A Comparative Analysis of Cold Brew Coffee Aroma Using the Gas Chromatography–Olfactometry–Mass Spectrometry Technique: Headspace–Solid-Phase Extraction and Headspace Solid-Phase Microextraction Methods for the Extraction of Sensory-Active Compounds
Abstract
:1. Introduction
2. Results
2.1. Optimization of the HS-SPME Technique
2.1.1. Type of Fiber
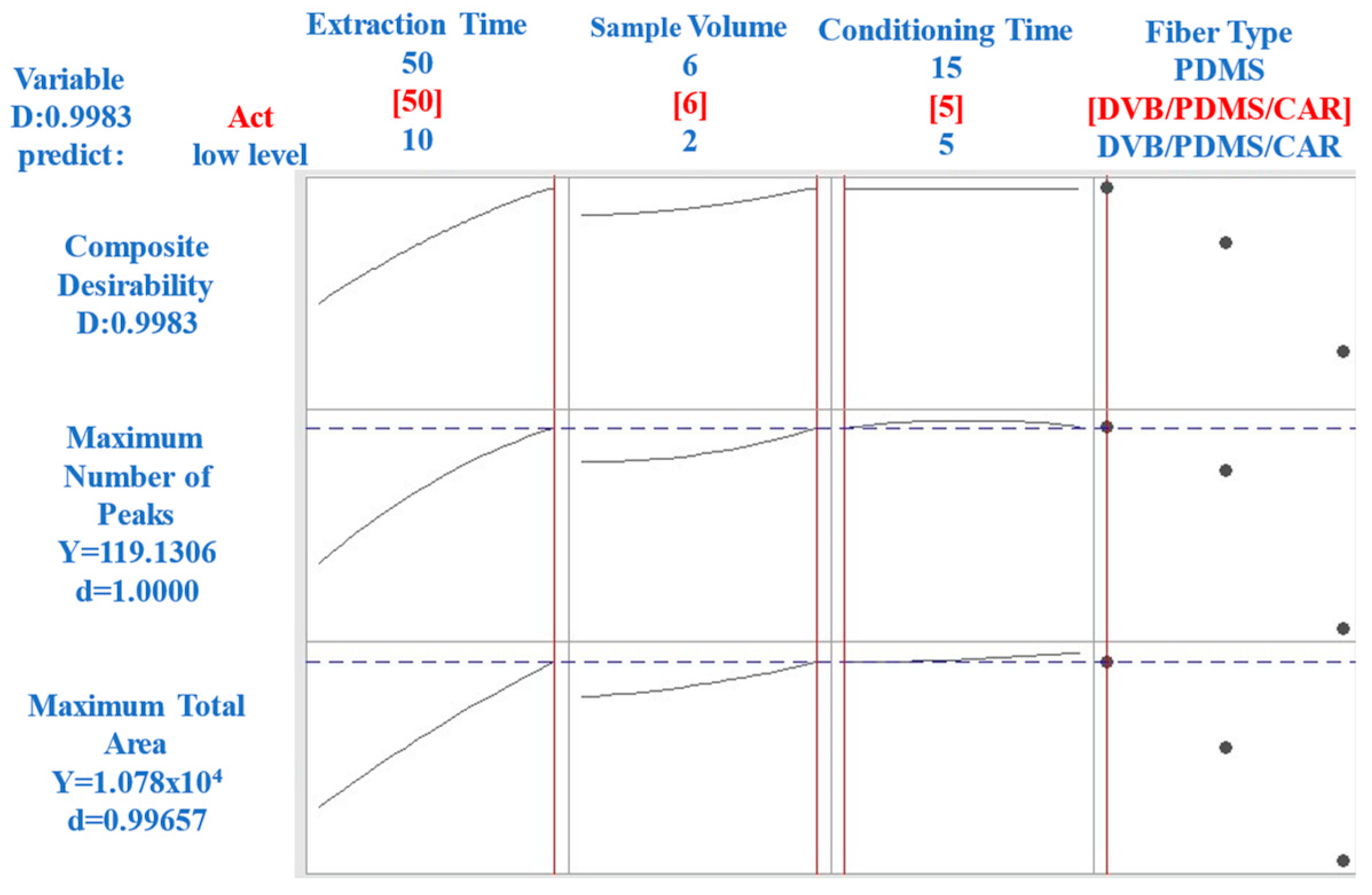
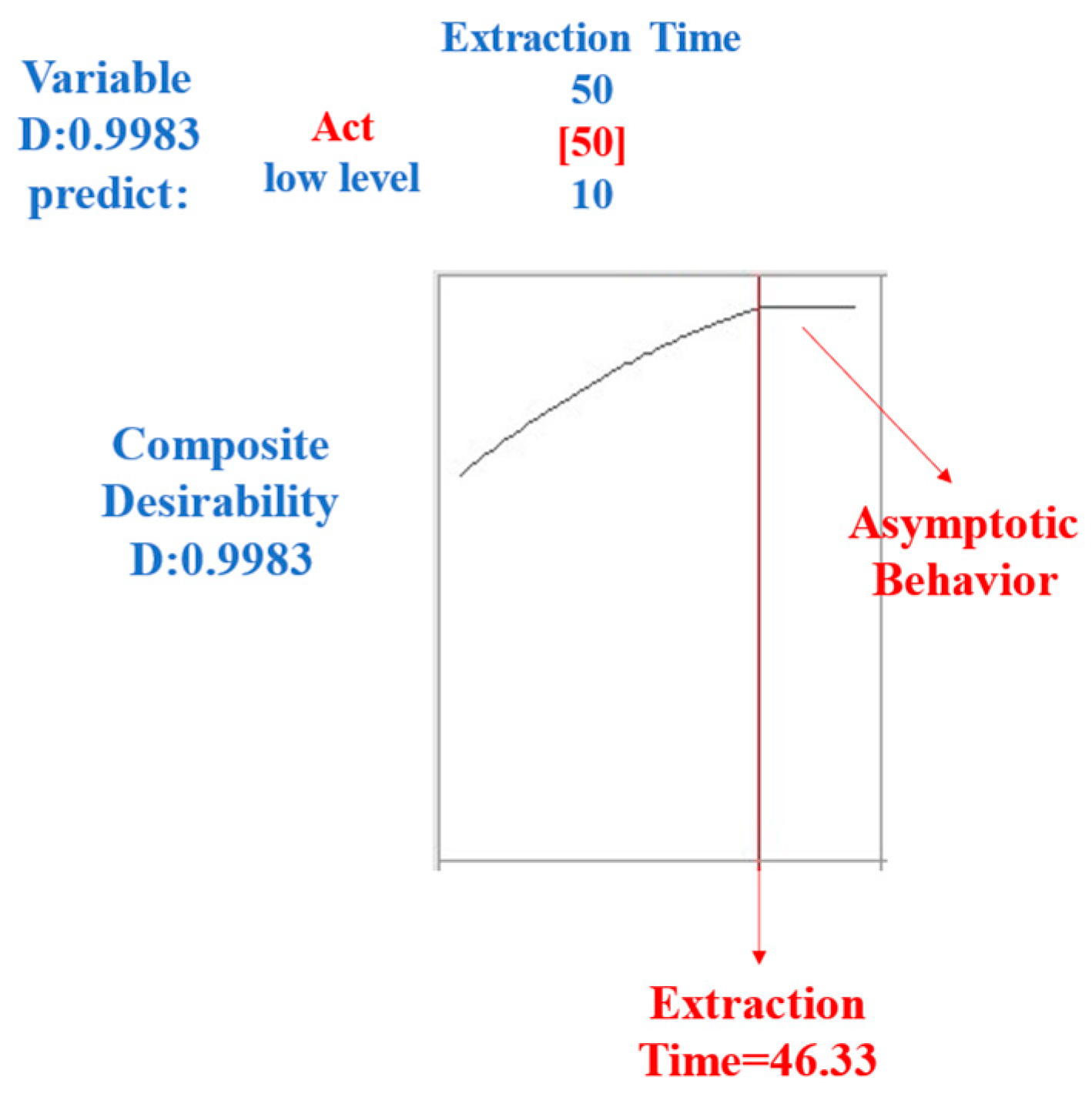
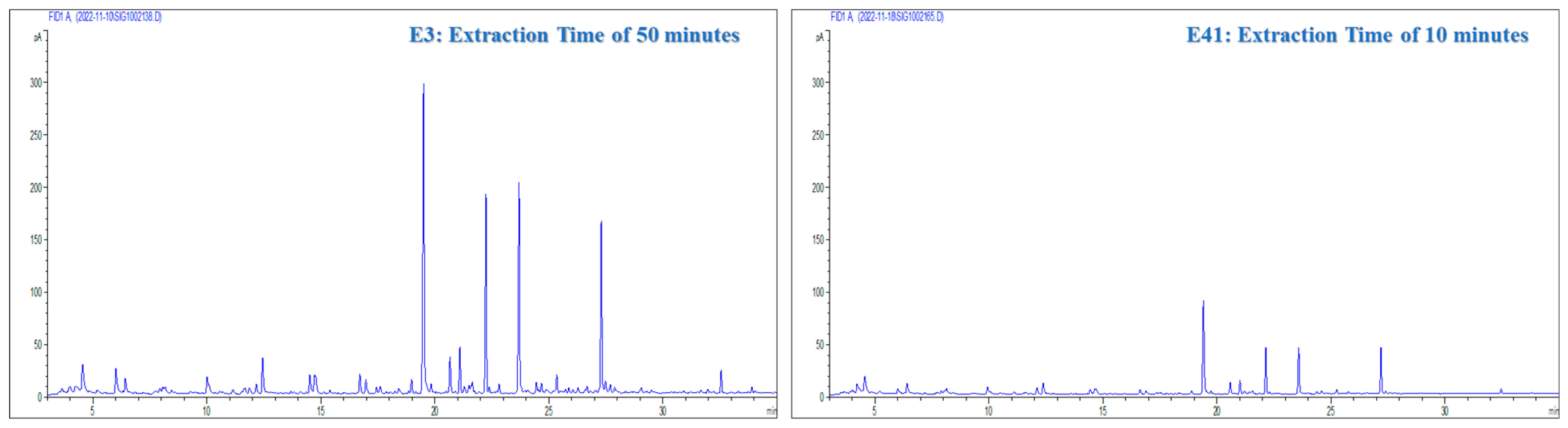
2.1.2. Extraction Time
2.1.3. Sample Volume Analysis
2.1.4. Conditioning Time
2.2. Verification of Aroma Extraction Methodology through HS-SPE
2.2.1. Extraction Time
2.2.2. Purge Gas Flow
2.2.3. Solvent Ratio in the Elution of Target Analytes from the Sorbent Material
2.3. Identification of Compounds with Sensory Activity Using GC-O-MS
3. Materials and Methods
3.1. Raw Materials
3.2. Sample Preparation
3.3. Reagents and Supplies
3.4. Chromatographic Conditions (GC-MS and GC-O-FID)
3.5. Sensory Assessment and Panel Training
3.6. Sample Preparation Methodology
3.6.1. HS-SPME Technique Optimization
3.6.2. Verification of Aroma Extraction Methodology Using HS-SPE
3.7. Identification of Compounds with HS-GC-O-MS Sensory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Córdoba, N. Chemical and Sensory Evaluation of Cold Brew Coffees Using Different Roasting Profiles and Brewing Methods. Food Res. Int. 2021, 13, 110141. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Yu, R.; Wang, F.; Wang, W.; Zhang, D.; Zhang, J. Changes in the Aroma Characteristics during the Different Processes of Dry Cabernet Sauvignon Wine Production. Eur. Food Res. Technol. 2022, 248, 3025–3036. [Google Scholar] [CrossRef]
- McCain-Keefer, H.R.; Meals, S.; Drake, M. The Sensory Properties and Consumer Acceptance of Cold Brew Coffee. J. Sens. Stud. 2020, 35, E12604. