Recent Advances and New Insights in Genome Analysis and Transcriptomic Approaches to Reveal Enzymes Associated with the Biosynthesis of Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) from the Last Decade
Abstract
:1. Introduction
2. The Resources for Obtaining Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs)
2.1. Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) Extracted from Dendrobium Species
2.2. Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) Extracted from Endophytes
2.3. Various Strategies to Increase DTSAs Production in Endophytes
3. Genomics and Transcriptomics Used to Elucidate Associated Genes
3.1. Genes Identified in Dendrobium spp. through Genomic and Transcriptomic Sequencing
3.2. Genes Identified in Endophytes through Genomic and Transcriptomic Sequencing
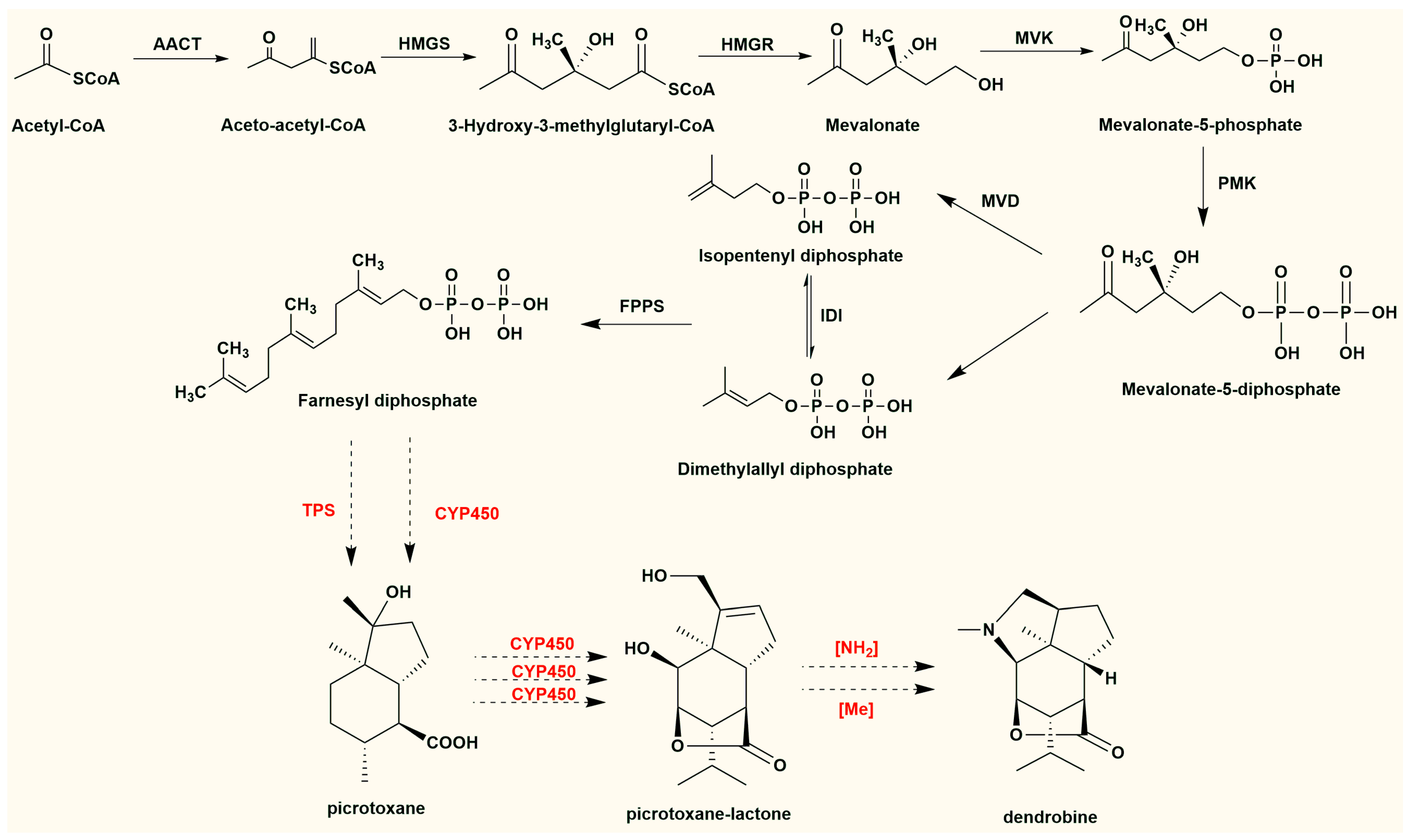
4. The Biosynthetic Pathways of DTSAs
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.L.; Huang, Y.; Linghu, C.; Xiao, J.F.; Gu, R.H. Metabolic profiling, in-situ spatial distribution, and biosynthetic pathway of functional metabolites in Dendrobium nobile stem revealed by combining UPLC-QTOF-MS with MALDI-TOF-MSI. Front. Plant. Sci. 2023, 13, 1128572. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Liu, J.M.; Sun, J.; Huang, Y.T.; Jin, N.; Li, M.M.; Liang, Y.T.; Fan, B.; Wang, F.Z. Analysis of endophytic bacterial diversity from different Dendrobium stems and discovery of an endophyte produced dendrobine-type sesquiterpenoid alkaloids. Front. Microbiol. 2022, 12, 775665. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Q.; Tang, J.J.; Gao, J.M. Picrotoxane sesquiterpenoids: Chemistry, chemo- and bio-syntheses and biological activities. Nat. Prod. Rep. 2022, 39, 2096–2131. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Chen, A.L. The alkaloid of Chin-Shih-Hu. J. Biol. Chem. 1935, 111, 653–658. [Google Scholar] [CrossRef]
- Xu, J.; Han, Q.B.; Li, S.L.; Chen, X.J.; Wang, X.N.; Zhao, Z.Z.; Chen, H.B. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- Li, K.Q.; Wu, F.J.; Chen, M.Z.; Xiao, Z.H.; Xu, Y.; Xu, M.W.; Liu, J.Y.; Xu, D.L. Identification, Biological Function Profiling and Biosynthesis of Secondary Metabolites in Medicinal Orchids. Metabolites 2023, 13, 829. [Google Scholar] [CrossRef]
- Lam, Y.; Ng, T.B.; Yao, R.M.; Shi, J.; Xu, K.; Sze, S.C.W.; Zhang, K.Y. Evaluation of Chemical Constituents and Important Mechanism of Pharmacological Biology in Dendrobium Plants. Evid. Based Comp. Alt. 2015, 2015, 841752. [Google Scholar] [CrossRef]
