Study of Mesoporous Zr-TiO2 Catalyst with Rich Oxygen Vacancies for N-Methylmorpholine Oxidation to N-Methylmorpholine-N-oxide
Abstract
1. Introduction
2. Results and Discussion
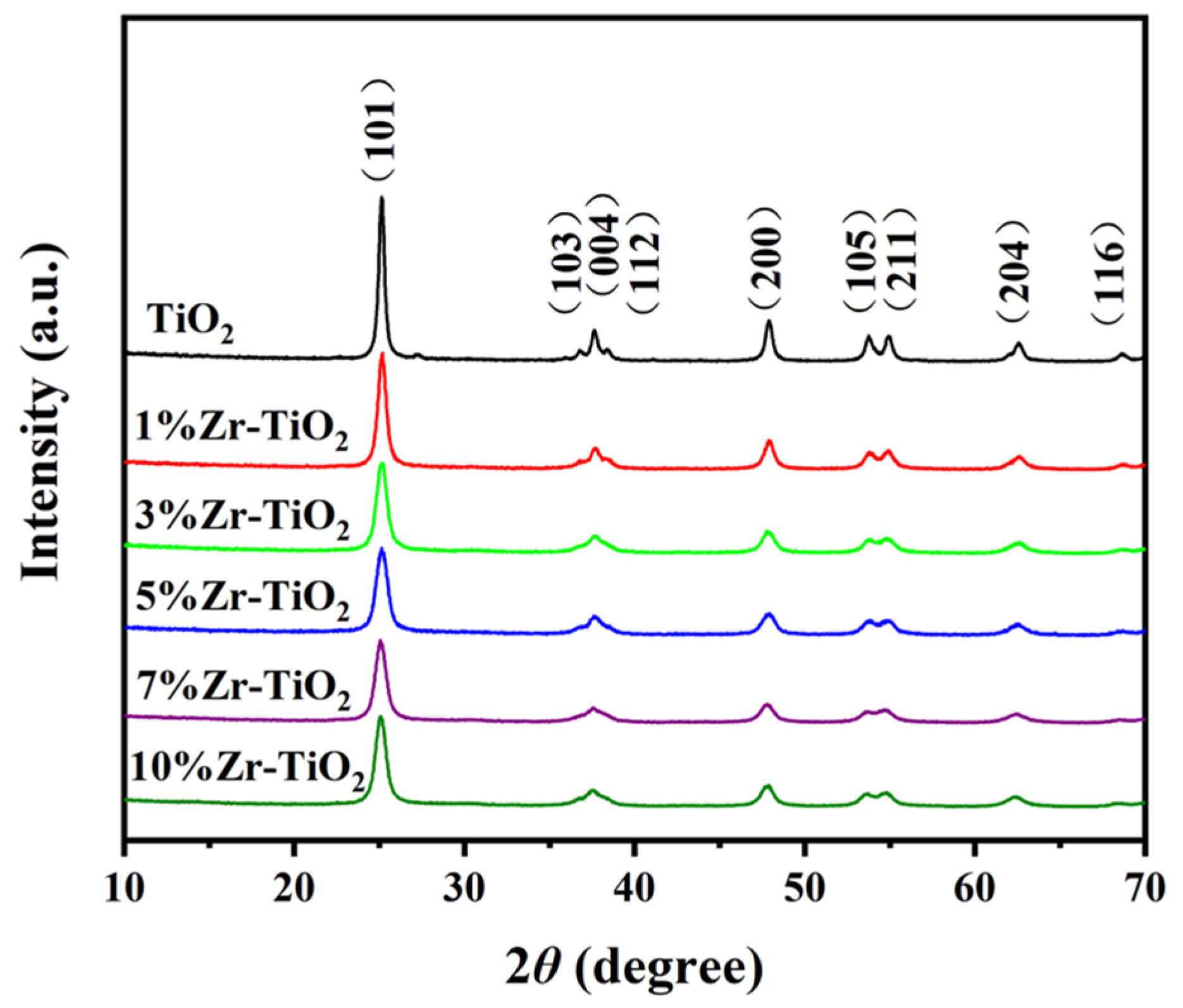
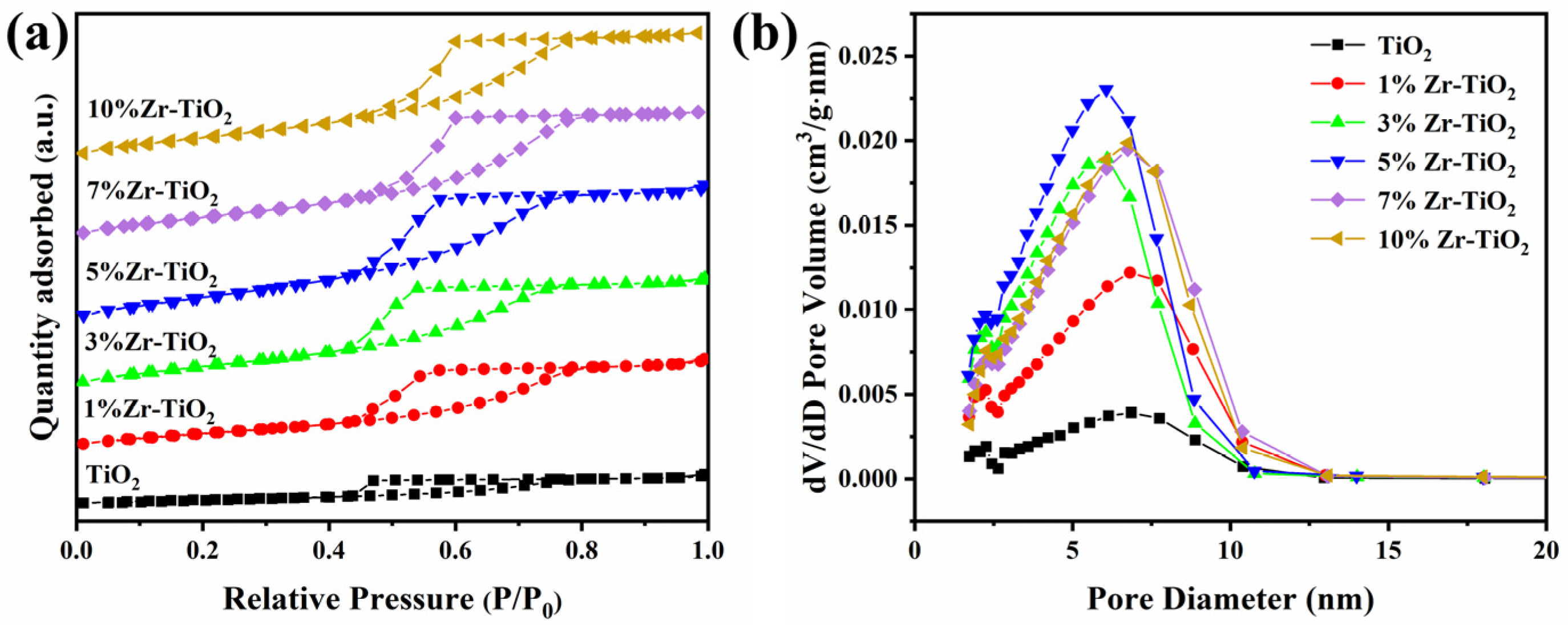
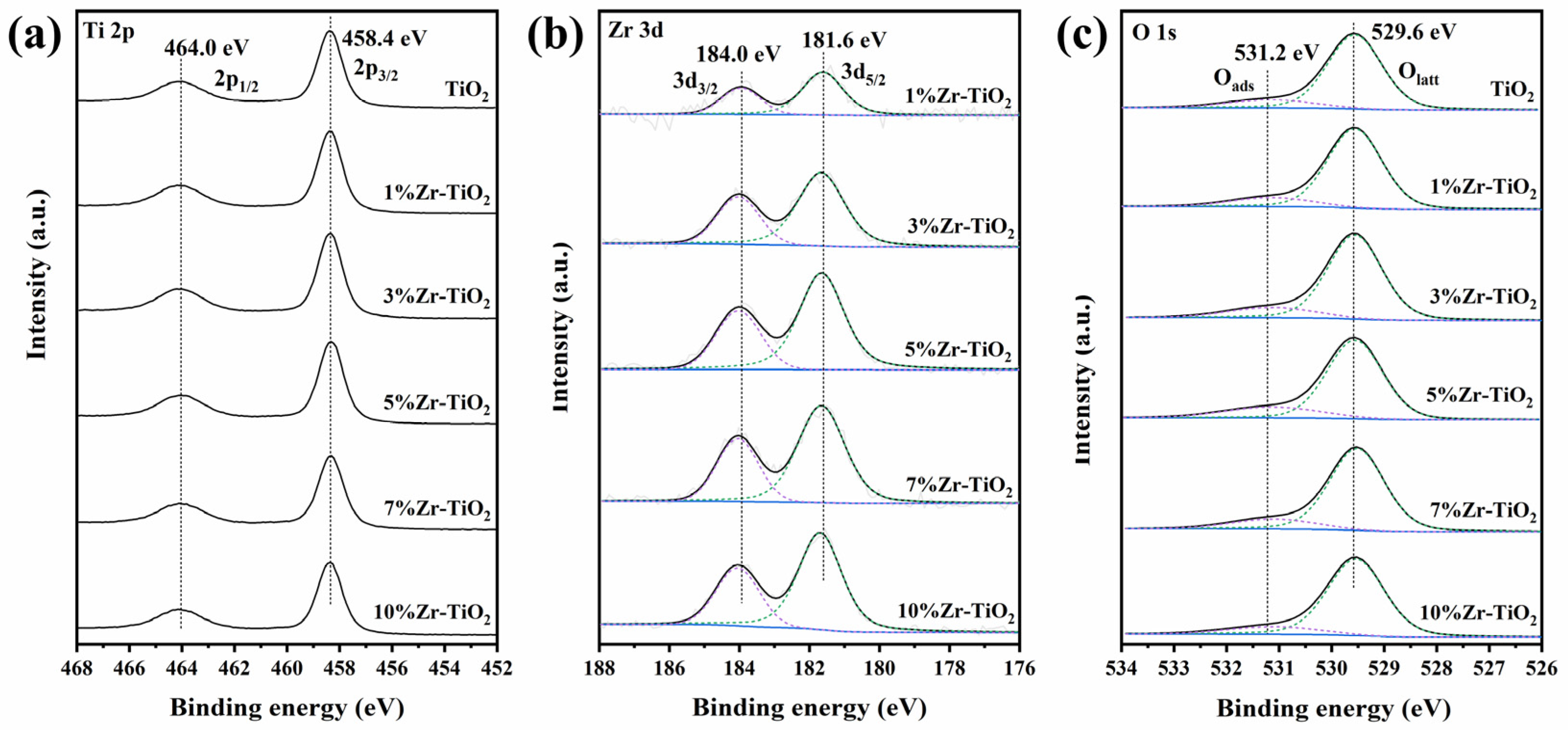
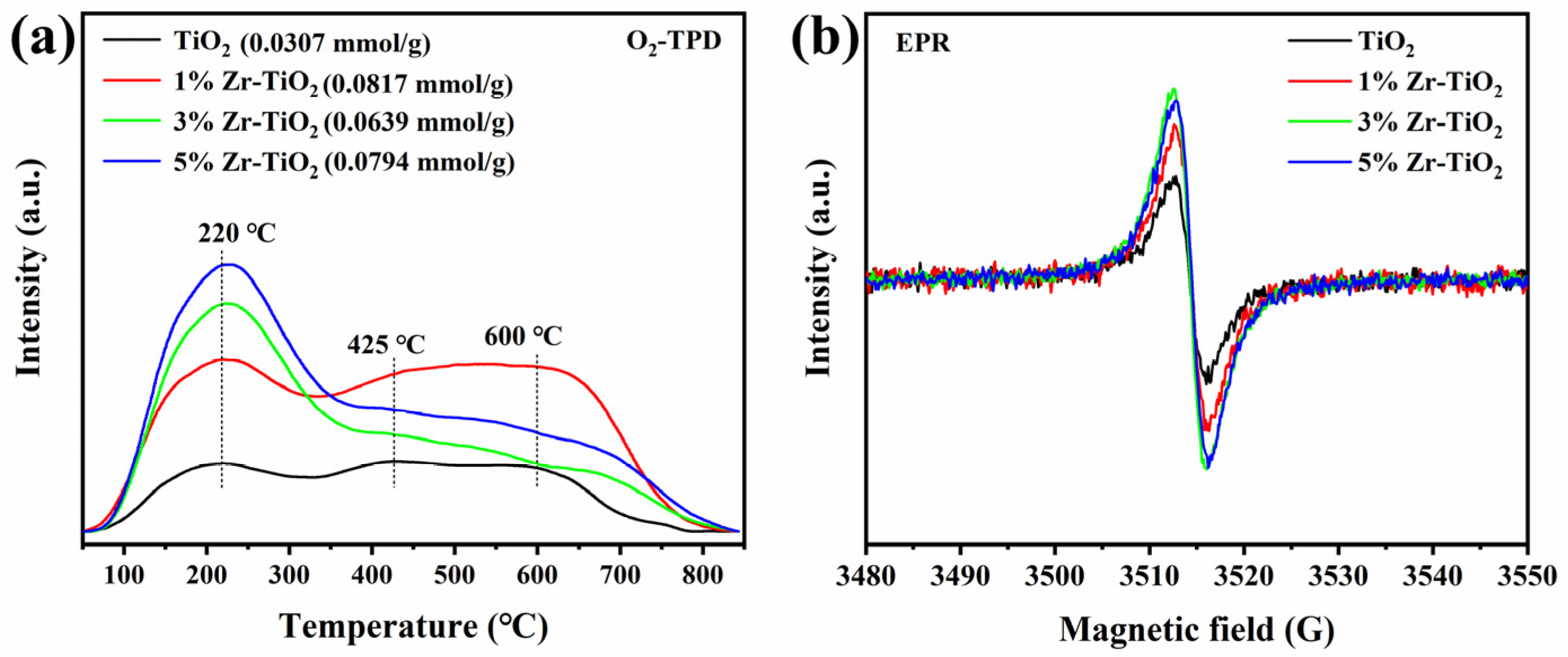
2.1. Structural Properties of the Catalysts
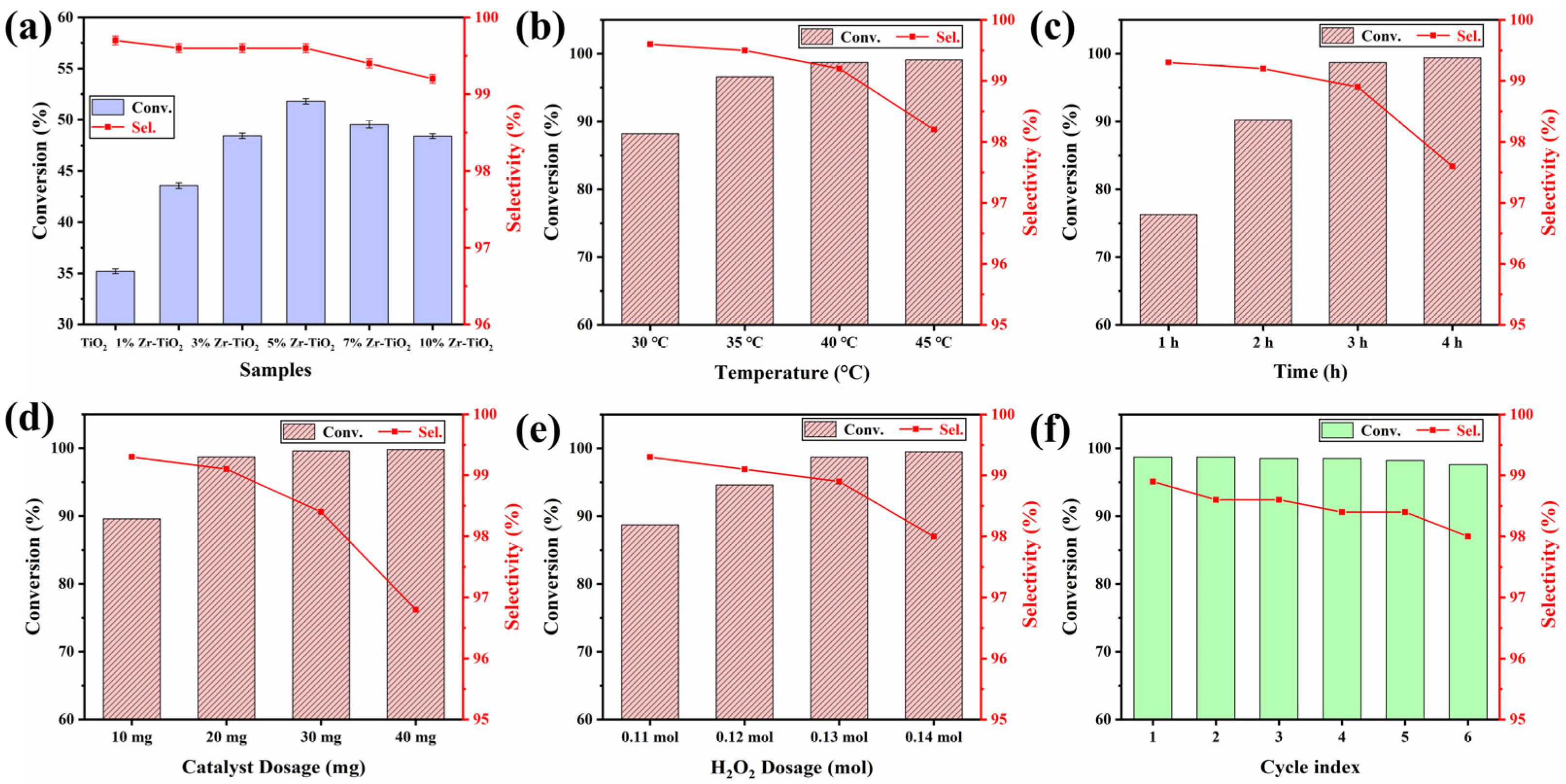
2.2. Catalytic Performance
2.3. Reaction Process
3. Experimental Section
3.1. Chemical Reagents
3.2. Zr-TiO2 Catalyst Preparation
3.3. Characterization
3.4. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R. A review on the modification of cellulose and its applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A review of water interactions, applications in composites, and water treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- D’Acierno, F.; Michal, C.A.; MacLachlan, M.J. Thermal stability of cellulose nanomaterials. Chem. Rev. 2023, 123, 7295–7325. [Google Scholar] [CrossRef]
- Agarwala, P.; Kanthale, P.; Thakre, S. Flocculation of spin bath NMMO solution for removal of colloidal impurities in lyocell process. Sep. Sci. Technol. 2019, 54, 2516–2527. [Google Scholar] [CrossRef]
- Jadhav, S.; Lidhure, A.; Thakre, S.; Ganvir, V. Modified Lyocell process to improve dissolution of cellulosic pulp and pulp blends in NMMO solvent. Cellulose 2021, 28, 973–990. [Google Scholar] [CrossRef]
- Oliva, A.; Tan, L.; Papirio, S.; Esposito, G.; Lens, P. Use of N-Methylmorpholine-N-oxide (NMMO) pretreatment to enhance the bioconversion of ligno-cellulosic residues to methane. Biomass Convers. Biorefin. 2024, 14, 11113–11130. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, A.; Baghdasaryan, G.; Hovhannisyan, G.; Attaryan, H.; Hasratyan, G. Waste-free technology for N-methylmorpholine synthesis. Russ. J. Appl. Chem. 2013, 86, 845–847. [Google Scholar] [CrossRef]