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of Grinding, Extraction Time and Type of Coffee on the Physicochemical and Flavour Characteristics of Cold Brew Coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Development of SPME Devices and Coatings. In Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012; pp. 61–97. ISBN 978-0-12-416017-0. [Google Scholar]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a Psychomotor Stimulant: Mechanism of Action. Cell. Mol. Life Sci. (CMLS) 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Loukri, A.; Tsitlakidou, P.; Goula, A.; Assimopoulou, A.N.; Kontogiannopoulos, K.N.; Mourtzinos, I. Green Extracts from Coffee Pulp and Their Application in the Development of Innovative Brews. Appl. Sci. 2020, 10, 6982. [Google Scholar] [CrossRef]
- Kang, D.; Lee, H.-U.; Davaatseren, M.; Chung, M.-S. Comparison of Acrylamide and Furan Concentrations, Antioxidant Activities, and Volatile Profiles in Cold or Hot Brew Coffees. Food Sci. Biotechnol. 2020, 29, 141–148. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi)Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- Egea, M.B.; Bertolo, M.R.V.; Oliveira Filho, J.G.D.; Lemes, A.C. A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules 2021, 26, 5181. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.H.; Zapata, J.; Dereix, J.D.; Escobar, J.; De Almeida, A.B.; Silva, F.G.; Egea, M.B. The Active Aroma of “Cerrado” Cashew and Cagaita Fruits: Comparison between Two Extraction Methods. Appl. Sci. 2022, 12, 3330. [Google Scholar] [CrossRef]
- Yu, J.-M.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of Volatile Compounds in Coffee Prepared by Various Brewing and Roasting Methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef]
- Wang, E.S.-T.; Yu, J.-R. Effect of Product Attribute Beliefs of Ready-to-Drink Coffee Beverages on Consumer-Perceived Value and Repurchase Intention. BFJ 2016, 118, 2963–2980. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee Fermentation and Flavor—An Intricate and Delicate Relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Flament, I. Coffee Flavor Chemistry; Wiley-Blackwell: Hoboken, NJ, USA, 2001. [Google Scholar]