- Mou, Z.M.; Zhao, Y.; Ye, F.; Shi, Y.N.; Kennelly, E.J.; Chen, S.Y.; Zhao, D.K. Identification, Biological Activities and Biosynthetic Pathway of Dendrobium Alkaloids. Front. Pharmacol. 2021, 12, 605994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, X.J.; Liu, H.; Chen, Y.X.; Chen, S.W.; Niu, H.; Luo, Y.; Lei, H.; Zhang, D. A systematical review on ethnobotanical, phytochemical and pharmacological aspects of Dendrobium nobile Lindl. Phytochem. Rev. 2023, 22, 743–780. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, F.J.; Yu, H.Y.; Wang, N.L.; Wang, Z.; Yao, X.S. Copacamphane, Picrotoxane and Cyclocopacamphane Sesquiterpenes from Dendrobium nobile. Chem. Pharm. Bull. 2008, 56, 854–857. [Google Scholar] [CrossRef]
- Ye, Q.H.; Zhao, W.M. New alloaromadendrane, cadinene and cyclocopacamphane type sesquiterpene derivatives and bibenzyls from Dendrobium nobile. Planta Medica 2002, 68, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Tanaka, A.; Yamada, K. Dendrobine, an antagonist of beta-alanine, taurine and of presynaptic inhibition in the frog spinal cord. Br. J. Pharmacol. 1983, 78, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Hirata, Y. Structures of nobiline and dendrobine. Tetrahedron Lett. 1964, 5, 79–87. [Google Scholar] [CrossRef]
- Meng, C.W.; He, Y.L.; Peng, C.; Ding, X.J.; Guo, L.; Xiong, L. Picrotoxane sesquiterpenoids from the stems of Dendrobium nobile and their absolute configurations and angiogenesis effect. Fitoterapia 2017, 121, 206–211. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, D.M.; Guo, S.X. A new sesquiterpene glycoside from Dendrobium nobile Lindl. J. Asian Nat. Prod. Res. 2004, 6, 311–314. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Cardoso, J.C.; Dobránszki, J.; Zeng, S.J. Dendrobium micropropagation: A review. Plant Cell Rep. 2015, 34, 671–704. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhao, X.W.; Li, Y.Y.; Ke, S.J.; Yin, W.L.; Lan, S.R.; Liu, Z.J. Advances and prospects of orchid research and industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Peng, Y.; Li, W.D. Advances on the total synthesis of sesquiterpenoid alkaid dendrobine. Chin. J. Org. Chem. 2021, 41, 2636–2649. [Google Scholar] [CrossRef]
- Duan, H.T.; Er-Bu, A.; Dongzhi, Z.M.; Xie, H.J.; Ye, B.G.; He, J. Alkaloids from Dendrobium and their biosynthetic pathway, biological activity and total synthesis. Phytomedicine 2022, 102, 154132. [Google Scholar] [CrossRef]
- Li, D.D.; Huang, M.J.; Han, Q.W.; Wang, D.C.; Li, K.J.; Yang, Q.Y.; Gu, R.H.; Zhou, G.C.; He, S.T.; Yu, H.L.; et al. A high-quality chromosomal-level reference genome of Dendrobium nobile Lindl. provides new insights into the biosynthesis and accumulation of picrotoxane-type sesquiterpenoid alkaloids. Ind. Crop. Prod. 2024, 211, 118243. