Precisely Designed Morphology and Surface Chemical Structure of Fe-N-C Electrocatalysts for Enhanced Oxygen Reaction Reduction Activity
Abstract
1. Introduction
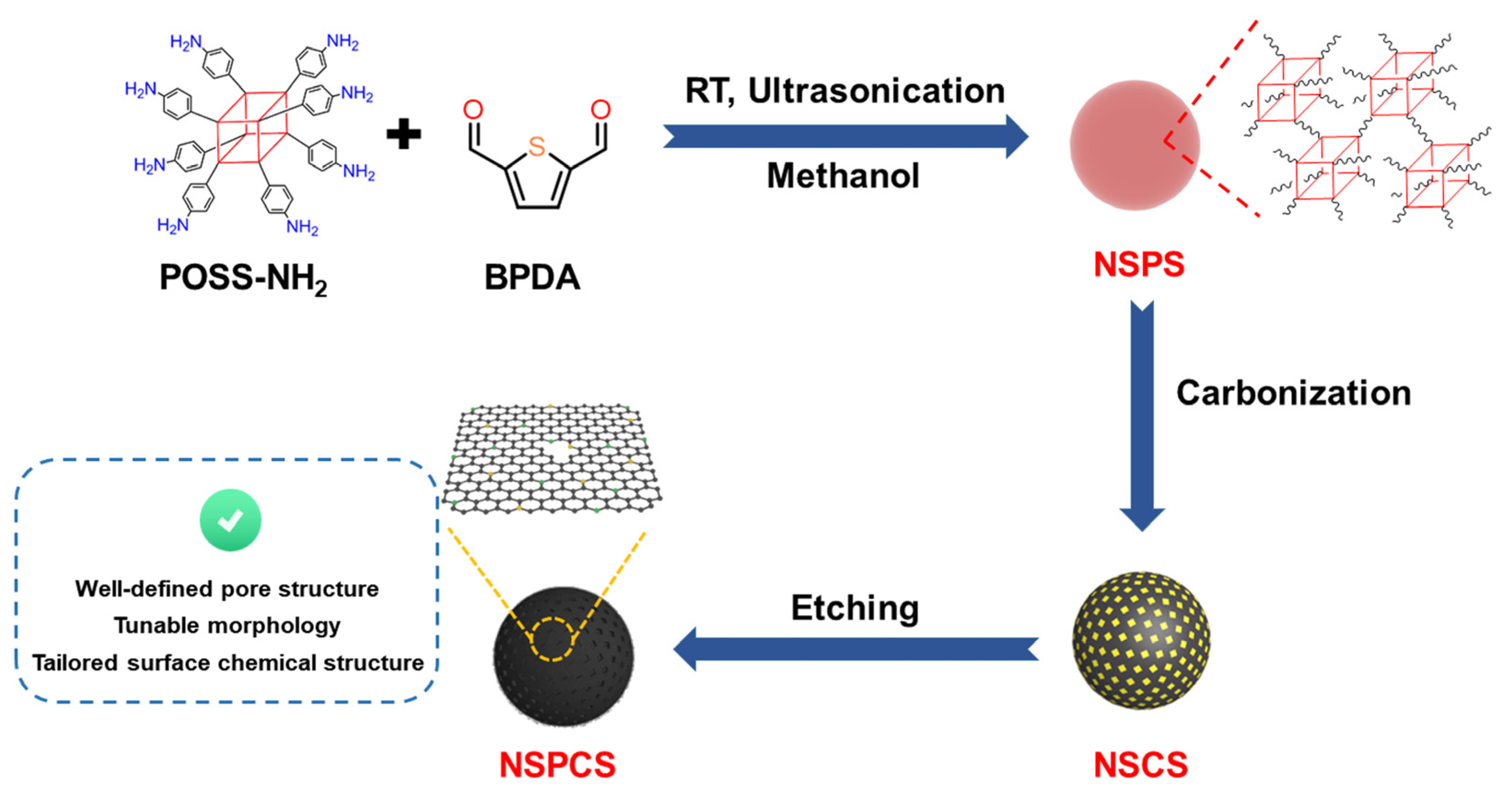
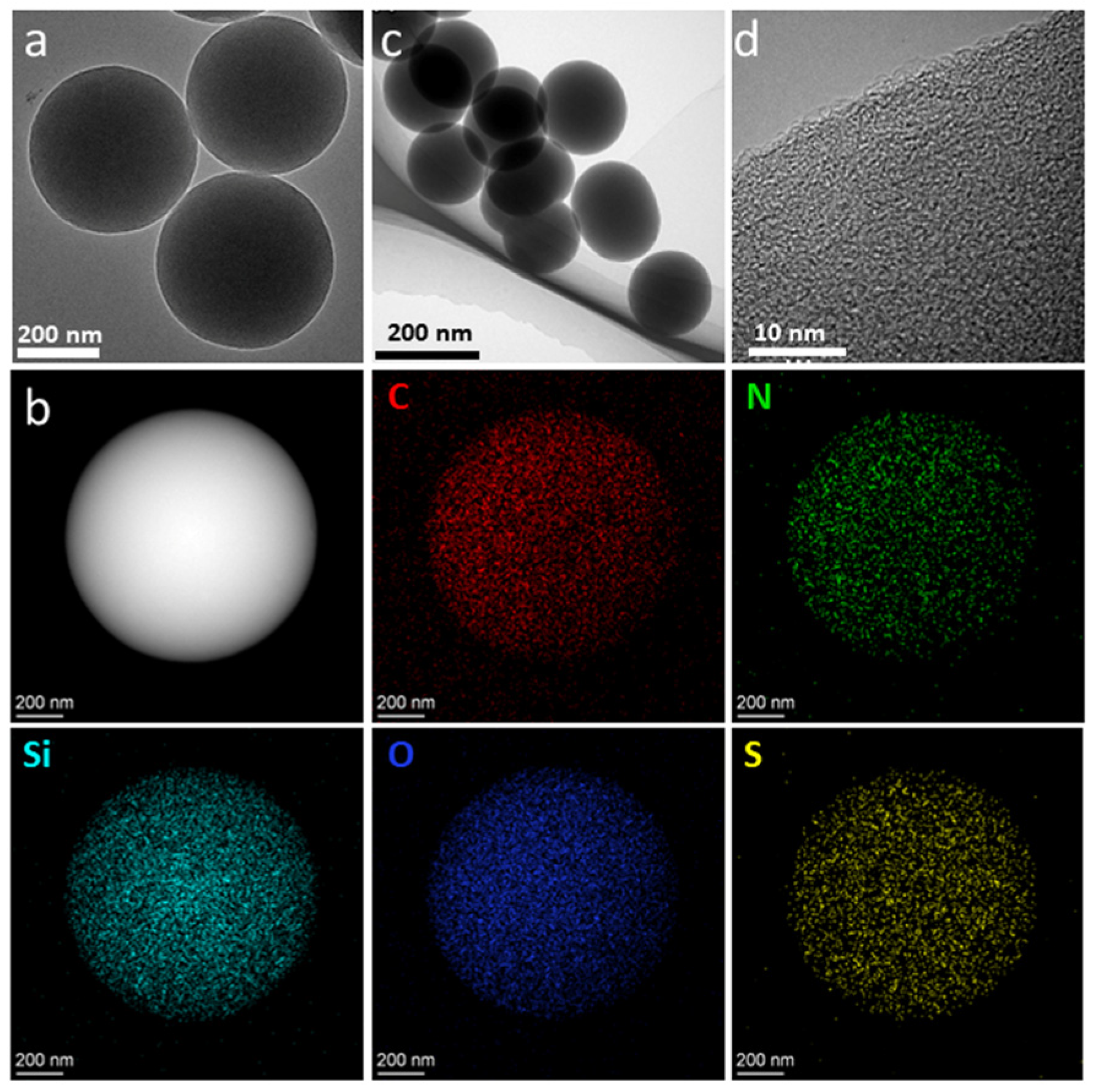
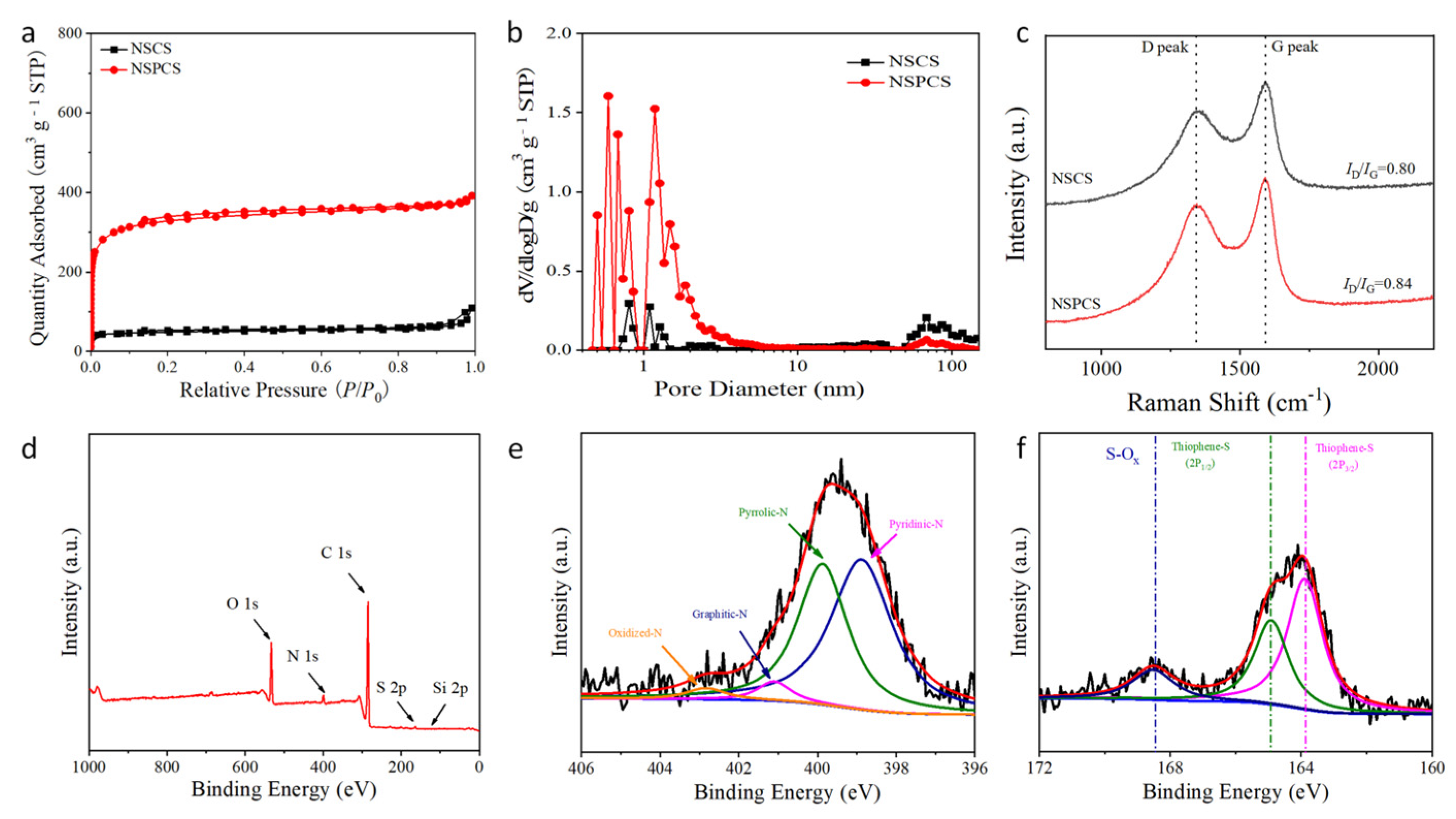
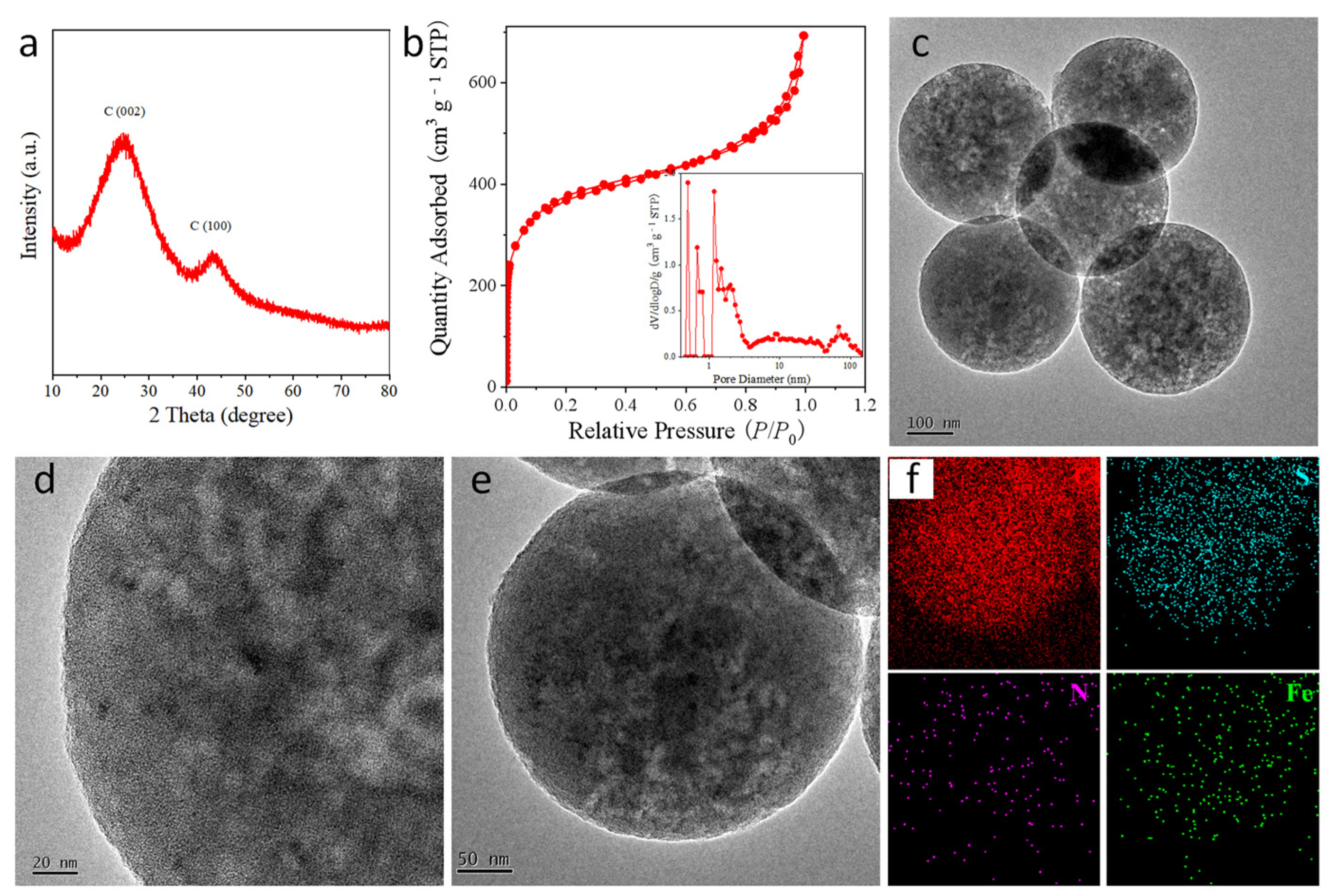
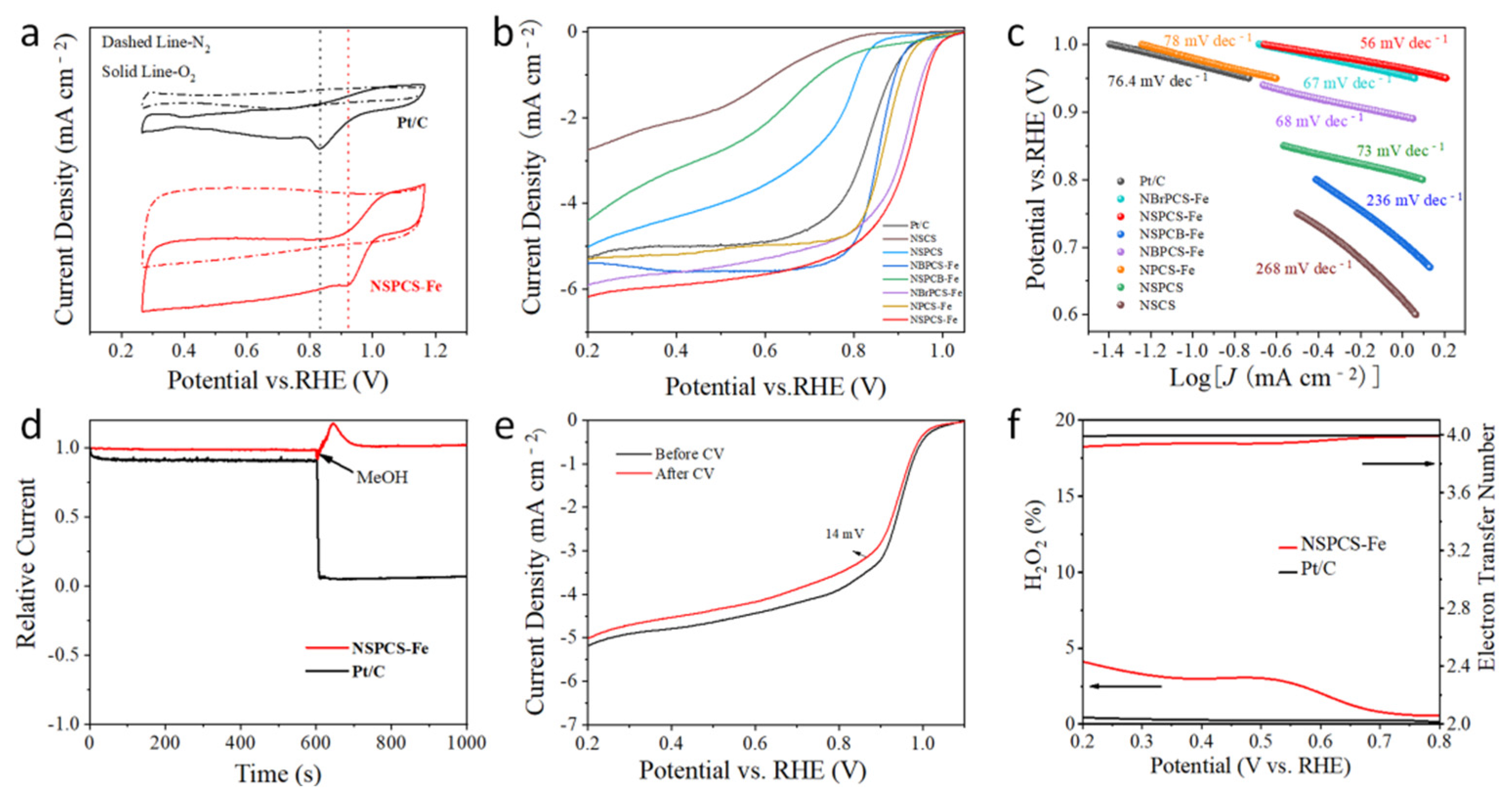
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of NSPCS
3.2.2. Synthesis of NSPCS-Fe
3.3. Characterization
3.4. Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, C.; Xing, G.; Han, W.; Hao, Y.; Zhang, C.; Zhang, Y.; Kuo, C.H.; Chen, H.Y.; Hu, F.; Li, L.; et al. Inhibiting Demetalation of Fe─N─C via Mn Sites for Efficient Oxygen Reduction Reaction in Zinc-Air Batteries. Adv. Mater. 2024, 2405763. [Google Scholar] [CrossRef]
- Poudel, M.B.; Balanay, M.P.; Lohani, P.C.; Sekar, K.; Yoo, D.J. Atomic Engineering of 3D Self-Supported Bifunctional Oxygen Electrodes for Rechargeable Zinc-Air Batteries and Fuel Cell Applications. Adv. Energy Mater. 2024, 2400347. [Google Scholar] [CrossRef]
- Jang, I.; Lee, S.; Kim, D.G.; Paidi, V.K.; Lee, S.; Kim, N.D.; Jung, J.Y.; Lee, K.S.; Lim, H.K.; Kim, P.; et al. Instantaneous Thermal Energy for Swift Synthesis of Single-Atom Catalysts for Unparalleled Performance in Metal–Air Batteries and Fuel Cells. Adv. Mater. 2024, 2403273. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horizons 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Zhao, S.N.; Li, J.K.; Wang, R.; Cai, J.; Zang, S.Q. Electronically and Geometrically Modified Single-Atom Fe Sites by Adjacent Fe Nanoparticles for Enhanced Oxygen Reduction. Adv. Mater. 