- Romano, R.; Santini, A.; Le Grottaglie, L.; Manzo, N.; Visconti, A.; Ritieni, A. Identification Markers Based on Fatty Acid Composition to Differentiate between Roasted Arabica and Canephora (Robusta) Coffee Varieties in Mixtures. J. Food Compos. Anal. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Dravnieks, A. Atlas of Odor Character Profiles/Sponsored by Section E-18.04.12 on Odor Profiling of Subcommittee E-18.04 on Instrumental-Sensory Relationships, ASTM Committee E-18 on Sensory Evaluation of Materials and Products; Compiled by Andrew Dravnieks; ASTM data series publication; DS 61; ASTM: Philadelphia, PA, USA, 1985. [Google Scholar]
- Zhang, Z.; Pawliszyn, J. Headspace Solid-Phase Microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Spencer, M.; Sage, E.; Velez, M.; Guinard, J. Using Single Free Sorting and Multivariate Exploratory Methods to Design a New Coffee Taster’s Flavor Wheel. J. Food Sci. 2016, 81, S2997–S3005. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas Chromatography–Olfactometry in Food Flavour Analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of Aroma Compounds Present in an Industrial Recovery Concentrate of Coffee Flavour. CyTA-J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef]
- Alegre, Y.; Sáenz-Navajas, M.-P.; Hernández-Orte, P.; Ferreira, V. Sensory, Olfactometric and Chemical Characterization of the Aroma Potential of Garnacha and Tempranillo Winemaking Grapes. Food Chem. 2020, 331, 127207. [Google Scholar] [CrossRef] [PubMed]
- Osorio Monsalve, J.; López Córdoba, C.A.; Zapata Ochoa, J.A. Caracterización de los compuestos del aroma en rones colombianos por HS-SPME-GC-MS-O. Rev. Colomb. Quim. 2016, 45, 48. [Google Scholar] [CrossRef]
- Monroy, L.; López Córdoba, C.; Araque Marín, P.; Torijano Gutiérrez, S.; Zapata Ochoa, J.A. Caracterización de los compuestos de aroma del aceite de sacha inchi (Plukenetia volubilis L.) por HS-SPME-GC-MS-O. Rev. Colomb. Quim. 2019, 48, 45–50. [Google Scholar] [CrossRef]