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, S.C.; Li, K.L.; Zheng, P.J.; Zhang, X.J.; Jia, Y.; Liu, Y.; Niu, Y.X.; Yu, L.H.; Chen, D.F.; et al. Chromosome-scale assembly of the Dendrobium nobile genome provides insights into the molecular mechanism of the biosynthesis of the medicinal active ingredient of Dendrobium. Front. Genet. 2022, 13, 844622. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Li, C.F.; Luo, H.M.; Wang, L.Z.; Qian, J.; Luo, X.; Xiang, L.; Song, J.Y.; Sun, C.; et al. Analysis of the Dendrobium officinale transcriptome reveals putative alkaloid biosynthetic genes and genetic markers. Gene 2013, 527, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, G.; Li, B.; Guo, S.X. Transcriptome analysis of genes involved in dendrobine biosynthesis in Dendrobium nobile Lindl. infected with mycorrhizal fungus MF23 (Mycena sp.). Sci. Rep. 2017, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.Z.; Lyu, P.; Chen, L.P.; Shen, C.J.; Sun, C.B. Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J. Plant Res. 2019, 132, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Guo, H.; Chen, H.L.; Shi, Y.J.; Meng, Y.J.; Lu, J.J.; Feng, S.G.; Wang, H.Z. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci. Rep. 2017, 7, 187. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Fan, X.; Jia, Q.; Xu, Q.; Shu, F.X.; Zhou, Q.N.; Shi, J.S.; Chen, J.S. New insights into detection of a dendrobine compound from a novel endophytic Trichoderma longibrachiatum strain and its toxicity against phytopathogenic bacteria. Front. Microbiol. 2020, 11, 337. [Google Scholar] [CrossRef]
- Qian, X.; Qin, Y.T.; Sarasiya, S.; Chen, J.S. Transcriptomic profiling of adding cobalt chloride to improve dendrobine-type total alkaloid production. Appl. Microbiol. Biot. 2024, 108, 26. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Wang, L.N.; Qian, X.; Jin, H.; Shu, F.X.; Sarsaiya, S.; Jin, L.L.; Chen, J.S. Transcriptome analysis of dendrobine biosynthesis in Trichoderma longibrachiatum MD33. Front. Microbiol. 2022, 13, 890733. [Google Scholar] [CrossRef]
- Qian, X.; Jin, H.; Chen, Z.J.; Dai, Q.Q.; Sarsaiya, S.; Qin, Y.T.; Jia, Q.; Jin, L.L.; Chen, J.S. Comparative transcriptome analysis of genes involved in sesquiterpene alkaloid biosynthesis in Trichoderma longibrachiatum MD33 and UN32. Front. Microbiol. 2021, 12, 800125. [Google Scholar] [CrossRef]
- Inubushi, Y.; Sasaki, Y.; Tsuda, Y.; Yasui, B.; Konita, T.; Matsumoto, J.; Katarao, E.; Nakano, J. Structure of Dendrobine. Yakugaku Zasshi 1963, 83, 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Behr, D.; Leander, K. Studies on orchidaceae alkaloids. 28. The absolute configuration of the dendrobine alkaloids. Acta Chem. Scand. 1972, 26, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Kamata, S.; Maeda, T.; Kawazoe, Y.; Natsume, M.; Okamoto, T.; Uchimaru, F.; Shimizu, M. The Structure of Dendrobine. Chem. Pharm. Bull. 1964, 12, 506–512. [Google Scholar] [CrossRef]