- El-Wakil, N.; Taha, M.; Abouzeid, R.; Dufresne, A. Dissolution and regeneration of cellulose from N-methylmorpholine N-oxide and fabrication of nanofibrillated cellulose. Biomass Convers. Biorefin. 2024, 14, 5399–5410. [Google Scholar] [CrossRef]
- Balagam, B.; Richardson, D.E. The mechanism of carbon dioxide catalysis in the hydrogen peroxide N-oxidation of amines. Inorg. Chem. 2008, 47, 1173–1178. [Google Scholar] [CrossRef]
- Prasad, M.R.; Kamalakar, G.; Madhavi, G.; Kulkarni, S.; Raghavan, K. An efficient synthesis of heterocyclic N-oxides over molecular sieve catalysts. J. Mol. Catal. A 2002, 186, 109–120. [Google Scholar] [CrossRef]
- Bouzakher-Ghomrasni, N.; Tache, O.; Leroy, J.; Feltin, N.; Testard, F.; Chivas-Joly, C. Dimensional measurement of TiO2 (Nano) particles by SAXS and SEM in powder form. Talanta 2021, 234, 122619. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, S.; Chang, X.; Wang, X.; Sun, G.; Lu, Z.; Zhao, Z.-J.; Pei, C.; Gong, J. Ultrathin TiOx nanosheets rich in tetracoordinated Ti sites for propane dehydrogenation. ACS Catal. 2023, 13, 6104–6113. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, D.-K.; Park, D.-W. Synthesis and characterization of zirconium-doped mesoporous nano-crystalline TiO2. Nanoscale 2010, 2, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xie, Z.; Song, S.; Zhao, Z.; Ke, M.; Song, W.; Zhao, Z.; Liu, J. Descriptor-guided design and experimental synthesis of metal-doped TiO2 for propane dehydrogenation. Ind. Eng. Chem. Res. 2021, 60, 1200–1209. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Zhang, Y.; Liu, D.; Ma, A.; Sun, Y.; Li, X.; Cai, Q.; Gui, J. Ag-induced anatase-rutile TiO2−x heterojunction facilitating the photogenerated carrier separation in visible-light irradiation. J. Alloys Compd. 2022, 909, 164815. [Google Scholar] [CrossRef]
- Wang, C.-T.; Lin, J.-C. Surface nature of nanoparticle zinc-titanium oxide aerogel catalysts. Appl. Surf. Sci. 2008, 254, 4500–4507. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Song, J.; Wu, L.; Zhou, B.; Zhou, H.; Fan, H.; Yang, Y.; Meng, Q.; Han, B. A new porous Zr-containing catalyst with a phenate group: An efficient catalyst for the catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone. Green Chem. 2015, 17, 1626–1632. [Google Scholar] [CrossRef]
- Yang, J.; Huang, W.; Liu, Y.; Zhou, T. Enhancing the conversion of ethyl levulinate to γ-valerolactone over Ru/UiO-66 by introducing sulfonic groups into the framework. RSC Adv. 2018, 8, 16611–16618. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Javed, H.M.A.; Ghaffar, A.; Majeed, M.I. Investigations of Zr/TiO2-based nanocomposites for efficient plasmonic dye-sensitized solar cells. Int. J. Mod. Phys. B 2023, 37, 2350042. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer hydrogenation of methyl and ethyl levulinate promoted by a ZrO2 catalyst: Comparison of batch vs continuous gas-flow conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Xue, Z.; Jiang, J.; Li, G.; Zhao, W.; Wang, J.; Mu, T. Zirconium-cyanuric acid coordination polymer: Highly efficient catalyst for conversion of levulinic acid to γ-valerolactone. Catal. Sci. Technol. 2016, 6, 5374–5379. [Google Scholar] [CrossRef]