- San-Juan, F.; Pet’ka, J.; Cacho, J.; Ferreira, V.; Escudero, A. Producing Headspace Extracts for the Gas Chromatography–Olfactometric Evaluation of Wine Aroma. Food Chem. 2010, 123, 188–195. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Zhou, X.; Gao, K.; Liu, X. Interactions of Heat-Induced Myosin with Hsian-Tsao Polysaccharide to Affect the Fishy Odor Adsorption Capacity. Food Hydrocoll. 2023, 136, 108282. [Google Scholar] [CrossRef]
- Escudero, A.; San-Juan, F.; Franco-Luesma, E.; Cacho, J.; Ferreira, V. Is Orthonasal Olfaction an Equilibrium Driven Process? Design and Validation of a Dynamic Purge and Trap System for the Study of Orthonasal Wine Aroma. Flavour Fragr. J. 2014, 29, 296–304. [Google Scholar] [CrossRef]


| Extraction Time (h) | Total Area | Number of Peaks |
|---|---|---|
| 1 | 392.0 | 38 |
| 2.5 | 435.7 | 42 |
| 4 | 735.1 | 62 |
| 6 | 907.0 | 43 |
| Flow (mL/min) | Total Area | Number of Peaks |
|---|---|---|
| 80 | 745.9 | 60 |
| 100 | 783.0 | 60 |
| 120 | 789.1 | 61 |
| Solvent Ratio | Total Area | Number of Peaks |
|---|---|---|
| 95/5 | 735.1 | 62 |
| 90/10 | 418.4 | 37 |
| Compound | CAS | HS-SPME | HS-SPE | RI | Aroma Descriptor | ||
|---|---|---|---|---|---|---|---|
| MF | RI(E) | MF | RI(E) | ||||
| N.I | N.I | 77.5 | 737.8 | N.I | N.I | N.I | Green, sweet, vanilla, smooth |
| butanal | 123-72-8 | 58.9 | 886.4 | N.D | N.D | 886 | Sweet |
| 1,5-Octadien-3-one | 65213-86-7 | 73.0 | 996.9 | N.D | N.D | 981 | Floral |
| phenylacetaldehyde | 122-78-1 | 63.2 | 1040.8 | N.D | N.D | 1048 | Floral, caramel, green |
| pyrazine | 290-37-9 | 49.0 | 1201.0 | N.D | N.D | 1219 | Walnut, spicy, sweet corn, toasted |
| 2-(methoxymethyl)furan | 13679-46-4 | 28.3 | 1231.9 | N.D | N.D | 1251 | Coffee |
| 2-methyloxolan-3-one | 3188-00-9 | 73.0 | 1268.7 | N.D | N.D | 1269 | Sweet |
| 2-Methylpyrazine | 109-08-0 | 44.7 | 1269.5 | 57.7 | 1285.4 | 1267 | Chocolate |
| N.I | N.I | 60.6 | 1305.1 | N.I | N.I | N.I | Floral, sweet, woody, burnt |