- Inubushi, Y.; Sasaki, Y.; Tsuda, Y.; Yasui, B.; Konita, T.; Matsumoto, J.; Katarao, E.; Nakano, J. Structure of dendrobine. Tetrahedron 1964, 20, 2007–2023. [Google Scholar] [CrossRef]
- Inubushi, Y.; Ishii, H.; Yasui, B.; Konita, T.; Harayama, T. Isolation and Characterization of Alkaloids of the Chinese Drug “Chin-Shih-Hu”. Chem. Pharm. Bull. 1964, 12, 1175–1180. [Google Scholar] [CrossRef]
- Inubushi, Y.; Tsuda, Y.; Katarao, E. The Structure of Dendramine. Chem. Pharm. Bull. 1966, 14, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Natsume, M.; Onaka, T.; Uchimaru, F.; Shimizu, M. The Structure of Dendramine (6-Oxydendrobine) and 6-Oxydendroxine The Fourth and Fifth Alkaloid from Dendrobium nobile. Chem. Pharm. Bull. 1966, 14, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Fujiwara, M.; Yoshida, N.; Kobayashi, J.i. New Picrotoxinin-type and Dendrobine-type Sesquiterpenoids from Dendrobium Snowflake ‘Red Star’. Tetrahedron 2000, 56, 5801–5805. [Google Scholar] [CrossRef]
- Lynch, V.M.; Li, W.; Martin, S.F.; Davis, B.E. Structure of the ABC ring subunit of 3-hydroxy-2-oxodendrobine. Acta. Crystallogr. C. 1990, 46, 1159–1161. [Google Scholar] [CrossRef]
- Yi, T.P.; Li, X.; Wang, Z.; Wang, Y.R.; Wang, M. A review on spectral characteristics of dendrobines from the Dendrobium plants. Chinses J. Magn. Reson. 2020, 37, 381–389. [Google Scholar]
- Gu, R.H.; Rybalov, L.; Negrin, A.; Morcol, T.; Long, W.W.; Myers, A.K.; Isaac, G.; Yuk, J.; Kennelly, E.J.; Long, C.L. Metabolic Profiling of different parts of Acer truncatum from the Mongolian Plateau Using UPLC-QTOF-MS with Comparative Bioactivity Assays. J. Agric. Food Chem. 2019, 67, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Zhang, C.; Tu, Y.; Chen, J.; Li, Q.; Zeng, Z.; Wang, F.Y.; Sun, L.N.; Huang, D.D.; Li, M.M.; et al. Biosynthesis-based spatial metabolome of Salvia miltiorrhiza Bunge by combining metabolomics approaches with mass spectrometry-imaging. Talanta 2022, 238, 123045. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, C.; Zhao, H.W.; Zhou, G.Z.; Qin, L.; Li, X.Y.; Chen, L.L.; Wang, X.D.; Wan, Y.L. In-situ detection and imaging of Areca catechu fruit alkaloids by MALDI-MSI. Ind. Crop. Prod. 2022, 188, 115533. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Jain, A.; Shu, F.X.; Yang, M.F.; Pu, M.X.; Jia, Q.; Gong, Q.H.; Wu, Q.; Qian, X.; Shi, J.S.; et al. Enhancing dendrobine production in Dendrobium nobile through mono-culturing of endophytic fungi, Trichoderma longibrachiatum (MD33) in a temporary immersion bioreactor system. Front. Plant. Sci. 2024, 15, 1302817. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.B.; Wen, W.E.; Li, Q.Q.; Pan, T.T.; Li, Z.G.; Qian, G.; He, Y.Q.; Xu, D.L. Dendrobine biosynthesis in Dendrobium nobile in four different habitats is affected by the variations in the endophytic fungal community. Front. Microbiol. 2022, 13, 981070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Ji, X.L.; Liu, X.Q.; Qin, L.; Tan, D.P.; Wu, D.; Bai, C.J.; Yang, J.Y.; Xie, J.; He, Y.Q. Age-dependent dendrobine biosynthesis in Dendrobium nobile: Insights into endophytic fungal interactions. Front. Microbiol. 2023, 14, 1294402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.M.; Chen, H.H.; Si, J.P.; Wu, L.S. Effect of cultivation mode on bacterial and fungal communities of Dendrobium catenatum. BMC Microbiol. 2022, 22, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.X.; Zheng, S.G.; Hu, Y.D.; Li, H.J.; Chen, Y.Y.; Chun, Z. Endophytic bacterial diversity of the medicinal orchid Dendrobium nobile. S. Afr. J. Bot. 2023, 158, 90–97. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, J.M.; Sun, J.; Sun, Y.F.; Liu, J.N.; Jia, N.; Fan, B.; Dai, X.F. Diversity of culture-independent bacteria and antimicrobial activity of culturable endophytic bacteria isolated from different Dendrobium stems. Sci. Rep. 2019, 9, 10389. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Jin, J.J.; Sarojam, R.; Ramachandran, S. A comprehensive survey on the terpene synthase gene family provides new insight into its evolutionary patterns. Genome Biol. Evol. 2019, 11, 2078–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zhang, G.Q.; Zhang, D.Y.; Liu, X.D.; Xu, X.Y.; Sun, W.H.; Yu, X.; Zhu, X.E.; Wang, Z.W.; Zhao, X.; et al. Chromosome-scale assembly of the Dendrobium chrysotoxum genome enhances the understanding of orchid evolution. Hortic. Res. 2021, 8, 183. [Google Scholar] [CrossRef]
- Liu, Q.; Manzano, D.; Tanić, N.; Pesic, M.; Bankovic, J.; Pateraki, I.; Ricard, L.; Ferrer, A.; de Vos, R.; de Krol, S.v.; et al. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab. Eng. 2014, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.N.; Okamoto, S.; Harada, H.; Yamasaki, K.; Misawa, N.; Utsumi, R. Zingiber zerumbet CYP71BA1 catalyzes the conversion of α-humulene to 8-hydroxy-α-humulene in zerumbone biosynthesis. Cell. Mol. Life Sci. 2011, 68, 1033–1040. [Google Scholar] [CrossRef]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.U.; O’Maille, P.E.; Spring, O.; Ro, D.K. Lettuce Costunolide Synthase (CYP71BL2) and Its Homolog (CYP71BL1) from Sunflower Catalyze Distinct Regio- and Stereoselective Hydroxylations in Sesquiterpene Lactone Metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Liang, Y.M.; Chen, Z.; Zheng, P.J.; Zhang, G.Q.; Yan, B.H.; Elshikh, M.S.; Rizwana, H.; Chen, B.J.; Xu, Q. Genome-wide identification of the alkaloid synthesis gene family CYP450, gives new insights into alkaloid resource utilization in medicinal Dendrobium. Int. J. Biol. Macromol. 2024, 259, 129229. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Li, S. Notes on symbiotic relationship between Cypripedium flavum and its mycorrhizal fungi. Acta Bot. Yunnanica 2004, 26, 445–450. [Google Scholar]