2022, 34, 2107291. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, D.; Hu, F.; Huang, S.C.; Song, J.; Chen, H.Y.; Li, L.L.; Peng, S. Clusters Induced Electron Redistribution to Tune Oxygen Reduction Activity of Transition Metal Single-Atom for Metal–Air Batteries. Angew. Chem. Int. Ed. 2022, 61, e202116068. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Chen, Y. Recent Design Strategies for M-N-C Single-Atom Catalysts in Oxygen Reduction: An Entropy Increase Perspective. Adv. Funct. Mater. 2024, 2401027. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.H.; Zheng, H.; Li, R.; Zhou, K. Oxygen-Rich Cobalt–Nitrogen–Carbon Porous Nanosheets for Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2200763. [Google Scholar] [CrossRef]
- Tong, M.; Sun, F.; Xing, G.; Tian, C.; Wang, L.; Fu, H. Potential Dominates Structural Recombination of Single Atom Mn Sites for Promoting Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2023, 62, e202314933. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Geng, S.; Tian, Y.; Chen, R.; Wang, K.; Wang, Y.; Song, S. PtFe Nanoalloys Supported on Fe-Based Cubic Framework as Efficient Oxygen Reduction Electrocatalysts for Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2024, 34, 2307297. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, T.; Kou, Z.; Shen, L.; Zhao, Y.; Wang, Z.; Wang, X.; Yang, Z.; Du, J.; Xu, J.; et al. Negative Pressure Pyrolysis Induced Highly Accessible Single Sites Dispersed on 3D Graphene Frameworks for Enhanced Oxygen Reduction. Angew. Chem. Int. Ed. 2020, 59, 