| 2,5-dimethylpyrazine | 109-08-0 | 54.2 | 1319.9 | 51.6 | 1308.6 | 1320 | Green, wood, tan |
| 2,6-dimethylpyrazine | 108-50-9 | N.D | N.D | 60.6 | 1321.0 | 1322 | Chocolate |
| 2-ethyl-3,5-dimethylpyrazine | 13925-07-0 | 70.5 | 1329.3 | N.D | ND | 1328 | Toasted, sweet |
| 2-ethylpyrazine | 13925-00-3 | 40.0 | 1341.7 | 40.7 | 1338.0 | 1334 | Toasted, roasted |
| 2-ethyl-6-methylpyrazine | 13925-03-6 | N.D | N.D | 51.6 | 1368.0 | 1363 | Floral, green |
| 2-ethyl-5-methylpyrazine | 13360-64-0 | 63.2 | 1382.9 | 75.3 | 1396.7 | 1387 | Coffee, green |
| linalool oxide/(Z)-linalool oxide | 1365-19-1/5989-33-3 | 83.7 | 1422.2 | 85.6 | 1451.2 | 1451 | Floral, sweet |
| 3-ethyl-2,5-dimethylpyrazine | 13360-65-1 | 20.0 | 1442.3 | N.D | N.D | 1447 | Toasted |
| acetic acid | 64-19-7 | 87.6 | 1457.5 | 85.0 | 1466.4 | 1460 | Sharp, pungent, sour, vinegar |
| furfural | 98-01-1 | 52.9 | 1463.8 | 50.0 | 1474.3 | 1473 | Sweet, woody, bready, caramel |
| 2,3-diethyl-5-methylpyrazine | 18138-04-0 | 52.9 | 1481.8 | 50.0 | 1494.3 | 1492 | Green, tan, wood |
| ethanone, 1-(2-furanyl)- | 1192-62-7 | 69.3 | 1503.3 | N.D | N.D | 1499 | Sweet, cocoa |
| pyrrole | 109-97-7 | N.D | N.D | 40.0 | 1507.2 | 1507 | Dust, humidity |
| benzaldehyde | 100-52-7 | 75.3 | 1524.4 | N.D | N.D | 1528 | Sweet, bitter, almond |
| furan-2-ylmethyl acetate | 623-17-6 | 52.9 | 1537.5 | 43.0 | 1533.2 | 1531 | Sweet, savory, banana |
| linalool | 78-70-6 | 100.0 | 1550.9 | 100.0 | 1550.9 | 1547 | Woody, green, citric, floral, green, patchouli |
| 5-methylfuran-2-carbaldehyde | 620-02-0 | 75.3 | 1567.5 | N.D | N.D | 1570 | Sweet, caramel, pungent |
| furan-2-ylmethyl propanoate | 623-19-8 | 81.6 | 1594.2 | 51.6 | 1599.7 | 1602 | Sweet savory, green, banana, coffee |
| 2-(furan-2-methyl)furan | 1197-40-6 | 68.3 | 1600.4 | 65.4 | 1609.7 | 1615 | Toasted |
| butyrolactone | 96-48-0 | 49.0 | 1629.2 | 68.3 | 1631.7 | 1632 | Caramelized |
| furfuryl Alcohol | 98-00-0 | N.D | N.D | 44.7 | 1662.1 | 1666 | Earthy, sulfurous |
