Influence of Synthesis Conditions on Catalytic Performance of Ni/CeO2 in Aqueous-Phase Hydrogenolysis of Glycerol without External Hydrogen Input
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization Results before the Reaction
2.1.1. Chemical Analysis and Textural Properties
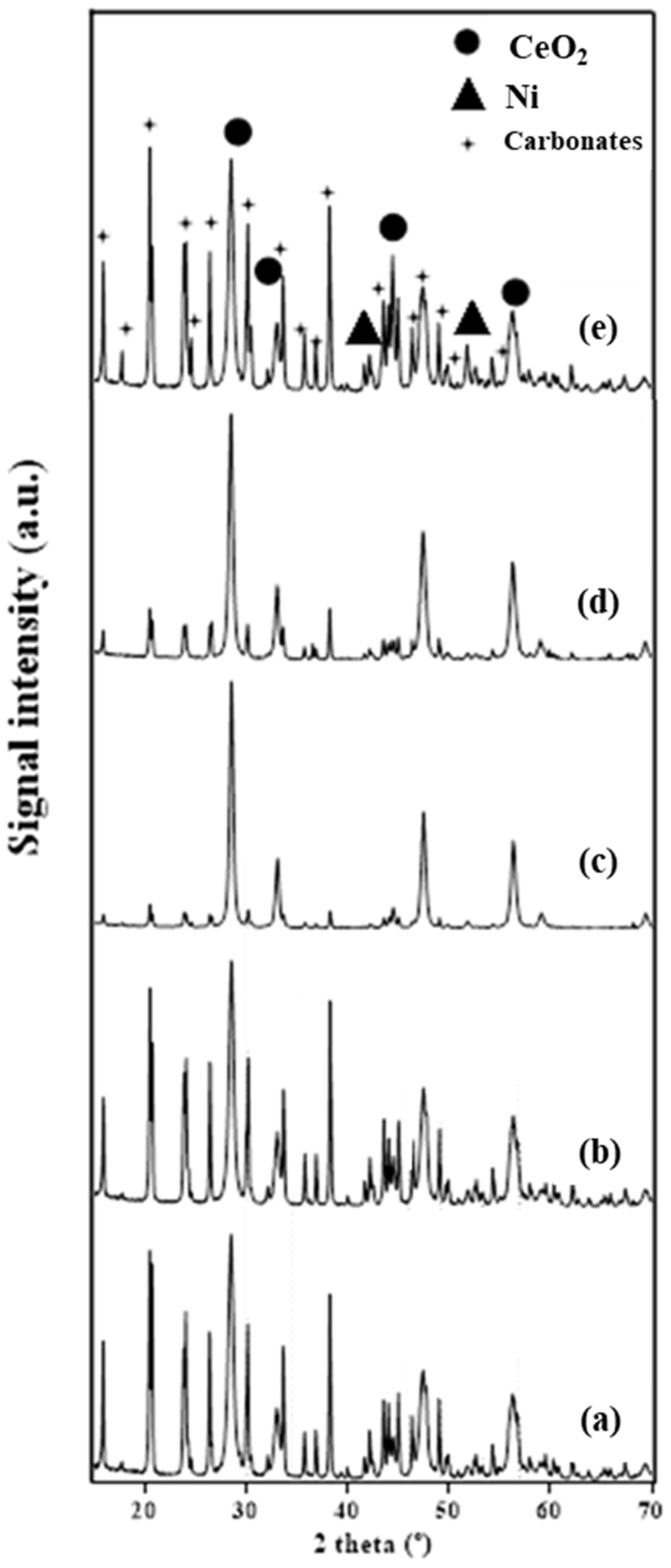
2.1.2. X-ray Diffraction
2.1.3. H2-TPR Results
2.1.4. NH3-TPD Results
2.2. Catalytic Activity
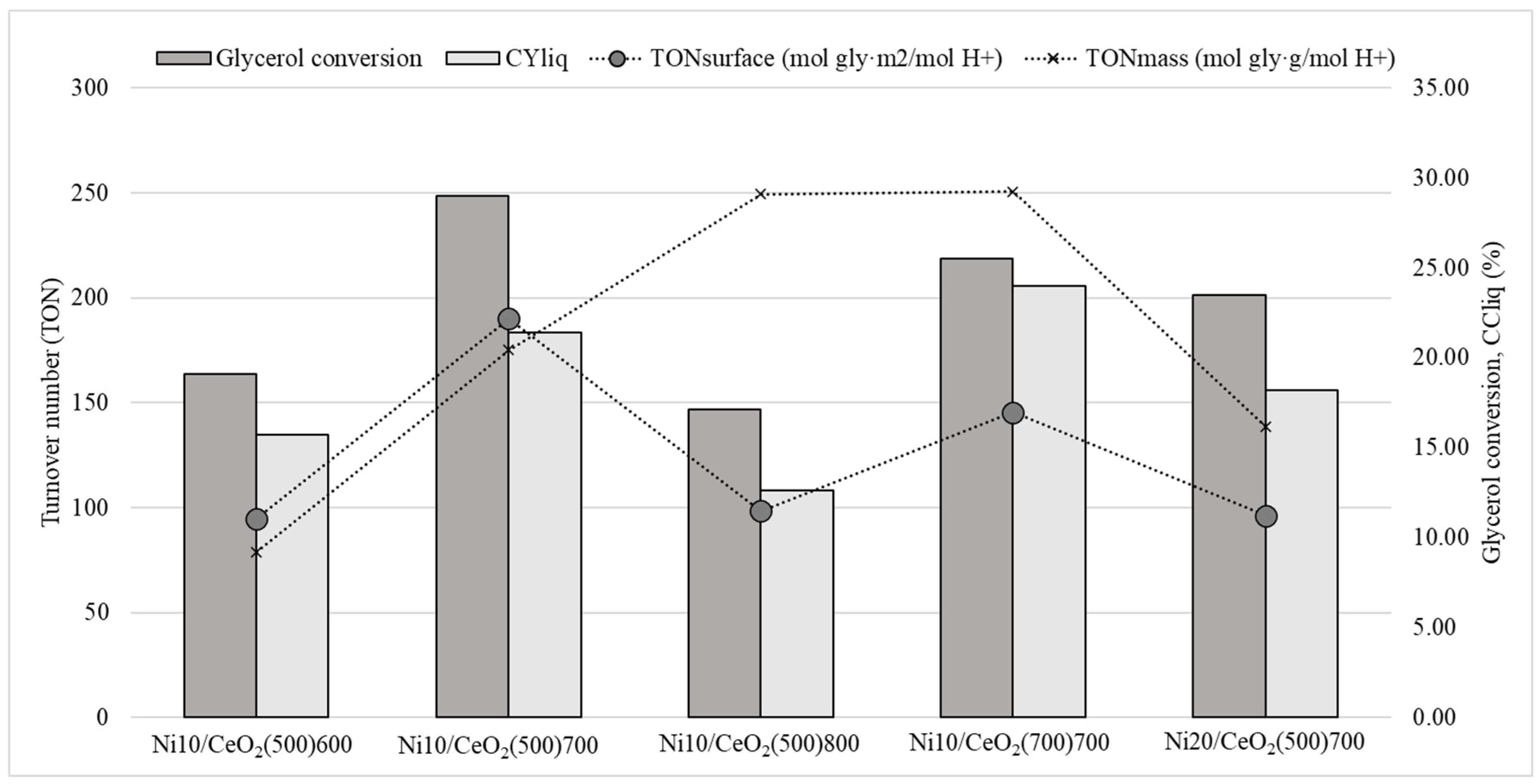
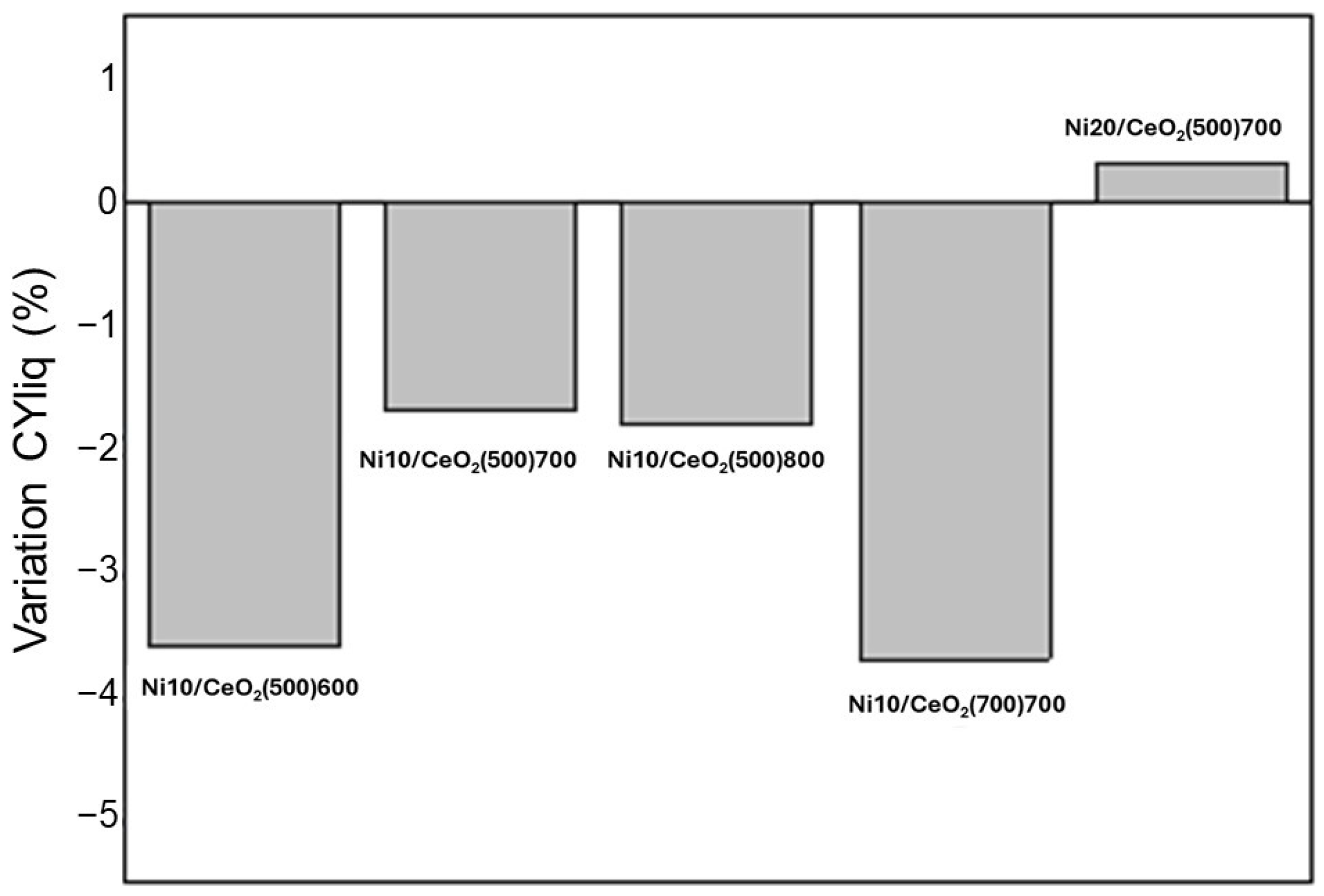
2.2.1. Global Activity Results
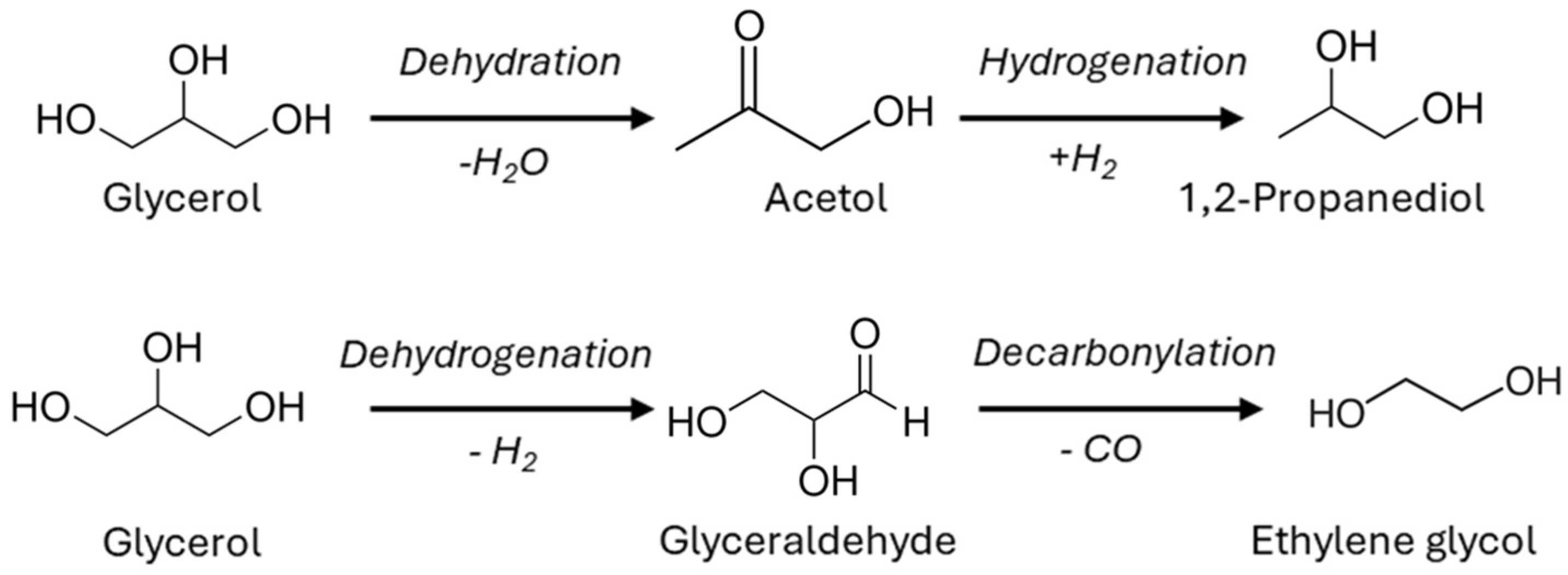
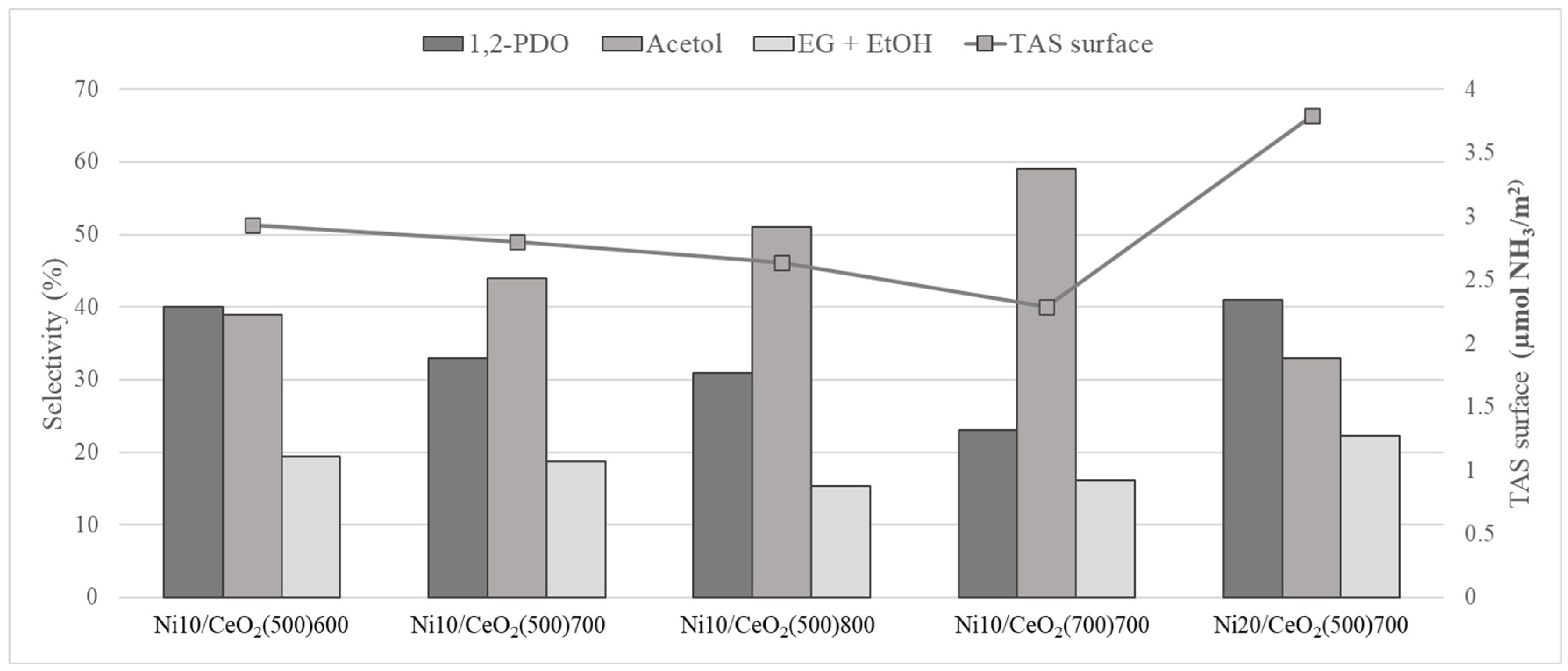
2.2.2. Products Distribution
2.3. Catalyst Characterization Results after the Reaction
2.3.1. X-ray Diffraction
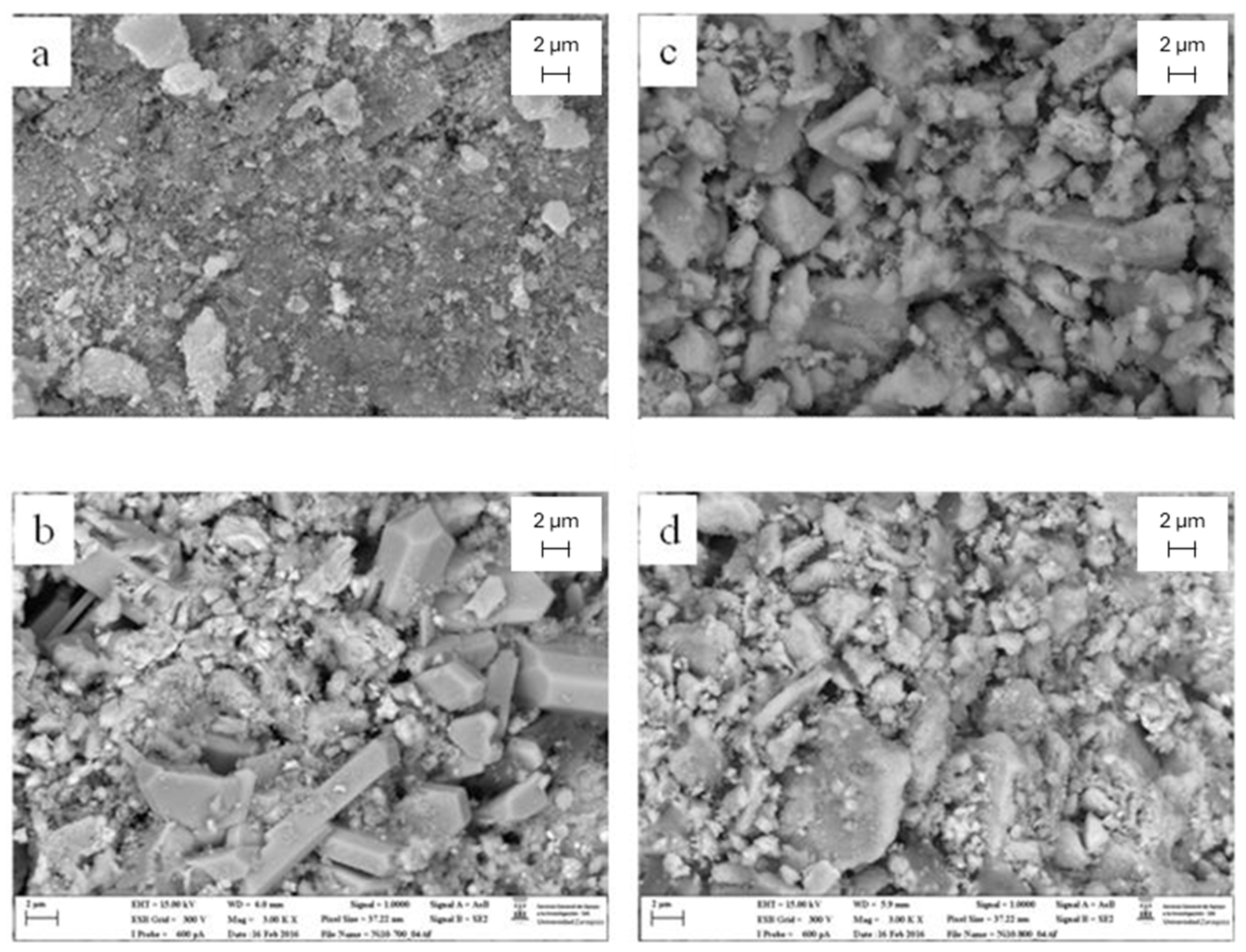
2.3.2. SEM Microscopy
2.3.3. Elemental Analysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norouzi, N.; Fani, M.; Ziarani, Z. The fall of oil Age:A scenario planning approach over the last peak oil of human history by 2040. J. Pet. Sci. Eng. 2020, 188, 106827. [Google Scholar] [CrossRef]
- Brandt, A. Review of mathematical models of future oil supply: Historical overview and synthesizing critique. Energy 2010, 35, 3958–3974. [Google Scholar] [CrossRef]
- Tomatis, M.; Jeswani, H.; Azapagic, A. Environmental impacts of valorisation of crude glycerol from biodiesel production—A life cycle perspective. Waste Manag. 2024, 179, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, H.; Tong, D.; Wu, L.; Yu, W. Recent Advances in Catalytic Conversion of Glycerol. Catal. Rev.-Sci. Eng. 2013, 55, 369–453. [Google Scholar] [CrossRef]
- Kong, P.; Aroua, M.; Daud, W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Cortright, R.; Davda, R.; Dumesic, J. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Coronado, I.; Stekrova, M.; Reinikainen, M.; Simell, P.; Lefferts, L.; Lehtonen, J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions. Int. J. Hydrog. Energy 2016, 41, 11003–11032. [Google Scholar] [CrossRef]
