Enhanced TiO2-Based Photocatalytic Volatile Organic Compound Decomposition Combined with Ultrasonic Atomization in the Co-Presence of Carbon Black and Heavy Metal Nanoparticles
Abstract
:1. Introduction
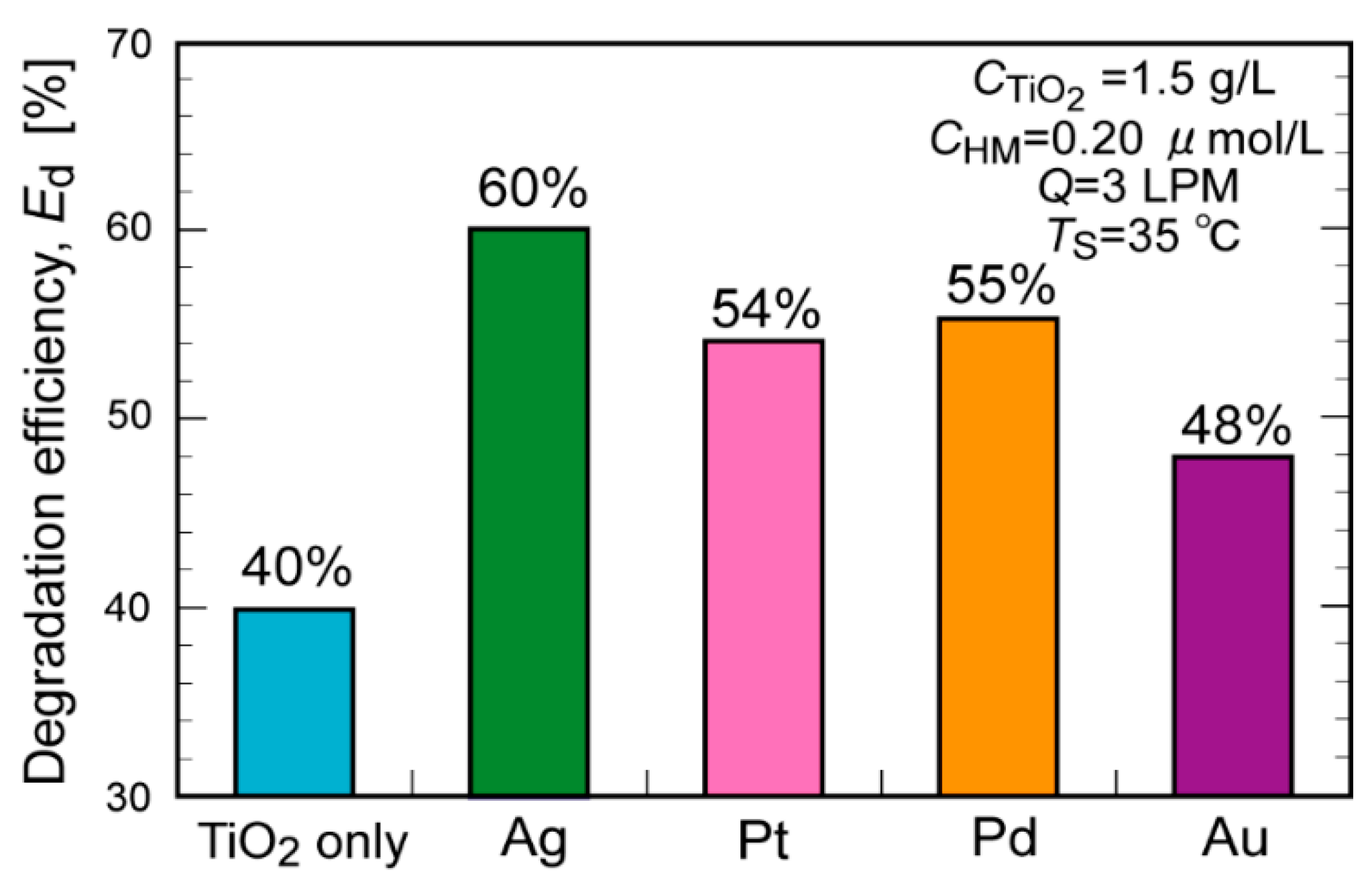
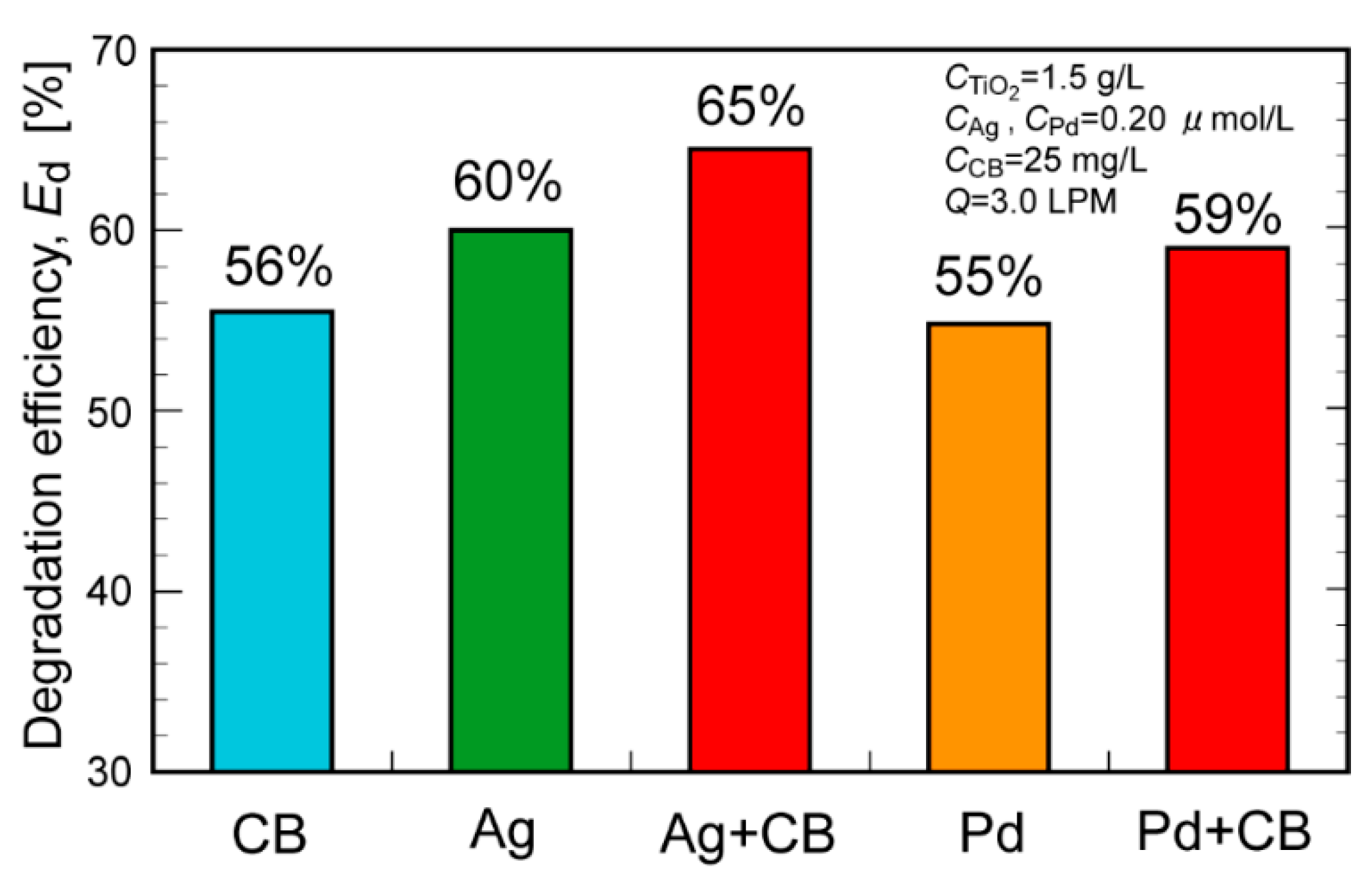
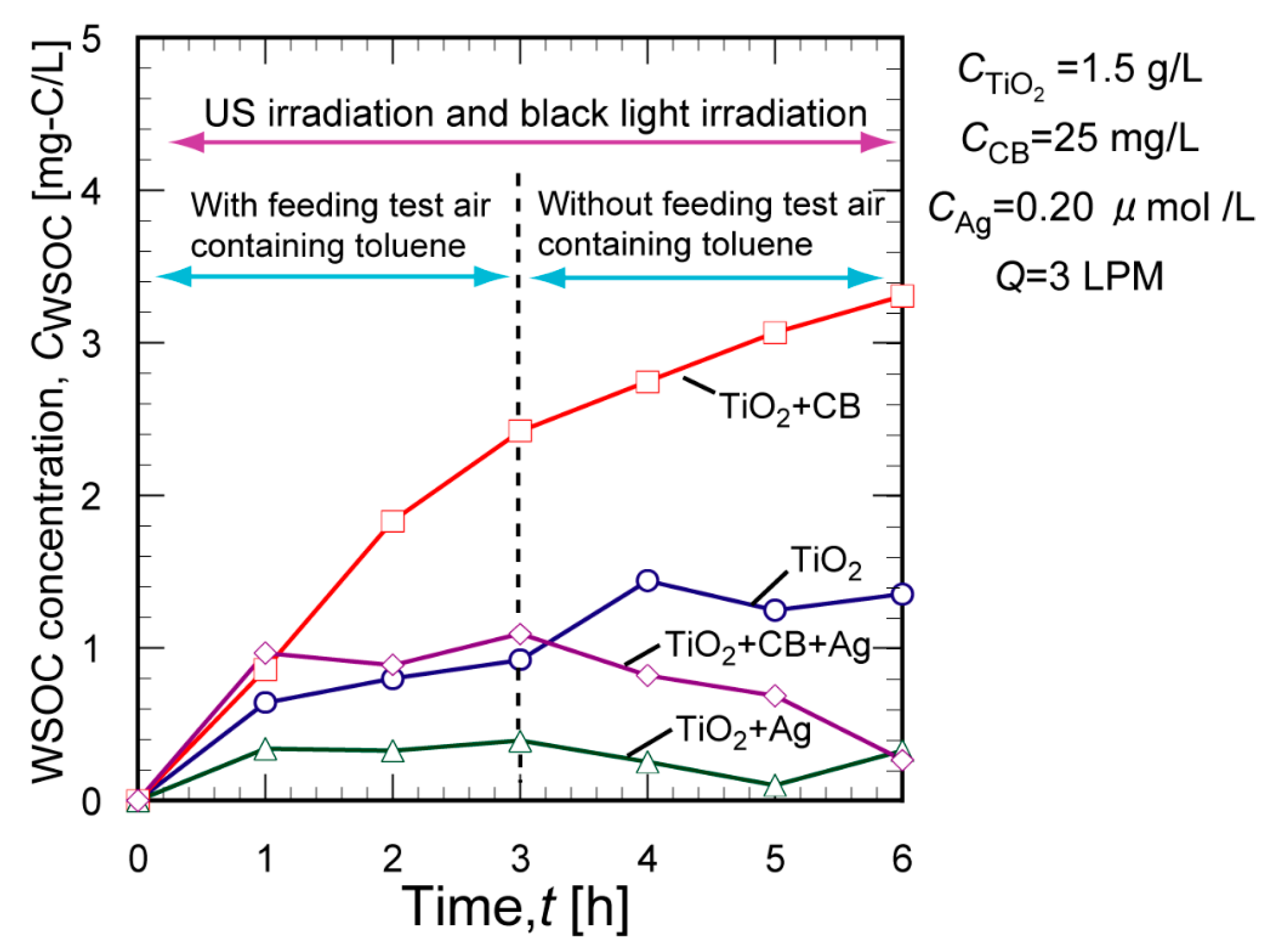
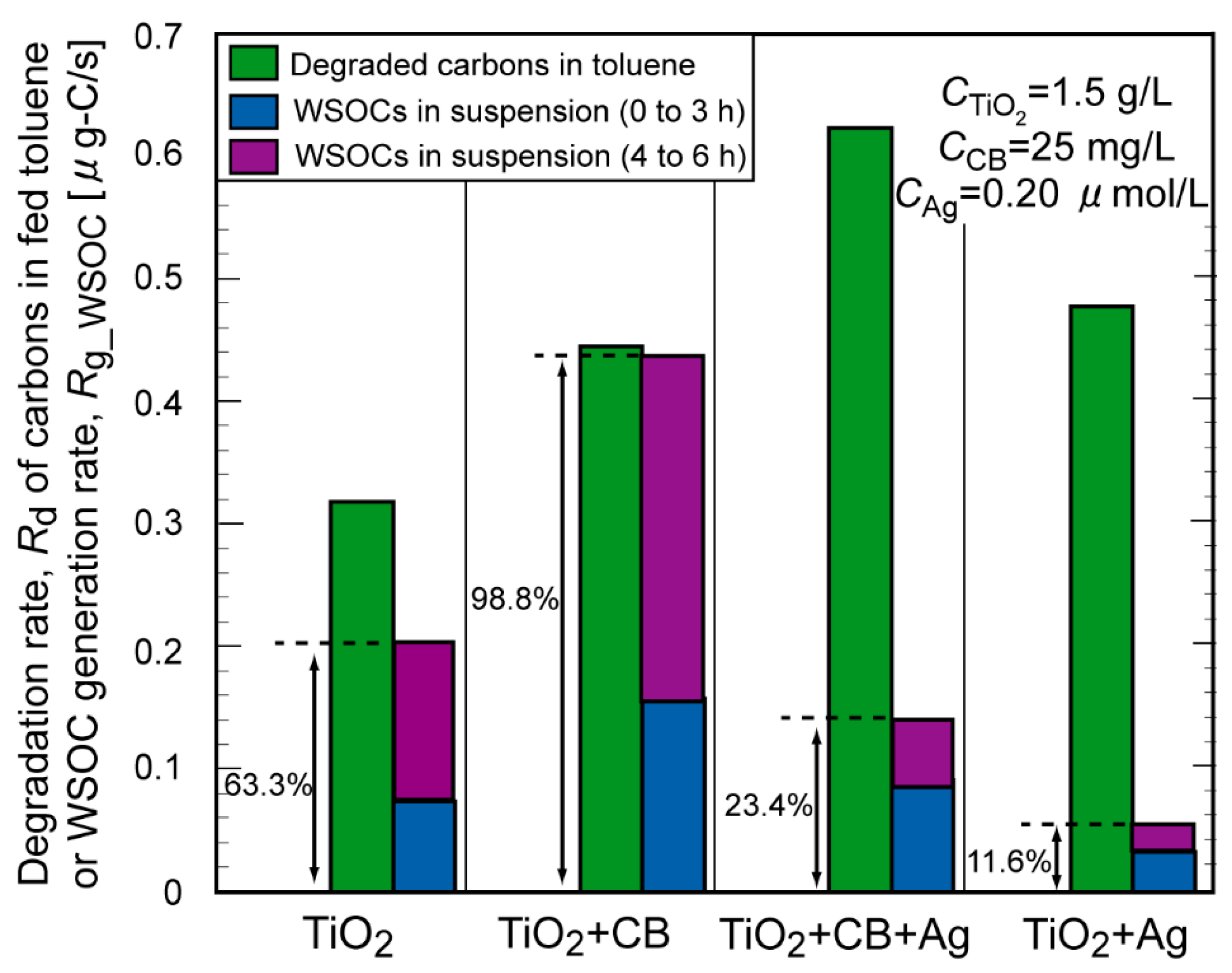
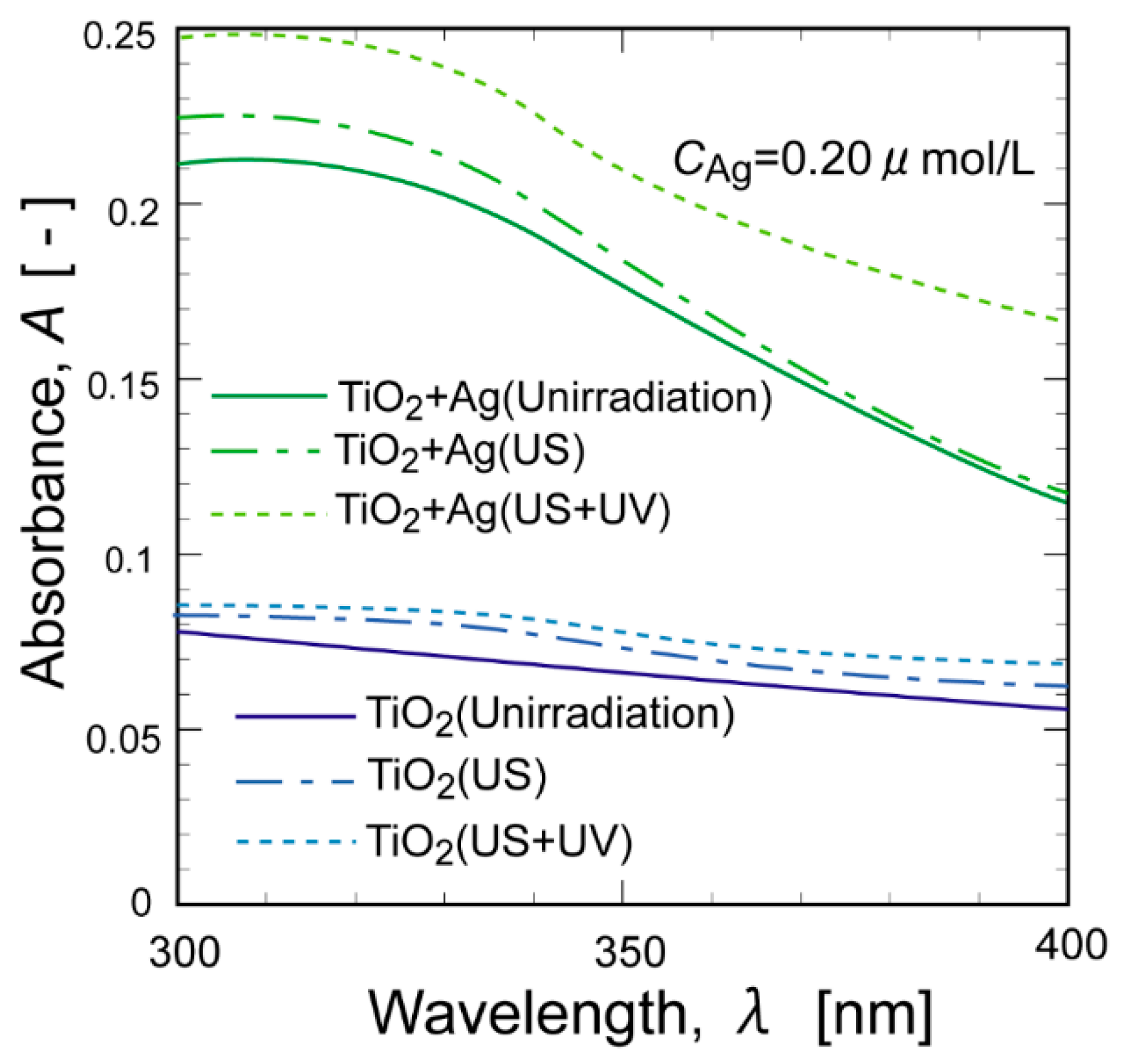
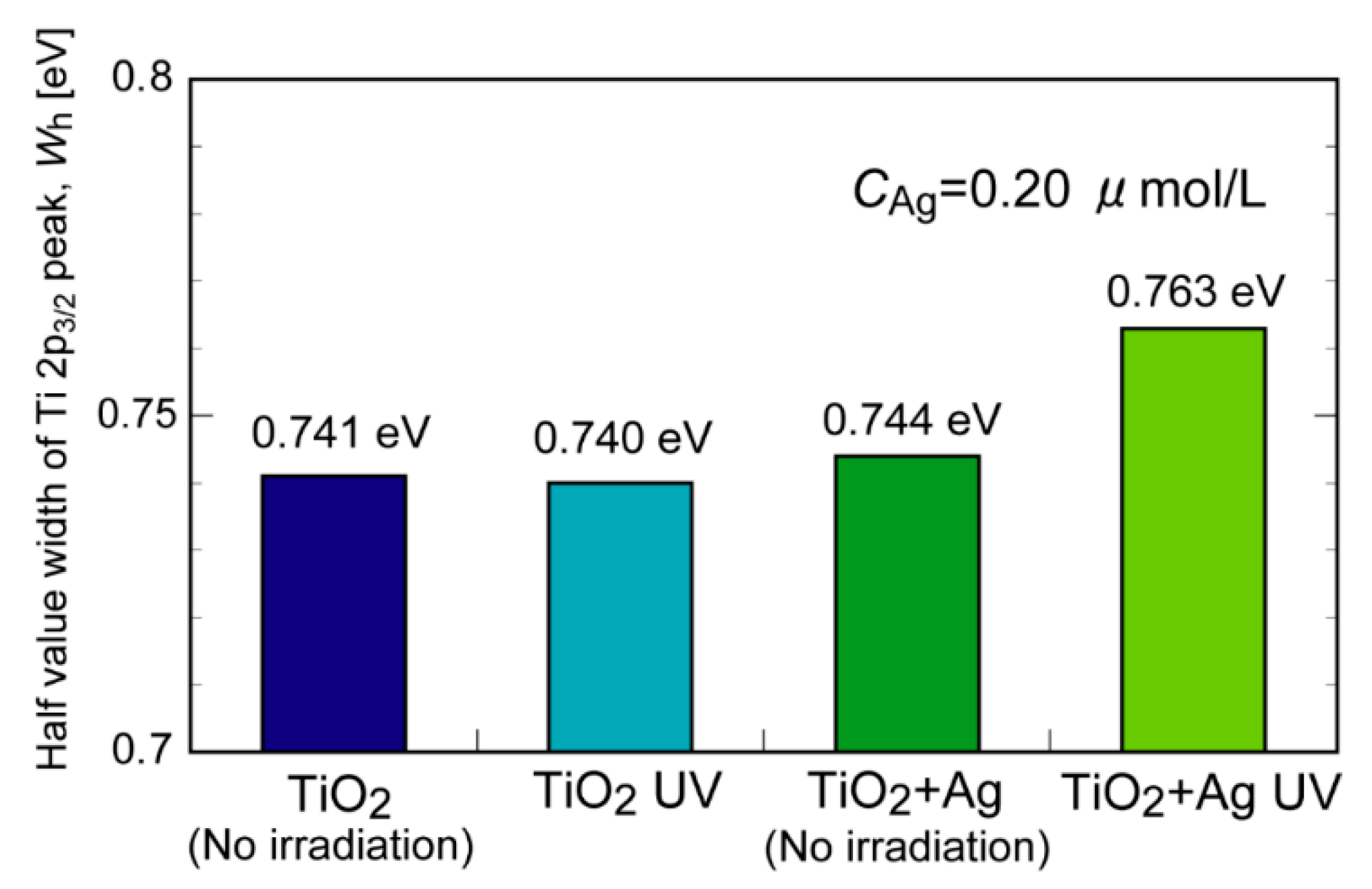
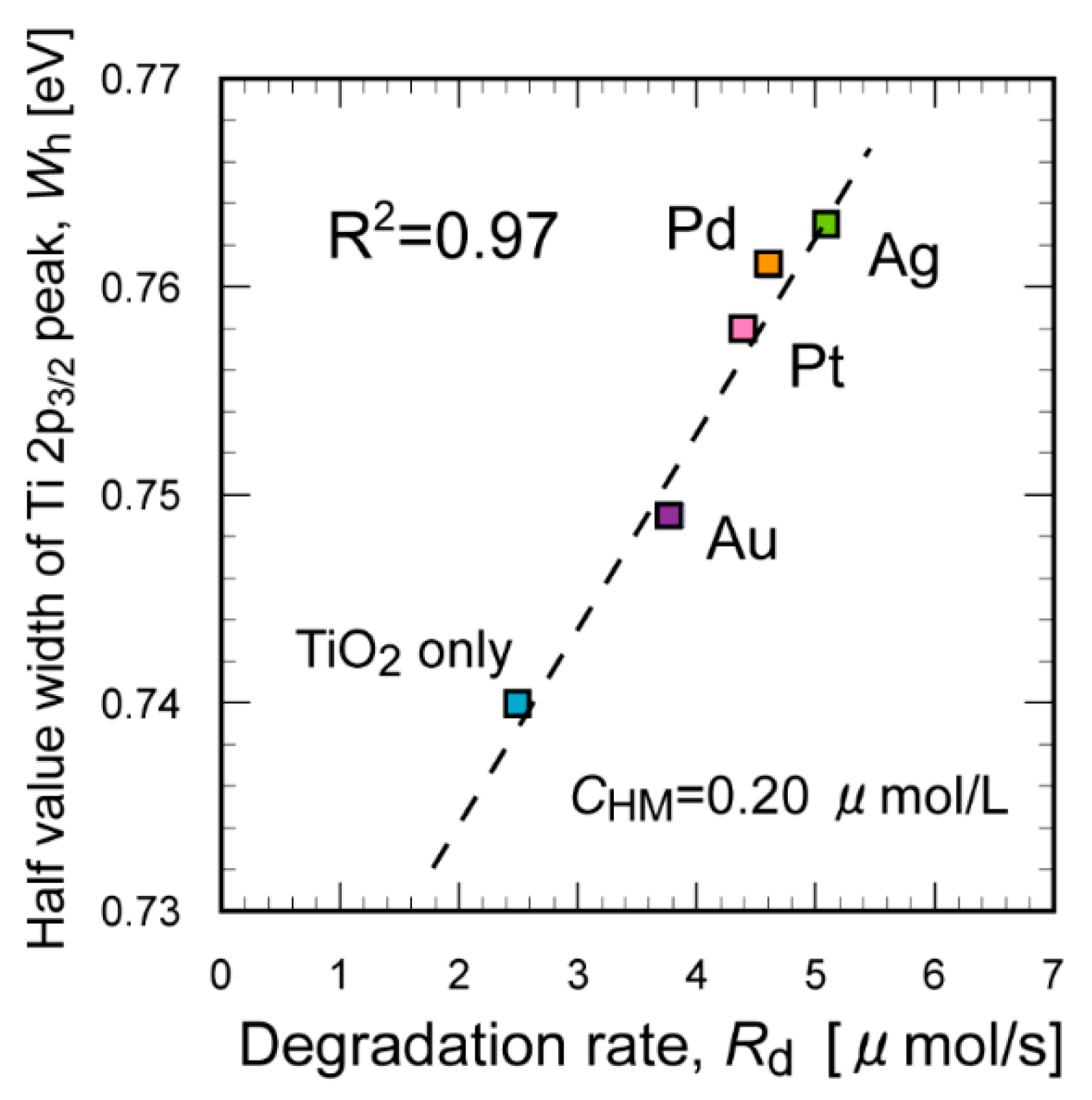
2. Results and Discussion
3. Materials and Methods
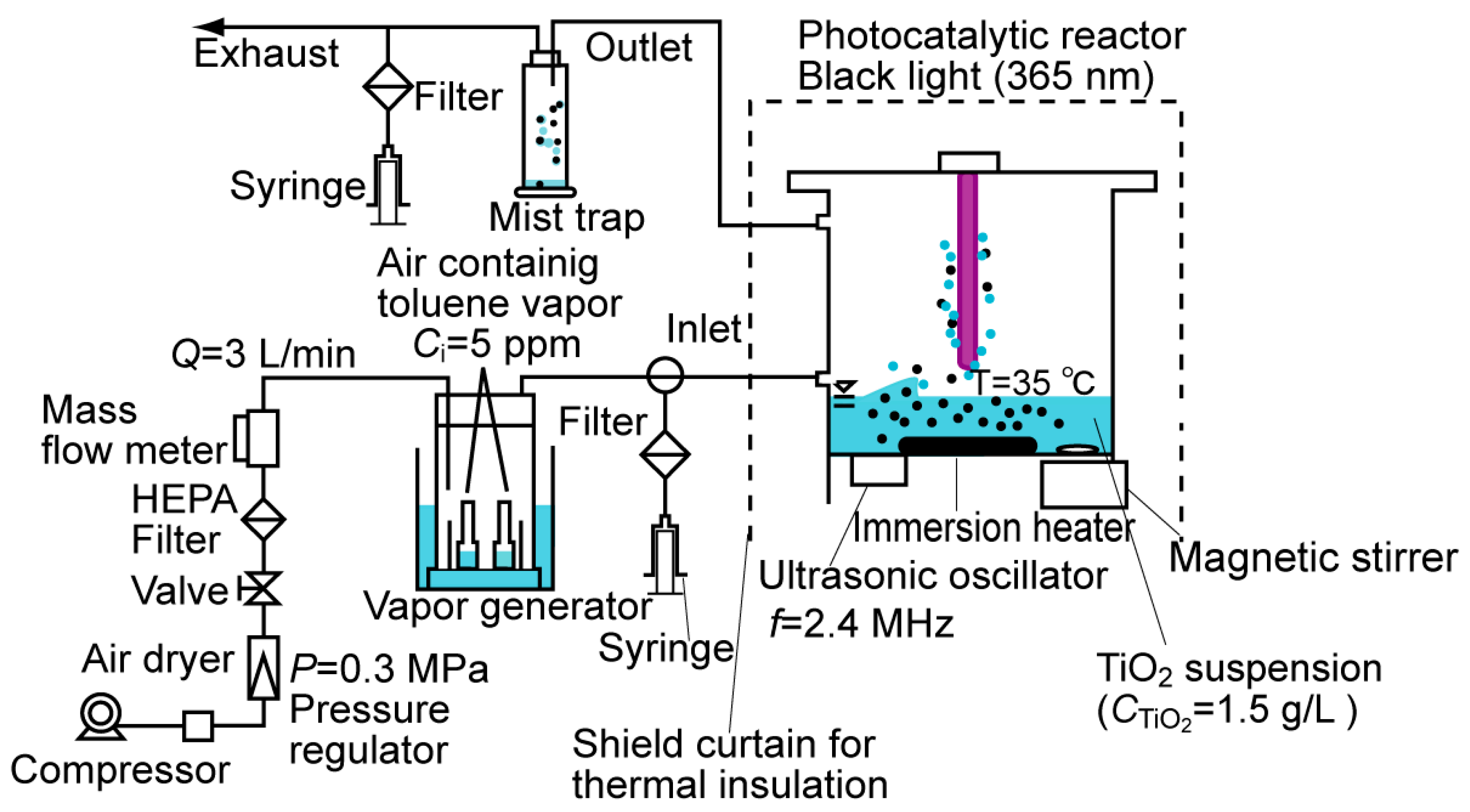
3.1. Photocatalytic Degradation of Toluene
3.2. Determination of the Amount of WSOCs
3.3. XPS Analysis of the TiO2 Suspension
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Repace, J.L. Indoor air pollution. Environ. Int. 1982, 8, 21–36. [Google Scholar] [CrossRef]