| 2-thiophenecarboxaldehyde | 98-03-3 | 49.0 | 1717.2 | 48.9 | 1685.7 | 1684 | Walnut, hazelnut, walnut |
| ethanone, 1-(2-thienyl) | 88-15-3 | 77.5 | 1747.5 | 54.8 | 1755.5 | 1763 | Sweet, earthy, green, floral |
| 2-buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- | 23696-85-7 | 63.2 | 1793.9 | N.D | N.D | 1801 | Tasty, coffee |
| 1H-Pyrrole, 1-(2-furanylmethyl)- | 1438-94-4 | 79.6 | 1832.6 | 75.9 | 1811.9 | 1824 | Phenolic, smoky, spicy |
| phenol, 2-methoxy- | 90-05-1 | 60.6 | 1862.8 | 60.6 | 1856.2 | 1861 | Floral, rose, phenolic. |
| benzyl alcohol | 100-51-6 | 58.9 | 1881.2 | N.D | N.D | 1870 | Sweet, caramel |
| 2-cyclopenten-1-one, 3-ethyl-2-hydroxy | 21835-01-8 | 61.1 | 1893.3 | N.D | N.D | 1894 | Herbal, hospital |
| 2-Thiophenemethanol | 636-72-6 | 31.6 | 1908.1 | N.D | N.D | 1930 | Coffee, roasted |
| furan, 2,2′-[oxybis(methylene)]bis- | 4437-22-3 | 51.6 | 1971.7 | 71.8 | 1949.8 | 1986 | Fusty, coffee |
| 1H-pyrrole-2-carboxaldehyde | 1003-29-8 | 77.5 | 2035.3 | N.D | N.D | 2030 | Earthy, sulfurous |
| furaneol | 3658-77-3 | 58.9 | N.D | 56.6 | 2038.0 | 2039 | Caramel, sweet |
| 4-ethylguaiacol | 2785-89-9 | 73.0 | N.D | 44.7 | 2070.2 | 2055 | Vanilla, sweet |
| 2-methoxy-4-vinylphenol | 7786-61-0 | 63.2 | 2096.6 | 40.8 | 2163.7 | 2188 | Phenolic, spicy, smoky, woody, powdery |
| indole | 120-72-9 | N.D | N.D | 36.5 | 2405.1 | 2448 | Floral |
| vanillin | 121-33-5 | 28.3 | 2526.7 | 31.6 | 2467.0 | 2566 | Vanilla, sweet |
| HS-SPE | ||
| Compound | %MF | Aroma Descriptor |
| 2,6-dimethylpyrazine | 60.6 | Chocolate |
| 2-ethyl-6-methylpyrazine | 51.6 | Floral, green |
| Pyrrole | 40.0 | Sweet |
| furfuryl Alcohol | 44.7 | Coffee |
| Furaneol | 56.6 | Caramel, sweet |
| 4-ethylguaiacol | 44.7 | Vanilla, sweet |
| Indole | 36.5 | Floral |
| HS-SPME | ||
| Compound | %FM | Aroma Descriptor |
| Butanal | 58.9 | Sweet |
| 1,5-octadien-3-one | 73.0 | Floral |
| Phenylacetaldehyde | 63.2 | Floral, caramel, green |
| Pyrazine | 49.0 | Walnut, spicy, sweet corn, toasted |
