Preparation of Perovskite-Type LaMnO3 and Its Catalytic Degradation of Formaldehyde in Wastewater
Abstract
1. Introduction
2. Results
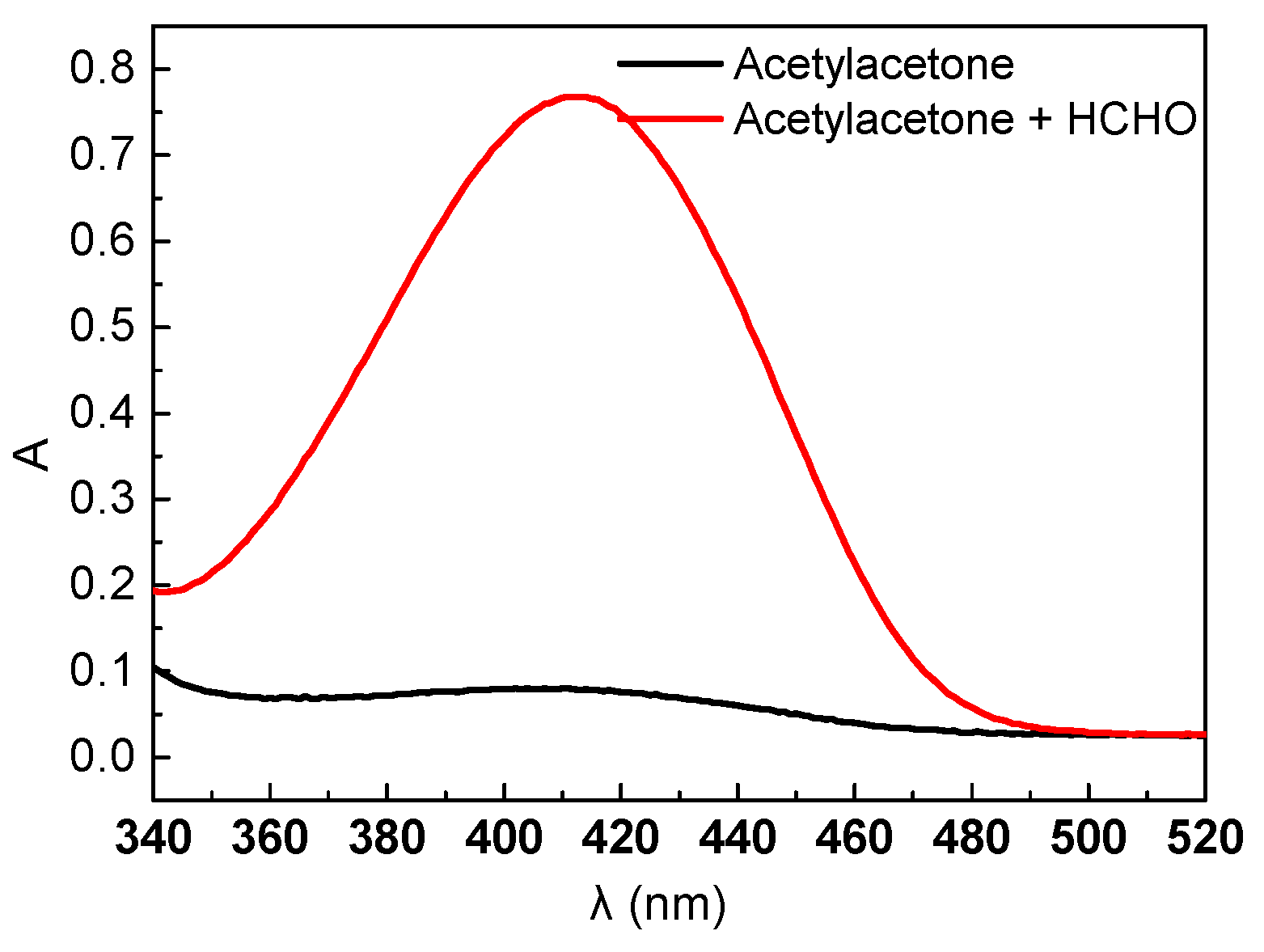
2.1. Draw HCHO Standard Curve
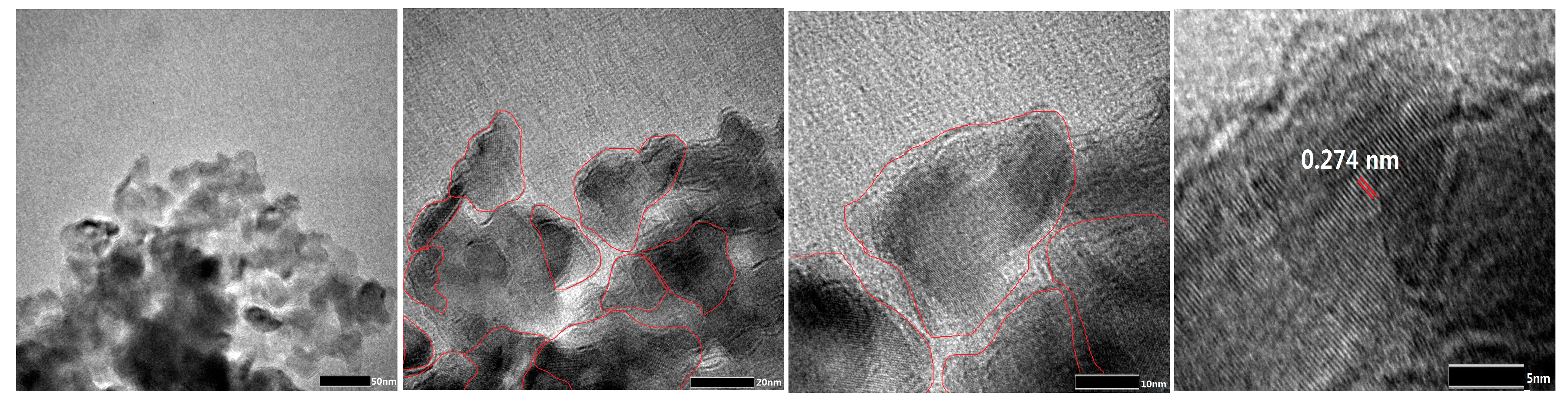
2.2. Catalyst Characterization
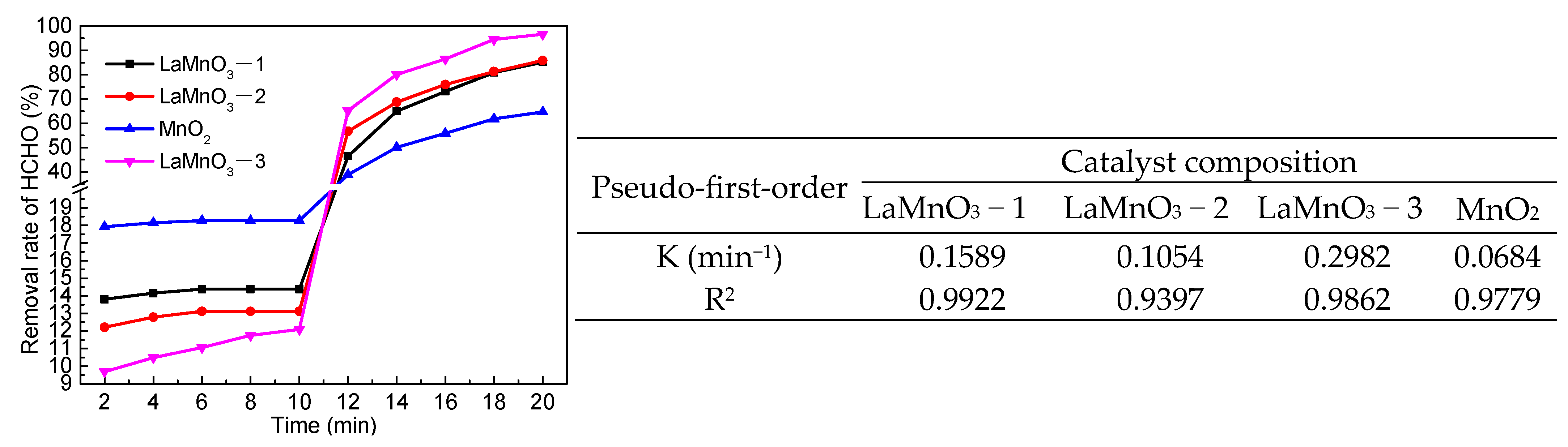
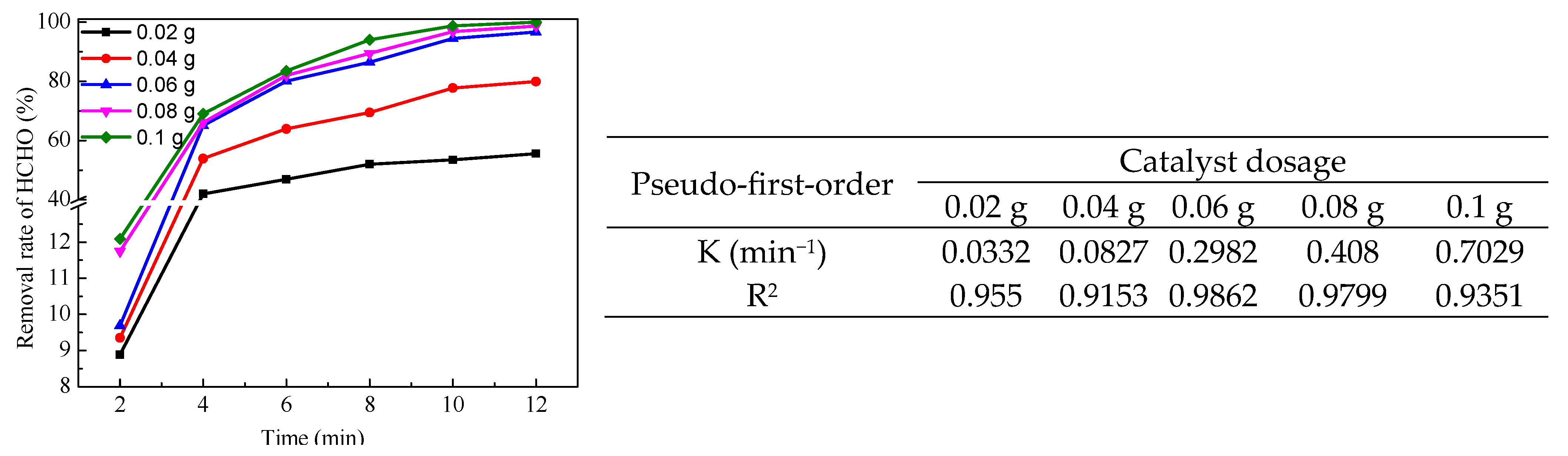
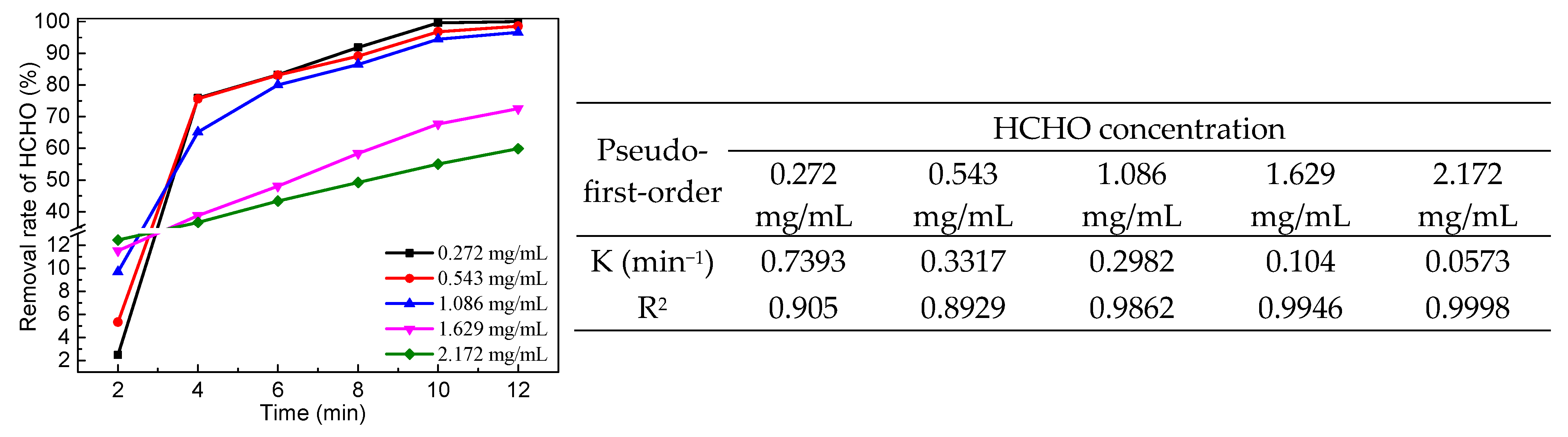
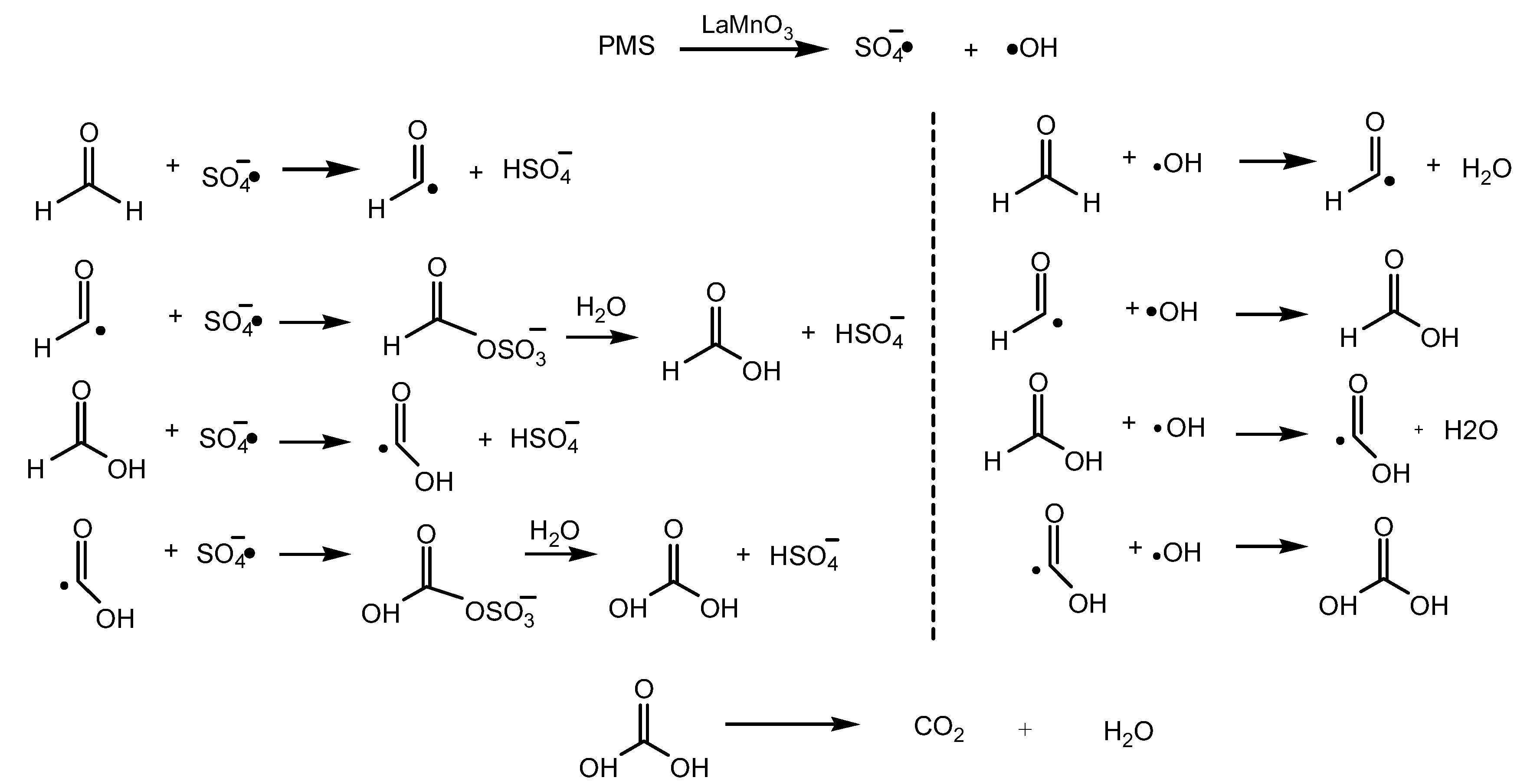
2.3. Catalytic Performance of LaMnO3 for Degradation of HCHO
3. Discussion
4. Materials and Methods
4.1. Preparation of HCHO Standard Solution
4.2. Draw the HCHO Standard Curve
4.3. Preparation of Catalyst
4.4. Catalyst Characterization
4.5. Catalytic Activity Experiment
4.6. Analysis of Degradation Products
4.7. Measurement of Chemical Oxygen Demand (COD)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Zhao, H.; Guo, X.; Jin, X.; Wang, L.; Dong, S.; Chen, J. Enhanced Electrochemical Performance of LaMnO3 Nanoparticles by Ca/Sr Doping. Coatings 2024, 14, 20. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Taranu, B.; Poienar, M.; Vlazan, P. Addressing electrocatalytic activity of metal-substituted lanthanum manganite for the hydrogen evolution reaction. Surf. Interfaces 2023, 39, 102881. [Google Scholar] [CrossRef]
- Aljurays, R.K.; Loucif, A.; Albadri, A.M. Synthesis of LaXO3 (X = Fe, Mn, Cr, Ni) Thin Films Using a Simple Spin Coating Set-Up for Resistive Switching Memory Devices. Electronics 2023, 12, 4141. [Google Scholar] [CrossRef]
- Zhu, T.; Zheng, K.; Wang, P.; Cai, X.; Wang, X.; Gao, D.; Yu, D.; Chen, C.; Liu, Y. A new zinc-ion battery cathode with high-performance: Loofah-like lanthanum manganese perovskite. J. Colloid Interface Sci. 2022, 610, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A. Distinct dominant dielectric relaxation mechanisms in CaCu3Ti4O12–LaMO3 (M = Mn and Fe) perovskite oxide solid solution. J. Solid State Chem. 2023, 325, 124182. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Xing, Z.; Chen, H.; Li, Y.; Song, D.; He, F. Ce-Doped LaMnO3 Redox Catalysts for Chemical Looping Oxidative Dehydrogenation of Ethane. Catalysts 2023, 13, 131. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Liu, J.; Zhou, B.; Guo, M.; Liu, L. Catalytic Degradation of Toluene over MnO2/LaMnO3: Effect of Phase Type of MnO2 on Activity. Catalysts 2022, 12, 1666. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Zhang, Q.; Zhang, C.; Li, Z.; Niu, J.; He, Z.; Xing, Q.; Tian, Z.; Ma, W.; et al. Novel porous perovskite composite CeO2@LaMnO3/3DOM SiO2 as an effective catalyst for activation of PMS toward oxidation of urotropine. Adv. Powder Technol. 2022, 33, 103802. [Google Scholar]
- Nie, Y.; Zhou, H.; Tian, S.; Tian, X.; Yang, C.; Li, Y.; Tian, Y.; Wang, Y. Anionic ligands driven efficient ofloxacin degradation over LaMnO3 suspended particles in water due to the enhanced peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 130998. [Google Scholar] [CrossRef]
- Evans, C.D.; Bartley, J.K.; Taylor, S.H.; Hutchings, G.J.; Kondrat, S.A. Perovskite Supported Catalysts for the Selective Oxidation of Glycerol to Tartronic Acid. Catal. Lett. 2023, 153, 2026–2035. [Google Scholar] [CrossRef]
- Wu, P.; Chen, T.; Jin, X.; Zhao, S.; Chong, Y.; Li, Y.; Lin, J.; Li, A.; Zhao, Y.; Qiu, Y. Quenching-induced surface modulation of perovskite oxides to boost catalytic oxidation activity. J. Hazard. Mater. 2022, 433, 128765. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Cheng, X.; Wan, T.; Gong, J.; Liang, T.; Wu, G. The role of modified manganese perovskite oxide for selective oxidative dehydrogenation of ethane: Not only selective H2 combustion but also ethane activation. Catal. Commun. 2022, 172, 106531. [Google Scholar] [CrossRef]
