Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Characteristics of Activated Carbon Samples
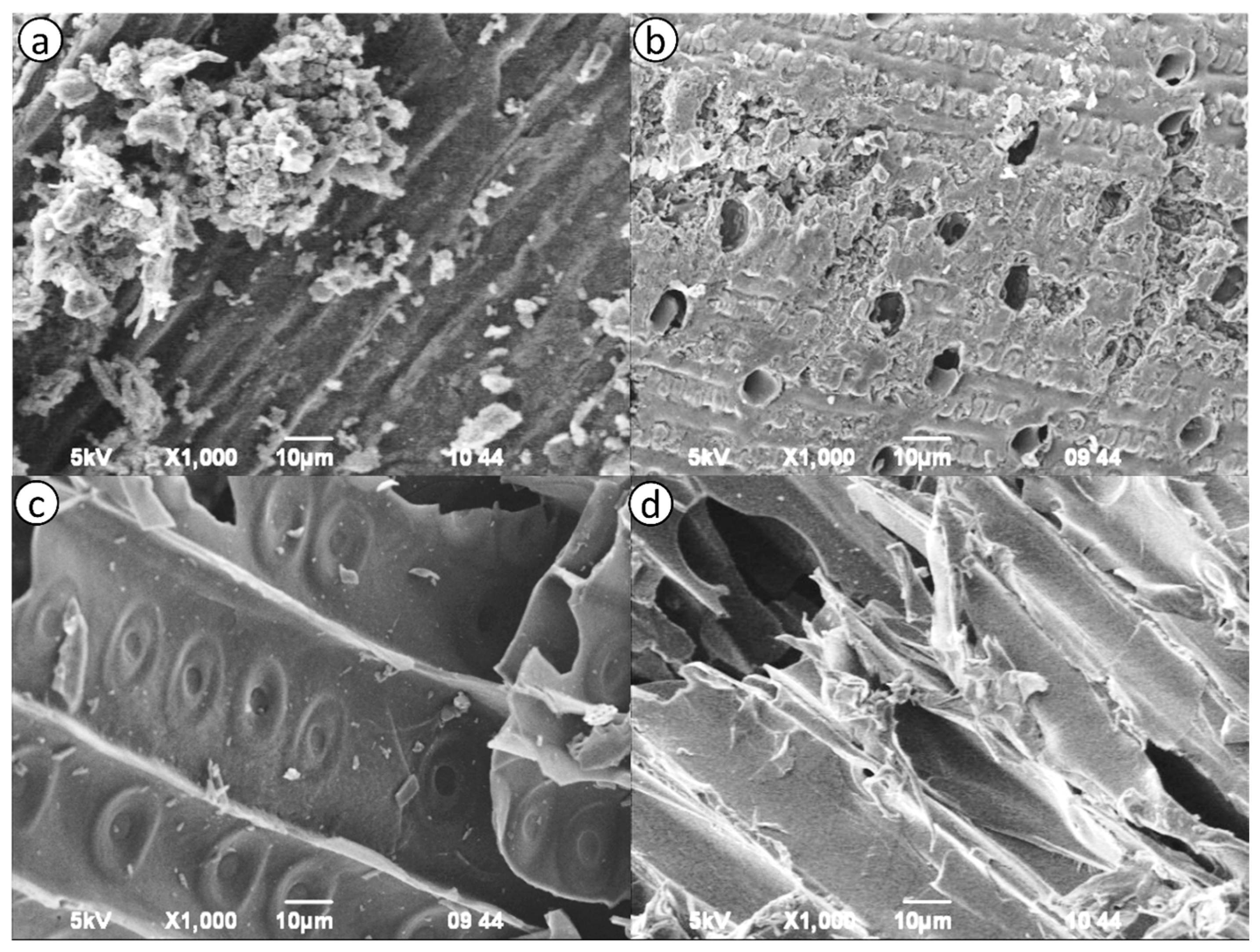
2.2. Structural Characteristics of Activated Carbon Samples
2.3. Optimization of Activation Parameters for Enhancing the Adsorption Capacity of Biomass-Derived Activated Carbons
2.4. Characterization of Biomass-Derived Activated Carbons: Insights from FTIR and Raman Spectroscopy
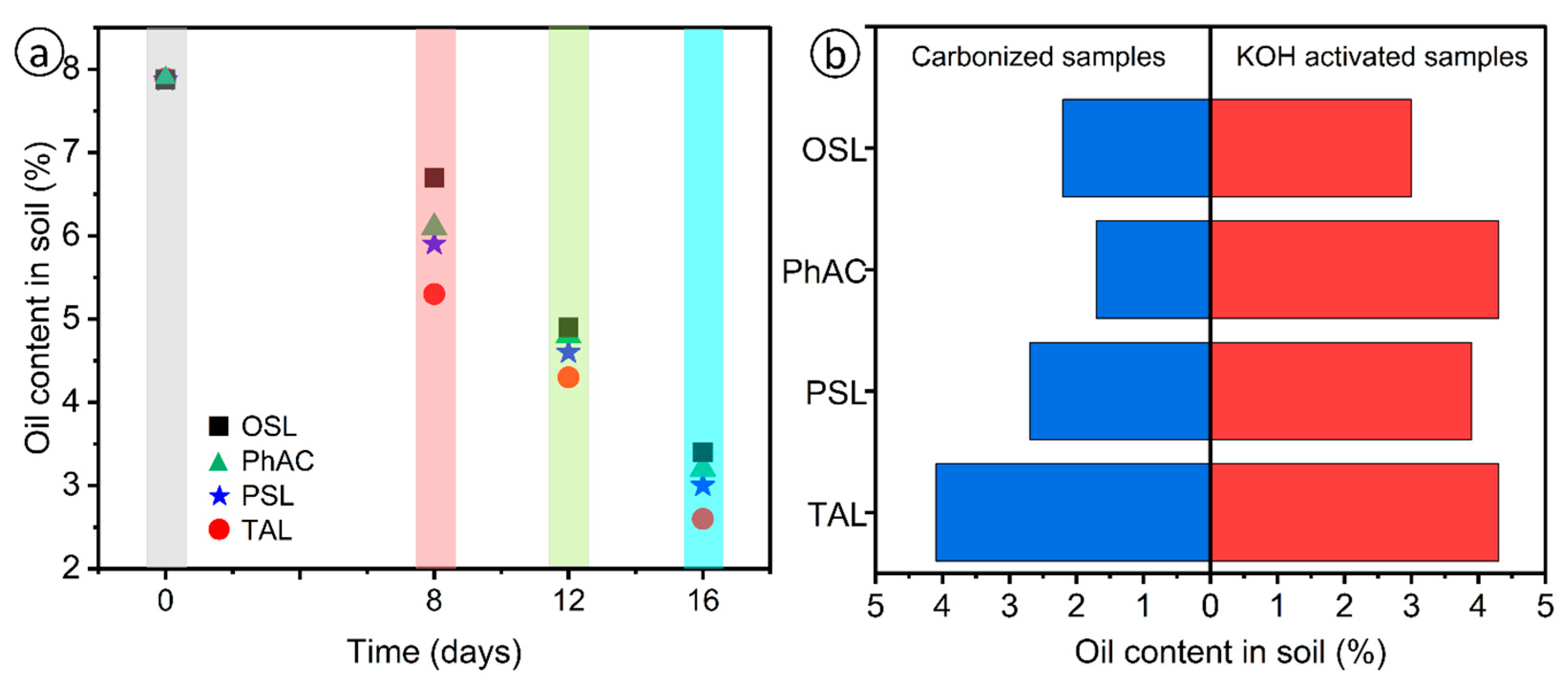
2.5. Investigation and Field Application of Activated Carbon for Hydrocarbon Sorption
3. Materials and Methods
3.1. Preparation of Materials
3.2. Activated Carbon Production from Agricultural Wastes
3.3. Study of Physico-Chemical, Technological Properties of Sorbents
3.4. Sample Characteristics
3.5. Research of the Sorption Capacity of Activated Carbon in Relation to Hydrocarbons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Rincon, J.; Gatsios, E.; Lenhard, R.J.; Atekwana, E.A.; Naidu, R. Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- da Silva Correa, H.; Blum, C.T.; Galvao, F.; Maranho, L.T. Effects of oil contamination on plant growth and development: A re-view. Environ. Sci. Pollut. Res. 2022, 29, 43501–43515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, X.; Li, X. Interfacial Interaction of Clay and Saturates in Petroleum-Contaminated Soil: Effect of Clay Surface Heterogeneity. Molecules 2022, 27, 7950. [Google Scholar] [CrossRef] [PubMed]
- Taurbekov, A.; Abdisattar, A.; Atamanov, M.; Kaidar, B.; Yeleuov, M.; Joia, R.; Amrousse, R.; Atamanova, T. Investigations of Activated Carbon from Different Natural Sources for Preparation of Binder-Free Few-Walled CNTs/Activated Carbon Electrodes. J. Compos. Sci. 2023, 7, 452. [Google Scholar] [CrossRef]
- Mohan, P.R. Effective acrylamide adsorption in aqueous environments using maize straw nanobiochar (MNBC). Nanotechnol. Environ. Eng. 2024, 7, 1–15. [Google Scholar] [CrossRef]
- Ngo, T.C.Q.; Tran, T.K.N.; Chau, H.D.; Hoang, B.N. The material potential and application of activated carbon from nut shells: Mini review. Mater. Today Proc. 2023, 3, 511. [Google Scholar] [CrossRef]
- Seitzhanova, M.; Azat, S.; Yeleuov, M.; Taurbekov, A.; Mansurov, Z.; Doszhanov, E.; Berndtsson, R. Production of Graphene Membranes from Rice Husk Biomass Waste for Improved Desalination. Nanomaterials 2024, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Saka, C. BET, TG-DTG, FT-IR, SEM, Iodine Number Analysis and Preparation of Activated Carbon from Acorn Shell by Chemical Activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Sahin, O.; Saka, C. Preparation and characterization of activated carbon from acorn shell by physical activation with H2O-CO2 in two-step pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Robles, E.; Izaguirre, N.; Martin, A.; Moschou, D.; Labidi, J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules 2021, 26, 1371. [Google Scholar] [CrossRef]