| 2-(methoxymethyl)furan | 28.3 | Coffee |
| 2-methyloxolan-3-one | 73.0 | Sweet |
| 2-ethyl-3,5-dimethylpyrazine | 77.5 | Toasted, sweet |
| 3-ethyl-2,5-dimethylpyrazine | 20.0 | Toasted |
| ethanone, 1-(2-furanyl)- | 69.3 | Sweet, cocoa |
| Benzaldehyde | 75.3 | Sweet, almond |
| 5-methylfuran-2-carbaldehyde | 75.3 | Sweet, caramel, pungent |
| 2-buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- | 63.2 | Sweet, earthy, green, floral |
| benzyl alcohol | 58.9 | Floral, rose, phenolic |
| 2-Thiophenemethanol | 31.6 | Coffee, roasted |
| 2-cyclopenten-1-one, 3-ethyl-2-hydroxy | 61.1 | Sweet, caramelized, maple |
| 1H-pyrrole-2-carboxaldehyde | 25.8 | Fusty, coffee |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narváez, E.; Zapata, E.; Dereix, J.D.; Lopez, C.; Torijano-Gutiérrez, S.; Zapata, J. A Comparative Analysis of Cold Brew Coffee Aroma Using the Gas Chromatography–Olfactometry–Mass Spectrometry Technique: Headspace–Solid-Phase Extraction and Headspace Solid-Phase Microextraction Methods for the Extraction of Sensory-Active Compounds. Molecules 2024, 29, 3791. https://doi.org/10.3390/molecules29163791
Narváez E, Zapata E, Dereix JD, Lopez C, Torijano-Gutiérrez S, Zapata J. A Comparative Analysis of Cold Brew Coffee Aroma Using the Gas Chromatography–Olfactometry–Mass Spectrometry Technique: Headspace–Solid-Phase Extraction and Headspace Solid-Phase Microextraction Methods for the Extraction of Sensory-Active Compounds. Molecules. 2024; 29(16):3791. https://doi.org/10.3390/molecules29163791
Chicago/Turabian StyleNarváez, Esteban, Esteban Zapata, Juan David Dereix, Carlos Lopez, Sandra Torijano-Gutiérrez, and Julián Zapata. 2024. "A Comparative Analysis of Cold Brew Coffee Aroma Using the Gas Chromatography–Olfactometry–Mass Spectrometry Technique: Headspace–Solid-Phase Extraction and Headspace Solid-Phase Microextraction Methods for the Extraction of Sensory-Active Compounds" Molecules 29, no. 16: 3791. https://doi.org/10.3390/molecules29163791