- Hodgson, M.; Levin, H.; Wolkoff, P. Volatile organic compounds and indoor air. J. Allergy Clin. Immunol. 1994, 94, 296–303. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Nakajima, A.; Obata, H.; Kameshima, Y.; Okada, K. Photocatalytic destruction of gaseous toluene by sulfated TiO2 powder. Catal. Commun. 2005, 6, 716–720. [Google Scholar] [CrossRef]
- Bosc, F.; Edwards, D.; Keller, N.; Keller, V.; Ayral, A. Mesoporous TiO2-based photocatalysts for UV and visible light gas-phase toluene degradation. Thin Solid. Films 2006, 495, 272–279. [Google Scholar] [CrossRef]
- Zuo, G.-M.; Cheng, Z.-X.; Chen, H.; Li, G.-W.; Miao, T.J. Study on photocatalytic degradation of several volatile organic compounds. Hazard. Mater. 2006, 128, 158–163. [Google Scholar] [CrossRef]
- Dutschke, M.; Schnabel, T.; Schütz, F.; Springer, C.J. Degradation of chlorinated volatile organic compounds from contaminated ground water using a carrier-bound TiO2/UV/O3-system. Environ. Manag. 2022, 304, 114236. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Tsoukleris, D.S.; Maggos, T.; Vassilakos, C.; Falaras, P. Photocatalytic degradation of volatile organics on TiO2 embedded glass spherules. Catal. Today 2007, 129, 96–101. [Google Scholar] [CrossRef]
- Cao, L.; Gao, Z.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Photocatalytic Oxidation of Toluene on Nanoscale TiO2 Catalysts: Studies of Deactivation and Regeneration. J. Catal. 2000, 196, 253–261. [Google Scholar] [CrossRef]
