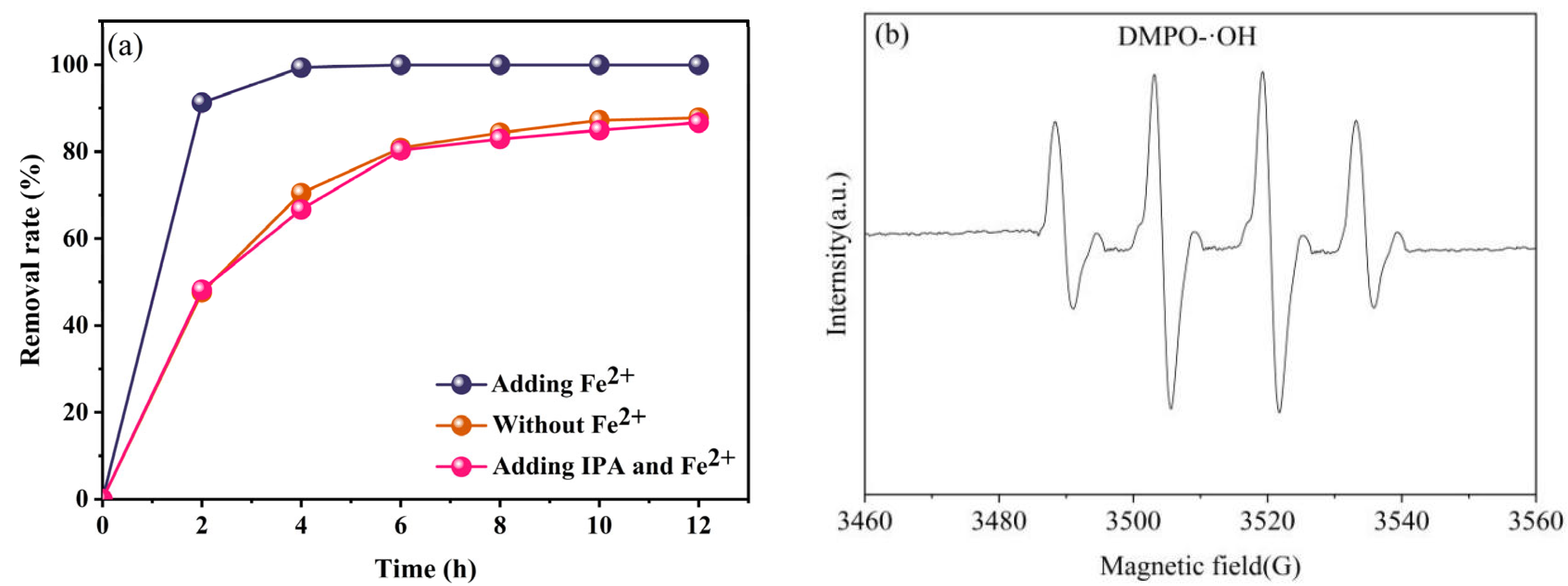
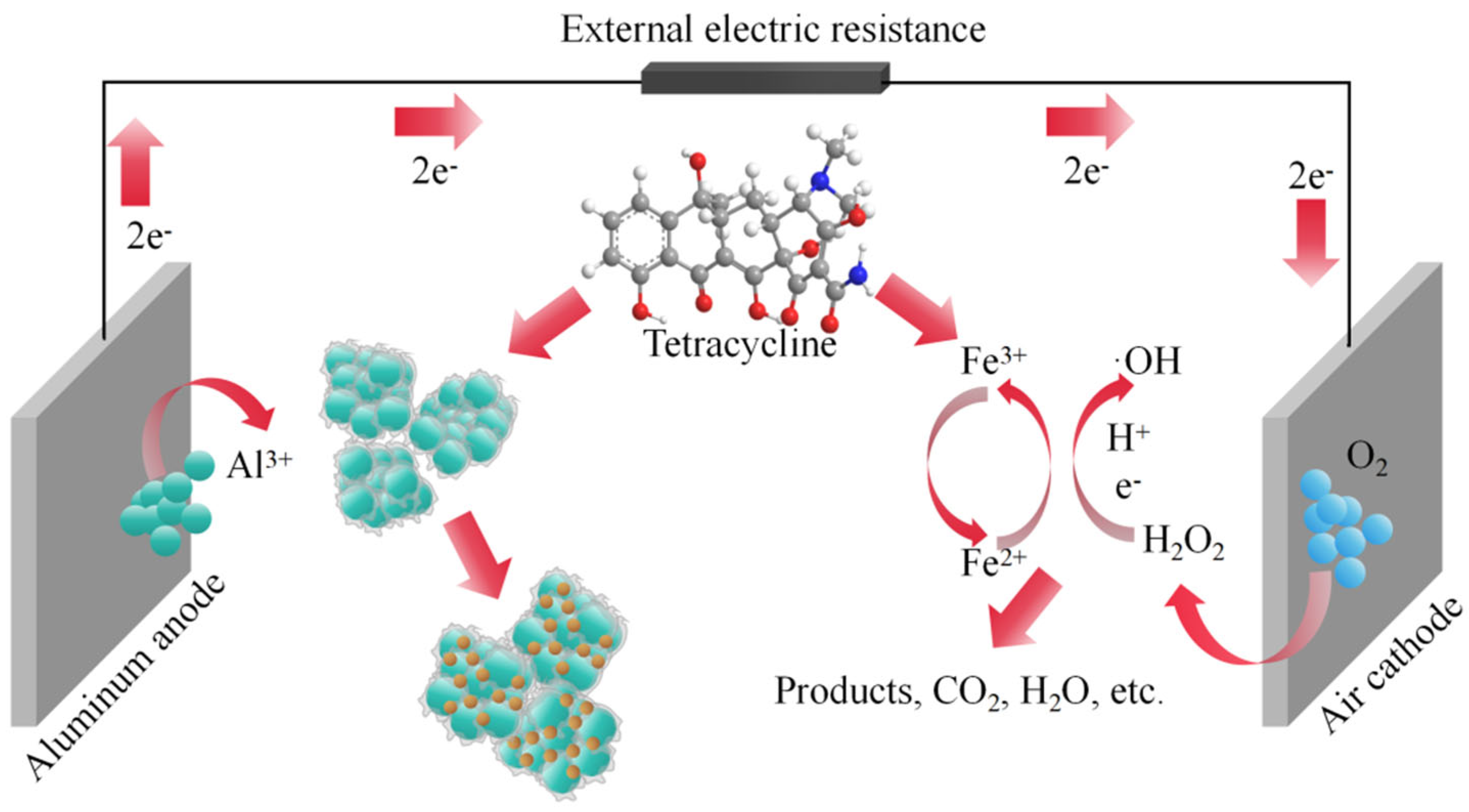
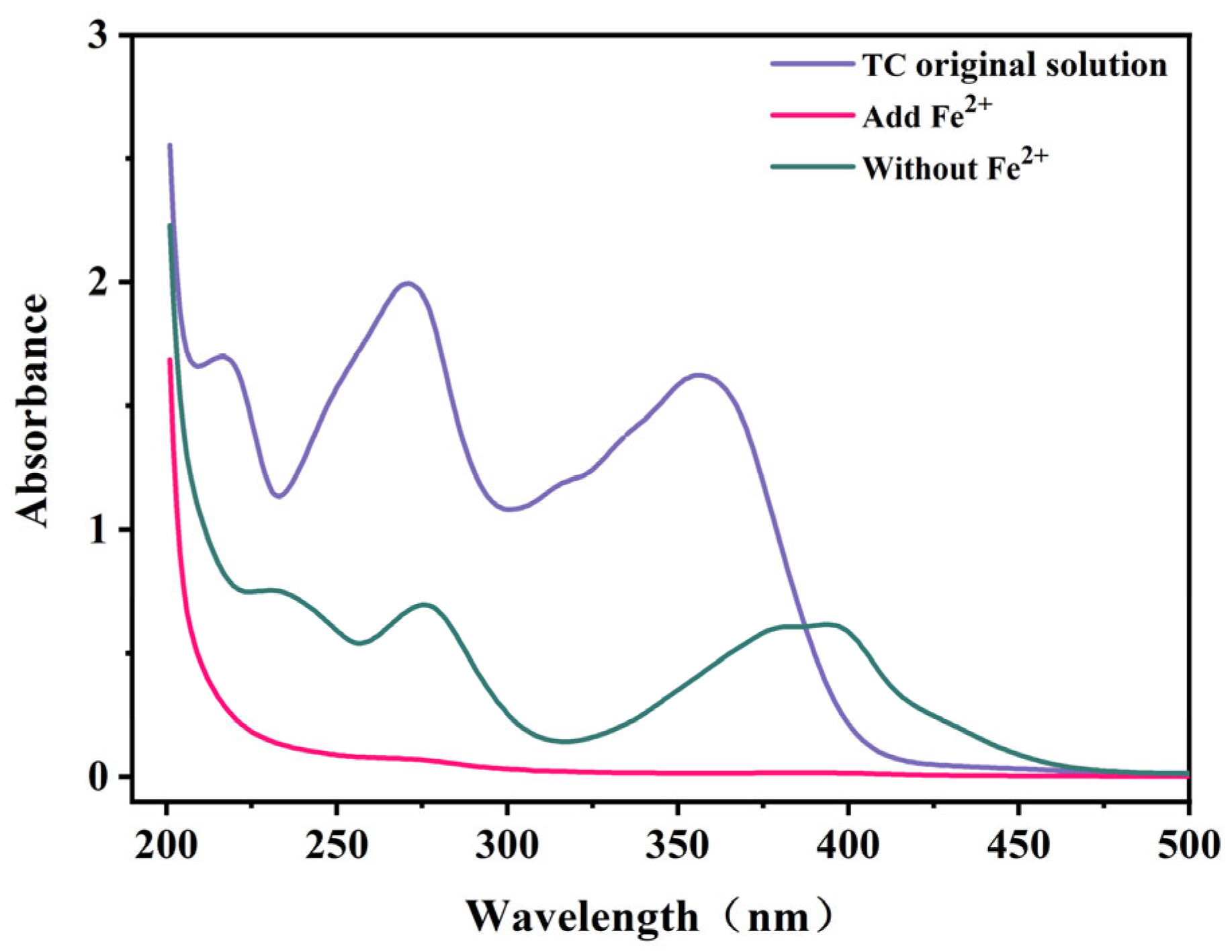
2.3. Mechanism of TC Removal
3. Materials and Methods
3.1. Air Cathode Fabrication
3.2. Setup and Operation
3.3. Material Characterization
3.4. Electrochemical and Chemical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Baldawi, I.A.; Mohammed, A.A.; Mutar, Z.H.; Abdullah, S.R.S.; Jasim, S.S.; Almansoory, A.F.; Ismail, N.I. Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis. J. Clean. Prod. 2021, 319, 128584. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Zhang, R.; Teng, H.H.; Padhye, L.P.; Sun, P. Transformation of tetracycline antibiotics with goethite: Mechanism, kinetic modeling and toxicity evaluation. Water Res. 2021, 199, 117196. [Google Scholar] [CrossRef] [PubMed]
- Rameshwar, S.S.; Sivaprakash, B.; Rajamohan, N.; Mohamed, B.A.; Vo, D.-V.N. Remediation of tetracycline pollution using MXene and nano-zero-valent iron materials: A review. Environ. Chem. Lett. 2023, 21, 2995–3022. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Nie, Z.; Wen, L.; Chen, B.; Tang, J.; Gao, M.; Chen, J.; Liu, J. Insights into the effects of natural pyrite-activated sodium percarbonate on tetracycline removal from groundwater: Mechanism, pathways, and column studies. Sci. Total. Environ. 2023, 902, 165883. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Song, W.; Ma, R.; Yang, J.; Zhang, X.; Huang, F.; Dong, W. Rapid degradation of atrazine by a novel advanced oxidation process of bisulfite/chlorine dioxide: Efficiency, mechanism, pathway. Chem. Eng. J. 2022, 445, 136558. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, X.; Ai, D.; Wei, T.; Fan, Z.; Wang, B. Sulfur-doped zero-valent iron supported on biochar for tetracycline adsorption and removal. J. Clean. Prod. 2022, 379, 134769. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zhuang, G.-L.; Tasi, P.-F.; Tseng, H.-H. Removal of protein, histological dye and tetracycline from simulated bioindustrial wastewater with a dual pore size PPSU membrane. J. Hazard. Mater. 2022, 431, 128525. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, K.; Yin, X.; Liu, Y.; Khan, I.; Liu, S.-Y. Photocatalytic Degradation of Tetracycline Hydrochloride with G-C3N4/Ag/AgBr Composites. Front. Chem. 2022, 10, 1069816. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, J.; He, Z.-C.; Ji, X.; Zhang, Y.-F.; Sun, H.-L.; Wang, K.; Su, Z.-W. Accelerated Photocatalytic Degradation of Tetracycline Hydrochloride over CuAl2O4/g-C3N4 p-n Heterojunctions under Visible Light Irradiation. Sep. Purif. Technol. 2021, 277, 119461. [Google Scholar] [CrossRef]