- Kim, J.; Song, K.C.; Foncillas, S.; Pratsinis, S.E. Dopants for synthesis of stable bimodally porous titania. J. Eur. Ceram. Soc. 2001, 21, 2863–2872. [Google Scholar] [CrossRef]
- Gnatyuk, Y.; Smirnova, N.; Korduban, O.; Eremenko, A. Effect of zirconium incorporation on the stabilization of TiO2 mesoporous structure. Surf. Interface Anal. 2010, 42, 1276–1280. [Google Scholar] [CrossRef]
- Zhao, D.; Su, T.; Rodríguez-Padrón, D.; Lü, H.; Len, C.; Luque, R.; Yang, Z. Efficient transfer hydrogenation of alkyl levulinates to γ-valerolactone catalyzed by simple Zr-TiO2 metal oxide systems. Mater. Today Chem. 2022, 24, 100745. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kang, M.-S. Synthesis of Zr-incorporated TiO2 using a solvothermal method and its photovoltaic efficiency on dye-sensitized solar cells. Bull. Korean Chem. Soc. 2011, 32, 3317–3322. [Google Scholar] [CrossRef][Green Version]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol-gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, G.; Zhou, Q. Preparation and characterization of Zr doped TiO2 nanotube arrays on the titanium sheet and their enhanced photocatalytic activity. J. Solid State Chem. 2009, 182, 3238–3242. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fu, X. Hierarchical macro/mesoporous TiO2/SiO2 and TiO2/ZrO2 nano-composites for environmental photocatalysis. Energy Environ. Sci. 2009, 2, 872–877. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Enhanced photocatalytic degradation of 4-chlorophenol by Zr4+ doped nano TiO2. J. Mol. Catal. A Chem. 2007, 1, 158–165. [Google Scholar] [CrossRef]