20465–20469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, S.; Chen, L.; Huang, J.; Zheng, B.; Huang, W.; Li, S.; Lu, Y.; Fu, R. A scalable molecular-templating strategy toward well-defined microporous carbon aerogels for efficient water treatment and electrocatalysis. Chem. Eng. J. 2021, 418, 129315. [Google Scholar] [CrossRef]
- Song, K.; Yang, B.; Zou, X.; Zhang, W.; Zheng, W. Unified ORR mechanism criteria via charge–spin–coordination of Fe functional units. Energy Environ. Sci. 2024, 17, 27–48. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, X.; Wang, M.; Yang, X.; Liu, S.; Chen, H.C.; Zhuang, Z.; Zhang, Y.; Cheong, W.C.; Zhang, C.; et al. Construction of N, P Co-Doped Carbon Frames Anchored with Fe Single Atoms and Fe2P Nanoparticles as a Robust Coupling Catalyst for Electrocatalytic Oxygen Reduction. Adv. Mater. 2022, 34, e2203621. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, W.; Xiao, H.; Gong, Y.; Li, Z.; Zheng, L.; Zheng, X.; Yan, W.; Cheong, W.C.; Shen, R.; et al. Fe Isolated Single Atoms on S, N Codoped Carbon by Copolymer Pyrolysis Strategy for Highly Efficient Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1800588. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, R.; Ye, Q.; Jin, X.; Lu, Z.; Yang, Z.; Wang, Y.; Yan, T.; Liu, Y.; Pan, Z.; et al. Tailoring First Coordination Sphere of Dual-Metal Atom Sites Boosts Oxygen Reduction and Evolution Activities. Adv. Funct. Mater. 2024, 34, 2315376. [Google Scholar] [CrossRef]