- Zhou, Q.; Ji, Z.; Yu, H.; Lu, S.; Guo, J.; Wu, C. Photocatalytic Degradation of Tetracycline Hydrochloride Based on the Structure–Property Exploration of BiOCl. Langmuir 2024, 40, 7078–7086. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, C.-Y.; Liao, G.-Y. Degradation of antibiotic tetracycline by ultrafine-bubble ozonation process. J. Water Process. Eng. 2020, 37, 101463. [Google Scholar] [CrossRef]
- Moein, H.; Balarak, D.; Meshkinain, A.; Chandrika, K.; Yazdani, N. Effects of Operational Parameters on the Removal of Tetracycline from Aqueous Solutions by Electrocoagulation. Int. J. Pharm. Investig. 2021, 11, 23–26. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, L.; Sun, X.; Dong, H.; Yu, H.; Yu, H. Radical and non-radical cooperative degradation in metal-free electro-Fenton based on nitrogen self-doped biochar. J. Hazard. Mater. 2022, 435, 129063. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain Boundary Engineering: An Emerging Pathway toward Efficient Electrocatalysis. InfoMat 2024, e12608. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Elektorowicz, M.; Tawalbeh, M.; Al Bsoul, A.; El Gendy, A.; Kamyab, H.; Yusuf, M. Integrating of electrocoagulation process with submerged membrane bioreactor for wastewater treatment under low voltage gradients. Chemosphere 2023, 339, 139693. [Google Scholar] [CrossRef] [PubMed]
- Karmankar, S.B.; Sharma, A.; Ahirwar, R.C.; Mehra, S.; Pal, D.; Prajapati, A.K. Cost cutting approach of distillery effluent treatment using solar photovoltaic cell driven electrocoagulation: Comparison with conventional electrocoagulation. J. Water Process. Eng. 2023, 54, 103982. [Google Scholar] [CrossRef]
- Saeed, O.F.; Hameed, K.W.; Abbar, A.H. Treatment of vegetable oil refinery wastewater by sequential electrocoagulation-electrooxidation process. J. Environ. Manag. 2023, 342, 118362. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Suresh, B.K.; Anantha-Singh, T.S. A sequential aerated electrocoagulation and peroxicoagulation process for the treatment of municipal stabilized landfill leachate by iron and graphite electrodes. Chemosphere 2023, 339, 139692. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, M.; Rao, P.; Zhao, W.; Wang, Z.; Du, X.; Tian, J. Electro-coagulation coupled with solid carrier enhanced GDCMBR as pretreatment for NF dual membrane system during surface brackish water treatment. J. Clean. Prod. 2022, 380, 135021. [Google Scholar] [CrossRef]