- Qin, L.; Wu, Y.; Hou, Z.; Jiang, E. Influence of biomass components, temperature and pressure on the pyrolysis behavior and biochar properties of pine nut shells. Bioresour. Technol. 2020, 313, 123682. [Google Scholar] [CrossRef]
- Vuong, T.X.; Stephen, J.; Nguyen, T.T.T.; Cao, V.; Pham, D.T.N. Insight into the Speciation of Heavy Metals in the Contaminated Soil Incubated with Corn Cob-Derived Biochar and Apatite. Molecules 2023, 28, 2225. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Adsorption of pesticides from aqueous solution onto banana stalk activated carbon. Chem. Eng. J. 2011, 174, 41–48. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-chemical characterization of carbonized apricot kernel shell as precursor for activated carbon preparation in clean technology utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Usino, D.O.; Ylitervo, P.; Richards, T. Primary Products from Fast Co-Pyrolysis of Palm Kernel Shell and Sawdust. Molecules 2023, 28, 6809. [Google Scholar] [CrossRef] [PubMed]
- Ganaraja, A.Y.; Machado, A.A.; Mulky, L. A Comparative Study of Treatment Methods of Raw Sugarcane Bagasse for Adsorption of Oil and Diesel. Water Air Soil Pollut. 2023, 234, 213. [Google Scholar] [CrossRef]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Lesbayev, B.; Rakhymzhan, N.; Ustayeva, G.; Maral, Y.; Atamanov, M.; Auyelkhankyzy, M.; Zhamash, A. Preparation of Nanoporous Carbon from Rice Husk with Improved Textural Characteristics for Hydrogen Sorption. J. Compos. Sci. 2024, 8, 74. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Velasco, L.F.; Lodewyckx, P.; Doszhanov, E.O.; Azat, S. Modified Carbon Sorbents Based on Walnut Shell for Sorption of Toxic Gases. J. Eng. Phys. Thermophys. 2022, 95, 1383–1392. [Google Scholar] [CrossRef]
- Sun, Y.; Webley, P.A. Preparation of activated carbons from corncob with large specific surface area by a variety of chemical activators and their application in gas storage. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar] [CrossRef]
- Saka, C.; Sahin, Ö.; Küçük, M.M. Applications on Agricultural and Forest Waste Adsorbents for the Removal of Lead(II) from Contaminated Waters. Int. J. Environ. Sci. Technol. 2012, 9, 379–394. [Google Scholar] [CrossRef]