- Rossoni, L.; Hall, S.J.; Eastham, G.; Licence, P.; Stephens, G. The Putative Mevalonate Diphosphate Decarboxylase from Picrophilus torridus Is in Reality a Mevalonate-3-Kinase with High Potential for Bioproduction of Isobutene. Appl. Environ. Microbiol. 2015, 81, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Liu, C.B.; Huang, C.Y.; Wang, M.F.; Long, T.; Liu, J.Y.; Shi, J.H.; Shi, J.L.; Li, L.; He, Y.Q.; et al. Transcriptome and Metabonomics Analysis Revealed the Molecular Mechanism of Differential Metabolite Production of Dendrobium nobile Under Different Epiphytic Patterns. Front. Plant. Sci. 2022, 13, 868472. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhao, M.L.; Cui, H.Q.; Li, J.; Wang, M.N. Transcriptomic landscape of medicinal Dendrobium reveals genes associated with the biosynthesis of bioactive components. Front. Plant. Sci. 2020, 11, 391. [Google Scholar] [CrossRef]
- Zhao, M.L.; Zhao, Y.C.; Yang, Z.Y.; Ming, F.; Li, J.; Kong, D.M.; Wang, Y.; Chen, P.; Wang, M.N.; Wang, Z.C. Metabolic pathway engineering improves dendrobine production in Dendrobium Catenatum. Int. J. Mol. Sci. 2023, 25, 397. [Google Scholar] [CrossRef]
- Gao, C.X.; Wu, X.D.; Yang, Z.; Qin, L.; Wu, D.; Fan, Q.J.; Zhao, Y.X.; Tan, D.P.; Li, J.Y.; Zhang, J.Y.; et al. Quantitative analysis of six sesquiterpene glycosides from Dendrobium nobile Lindl. under different growth conditions by high-performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry in MRM mode. Phytochem. Anal. 2024, 35, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.J.; Jiang, Y.; Wu, J.; Tan, D.P.; Qin, L.; Lu, Y.L.; Qian, Y.; Bai, C.J.; Yang, J.Y.; Ling, H.; et al. Opposite trends of glycosides and alkaloids in Dendrobium nobile of different age based on UPLC-Q/TOF-MS combined with multivariate statistical analyses. Phytochem. Anal. 2022, 33, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.P.; Wang, J.M.; Cao, L.G.; Zhao, Y.X.; Fan, Q.J.; Wu, X.D.; Wu, D.; Lu, Y.L.; Qin, L.; He, Y.Q. Transcriptome-based analysis reveals the key genes of sesquiterpene glycosylation in Dendrobium nobile. Food Sci. Technol. 2023, 43, e122122. [Google Scholar] [CrossRef]
- Yamazaki, M.; Matsuo, M.; Arai, K. Biosynthesis of Dendrobine. Chem. Pharm. Bull. 1966, 14, 1058–1059. [Google Scholar] [CrossRef]