- Hu, L.; Dai, C.; Chen, L.; Zhu, Y.; Hao, Y.; Zhang, Q.; Gu, L.; Feng, X.; Yuan, S.; Wang, L.; et al. Metal-Triazolate-Framework-Derived FeN4Cl1 Single-Atom Catalysts with Hierarchical Porosity for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 27324–27329. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Yu, L.; Hou, J.; Zhou, Z.; Lv, R. Atomic Fe-N4/C in flexible carbon fiber membrane as binder-free air cathode for Zn-air batteries with stable cycling over 1000 hours. Adv. Mater. 2022, 34, 2105410. [Google Scholar] [CrossRef]
- Wei, S.; Yang, R.; Wang, Z.; Zhang, J.; Bu, X.H. Planar Chlorination Engineering: A Strategy of Completely Breaking the Geometric Symmetry of Fe-N4 Site for Boosting Oxygen Electroreduction. Adv. Mater. 2024, 36, 2404692. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, X.; Zhao, Z.; Zuo, W.; Chen, S.; Li, Z.; He, Y.; Liang, J.; Ma, F.; Wang, H.L.; et al. Improving the Stability of Non-Noble-Metal M–N–C Catalysts for Proton-Exchange-Membrane Fuel Cells through M–N Bond Length and Coordination Regulation. Adv. Mater. 2021, 33, 2006613. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Li, L.; Liu, X.; Wang, N.; Liu, J.; Zhou, W.; Tang, Z.; Chen, S. Mesoporous N-Doped Carbons Prepared with Thermally Removable Nanoparticle Templates: An Efficient Electrocatalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, Q.; Xu, B.Z.; Fu, S.; Wan, G.; Yang, C.; Yao, S.; Song, J.; Zhou, H.; Du, D.; et al. Hierarchically Porous M–N–C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MNx Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Gao, Y.; Jiang, C.; Jia, Y.; Chen, T.; Hou, Z.; Qi, R.; Jiang, D.; et al. Metal–Organic Framework-Derived Graphene Mesh: A Robust Scaffold for Highly Exposed Fe–N4 Active Sites toward an Excellent Oxygen Reduction Catalyst in Acid Media. J. Am. Chem. Soc. 2022, 144, 9280–9291. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, Y.; Yang, J.; Liu, J.; Yang, S.; Cheng, X.; Huang, H.; Huang, R.; Wang, C.T.; Jiang, Y.; et al. Unveiling Low Temperature Assembly of Dense Fe-N4 Active Sites via Hydrogenation in Advanced Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2024, 63, e202404766. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gong, Z.; Liu, J.; Ye, G.; Fei, H. General and Ultrafast Photothermal Synthesis of Atomic Metal-Nitrogen-Carbon Catalysts for H2O2 Electrosynthesis. Adv. Funct. Mater. 2024, 34, 2316438. [Google Scholar] [CrossRef]
- Meng, R.; Zhang, C.; Lu, Z.; Xie, X.; Liu, Y.; Tang, Q.; Li, H.; Kong, D.; Geng, C.N.; Jiao, Y.; et al. An Oxygenophilic Atomic Dispersed Fe-N-C Catalyst for Lean-Oxygen Seawater Batteries. Adv. Energy Mater. 2021, 11, 2100683. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Kim, K.; Kim, S.; Lee, S.; Han, J.W.; Lee, J. Versatile Strategy for Tuning ORR Activity of a Single Fe-N4 Site by Controlling Electron-Withdrawing/Donating Properties of a Carbon Plane. J. Am. Chem. Soc. 2019, 141, 6254–6262. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Yuan, S.; Liu, T.; Wang, Q. High-coordination Fe–N4SP single-atom catalysts via the multi-shell synergistic effect for the enhanced oxygen reduction reaction of rechargeable Zn–air battery cathodes. Energy Environ. Sci. 2024, 17, 249–259. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Liu, S.; Hu, Y.; Zhang, J.; Xia, M.; Hou, Y.; Tse, J.; Zhang, J.; Zhao, Y. Turning on Zn 4s Electrons in a N2-Zn-B2 Configuration to Stimulate Remarkable ORR Performance. Angew. Chem. Int. Ed. 2021, 60, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, Q.; Xu, Y.; Guo, S.; Sun, W.; Liu, H.; Lv, L.P.; Zhang, Y.; Wang, Y. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, F.; Cao, L.; Li, B.; Yuan, K.; Lei, S.; Hu, W. A Robust Nonvolatile Resistive Memory Device Based on a Freestanding Ultrathin 2D Imine Polymer Film. Adv. Mater. 2019, 31, e1902264. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.F.; Li, Y.C.; Yu, R.H.; Zheng, L.R.; Yan, W.S.; Wang, H.; Xu, M.; Shui, J.L. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Chen, G.; An, Y.; Liu, S.; Sun, F.; Qi, H.; Wu, H.; He, Y.; Liu, P.; Shi, R.; Zhang, J.; et al. Highly accessible and dense surface single metal FeN4 active sites for promoting the oxygen reduction reaction. Energy Environ. Sci. 2022, 15, 2619–2628. [Google Scholar] [CrossRef]
- Wang, H.; Yin, F.X.; Liu, N.; Kou, R.H.; He, X.B.; Sun, C.J.; Chen, B.H.; Liu, D.J.; Yin, H.Q. Engineering Fe–Fe3C@Fe–N–C Active Sites and Hybrid Structures from Dual Metal–Organic Frameworks for Oxygen Reduction Reaction in H2–O2 Fuel Cell and Li–O2 Battery. Adv. Funct. Mater. 2019, 29, 1901531. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Huang, J.; Huang, W.; Chen, L.; Cui, Y.; Du, Y.; Fu, R. Molecular Level Design of Nitrogen-Doped Well-Defined Microporous Carbon Spheres for Selective Adsorption and Electrocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 12025–12032. [Google Scholar] [CrossRef]