- Scelfo, S.; Geobaldo, F.; Pirone, R.; Russo, N. Catalytic wet air oxidation of d-glucose by perovskite type oxides (Fe, Co, Mn) for the synthesis of value-added chemicals. Carbohydr. Res. 2022, 514, 108529. [Google Scholar] [CrossRef] [PubMed]
- Tapia, P.J.; Cao, Y.; Gallego, J.; Guillén, J.M.O.; Morgan, D.; Espinal, J.F. CO Oxidation Catalytic Effects of Intrinsic Surface Defects in Rhombohedral LaMnO3. Chemphyschem 2022, 23, e202200152. [Google Scholar] [CrossRef]
- Wahba, M.A.; Sharmoukh, W.; Yakout, S.M.; Khalil, M.S. Fast and full spectrum sunlight photocatalysts: Fe/Co or Ni implanted multiferroic LaMnO3. Opt. Mater. 2022, 124, 111973. [Google Scholar] [CrossRef]
- Balta, Z.; Simsek, E.B. Boosted photocatalytic hydrogen production and photo-Fenton degradation of ciprofloxacin antibiotic over spherical LaMnO3 perovskites assembled boron nitride quantum dots. Int. J. Hydrogen Energy 2023, 48, 26781–26794. [Google Scholar] [CrossRef]
- Sfirloaga, P.; Ivanovici, M.; Poienar, M.; Ianasi, C.; Vlazan, P. Investigation of Catalytic and Photocatalytic Degradation of Methyl Orange Using Doped LaMnO3 Compounds. Processes 2022, 10, 2688. [Google Scholar] [CrossRef]
- Merkulov, D.Š.; Vlazan, P.; Poienar, M.; Bognár, S.; Ianasi, C.; Sfirloaga, P. Sustainable removal of 17α-ethynylestradiol from aqueous environment using rare earth doped lanthanum manganite nanomaterials. Catal. Today 2023, 424, 113746. [Google Scholar] [CrossRef]
- Zhan, M.; Fang, M.; Li, L.; Zhao, Y.; Yang, B.; Min, X.; Du, P.; Liu, Y.; Wu, X.; Huang, Z. Effect of Fe dopant on oxygen vacancy variation and enhanced photocatalysis hydrogen production of LaMnO3 perovskite nanofibers. Mater. Sci. Semicond. Process 2023, 166, 107697. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Wang, Y.; Wu, Z.; Lai, F.; Chao, G.; Zhang, N.; Zhang, L.; Liu, T. Perovskites with Enriched Oxygen Vacancies as a Family of Electrocatalysts for Efficient Nitrate Reduction to Ammonia. Small 2023, 19, 2205625. [Google Scholar] [CrossRef]