- Blount, M.C.; Falconer, J.L. Steady-state surface species during toluene photocatalysis. Appl. Catal. B 2002, 39, 39–50. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Gruss, A.F.; Rodriguez, R.; Mazyck, D.W. Mercury Oxidation by UV Irradiation: Effect of Contact Time, UV Wavelength, and Moisture Content. Ind. Eng. Chem. Res. 2017, 56, 6131–6135. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Ji, Y.; Li, G.; An, T.; Choi, W. OH radicals determined photocatalytic degradation mechanisms of gaseous styrene in TiO2 system under 254 nm versus 185 nm irradiation: Combined experimental and theoretical studies. Appl. Catal. B 2019, 257, 117912. [Google Scholar] [CrossRef]
- Jeong, J.; Sekiguchi, K.; Lee, W.; Sakamoto, K. Photodegradation of gaseous volatile organic compounds (VOCs) using TiO2 photoirradiated by an ozone-producing UV lamp: Decomposition characteristics, identification of by-products and water-soluble organic intermediates. J. Photochem. Photobiol. A Chem. 2005, 169, 279–287. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Noshiroya, D.; Handa, M.; Yamamoto, K.; Sakamoto, K.; Namiki, N. Degradation of organic gases using ultrasonic mist generated from TiO2 suspension. Chemosphere 2010, 81, 33–38. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Yamamoto, K.; Sakamoto, K. Photocatalytic degradation of gaseous toluene in an ultrasonic mist containing TiO2 particles. Catal. Commun. 2008, 9, 281–285. [Google Scholar] [CrossRef]
- Nii, S.; Oka, N. Size-selective separation of submicron particles in suspensions with ultrasonic atomization. Ultrason. Sonochem. 