- Remon, J.; Gimenez, J.R.; Valiente, A.; Garcia, L.; Arauzo, J. Production of gaseous and liquid chemicals by aqueous phase reforming of crude glycerol: Influence of operating conditions on the process. Energy Convers. Manag. 2016, 110, 90–112. [Google Scholar] [CrossRef]
- Garcia, L.; Valiente, A.; Oliva, M.; Ruiz, J.; Arauzo, J. Influence of operating variables on the aqueous-phase reforming of glycerol over a Ni/Al coprecipitated catalyst. Int. J. Hydrogen Energy 2018, 43, 20392–20407. [Google Scholar] [CrossRef]
- Davda, R.; Shabaker, J.; Huber, G.; Cortright, R.; Dumesic, J. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B-Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Remon, J.; Garcia, L.; Arauzo, J. Cheese whey management by catalytic steam reforming and aqueous phase reforming. Fuel Process. Technol. 2016, 154, 66–81. [Google Scholar] [CrossRef]
- Oliveira, A.; Baeza, J.; Calvo, L.; Alonso-Morales, N.; Heras, F.; Rodriguez, J.; Gilarranz, M. Production of hydrogen from brewery wastewater by aqueous phase reforming with Pt/C catalysts. Appl. Catal. B-Environ. 2019, 245, 367–375. [Google Scholar] [CrossRef]
- Oliveira, A.; Baeza, J.; Calvo, L.; Alonso-Morales, N.; Heras, F.; Lemus, J.; Rodriguez, J.; Gilarranz, M. Exploration of the treatment of fish-canning industry effluents by aqueous-phase reforming using Pt/C catalysts. Environ. Sci.-Water Res. Technol. 2018, 4, 1979–1987. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.; Requies, J.; Guemez, M.; Fierro, J. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B-Environ. 