- Ilyin, Y.V.; Kudaibergenov, K.K.; Sharipkhanov, S.D.; Mansurov, Z.A.; Zhaulybayev, A.A.; Atamanov, M.K. Surface Modifications of CuO Doped Carbonaceous Nanosorbents and their CO2 Sorption Properties. Eurasian Chem.-Technol. J. 2023, 25, 33–38. [Google Scholar] [CrossRef]
- El-Hendawy, A.A.; Alexander, A.J.; Andrews, R.J.; Forrest, G. Effects of activation schemes on porous, surface and thermal properties of activated carbons prepared from cotton stalks. J. Anal. Appl. Pyrolysis 2008, 82, 272–278. [Google Scholar] [CrossRef]
- Mopoung, S.; Dejang, N. Activated carbon preparation from eucalyptus wood chips using continuous carbonization-steam activation process in a batch intermittent rotary kiln. Sci. Rep. 2021, 11, 13948. [Google Scholar] [CrossRef] [PubMed]
- Yakaboylu, G.A.; Jiang, C.; Yumak, T.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2021, 163, 276–287. [Google Scholar] [CrossRef]
- Wen, Y.; Chi, L.; Wenelska, K.; Wen, X.; Chen, X.; Mijowska, E. Eucalyptus derived heteroatom-doped hierarchical porous carbons as electrode materials in supercapacitors. Sci. Rep. 2020, 10, 14631. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Garcia, C.A.; Moraes, J.C.G.; Nogami, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. Preparation and Characterization of Activated Carbon from a New Raw Lignocellulosic Material: Flamboyant (Delonix regia) Pods. J. Environ. Manag. 2011, 92, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, C.; Cheng, S.; Li, T. Microwave thermal regeneration characteristics of spent activated carbon based on a coupled electromagnetic, heat and mass transfer multiphase porous media model. Energy 2024, 292, 130437. [Google Scholar] [CrossRef]
- Roy, Z.; Barman, S.; Halder, G. Carbon dioxide sorptive cooling in substitute model refrigeration system for replacing halogenated refrigerants. Environ. Technol. Innov. 2021, 23, 101636. [Google Scholar] [CrossRef]
- Unger, R.; Killorn, R. Effect of the Application of Biochar on Selected Soil Chemical Properties, Corn Grain, and Biomass Yields in Iowa. Commun. Soil Sci. Plant Anal. 2011, 42, 2441–2451. [Google Scholar] [CrossRef]
- Gao, J.; Liu, D.; Xu, Y.; Chen, J.; Yang, Y.; Xia, D.; Ding, Y.; Xu, W. Effects of two types of activated carbon on the properties of vegetation concrete and Cynodon dactylongrowth. Sci. Rep. 2020, 10, 14483. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Ugulu, I.; Zafar, A.; Mehmood, N.; Bashir, H.; Ahmad, K.; Sana, M. Biomonitoring of heavy metals accumulation in wild plants growing at Soon valley, Khushab, Pakistan. Pak. J. Bot. 2021, 53, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Taurbekov, A.; Abdisattar, A.; Atamanov, M.; Yeleuov, M.; Daulbayev, C.; Askaruly, K.; Kaidar, B.; Mansurov, Z.; Castro-Gutierrez, J.; Celzard, A.; et al. Biomass Derived High Porous Carbon via CO2 Activation for Supercapacitor Electrodes. J. Compos. Sci. 2023, 7, 444. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Li, J.; Xue, N.; Meng, X.; Xie, L.; Yu, Y.; Liu, Z.; Qu, Z.; Gao, J.; et al. Efficient production of activated carbon with well-developed pore structure based on fast pyrolysis-physical activation. J Energy Inst. 2024, 11, 101685. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, G. Preparation and characterization of activated carbon fibers from liquefied wood by KOH activation. Holzforschung 2016, 70, 195–202. [Google Scholar] [CrossRef]
- Gao, B.; Zhou, H.; Chen, D.; Yang, J. Porous carbon with small mesoporesas an ultra-high capacity adsorption medium. Appl. Surf. Sci. 2017, 420, 535–541. [Google Scholar] [CrossRef]
- Zornitta, R.L.; Barcelos, K.M.; Nogueira, F.G.; Ruotolo, L.A. Understanding the mechanism of carbonization and KOH activation of polyaniline leading to enhanced electrosorption performance. Carbon 2020, 156, 346–358. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Muñoz, M.; Zazo, J.A.; Casas, J.A.; García, J. Synthesis of high surface area carbon adsorbents prepared from pine sawdust-Onopordum acanthium L. for nonsteroidal anti-inflammatory drugs adsorption. J. Environ. Manag. 2016, 183, 294–305. [Google Scholar] [CrossRef]
- Dujearic-Stephane, K.; Gupta, M.; Kumar, A.; Sharma, V.; Pandit, S.; Bocchetta, P.; Kumar, Y. The effect of modifications of activated carbon materials on the capacitive performance: Surface, microstructure, and wettability. J. Compos. Sci. 2021, 5, 66. [Google Scholar] [CrossRef]
- Zhang, J.; Jones, I.; Zhu, M.; Zhang, Z.; Preciado-Hernandez, J.; Zhang, D. Pore development during CO2 and steam activation of a spent tyre pyrolysis char. Waste Biomass Valorization 2021, 12, 2097–2108. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Román-Martínez, M.D.C.; Lillo-Ródenas, M.Á. Chemical activation of lignocellulosic precursors and residues: What else to consider? Molecules 2022, 27, 1630. [Google Scholar] [CrossRef] [PubMed]