- Soliman, S.S.M.; Raizada, M.N. Sites of biosynthesis and storage of Taxol in Taxus media (Rehder) plants: Mechanism of accumulation. Phytochemistry 2020, 175, 112369. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.W.; Jiang, Y.H.; Wang, C.Y.; Song, M.J.; Liu, Y.F.; Liu, J.; Jiang, Z.T.; Yang, Y.; Ren, X.L.; Ding, Y.D. Metabolomic and transcriptomic analysis reveal high solar irradiance inhibited the melanin formation in persimmon fruit peel. Environ. Exp. Bot. 2023, 207, 105218. [Google Scholar] [CrossRef]
- Cao, Y.W.; Qian, X.; Yu, T.F.; Jia, Q.; Sarsaiya, S.; Chen, J.S. Improving biomass and dendrobine-type total alkaloids (DTTAs) production of Dendrobium nobile through combining Temporary Immersion Bioreactor System (TIBS) with endophyte MD33 elicitation. Plant Cell Tiss. Org. 2024, 156, 9. [Google Scholar] [CrossRef]
- Gong, D.Y.; Wu, B.; Qin, H.T.; Fu, D.Z.; Guo, S.X.; Wang, B.C.; Li, B. Functional characterization of a farnesyl diphosphate synthase from Dendrobium nobile Lindl. AMB Express 2022, 12, 129. [Google Scholar] [CrossRef]
- Yu, W.B.; Pei, R.Q.; Zhou, J.Y.; Zeng, B.; Tu, Y.Y.; He, B. Molecular regulation of fungal secondary metabolism. World J. Microbiol. Biotechnol. 2023, 39, 204. [Google Scholar] [CrossRef]
- Xu, C.L.; Lin, W.X.; Chen, Y.N.; Gao, B.L.; Zhang, Z.B.; Zhu, D. Heat stress enhanced perylenequinones biosynthesis of Shiraia sp. Slf14(w) through nitric oxide formation. Appl. Microbiol. Biotechnol. 2023, 107, 3745–3761. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zeng, Y.; Chen, Y.M.; Shen, Y.L.; Wang, W. Induction of cellulase production by Sr2+ in Trichoderma reesei via calcium signaling transduction. Bioresour. Bioprocess. 2022, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Wang, J.J.; Lin, L.; Xu, X.Y.; Wei, W.; Shen, Y.L.; Wei, D.Z. Synergistic regulation of metabolism by Ca2+/Reactive Oxygen Species in Penicillium brevicompactum improves production of mycophenolic acid and investigation of the Ca2+ channel. ACS Synth. Biol. 2022, 11, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Wang, Y.; Ma, Y.J.; Wang, J.W.; Zheng, L.P. Nitric oxide and hydrogen peroxide signaling in extractive Shiraia fermentation by Triton X-100 for hypocrellin a production. Int. J. Mol. Sci. 2020, 21, 882. [Google Scholar] [CrossRef]
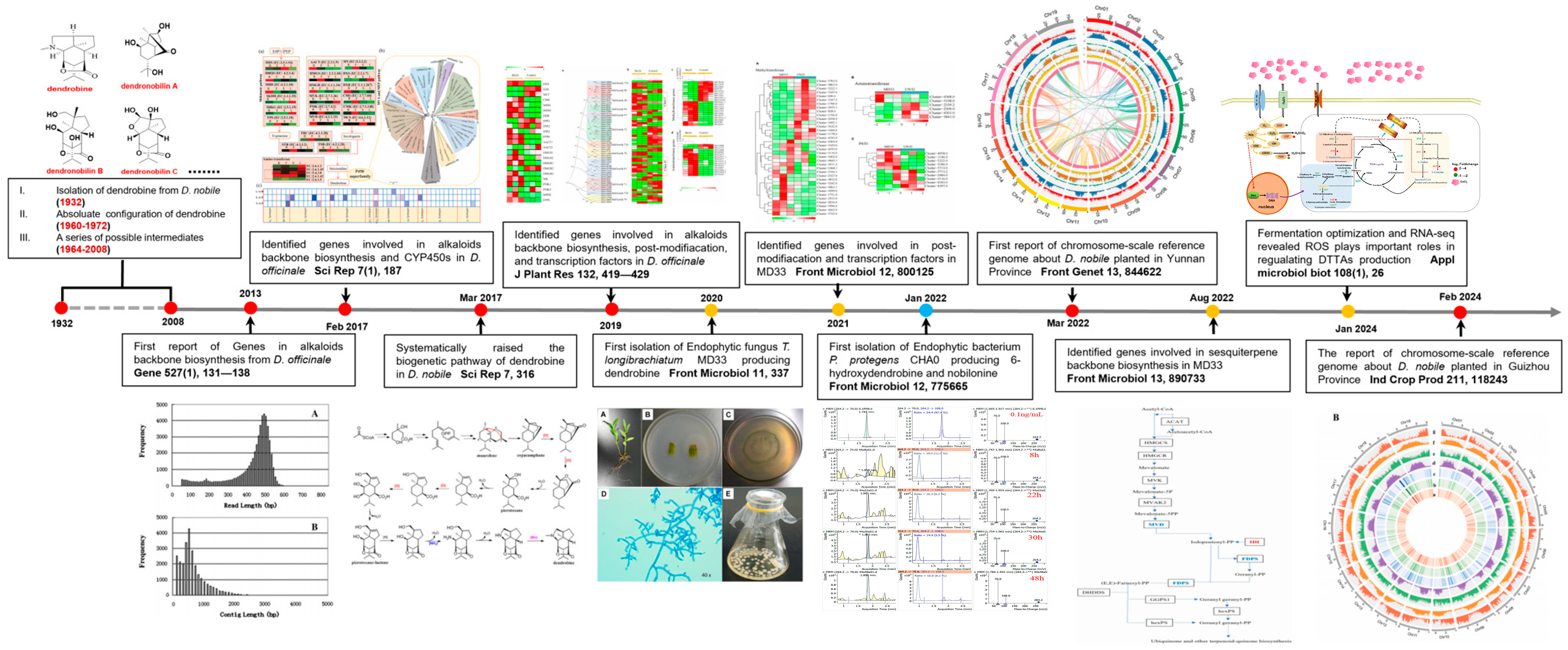
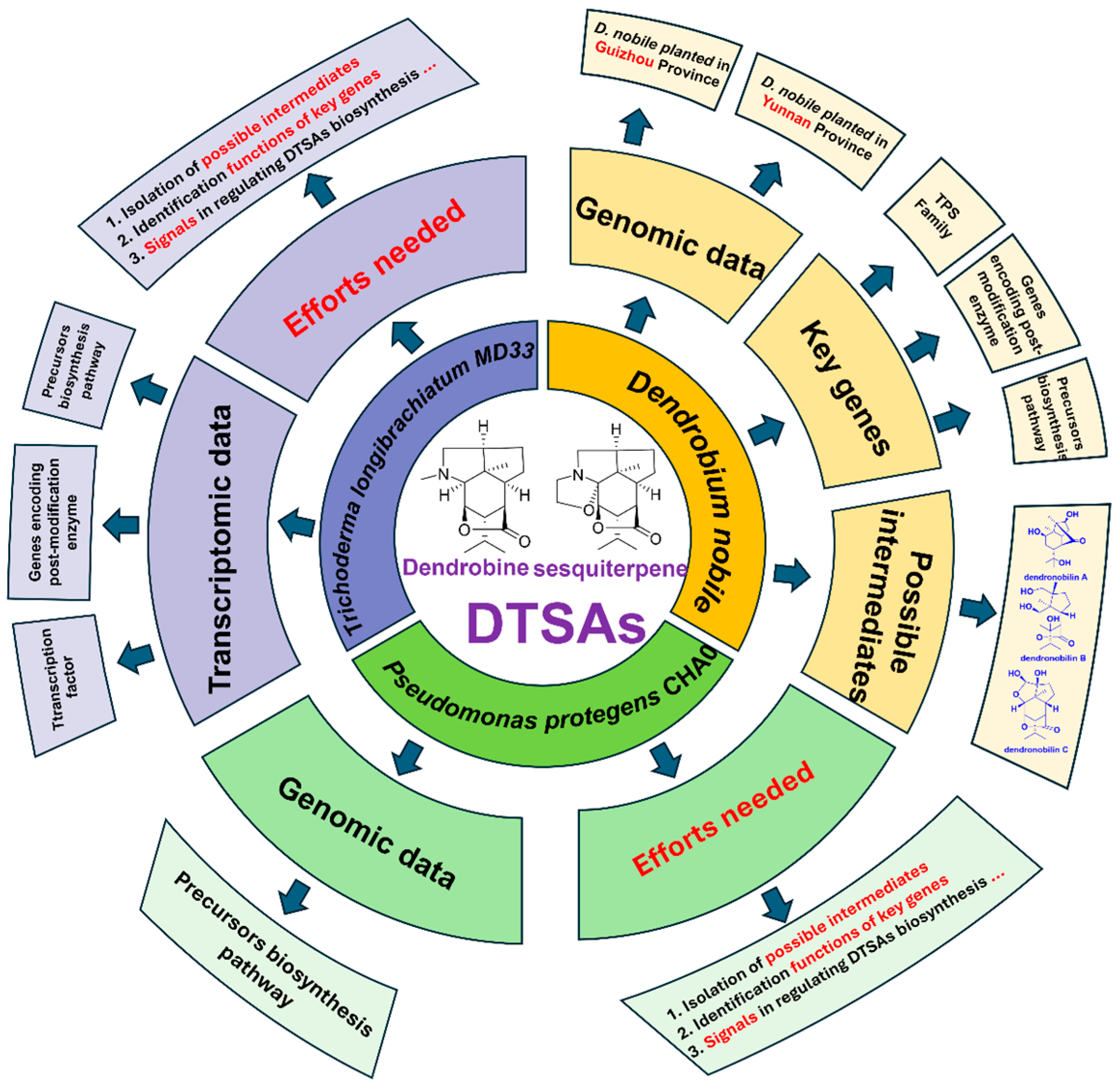
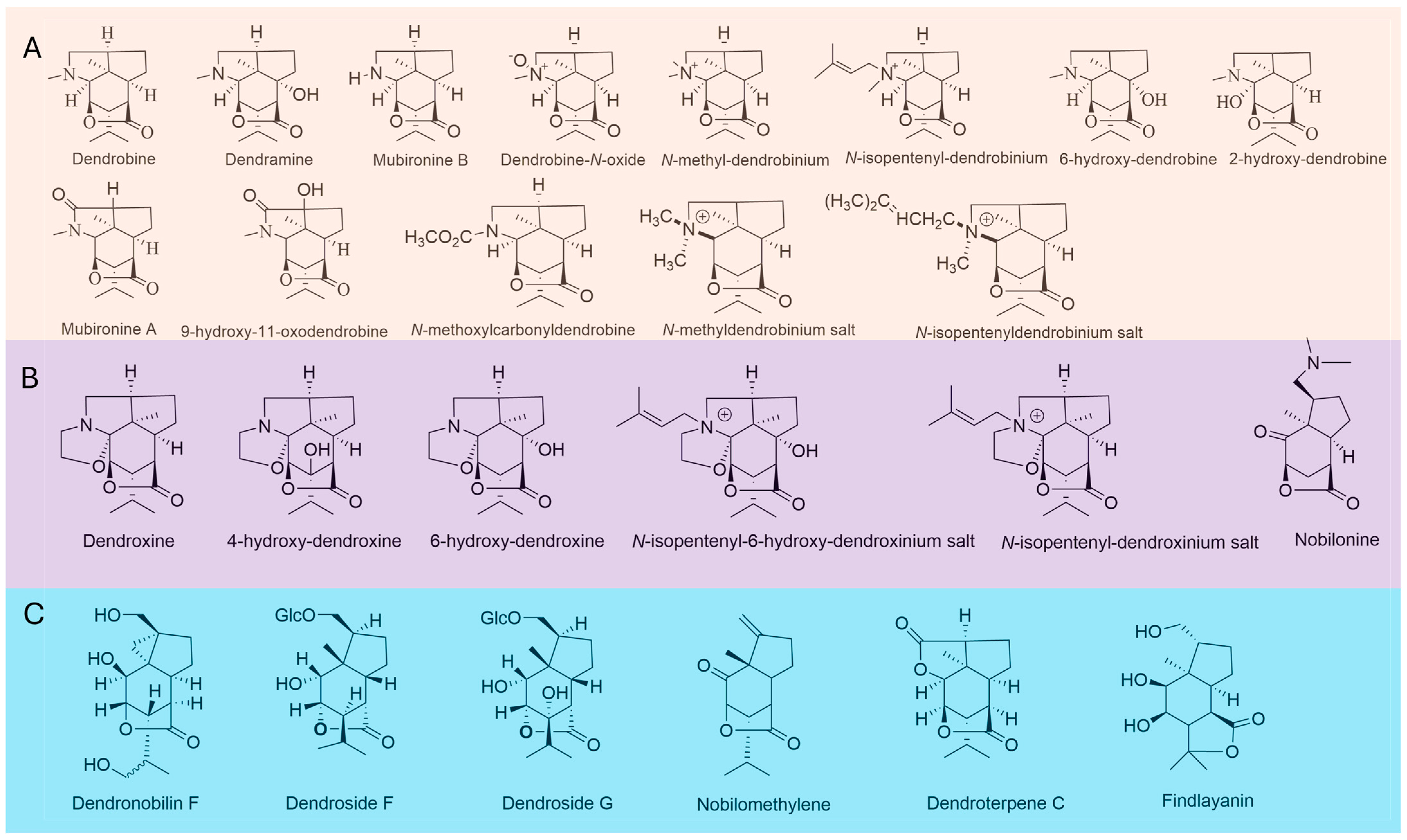
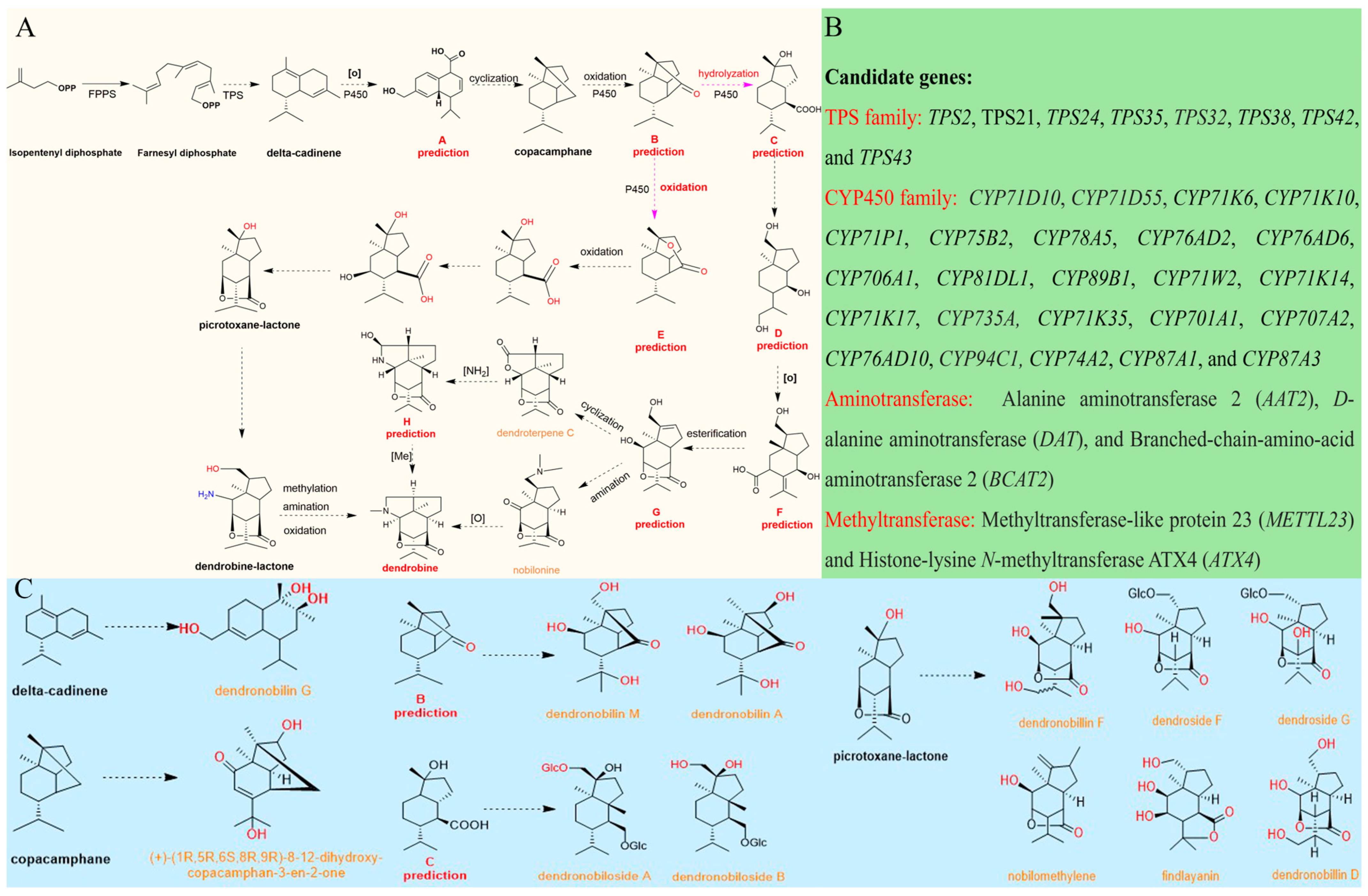
| Species | Publication Date | Materials | Sequencing Platforms | Annotated Genes | Data Type | Reference |
|---|---|---|---|---|---|---|
| P. protegens | 2022 | Mycelium | Illumina Miseq | MEP pathway | Genomic Data | [2] |
| D. nobile | 2024 | Young leaves | Illumina Hiseq X ten | (1) Terpenoid backbone (2) TPS family (3) CYP450 family | [21] | |