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Cai, F.; Pan, D.; Ibrahim, J.; Zhang, J.; Xiao, G. Hydrogenolysis of glycerol over supported bimetallic Ni/Cu catalysts with and without external hydrogen addition in a fixed-bed flow reactor. Appl. Catal. A-Gen. 2018, 564, 172–182. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Song, Q.; Xin, Q.; Xu, S.; Xu, J. Heterogeneous Ceria Catalyst with Water-Tolerant Lewis Acidic Sites for One-Pot Synthesis of 1,3-Diols via Prins Condensation and Hydrolysis Reactions. J. Am. Chem. Soc. 2013, 135, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Smith, L.; Dummer, N.; Douthwaite, M.; Willock, D.; Howard, M.; Knight, D.; Taylor, S.; Hutchings, G. Investigating the Influence of Reaction Conditions and the Properties of Ceria for the Valorisation of Glycerol. Energies 2019, 12, 1359. [Google Scholar] [CrossRef]
- Bimbela, F.; Abrego, J.; Puerta, R.; Garcia, L.; Arauzo, J. Catalytic steam reforming of the aqueous fraction of bio-oil using Ni-Ce/Mg-Al catalysts. Appl. Catal. B-Environ. 2017, 209, 346–357. [Google Scholar] [CrossRef]
- Coronado, I.; Stekrova, M.; Moreno, L.; Reinikainen, M.; Simell, P.; Karinen, R.; Lehtonen, J. Aqueous-phase reforming of methanol over nickel-based catalysts for hydrogen production. Biomass Bioenergy 2017, 106, 29–37. [Google Scholar] [CrossRef]
- Chary, K.; Rao, P.; Vishwanathan, V. Synthesis and high performance of ceria supported nickel catalysts for hydrodechlorination reaction. Catal. Commun. 2006, 7, 974–978. [Google Scholar] [CrossRef]
- Manfro, R.; da Costa, A.; Ribeiro, N.; Souza, M. Hydrogen production by aqueous-phase reforming of glycerol over nickel catalysts supported on CeO2. Fuel Process. Technol. 2011, 92, 330–335. [Google Scholar] [CrossRef]
- Roy, B.; Sullivan, H.; Leclerc, C. Aqueous-phase reforming of n-BuOH over Ni/Al2O3 and Ni/CeO2 catalysts. J. Power Sour. 2011, 196, 10652–10657. [Google Scholar] [CrossRef]