2014, 21, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Sekiguchi, K.; Sankoda, K.; Namiki, N.; Nii, S. Effect of ultrasonic frequency on size distributions of nanosized mist generated by ultrasonic atomization. Ultrason. Sonochem. 2017, 37, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B 2014, 210, 352–367. [Google Scholar] [CrossRef]
- Qian, J.; Hu, H.; Liang, Y.; Zhang, Z. Mesoporous TiO2 matrix embeded with Cs2CuBr4 perovskite quantum dots as a step-scheme-based photocatalyst for boosting charge separation and CO2 photoconversion. Appl. Surf. Sci. 2024, 648, 159084. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Chen, Y.-W. Facile Synthesis of Ag/TiO2 by Photoreduction Method and Its Degradation Activity of Methylene Blue under UV and Visible Light Irradiation. Mod. Res. Catal. 2020, 09, 1–19. [Google Scholar] [CrossRef]
- Khanna, A.; Shetty, V.K. Solar light induced photocatalytic degradation of Reactive Blue 220 (RB-220) dye with highly efficient Ag@TiO2 core–shell nanoparticles: A comparison with UV photocatalysis. Sol. Energy 2014, 99, 67–76. [Google Scholar] [CrossRef]
- Sellappan, R.; Nielsen, M.G.; González-Posada, F.; Vesborg, P.C.K.; Chorkendorff, I.; Chakarov, D. Effects of plasmon excitation on photocatalytic activity of Ag/TiO2 and Au/TiO2 nanocomposites. J. Catal. 2013, 307, 214–221. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2− and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeno, Z.; Nishitani, M.; Saito, T.; Sekiguchi, K.; Kagi, N.; Namiki, N. Enhanced TiO2-Based Photocatalytic Volatile Organic Compound Decomposition Combined with Ultrasonic Atomization in the Co-Presence of Carbon Black and Heavy Metal Nanoparticles. Molecules 2024, 29, 3819. https://doi.org/10.3390/molecules29163819
Maeno Z, Nishitani M, Saito T, Sekiguchi K, Kagi N, Namiki N. Enhanced TiO2-Based Photocatalytic Volatile Organic Compound Decomposition Combined with Ultrasonic Atomization in the Co-Presence of Carbon Black and Heavy Metal Nanoparticles. Molecules. 2024; 29(16):3819. https://doi.org/10.3390/molecules29163819
Chicago/Turabian StyleMaeno, Zen, Mika Nishitani, Takehiro Saito, Kazuhiko Sekiguchi, Naoki Kagi, and Norikazu Namiki. 2024. "Enhanced TiO2-Based Photocatalytic Volatile Organic Compound Decomposition Combined with Ultrasonic Atomization in the Co-Presence of Carbon Black and Heavy Metal Nanoparticles" Molecules 29, no. 16: 3819. https://doi.org/10.3390/molecules29163819