- Gerasimova, T.; Evdokimova, O.; Kraev, A.; Ivanov, V.; Agafonov, A. Micro-mesoporous anatase TiO2 nanorods with high specific surface area possessing enhanced adsorption ability and photocatalytic activity. Microporous Mesoporous Mater. 2016, 235, 185–194. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TiO2 new photo-chemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Qian, J.; Hou, X.; Qin, Z.; Li, B.; Tong, Z.; Dong, L.; Dong, L. Enhanced catalytic properties of Cu-based composites for NOx reduction with coexistence and intergrowth effect. Fuel 2018, 234, 296–304. [Google Scholar] [CrossRef]
- Qian, J.; Hu, Q.; Hou, X.; Qian, F.; Dong, L.; Li, B. Study of different Ti/Zr ratios on the physicochemical properties and catalytic activities for CuO/Ti-Zr-O composites. Ind. Eng. Chem. Res. 2018, 57, 12792–12800. [Google Scholar] [CrossRef]
- Suib, S.L. A review of recent developments of mesoporous materials. Chem. Rec. 2017, 17, 1169–1183. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2011, 6, 27. [Google Scholar] [CrossRef]
- Huang, J.; Kang, Y.; Wang, L.; Yang, T.; Wang, Y.; Wang, S. Mesoporous CuO/TixZr1−xO2 catalysts for low-temperature CO oxidation. Catal. Commun. 2011, 15, 41–45. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Asami, K.; Ito, S.; Yashima, M.; Sugimoto, T. XPS study of the phase transition in pure zirconium oxide nanocrystallites. Appl. Surf. Sci. 2005, 252, 1651–1656. [Google Scholar] [CrossRef]
- Nelson, A.E.; Schulz, K.H. Surface chemistry and microstructural analysis of CexZr1−xO2−y model catalyst surfaces. Appl. Surf. Sci. 2003, 210, 206–221. [Google Scholar] [CrossRef]