- Roy, B.; Sullivan, H.; Leclerc, C. Effect of variable conditions on steam reforming and aqueous phase reforming of n-butanol over Ni/CeO2 and Ni/Al2O3 catalysts. J. Power Sources 2014, 267, 280–287. [Google Scholar] [CrossRef]
- Roy, B.; Leclerc, C. Study of preparation method and oxidization/reduction effect on the performance of nickel-cerium oxide catalysts for aqueous-phase reforming of ethanol. J. Power Sour. 2015, 299, 114–124. [Google Scholar] [CrossRef]
- Lee, H.; Shin, G.; Kim, Y. Characterization of supported Ni catalysts for aqueous-phase reforming of glycerol. Korean J. Chem. Eng. 2015, 32, 1267–1272. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A. Aqueous-phase reforming of glycerol for production of alkanes over Ni/CexZr1-xO2 nano-catalyst: Effects of the support’s composition. Renew. Energy 2017, 108, 417–424. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Y.; Hill, M.; Xia, X.; Zhao, Y.; Jiang, Z. Highly efficient Ni/CeO2 catalyst for the liquid phase hydrogenation of maleic anhydride. Appl. Catal. A-Gen. 2014, 488, 256–264. [Google Scholar] [CrossRef]
- Tang, R.; Ullah, N.; Hui, Y.; Li, X.; Li, Z. Enhanced CO2 methanation activity over Ni/CeO2 catalyst by one-pot method. Mol. Catal. 2021, 508, 111602. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; de Leitenburg, C.; Giona, M. A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrog. Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M.; Tyagi, S. Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T. Structural defects in a nano-scale powder of CeO2 studied by pulsed neutron diffraction. J. Phys. Chem. Solids 2000, 61, 1345–1356. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.; Overbury, S. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.; Conesa, J.; Martinez-Arias, A. Characterization of surface defects in CeO2 modified by incorporation of precious metals from chloride salts precursors: An EPR study using oxygen as probe molecule. Colloids Surf. A-Physicochem. Eng. Asp. 1999, 158, 67–74. [Google Scholar] [CrossRef]