| D. nobile | 2022 | Young tenders | MGISEQ-2000 | (1) TPS family (2) CYP450 family | [22] | |
| T. longibrachiatum | 2024 | Strain UN32 treated with CoCl2 | Illumina Hiseq4000 | ROS signaling pathway | Transcriptomic Data | [28] |
| T. longibrachiatum | 2022 | Strain MD33 treated with MeJA | Illumina Hiseq4000 | (1) Alkaloid backbone (2) P450 superfamily, methyltransferase and aminotransferase | [29] | |
| D. officinale | 2013 | Stem | 454 pyrosequencing | Alkaloid backbone | [23] | |
| D. nobile | 2017 | Stems treated with mycorrhizal fungus | Illumina Hiseq4000 | Dendrobine biosynthesis pathway | [24] | |
| D. officinale | 2019 | Leaves treated with MeJA | Illumina Hiseq4000 | (1) Alkaloid backbone (2) P450 superfamily, methyltransferase and aminotransferase | [25] | |
| T. longibrachiatum | 2021 | MD33 with its mutant UN32 | Illumina Hiseq4000 | (1) Sesquiterpenoid Alkaloid backbone (2) P450 superfamily, methyltransferase and aminotransferase | [30] | |
| D. officinale | 2017 | Leaves, stems and roots | Illumina Hiseq2500 | (1) Alkaloid backbone (2) CYP450s | [26] |
| Items | Yunan Province [22] | Guizhou Province [21] |
|---|---|---|
| Sequencing platform | MGISEQ-2000 Pacbio sequel II Hi-C | Illumina Hiseq X ten Pacbio sequel II Hi-C |
| Genome size | 1.19 Gb | 1.19 Gb |
| Heterozygosity | 1.35% | 2.03% |
| Contig N50 | 1.61 Mb | 10.01 Mb |
| Assembly level | Chromosome | Chromosome |
| WGD | 2 | 0 |
| Repetitive ratio | 61.07% | 42.3% |
| SNPs | Not reported | Not reported |
| Protein-coding genes | 29,476 | 31,672 |
| Functionally annotated genes | 27,765 | 30,828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Sarsaiya, S.; Dong, Y.; Yu, T.; Chen, J. Recent Advances and New Insights in Genome Analysis and Transcriptomic Approaches to Reveal Enzymes Associated with the Biosynthesis of Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) from the Last Decade. Molecules 2024, 29, 3787. https://doi.org/10.3390/molecules29163787
Qian X, Sarsaiya S, Dong Y, Yu T, Chen J. Recent Advances and New Insights in Genome Analysis and Transcriptomic Approaches to Reveal Enzymes Associated with the Biosynthesis of Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) from the Last Decade. Molecules. 2024; 29(16):3787. https://doi.org/10.3390/molecules29163787
Chicago/Turabian StyleQian, Xu, Surendra Sarsaiya, Yuanyuan Dong, Tuifan Yu, and Jishuang Chen. 2024. "Recent Advances and New Insights in Genome Analysis and Transcriptomic Approaches to Reveal Enzymes Associated with the Biosynthesis of Dendrobine-Type Sesquiterpenoid Alkaloids (DTSAs) from the Last Decade" Molecules 29, no. 16: 3787. https://doi.org/10.3390/molecules29163787