- Wang, Z.; An, X.; Wang, P.; Du, X.; Hao, X.; Hao, X.; Ma, X. Removal of high concentration of chloride ions by electrocoagulation using aluminium electrode. Environ. Sci. Pollut. Res. 2023, 30, 50567–50581. [Google Scholar] [CrossRef]
- Kim, J.H.; Maitlo, H.A.; Park, J.Y. Treatment of synthetic arsenate wastewater with iron-air fuel cell electrocoagulation to supply drinking water and electricity in remote areas. Water Res. 2017, 115, 278–286. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Lv, T.; Chen, H.; Chika, A.O.; Xiang, C.; Guo, M.; Wu, M.; Li, J.; Jia, L. Energy-producing electro-flocculation for harvest of Dunaliella salina. Bioresour. Technol. 2017, 241, 1022–1026. [Google Scholar] [CrossRef]
- Li, F.; Wang, P.; Li, M.; Zhang, T.; Li, Y.; Zhan, S. Efficient photo-Fenton reaction for tetracycline and antibiotic resistant bacteria removal using hollow Fe-doped In2O3 nanotubes: From theoretical research to practical application. Water Res. 2023, 240, 120088. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Yong, T.; Cao, W.; Wu, J.; Shen, Y. Synergistic effects of oxidation, coagulation and adsorption in the integrated Fenton-based process for wastewater treatment: A review. J. Environ. Manag. 2022, 306, 114460. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, Q.; Li, C.; Tao, Z.; Hou, Z.; Zheng, T.; Wang, Q.; Ouyang, S.; Zhan, S. Diatomic catalysts for Fenton and Fenton-like reactions: A promising platform for designing/regulating reaction pathways. Chem. Sci. 2023, 14, 7818–7827. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, P.M.; Campos, S.A.; Calzadilla, W.; Marco, J.F.; Denardin, J.C.; Salazar-González, R.A. Pd-Based Nanoparticles as Catalysts for Improved Removal of Florfenicol via Heterogeneous Fenton and Photo-Fenton(-like) Processes. ACS Appl. Nano Mater. 2023, 6, 12177–12189. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, Y.; Yan, Y.; Bu, L.; Wei, Z.; Spinney, R.; Dionysiou, D.D.; Xiao, R. Mechanistic and quantitative profiling of electro-Fenton process for wastewater treatment. Water Res. 2023, 235, 119838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhou, P.; Sun, M.; Liu, Y.; Zhang, H.; Xiong, Z.; Liang, J.; Duan, X.; Lai, B. Nitrogen-doped carbon nanotubes enhanced Fenton chemistry: Role of near-free iron(III) for sustainable iron(III)/iron(II) cycles. Water Res. 2022, 210, 117984. [Google Scholar] [CrossRef]