- Zou, W.; Ge, C.; Lu, M.; Wu, S.; Wang, Y.; Sun, J.; Pu, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation. Rsc. Adv. 2015, 5, 98335–98343. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernandez, C.; O’Shea, V.; Pizarro, P.; Coronado, J.; Serrano, D. Effect of metal-support interaction on the selective hydrodeoxygenation of anisole to aromatics over Ni-based catalysts. Appl. Catal. B-Environ. 2014, 145, 91–100. [Google Scholar] [CrossRef]
- Kulthananat, T.; Kim-Lohsoontorn, P.; Seeharaj, P. Ultrasonically assisted surface modified CeO2 nanospindle catalysts for conversion of CO2 and methanol to DMC. Ultrason. Sonochem. 2022, 90, 106164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, Y.; Yang, Y.; Kong, T.; Song, Y.; Zhang, S.; Zheng, H. Elucidating the Role of Surface Ce4+ and Oxygen Vacancies of CeO2 in the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Molecules 2023, 28, 3785. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Subramaniam, B.; Chaudhari, R. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Dietrich, P.; Wu, T.; Sumer, A.; Dumesic, J.; Jellinek, J.; Delgass, W.; Ribeiro, F.; Miller, J. Aqueous Phase Glycerol Reforming with Pt and PtMo Bimetallic Nanoparticle Catalysts: The Role of the Mo Promoter. Top. Catal. 2013, 56, 1814–1828. [Google Scholar] [CrossRef]
- Ciftci, A.; Ligthart, D.; Hensen, E. Aqueous phase reforming of glycerol over Re-promoted Pt and Rh catalysts. Green Chem. 2014, 16, 853–863. [Google Scholar] [CrossRef]