- Duan, B.; Zhou, Y.; Huang, C.; Huang, Q.; Chen, Y.; Xu, H.; Shen, S. Impact of Zr-doped TiO2 photocatalyst on formaldehyde degradation by Na addition. Ind. Eng. Chem. Res. 2018, 57, 14044–14051. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Liu, D.; Zhang, J.; Hao, Y.; Zhang, W. Multi-layer and open three-dimensionally ordered macroporous TiO2-ZrO2 composite: Diversified design and the comparison of multiple mode photocatalytic performance. Mater. Des. 2015, 86, 818–828. [Google Scholar] [CrossRef]
- Divya, G.; Jaishree, G.; Sivarao, T.; Lakshmi, K.D. Microwave assisted sol-gel approach for Zr doped TiO2 as a benign photocatalyst for bismark brown red dye pollutant. RSC Adv. 2023, 13, 8692–8705. [Google Scholar] [CrossRef] [PubMed]
- Gurubasavaraj, P.M.; Roesky, H.W.; Sharma, P.M.V.; Oswald, R.B.; Dolle, V.; Herbst-Irmer, R.; Pal, A. Oxygen Effect in Heterobimetallic Catalysis: The Zr-O-Ti System as an Excellent Example for Olefin Polymerization. Organometallics 2007, 26, 3346–3351. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Wang, C.; Hou, H.; Zhang, Y.; Wang, C.; Zou, G.; Ji, X. Black anatase titania with ultrafast sodium-storage performances stimulated by oxygen vacancies. ACS Appl. Mater. Interfaces 2016, 8, 9142–9151. [Google Scholar] [CrossRef]
- Ma, J.; Jin, G.; Gao, J.; Li, Y.; Dong, L.; Huang, M.; Huang, Q.; Li, B. Catalytic effect of two-phase intergrowth and coexistence CuO-CeO2. J. Mater. Chem. A 2015, 3, 24358–24370. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, M.; Wei, Z.; Xin, Q.; Ying, P.; Li, C. Catalytic oxidation of chlorobenzene on supported manganese oxide catalysts. Appl. Catal. B Environ. 2001, 29, 61–67. [Google Scholar] [CrossRef]
- Hu, D.; Li, W.; Yin, K.; Huang, B. Promoting effect of Ru-doped Mn/TiO2 catalysts for catalytic oxidation of chlorobenzene. New J. Chem. 2022, 46, 10820–10828. [Google Scholar] [CrossRef]