- Alcamand, H.; Oliveira, H.; Gabriel, J.; Bruziquesi, C.; Oliveira, L.; Gonçalves, B.; Victória, H.; Krambrock, K.; Houmard, M.; Nunes, E. Environmentally friendly synthesis of imine using LaMnO3 as a catalyst under continuous flow conditions. Mater. Lett. 2022, 316, 132053. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, P.; Meng, J.; Zuo, S.; Wang, X.; Zhu, W.; Liu, W.; Liu, J.; Yao, C. Surface exposure engineering on LaMnO3@Co2MnO4 for high-efficiency ethane catalytic combustion. Appl. Surf. Sci. 2024, 653, 159393. [Google Scholar] [CrossRef]
- Liu, X.; He, L.; Han, G.; Sheng, J.; Yu, Y.; Yang, W. Design of rich defects carbon coated MnFe2O4/LaMnO3/LaFeO3 heterostructure nanocomposites for broadband electromagnetic wave absorption. Chem. Eng. J. 2023, 476, 146199. [Google Scholar] [CrossRef]
- Chen, Y.; He, L.; Li, J.; Zhang, S. Multi-criteria design of shale-gas-water supply chains and production systems towards optimal life cycle economics and greenhouse gas emissions under uncertainty. Comput. Chem. Eng. 2018, 109, 216–235. [Google Scholar] [CrossRef]
- Yamada, M.; Funaki, S.; Miki, S. Formaldehyde interacts with RNA rather than DNA: Accumulation of formaldehyde by the RNA-inorganic hybrid material. Int. J. Biol. Macromol. 2019, 122, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, H.; Ren, M.; Kong, F. A novel cellulose-based fluorescent probe for the quantitative detection of HCHO in real food samples and living cells. Ind. Crop. Prod. 2023, 204, 117406. [Google Scholar] [CrossRef]
- Kou, Y.; Zhao, H.; Cui, D.; Han, H.; Tong, Z. Formaldehyde toxicity in age-related neurological dementia. Ageing Res. Rev. 2022, 73, 101512. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, F.; Zhou, Q.; Wang, X.; Liao, C.; Zhou, L.; Wan, L.; An, J.; Wan, Y.; Nan, L. Unignorable toxicity of formaldehyde on electroactive bacteria in bioelectrochemical systems. Environ. Res. 2020, 183, 109143. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Agustina, L. The potential application of photocatalytic processes in the processing of wastewater in the leather industry: A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1253, 012025. [Google Scholar] [CrossRef]
- Mei, X.; Ma, M.; Guo, Z.; Shen, W.; Wang, Y.; Xu, L.; Zhang, Z.; Ding, Y.; Xiao, Y.; Yang, X.; et al. A novel clean and energy-saving system for urea-formaldehyde resin wastewater treatment: Combination of a low-aeration-pressure plate membrane-aerated biofilm reactor and a biological aerated filter. J. Environ. Chem. Eng. 2021, 9, 105955. [Google Scholar] [CrossRef]
- Pu, H.; Han, K.; Dai, R.; Shan, Z. Semi-liquefied bamboo modified urea-formaldehyde resin to synthesize composite adhesives. Int. J. Adhes. Adhes. 2022, 113, 103061. [Google Scholar] [CrossRef]
- Pervova, I.G.; Klepalova, I.A.; Lipunov, I.N. Recycling Phenolic Wastewater from Phenol-Formaldehyde Resin Production. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 042032. [Google Scholar] [CrossRef]
- Manna, S.; Bobde, P.; Roy, D.; Sharma, A.K.; Mondal, S.J. Separation of iodine using neem oil-cashew nut shell liquid based-phenol formaldehyde resin modified lignocellulosic biomatrices: Batch and column study. J. Taiwan Inst. Chem. E 2021, 122, 98–105. [Google Scholar] [CrossRef]
- Wang, H.; Song, T.; Li, Z.; Qiu, J.; Zhao, Y.; Zhang, H.; Wang, J. Exceptional High and Reversible Ammonia Uptake by Two Dimension Few-layer BiI3 Nanosheet. ACS Appl. Mater. Interfaces 2021, 13, 25918–25925. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Z.; Xiao, B.; Zhao, J.; Ma, H.; Tian, Y.; Pang, H.; Tan, L. Polyoxometalate-based metal–organic framework-derived bimetallic hybrid materials for upgraded electrochemical reduction of nitrogen. Green Chem. 2020, 22, 6157–6169. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef]