- Guo, Z.; Du, F.; Li, G.; Cui, Z. Synthesis and characterization of single-crystal Ce(OH)CO3 and CeO2 triangular microplates. Inorg. Chem. 2006, 45, 4167–4169. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, D.; Munuera, G.; Hungría, A.; Fernández-García, M.; Conesa, J.; Midgley, P.; Wang, X.; Hanson, J.; Rodríguez, J.; Martínez-Arias, A. Structure-activity relationship in nanostructured copper-ceria-based preferential CO oxidation catalysts. J. Phys. Chem. C 2007, 111, 11026–11038. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H. Formation and microstructural variation of cerium carbonate hydroxide prepared by the hydrothermal process. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2002, 90, 138–141. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
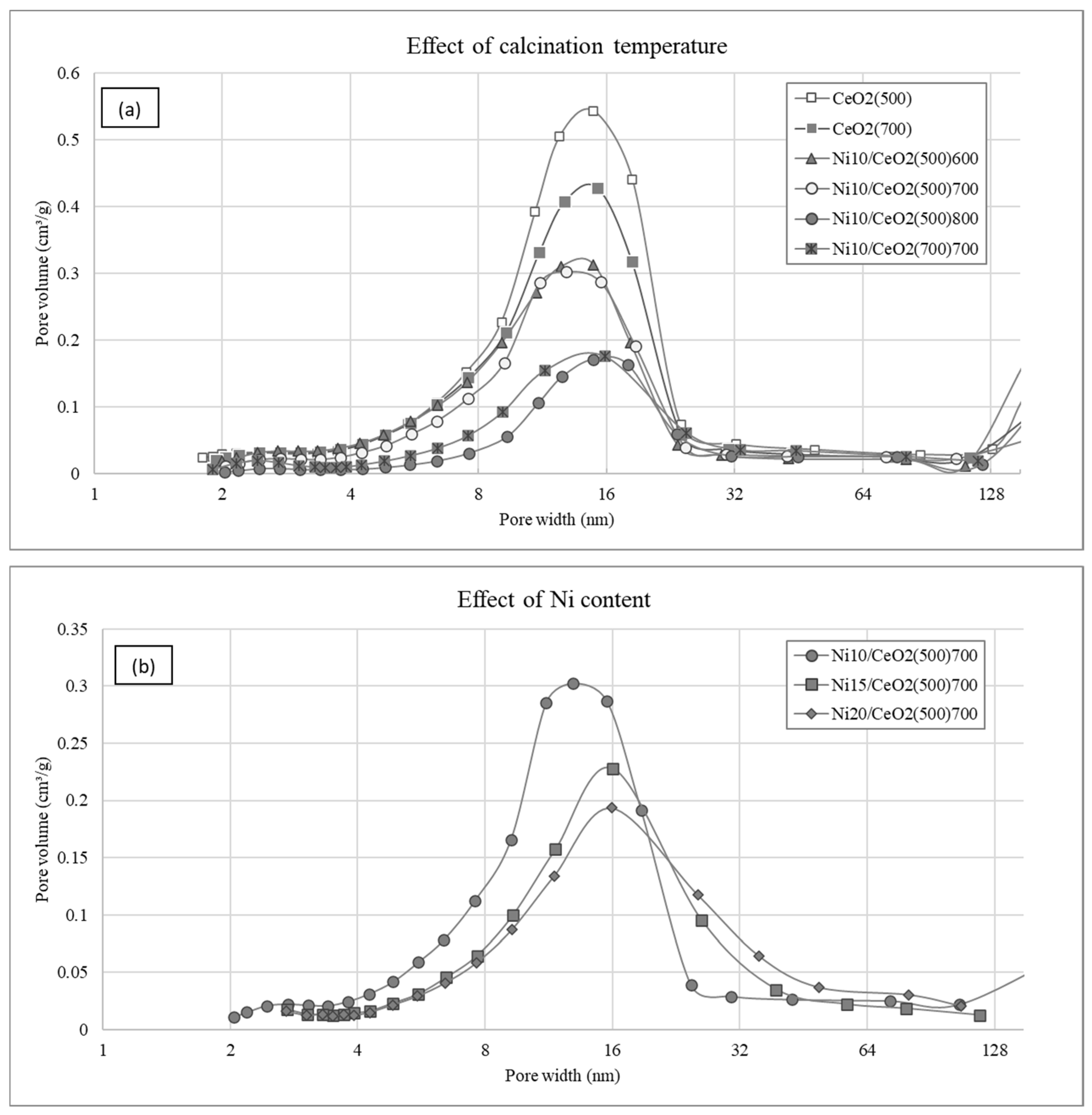

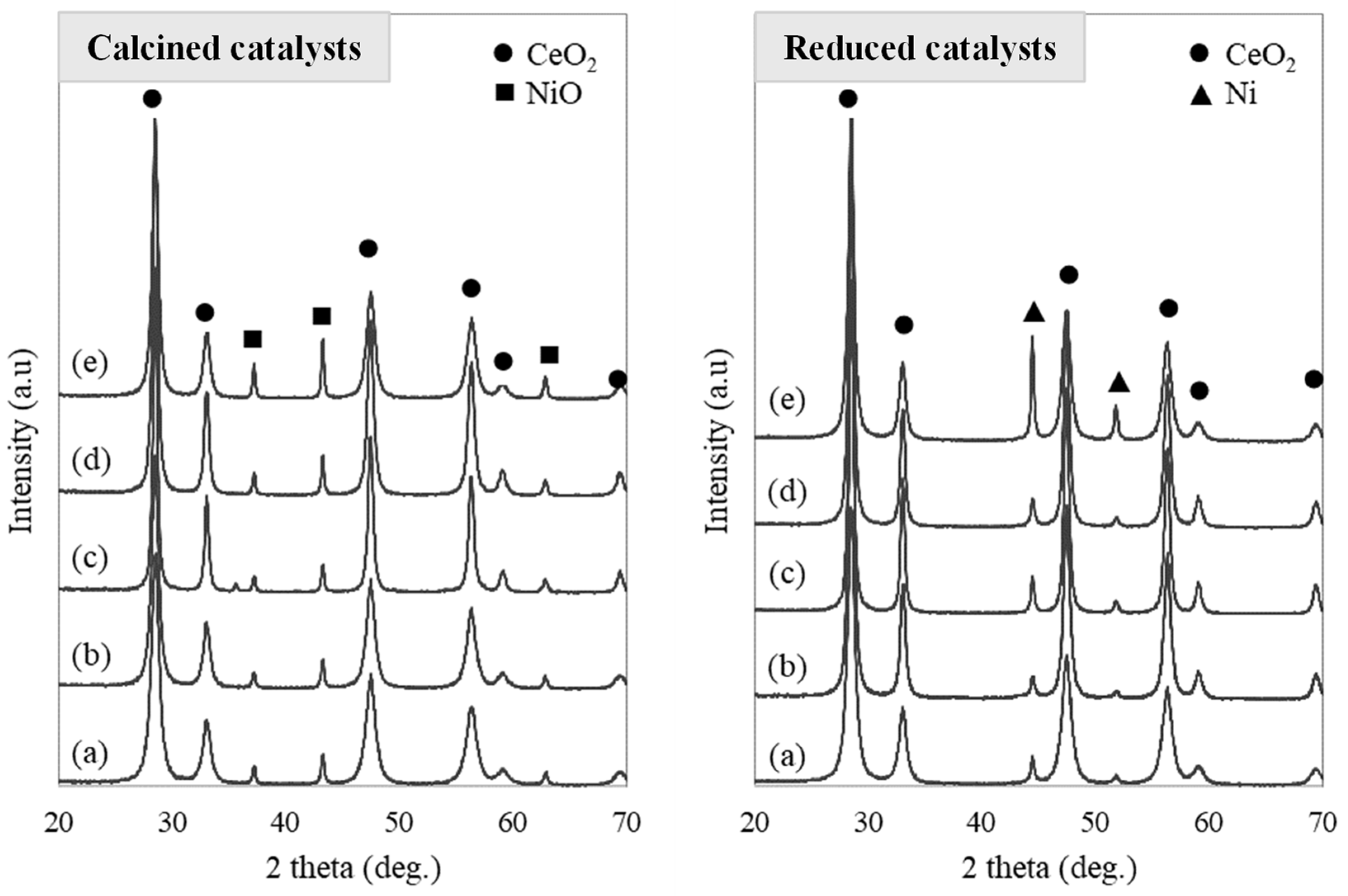

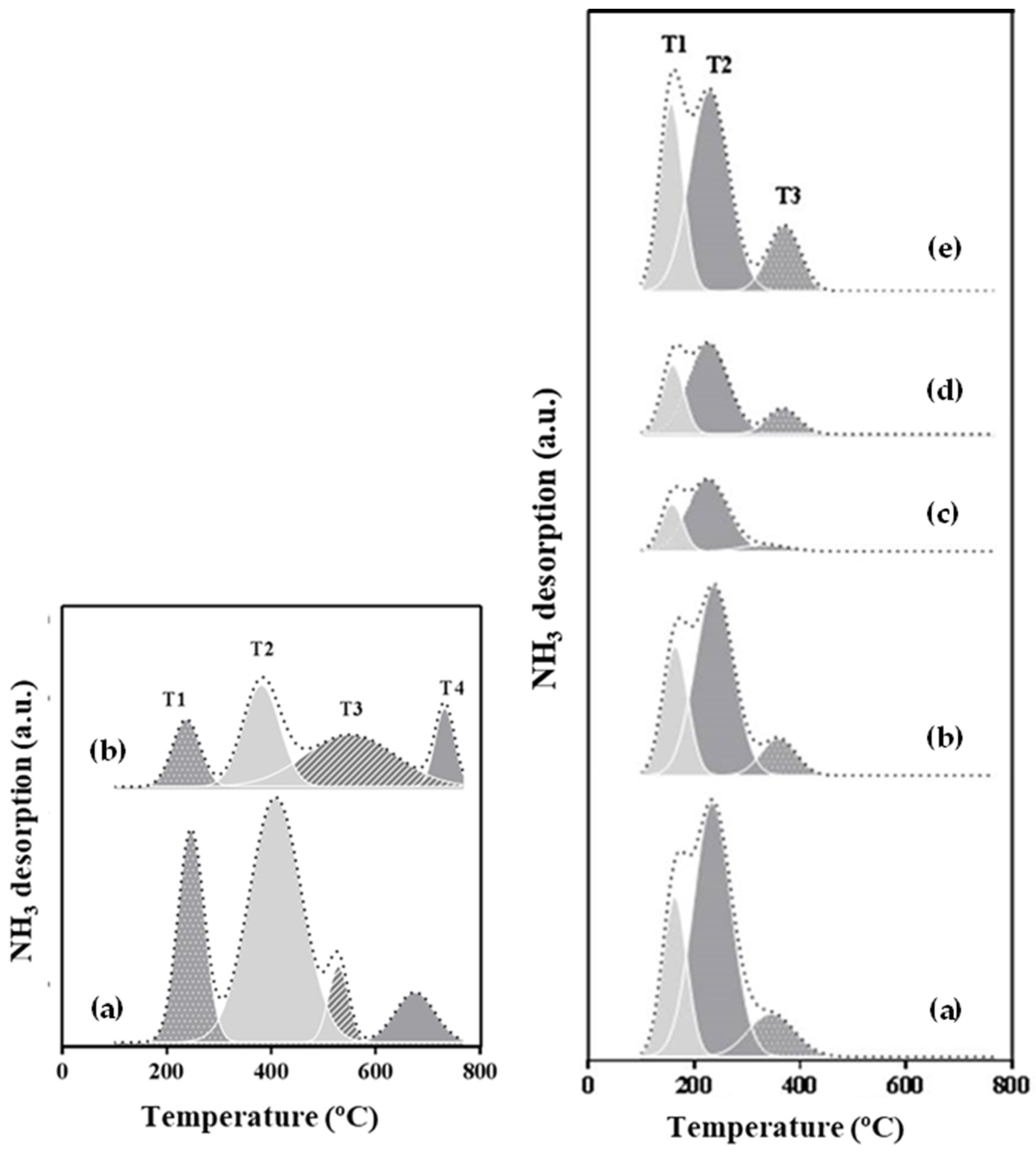

| Ni Content (wt.%) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Metallic Dispersion, Dexp (%) | |
|---|---|---|---|---|---|
| Ni10/CeO2(500)600 | 9.644 ± 0.085 | 67 | 0.15 | 8.8 | 1.90 |
| Ni10/CeO2(500)700 | 9.793 ± 0.098 | 54 | 0.14 | 10.0 | 2.72 |
| Ni10/CeO2(500)800 | 9.418 ± 0.066 | 22 | 0.07 | 12.7 | 1.13 |
| Ni10/CeO2(700)700 | 9.592 ± 0.013 | 34 | 0.11 | 12.9 | 1.85 |
| Ni20/CeO2(500)700 | 19.670 ± 0.089 | 38 | 0.13 | 13.4 | 1.62 |
| CeO2(500) | - | 98 | 0.23 | 9.4 | - |
| CeO2(700) | - | 61 | 0.19 | 11.5 | - |
| Temperatures (°C) | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Ni10/CeO2(500)600 | 172 | 218 | 309 | 335 | 798 |
| Ni10/CeO2(500)700 | 182 | 224 | 320 | 367 | 807 |
| Ni10/CeO2(500)800 | 196 | 204 | 308 | 491 | 810 |
| Ni10/CeO2(700)700 | 160 | 213 | 309 | 413 | 812 |
| Ni20/CeO2(500)700 | 171 | 227 | 341 | 444 | 823 |
| Temperatures (°C) | Strength of Acid Sites * (%) | TASmass | TASsurface | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | L | L-M | M | H | µmol NH3/g | µmol NH3/m2 | |