- Song, W.; Poyraz, A.S.; Meng, Y.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Mesoporous Co3O4 with controlled porosity: Inverse micelle synthesis and high-performance catalytic CO oxidation at −60 °C. Chem. Mater. 2014, 26, 4629–4639. [Google Scholar] [CrossRef]
- Chen, X.; Cai, S.; Yu, E.; Li, J.; Chen, J.; Jia, H. Photothermocatalytic performance of ACo2O4 type spinel with light-enhanced mobilizable active oxygen species for toluene oxidation. Appl. Surf. Sci. 2019, 484, 479–488. [Google Scholar] [CrossRef]
- Feng, X.; Tian, M.; He, C.; Li, L.; Shi, J.-W.; Yu, Y.; Cheng, J. Yolk-shell-like mesoporous CoCrOx with superior activity and chlorine resistance in dichloromethane destruction. Appl. Catal. B Environ. 2020, 264, 118493. [Google Scholar] [CrossRef]
- Beniya, A.; Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2019, 2, 590–602. [Google Scholar] [CrossRef]
- Yang, X.J.; Tian, P.F.; Wang, H.L.; Xu, J.; Han, Y.F. Catalytic decomposition of H2O2 over a Au/carbon catalyst: A dual intermediate model for the generation of hydroxyl radicals. J. Catal. 2016, 336, 126–132. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; He, Z.; Tan, W.; Zhu, J.; Yuan, P.; Zhu, R.; He, H. The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous Fenton and UV/Fenton reaction: From the perspective of hydroxyl radical generation. Appl. Catal. B Environ. 2014, 150, 612–618. [Google Scholar] [CrossRef]
- Sun, G.; Li, M.M.-J.; Nakagawa, K.; Li, G.; Wu, T.-S.; Peng, Y.-K. Bulk-to-nano regulation of layered metal oxide gears H2O2 activation pathway for its stoichiometric utilization in selective oxidation reaction. Appl. Catal. B Environ. 2022, 313, 121461. [Google Scholar] [CrossRef]


| Catalysts | Crystal Size (nm) | Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|---|