- Shawabkeh, R.A.; Aslam, Z.; Hussien, I.A. Thermochemical treatment of fly ash for synthesis of mesoporous activated carbon. J. Therm. Anal. Calorim. 2015, 122, 1191–1201. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Correa, C.R.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2017, 97, 53–64. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Abdennouri, M.; Barka, N. Activated carbon from Diplotaxis Harra biomass: Optimization of preparation conditions and heavy metal removal. J. Taiwan Inst. Chem. Eng. 2016, 59, 348–358. [Google Scholar] [CrossRef]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Loredo-Cancino, M.; Soto-Regalado, E.; Cerino-Córdova, F.J.; García-Reyes, R.B.; García-León, A.M.; Garza-González, M.T. Determining optimal conditions to produce activated carbon from barley husks using single or dual optimization. J. Environ. Manag. 2013, 125, 117–125. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, Z.; Sun, S.; Xie, J.; Zhang, K.; Zhai, Y.; Zuo, R.; Bi, E.; Tao, Y.; Song, Q. Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil by Using Activated Persulfate with Carbonylated Activated Carbon Supported Nanoscale Zero-Valent Iron. Catalysts 2024, 14, 311. [Google Scholar] [CrossRef]
- Sugashini, S.; Begum, K.M.M.S. Preparation of activated carbon from carbonized rice husk by ozone activation for Cr (VI) removal. New Carbon Mater. 2015, 30, 252–261. [Google Scholar] [CrossRef]
- Wang, S.; Lee, Y.R.; Won, Y.; Kim, H.; Jeong, S.E.; Hwang, B.W.; Cho, A.R.; Kim, J.Y.; Park, Y.C.; Nam, H.; et al. Development of high-performance adsorbent using KOH-impregnated rice husk-based activated carbon for indoor CO2 adsorption. J. Chem. Eng. 2022, 437, 135378. [Google Scholar] [CrossRef]
- Somasundaram, S.; Sekar, K.; Gupta, V.K.; Ganesan, S. Synthesis and characterization of mesoporous activated carbon from rice husk for adsorption of glycine from alcohol-aqueous mixture. J. Mol. Liq. 2013, 177, 416–425. [Google Scholar] [CrossRef]
- Phan, K.A.; Phihusut, D.; Tuntiwiwattanapun, N. Preparation of rice husk hydrochar as an atrazine adsorbent: Optimization, characterization, and adsorption mechanisms. J. Environ. Chem. Eng. 2022, 10, 107575. [Google Scholar] [CrossRef]
- Qureshi, U.A.; Khatri, Z.; Ahmed, F.; Ibupoto, A.S.; Khatri, M.; Mahar, F.K.; Brohi, R.Z.; Kim, I.S. Highly efficient and robust electrospun nanofibers for selective removal of acid dye. J. Mol. Liq. 2017, 244, 478–488. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascón, J.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Maltay, A.B.; Askaruly, K.; Assylkhanova, D.D.; Seitkazinova, A.R.; Mansurov, Z.A. Biomass-derived porous carbon materials for Li-ion battery. Nanomaterials 2022, 12, 3710. [Google Scholar] [CrossRef] [PubMed]
- GOST 1236-2013; Fertilizers. Method for Determining Bulk Density without Compaction: Moscow, Russia, 2013.