| CeO2(500) | 245 | 413 | 528 | 675 | - | 16 | 50 | 34 | 64 | 0.65 |
| CeO2(700) | 241 | 372 | 551 | 740 | - | 20 | 57 | 23 | 18 | 0.30 |
| Ni10/CeO2(500)600 | 163 | 223 | 346 | - | 24 | 67 | 8 | - | 196 | 2.93 |
| Ni10/CeO2(500)700 | 151 | 238 | 362 | - | 32 | 53 | 15 | - | 151 | 2.80 |
| Ni10/CeO2(500)800 | 145 | 219 | 337 | - | 27 | 68 | 5 | - | 58 | 2.64 |
| Ni10/CeO2(700)700 | 145 | 227 | 367 | - | 28 | 56 | 16 | - | 78 | 2.29 |
| Ni20/CeO2(500)700 | 146 | 231 | 361 | - | 34 | 49 | 17 | - | 144 | 3.79 |
| Ni10/CeO2(500)600 | Ni10/CeO2(500)700 | Ni10/CeO2(500)800 | Ni10/CeO2(700)700 | Ni20/CeO2(500)700 | |
|---|---|---|---|---|---|
| Global catalyst performance | |||||
| Gly. conv. (%) | 19.1 ± 0.9 | 29.0 ± 0.3 | 17.1 ± 0.1 | 25.5 ± 0.1 | 23.5 ± 0.9 |
| CYliq (%) | 15.7 ± 0.8 | 21.4 ± 0.5 | 12.6 ± 0.5 | 24.0 ± 0.1 | 18.2 ± 0.3 |
| CYgas (%) | 1.4 ± 0.2 | 1.1 ± 0.1 | 0.8 ± 0.5 | 1.5 ± 0.9 | 1.9 ± 0.1 |
| Cdef (%) | 2.0 | 6.5 | 3.7 | 0 | 3.4 |
| TONsurface (molgly·m2/molH+) | 95 | 190 | 98 | 145 | 96 |
| TONmass (molgly·g/molH+) | 79 | 175 | 249 | 251 | 139 |
| Selectivity (%) | |||||
| 1,2-PDO | 40 ± 1 | 33 ± 1 | 31 ± 1 | 23 ± 2 | 41 ± 1 |
| Acetol | 39 ± 1 | 44 ± 2 | 51 ± 1 | 59 ± 3 | 33 ± 1 |
| Acetone | 0.54 ± 0.05 | 0.54 ± 0.01 | 0.48 ± 0.01 | 0.82 ± 0.01 | 0.47 ± 0.03 |
| EG | 7.6 ± 0.1 | 6.3 ± 0.4 | 6.0 ± 0.1 | 4.5 ± 0.5 | 8.4 ± 0.2 |
| EtOH | 11.8 ± 0.3 | 12.4 ± 0.7 | 9.3 ± 0.1 | 11.6 ± 0.5 | 13.8 ± 0.7 |
| Acetic acid | 0.9 ± 0.1 | 3.1 ± 0.1 | 1.2 ± 0.2 | - | 2.8 ± 0.3 |
| MeOH | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.3 | 0.8 ± 0.1 |
| Yields (gA/gglycerol) | |||||
| 1,2-PDO | 0.0513 | 0.058 | 0.0327 | 0.0458 | 0.0613 |
| Acetol | 0.0486 | 0.0758 | 0.0514 | 0.1136 | 0.0484 |
| EG | 0.012 | 0.0136 | 0.0076 | 0.0109 | 0.0154 |
| EtOH | 0.0138 | 0.0198 | 0.0088 | 0.0209 | 0.0188 |