| TiO2 | 21.7 | 16 | 6.1 |
| 1%Zr-TiO2 | 14.6 | 49 | 5.7 |
| 3%Zr-TiO2 | 11.5 | 72 | 5.1 |
| 5%Zr-TiO2 | 10.6 | 85 | 5.0 |
| 7%Zr-TiO2 | 11.1 | 75 | 5.4 |
| 10%Zr-TiO2 | 11.4 | 74 | 5.5 |
| Catalysts | [Zr]/[Ti] | [O]/[Ti] | [C]/[Ti] | Oads/(Oads + Olatt)% |
|---|---|---|---|---|
| TiO2 | -- | 1.98 | 1.00 | 12.6 |
| 1%Zr-TiO2 | 0.007 | 1.99 | 0.72 | 13.5 |
| 3%Zr-TiO2 | 0.015 | 2.05 | 0.78 | 15.0 |
| 5%Zr-TiO2 | 0.021 | 2.06 | 0.75 | 18.3 |
| 7%Zr-TiO2 | 0.030 | 2.04 | 0.77 | 15.4 |
| 10%Zr-TiO2 | 0.045 | 2.15 | 0.72 | 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fang, Z.; Feng, L.; Liu, F.; Shi, Y.; Li, J.; Zhao, C. Study of Mesoporous Zr-TiO2 Catalyst with Rich Oxygen Vacancies for N-Methylmorpholine Oxidation to N-Methylmorpholine-N-oxide. Molecules 2024, 29, 3812. https://doi.org/10.3390/molecules29163812
Li Y, Fang Z, Feng L, Liu F, Shi Y, Li J, Zhao C. Study of Mesoporous Zr-TiO2 Catalyst with Rich Oxygen Vacancies for N-Methylmorpholine Oxidation to N-Methylmorpholine-N-oxide. Molecules. 2024; 29(16):3812. https://doi.org/10.3390/molecules29163812
Chicago/Turabian StyleLi, Yongwei, Zhihao Fang, Lijuan Feng, Fangfang Liu, Yucui Shi, Jiao Li, and Chao Zhao. 2024. "Study of Mesoporous Zr-TiO2 Catalyst with Rich Oxygen Vacancies for N-Methylmorpholine Oxidation to N-Methylmorpholine-N-oxide" Molecules 29, no. 16: 3812. https://doi.org/10.3390/molecules29163812
APA StyleLi, Y., Fang, Z., Feng, L., Liu, F., Shi, Y., Li, J., & Zhao, C. (2024). Study of Mesoporous Zr-TiO2 Catalyst with Rich Oxygen Vacancies for N-Methylmorpholine Oxidation to N-Methylmorpholine-N-oxide. Molecules, 29(16), 3812. https://doi.org/10.3390/molecules29163812