- Khaleghi, H.; Esmaeili, H.; Jaafarzadeh, N.; Ramavandi, B. Date seed activated carbon decorated with CaO and Fe3O4 nanoparticles as a reusable sorbent for removal of formaldehyde. Korean J. Chem. Eng. 2022, 39, 146–160. [Google Scholar] [CrossRef]
- An, S.; Zhao, Z.; Bu, J.; He, J.; Ma, W.; Lin, J.; Bai, R.; Shang, L.; Zhang, J. Multi-Functional Formaldehyde-Nitrate Batteries for Wastewater Refining, Electricity Generation, and Production of Ammonia and Formate. Angew. Chem. Int. Ed. 2024, 63, e202318989. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bao, S.X.; Zhang, Y.M.; Ren, L.Y. A novel and sustainable technique to precipitate vanadium from vanadium-rich solutions via efficient ultrasound irradiation. J. Clean. Prod. 2022, 339, 130755. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, C.; Zheng, S.; Di, Y.; Sun, Z.; Li, C. Design and controllable preparation of Bi2MoO6/attapulgite photocatalyst for the removal of tetracycline and formaldehyde. Appl. Clay Sci. 2021, 215, 106319. [Google Scholar] [CrossRef]
- Santos, S.; Jones, K.; Abdul, R.; Boswell, J.; Paca, J. Treatment of wet process hardboard plant VOC emissions by a pilot scale biological system. Biochem. Eng. J. 2007, 37, 261–270. [Google Scholar] [CrossRef]
- Athikaphan, P.; Wongsanga, K.; Klanghiran, S.; Lertna, N.; Neramittagapong, A.; Rood, S.C.; Nijpanich, S.; Neramittagapong, S. Degradation of formaldehyde by photo-Fenton process overn-ZVI/TiO2 catalyst. Environ. Sci. Pollut. R. 2023, 30, 90397–90409. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A Review on Approaches for Addressing the Limitations of Fenton Oxidation for Recalcitrant Wastewater Treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Wang, L.L.; Lan, X.; Peng, W.Y.; Wang, Z.H. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Zhao, H.; Qu, Z.; Tang, Z.; Yang, X.; Jiang, P.; Wang, Z. Promotion of formaldehyde degradation by electro-Fenton: Controlling the distribution of ⋅OH and formaldehyde near cathode to increase the reaction probability. Chemosphere 2022, 307, 135776. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shi, S.; Yang, F.; Zhang, X. Removal of formaldehyde in water with low concentration of hydrogen peroxide catalyzed by lanthanum-silicon oxide composite. Desalin. Water Treat. 2023, 300, 101–106. [Google Scholar] [CrossRef]
- Gao, C.; Feng, X.; Yi, L.; Wu, X.; Zheng, R.; Zhang, G.; Li, Y. Peroxymonosulfate activation based on Co9S8@N−C: A new strategy for highly efficient hydrogen production and synchronous formaldehyde removal in wastewater. J. Mater. Sci. Technol. 2022, 127, 256–267. [Google Scholar] [CrossRef]
- Wei, J.; Chen, J.; Li, F.; Han, D.; Gong, J. Anchoring CoTiO3/TiO2 heterostructure on fiber network actuates flow-through Fenton-like oxidation: Electronic modulation and rapid pollutants degradation. Sep. Purif. Technol. 2024, 336, 126231. [Google Scholar] [CrossRef]
- Ma, Q.; Hao, Y.; Xue, Y.; Niu, Y.; Chang, X. Removal of Formaldehyde from Aqueous Solution by Hydrogen Peroxide. J. Water Chem. Technol. 2022, 44, 297–303. [Google Scholar] [CrossRef]
- Moussavi, G.; Yazdanbakhsh, A.; Heidarizad, M. The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment. J. Hazard. Mater. 2009, 171, 907–913. [Google Scholar] [CrossRef]
- Kumar, R.D.; Sampath, S.; Thangappan, R.; Gosu, N.R.; Manthrammel, M.A.; Shkir, M. Citric acid mediated hydrothermal synthesis of LaMn1-xFexO3 nanoparticles for visible light driven photocatalytic applications. J. Mater. Sci. Mater. Electron. 2024, 35, 2. [Google Scholar] [CrossRef]
- Grundy, A.N.; Chen, M.; Hallstedt, B.; Gauckler, L.J. Assessment of the La-Mn-O System. J. Phase Equilib. Diff. 2005, 26, 131–151. [Google Scholar] [CrossRef]