- Fu, X.; Li, N.; Ren, B.; Jiang, G.; Liu, Y.; Hassan, F.M.; Su, D.; Zhu, J.; Yang, L.; Bai, Z.; et al. Tailoring FeN4 Sites with Edge Enrichment for Boosted Oxygen Reduction Performance in Proton Exchange Membrane Fuel Cell. Adv. Energy Mater. 2019, 9, 1803737. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yu, X.W.; Manthiram, A. “Wiring” Fe-Nx-Embedded Porous Carbon Framework onto 1D Nanotubes for Efficient Oxygen Reduction Reaction in Alkaline and Acidic Media. Adv. Mater. 2017, 29, 10. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Shen, H.; Gracia Espino, E.; Ma, J.; Zang, K.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T.; et al. Synergistic Effects between Atomically Dispersed Fe-N-C and C-S-C for the Oxygen Reduction Reaction in Acidic Media. Angew. Chem. Int. Ed. 2017, 56, 13800–13804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Zhu, Y.; Sun, D.; Liu, X.; Xu, L.; Tang, Y. Atomic Fe Dispersed on N-Doped Carbon Hollow Nanospheres for High-Efficiency Electrocatalytic Oxygen Reduction. Adv. Mater. 2019, 31, 1806312. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zou, S.; Li, J.; Li, C.; Liu, X.; Dong, D. Fe, B, and N Codoped Carbon Nanoribbons Derived from Heteroatom Polymers as High-Performance Oxygen Reduction Reaction Electrocatalysts for Zinc–Air Batteries. Langmuir 2021, 37, 13018–13026. [Google Scholar] [CrossRef] [PubMed]
- Vasu, D.; Ravi, P.V.; Subramaniyam, V.; Pichumani, M.; You, Y.F.; Chiu, T.W. Electrochemical Quantification of Dopamine Sensing in Human Samples Using Bromine Doped Layered gC3N4 Nanosheets. J. Electrochem. Soc. 2023, 170, 117503. [Google Scholar] [CrossRef]