- Gamaralalage, D.; Sawai, O.; Nunoura, T. Degradation behavior of palm oil mill effluent in Fenton oxidation. J. Hazard. Mater. 2019, 364, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhen, L.; Zhang, H.; Zhang, Y.; Wang, C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J. Hazard. Mater. 2012, 229–230, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Li, G.; Wu, Y.; Zhang, H.; Yuan, Y.; Zhang, H.; Liu, B.; Zhang, F. Tradeoff between groundwater arsenite removal efficiency and current production in the self-powered air cathode electrocoagulation with different oxygen reduction pathways. J. Hazard. Mater. 2018, 357, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Li, K.; Li, Z.; Jiang, M.; Wang, F.; Yang, Z.; Zhao, T.; Meng, P.; Fu, C. Rational design of boron-nitrogen coordinated active sites towards oxygen reduction reaction in aluminum-air batteries with robust integrated air cathode. J. Power Sources 2023, 556, 232476. [Google Scholar] [CrossRef]
- Li, L.; Bai, J.; Jiang, P.; Zhang, Y.; Zhou, T.; Wang, J.; Zhou, C.; Li, J.; Zhou, B. Efficient H2O2 Electrosynthesis and Its Electro-Fenton Application for Refractory Organics Degradation. Engineering 2023, 30, 131–143. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Zhu, Y.; Zhao, P.; Chen, B.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting Reaction Kinetics and Mass Transfer of Bifunctional Co-Based Oxygen Electrocatalyst Prepared from CoAl-LDH. Adv. Energy Mater. 2023, 13, 2301580. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Cheng, C. Three-dimensionally ordered Co3O4@WO3 composite arrays as a binder-free air cathode for high-performance Zn-air batteries. Mater. Today Nano 2023, 22, 100339. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Ali, R.H.; Kim, H.Y.; Nassar, M.M.; Fadali, O.A.; Tolba, G.M.K.; Moustafa, H.M.; Ali, M.A. Carbon Nanofibers-Sheathed Graphite Rod Anode and Hydrophobic Cathode for Improved Performance Industrial Wastewater-Driven Microbial Fuel Cells. Nanomaterials 2022, 12, 3961. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.K.; Dziegielowski, J.; Kundu, P.P.; Di Lorenzo, M. Functionalised graphite felt anodes for enhanced power generation in membrane-less soil microbial fuel cells. RSC Sustain. 2023, 1, 310–325. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure. Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kumagai, S.; Ishizawa, H.; Aoki, Y.; Toida, Y. Molded micro- and mesoporous carbon/silica composite from rice husk and beet sugar. Chem. Eng. J. 2010, 156, 270–277. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, X. Catalysis Kinetics and Porous Analysis of Rolling Activated Carbon-PTFE Air-Cathode in Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 13009–13015. [Google Scholar] [CrossRef]
- Li, N.; An, J.; Zhou, L.; Li, T.; Li, J.; Feng, C.; Wang, X. A novel carbon black graphite hybrid air-cathode for efficient hydrogen peroxide production in bioelectrochemical systems. J. Power Sources 2016, 306, 495–502. [Google Scholar] [CrossRef]
- Chen, J.; Li, N.; Zhao, L. Three-dimensional electrode microbial fuel cell for hydrogen peroxide synthesis coupled to wastewater treatment. J. Power Sources 2014, 254, 316–322. [Google Scholar] [CrossRef]
- Huang, J.; Sun, B.; Zhang, X. Electricity generation at high ionic strength in microbial fuel cell by a newly isolated Shewanella marisflavi EP1. Appl. Microbiol. Biotechnol. 2009, 85, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Maitlo, H.A.; Kim, J.H.; An, B.M.; Park, J.Y. Effects of supporting electrolytes in treatment of arsenate-containing wastewater with power generation by aluminumair fuel cell electrocoagulation. J. Ind. Eng. Chem. 2018, 57, 254–262. [Google Scholar] [CrossRef]
- Huang, A.; Zhi, D.; Tang, H.; Jiang, L.; Luo, S.; Zhou, Y. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage. Sci. Total. Environ. 2020, 720, 137560. [Google Scholar] [CrossRef]
- Dai, C.; Shi, S.; Chen, D.; Liu, J.; Huang, L.; Zhang, J.; Feng, Y. Study on the mechanism of tetracycline removal in electrocoagulation coupled with electro-Fenton reaction system with Fe anode and carbon nanotube cathode. Chem. Eng. J. 2022, 428, 131045. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior Fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Feng, H.; Zhou, Y.; Zeng, G.; Lu, Y.; Yu, J.; Ren, X.; Peng, B.; Liu, X. Carbon felt cathodes for electro-Fenton process to remove tetracycline via synergistic adsorption and degradation. Sci. Total. Environ. 2019, 670, 921–931. [Google Scholar] [CrossRef]