- Mao, M.; Xu, J.; Li, Y.; Liu, Z. Hydrogen evolution from photocatalytic water splitting by LaMnO3 modified with amorphous CoSx. J. Mater. Sci. 2020, 55, 3521–3537. [Google Scholar] [CrossRef]
- He, Y.; Qian, J.; Wang, P.; Wu, J.; Lu, B.; Tang, S.; Gao, P. Acceleration of levofloxacin degradation by combination of multiple free radicals via MoS2 anchored in manganese ferrite doped perovskite activated PMS under visible light. Chem. Eng. J. 2022, 431, 133933. [Google Scholar] [CrossRef]
- Hauback, B.C.; Fjellvag, H.; Sakai, N. Effect of Nonstoichiometry on Properties of La1-tMnO3+δ. J. Solid State Chem. 1996, 124, 43–51. [Google Scholar] [CrossRef]
- Kekade, S.S.; Yadav, P.A.; Thombare, B.R.; Dusane, P.R.; Phase, D.M.; Choudhari, R.J.; Pati, S.I. Effect of sintering temperature on electronic properties of nanocrystalline La0.7Sr0.3MnO3. Mater. Res. Express 2019, 6, 096108. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Huo, P.; Wang, K.; Yuan, Y.; Bai, S.; Zhao, C.; Li, W. Preparation of Perovskite-Type LaMnO3 and Its Catalytic Degradation of Formaldehyde in Wastewater. Molecules 2024, 29, 3822. https://doi.org/10.3390/molecules29163822
Ma Q, Huo P, Wang K, Yuan Y, Bai S, Zhao C, Li W. Preparation of Perovskite-Type LaMnO3 and Its Catalytic Degradation of Formaldehyde in Wastewater. Molecules. 2024; 29(16):3822. https://doi.org/10.3390/molecules29163822
Chicago/Turabian StyleMa, Qingguo, Pengcheng Huo, Kesong Wang, Ye Yuan, Songjiang Bai, Chentong Zhao, and Wenzhuo Li. 2024. "Preparation of Perovskite-Type LaMnO3 and Its Catalytic Degradation of Formaldehyde in Wastewater" Molecules 29, no. 16: 3822. https://doi.org/10.3390/molecules29163822
APA StyleMa, Q., Huo, P., Wang, K., Yuan, Y., Bai, S., Zhao, C., & Li, W. (2024). Preparation of Perovskite-Type LaMnO3 and Its Catalytic Degradation of Formaldehyde in Wastewater. Molecules, 29(16), 3822. https://doi.org/10.3390/molecules29163822