- GOST 33618-2015; Activated Carbon. Standard Method for Determination of Iodine Value: Moscow, Russia, 2016.
- Gargiulo, V.; Alfè, M.; Raganati, F.; Zhumagaliyeva, A.; Doszhanov, Y.; Ammendola, P.; Chirone, R. CO2 Adsorption under Dynamic Conditions: An Overview on Rice Husk-Derived Sorbents and Other Materials. Combust. Sci. Technol. 2019, 191, 1484–1498. [Google Scholar] [CrossRef]
- Regadera-Macias, A.M.; Morales-Torres, S.; Pastrana-Martinez, L.M.; Maldonado-Hódar, F.J. Optimizing filters of activated carbons obtained from biomass residues for ethylene removal in agro-food industry devices. Environ. Res. 2024, 248, 118247. [Google Scholar] [CrossRef]



| Activated Carbon | Bulk Density, kg/m3 | Average Particle Size, mm | Specific Surface Area According to BET, m2/g | Volume of Micropores, cm3/g | Volume of Mesopores, cm3/g |
|---|---|---|---|---|---|
| OSL | 0.482 | 0.25 | 2790 | 0.46 | 0.16 |
| PhAC | 0.396 | 0.15 | 1330 | 0.06 | 0.08 |
| PSL | 0.423 | 0.30 | 2305 | 0.26 | 0.09 |
| TAL | 0.388 | 0.20 | 1755 | 0.08 | 0.06 |
| Activated Carbon | Volume of Pores According to (D-R), cm3/g | Volume of Pores According to (B-J-H), cm3/g | Volume of Pores According to (D-A), cm3/g | Relative Pressure, P/P0 |
|---|---|---|---|---|
| OSL | 0.665 | 1.19 | 0.74 | 0–0.5 |
| PhAC | 0.490 | 0.43 | 0.59 | 0–0.5 |
| PSL | 0.712 | 1.46 | 0.84 | 0–0.5 |
| TAL | 0.576 | 0.88 | 0.65 | 0–0.5 |
| Activated Carbon | Sorption Capacity | ||
|---|---|---|---|
| Gasoline, g/g | Kerosene, g/g | Diesel Fuel, g/g | |
| OSL | 9.30 | 9.00 | 10.10 |
| PhAC | 6.10 | 6.50 | 7.00 |
| PSL | 4.70 | 4.60 | 5.10 |
| TAL | 3.80 | 3.80 | 4.50 |
| Number of Days | Activated Carbon | Degree of Soil Purification, % |
|---|---|---|
| 0 | - | 0 |
| 8 | OSL | 27.8 |
| PhAC | 25.3 | |
| PSL | 22.8 | |
| TAL | 15.2 | |
| 16 | OSL | 67.1 |
| PhAC | 60.8 | |
| PSL | 59.5 | |
| TAL | 58.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabitov, A.; Atamanov, M.; Doszhanov, O.; Saurykova, K.; Tazhu, K.; Kerimkulova, A.; Orazbayev, A.; Doszhanov, Y. Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils. Molecules 2024, 29, 3786. https://doi.org/10.3390/molecules29163786
Sabitov A, Atamanov M, Doszhanov O, Saurykova K, Tazhu K, Kerimkulova A, Orazbayev A, Doszhanov Y. Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils. Molecules. 2024; 29(16):3786. https://doi.org/10.3390/molecules29163786
Chicago/Turabian StyleSabitov, Aitugan, Meiram Atamanov, Ospan Doszhanov, Karina Saurykova, Kairat Tazhu, Almagul Kerimkulova, Adilkhan Orazbayev, and Yerlan Doszhanov. 2024. "Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils" Molecules 29, no. 16: 3786. https://doi.org/10.3390/molecules29163786