- Hou, Y.; Qu, J.; Zhao, X.; Lei, P.; Wan, D.; Huang, C.P. Electro-photocatalytic degradation of acid orange II using a novel TiO2/ACF photoanode. Sci. Total. Environ. 2009, 407, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and stable photocatalytic degradation of tetracycline wastewater by 3D Polyaniline/Perylene diimide organic heterojunction under visible light irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020, 277, 119122. [Google Scholar] [CrossRef]


| Cathodes | |||
|---|---|---|---|
| Characteristic | AC | CB | G |
| BET surface area (m2/g) | 355.3996 | 177.8102 | 2.9440 |
| t-Plot micropore area (m2/g) | 146.2380 | 2.7059 | 0.5463 |
| t-Plot micropore volume (cm3/g) | 0.060737 | 0.000224 | 0.000041 |
| BJH desorption cumulative volume (cm3/g) | 0.278715 | 0.879021 | 0.037034 |
| BJH desorption average pore diameter (nm) | 5.6651 | 17.1297 | 31.2793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Wei, W.; Wu, H.; Gong, H.; Zhou, K.; Zheng, Q.; Liu, S.; Gui, L.; Jiang, Z.; Zhu, S. Coupling Electro-Fenton and Electrocoagulation of Aluminum–Air Batteries for Enhanced Tetracycline Degradation: Improving Hydrogen Peroxide and Power Generation. Molecules 2024, 29, 3781. https://doi.org/10.3390/molecules29163781
Zhou Z, Wei W, Wu H, Gong H, Zhou K, Zheng Q, Liu S, Gui L, Jiang Z, Zhu S. Coupling Electro-Fenton and Electrocoagulation of Aluminum–Air Batteries for Enhanced Tetracycline Degradation: Improving Hydrogen Peroxide and Power Generation. Molecules. 2024; 29(16):3781. https://doi.org/10.3390/molecules29163781
Chicago/Turabian StyleZhou, Zhenghan, Wei Wei, Houfan Wu, Haoyang Gong, Kai Zhou, Qiyuan Zheng, Shaogen Liu, Ling Gui, Zhongqi Jiang, and Shuguang Zhu. 2024. "Coupling Electro-Fenton and Electrocoagulation of Aluminum–Air Batteries for Enhanced Tetracycline Degradation: Improving Hydrogen Peroxide and Power Generation" Molecules 29, no. 16: 3781. https://doi.org/10.3390/molecules29163781
APA StyleZhou, Z., Wei, W., Wu, H., Gong, H., Zhou, K., Zheng, Q., Liu, S., Gui, L., Jiang, Z., & Zhu, S. (2024). Coupling Electro-Fenton and Electrocoagulation of Aluminum–Air Batteries for Enhanced Tetracycline Degradation: Improving Hydrogen Peroxide and Power Generation. Molecules, 29(16), 3781. https://doi.org/10.3390/molecules29163781