| Carbon (wt.%) | Ni (wt.%) | Ce (wt.%) | |
|---|---|---|---|
| Ni10/CeO2(500)600 | 3.83 | 0.39 | 0.35 |
| Ni10/CeO2(500)700 | 3.88 | 0.30 | 0.08 |
| Ni10/CeO2(500)800 | 2.38 | 0.25 | 0.07 |
| Ni10/CeO2(700)700 | 1.88 | 0.35 | 0.40 |
| Ni20/CeO2(500)700 | 3.96 | 0.13 | 0.04 |
| Catalyst | T1 (°C) | T2 (°C) | Nickel Load (wt.%) | Reduction Temp. (°C) |
|---|---|---|---|---|
| Ni10/CeO2(500)600 | 500 | 600 | 10 | 500 |
| Ni10/CeO2(500)700 | 500 | 700 | 10 | 500 |
| Ni10/CeO2(500)800 | 500 | 800 | 10 | 550 |
| Ni10/CeO2(700)700 | 700 | 700 | 10 | 500 |
| Ni20/CeO2(500)700 | 500 | 700 | 20 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarauta-Córdoba, C.; García, L.; Ruiz, J.; Oliva, M.; Arauzo, J. Influence of Synthesis Conditions on Catalytic Performance of Ni/CeO2 in Aqueous-Phase Hydrogenolysis of Glycerol without External Hydrogen Input. Molecules 2024, 29, 3797. https://doi.org/10.3390/molecules29163797
Jarauta-Córdoba C, García L, Ruiz J, Oliva M, Arauzo J. Influence of Synthesis Conditions on Catalytic Performance of Ni/CeO2 in Aqueous-Phase Hydrogenolysis of Glycerol without External Hydrogen Input. Molecules. 2024; 29(16):3797. https://doi.org/10.3390/molecules29163797
Chicago/Turabian StyleJarauta-Córdoba, Clara, Lucía García, Joaquín Ruiz, Miriam Oliva, and Jesús Arauzo. 2024. "Influence of Synthesis Conditions on Catalytic Performance of Ni/CeO2 in Aqueous-Phase Hydrogenolysis of Glycerol without External Hydrogen Input" Molecules 29, no. 16: 3797. https://doi.org/10.3390/molecules29163797
APA StyleJarauta-Córdoba, C., García, L., Ruiz, J., Oliva, M., & Arauzo, J. (2024). Influence of Synthesis Conditions on Catalytic Performance of Ni/CeO2 in Aqueous-Phase Hydrogenolysis of Glycerol without External Hydrogen Input. Molecules, 29(16), 3797. https://doi.org/10.3390/molecules29163797