- Naveen, M.H.; Shim, K.; Hossain, M.S.A.; Kim, J.H.; Shim, Y.B. Template Free Preparation of Heteroatoms Doped Carbon Spheres with Trace Fe for Efficient Oxygen Reduction Reaction and Supercapacitor. Adv. Energy Mater. 2017, 7, 1602002. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Wu, H.; Liu, X.; Wang, L.; Yu, Q.; Li, A.; Wang, H.; Song, C.; Gao, Z.; et al. Construction of a sp3/sp2 Carbon Interface in 3D N-Doped Nanocarbons for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 15089–15097. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xiong, Y.; Liu, Y.; Wang, Z.; Zhang, B.; Liang, X.; Chen, X.; Yin, Y. Precisely Designed Morphology and Surface Chemical Structure of Fe-N-C Electrocatalysts for Enhanced Oxygen Reaction Reduction Activity. Molecules 2024, 29, 3785. https://doi.org/10.3390/molecules29163785
Chen Z, Xiong Y, Liu Y, Wang Z, Zhang B, Liang X, Chen X, Yin Y. Precisely Designed Morphology and Surface Chemical Structure of Fe-N-C Electrocatalysts for Enhanced Oxygen Reaction Reduction Activity. Molecules. 2024; 29(16):3785. https://doi.org/10.3390/molecules29163785
Chicago/Turabian StyleChen, Zirun, Yuang Xiong, Yanling Liu, Zhanghongyuan Wang, Binbin Zhang, Xingtang Liang, Xia Chen, and Yanzhen Yin. 2024. "Precisely Designed Morphology and Surface Chemical Structure of Fe-N-C Electrocatalysts for Enhanced Oxygen Reaction Reduction Activity" Molecules 29, no. 16: 3785. https://doi.org/10.3390/molecules29163785
APA StyleChen, Z., Xiong, Y., Liu, Y., Wang, Z., Zhang, B., Liang, X., Chen, X., & Yin, Y. (2024). Precisely Designed Morphology and Surface Chemical Structure of Fe-N-C Electrocatalysts for Enhanced Oxygen Reaction Reduction Activity. Molecules, 29(16), 3785. https://doi.org/10.3390/molecules29163785






