Exploring Aromaticity Effects on Electronic Transport in Cyclo[n]carbon Single-Molecule Junctions
Abstract
1. Introduction
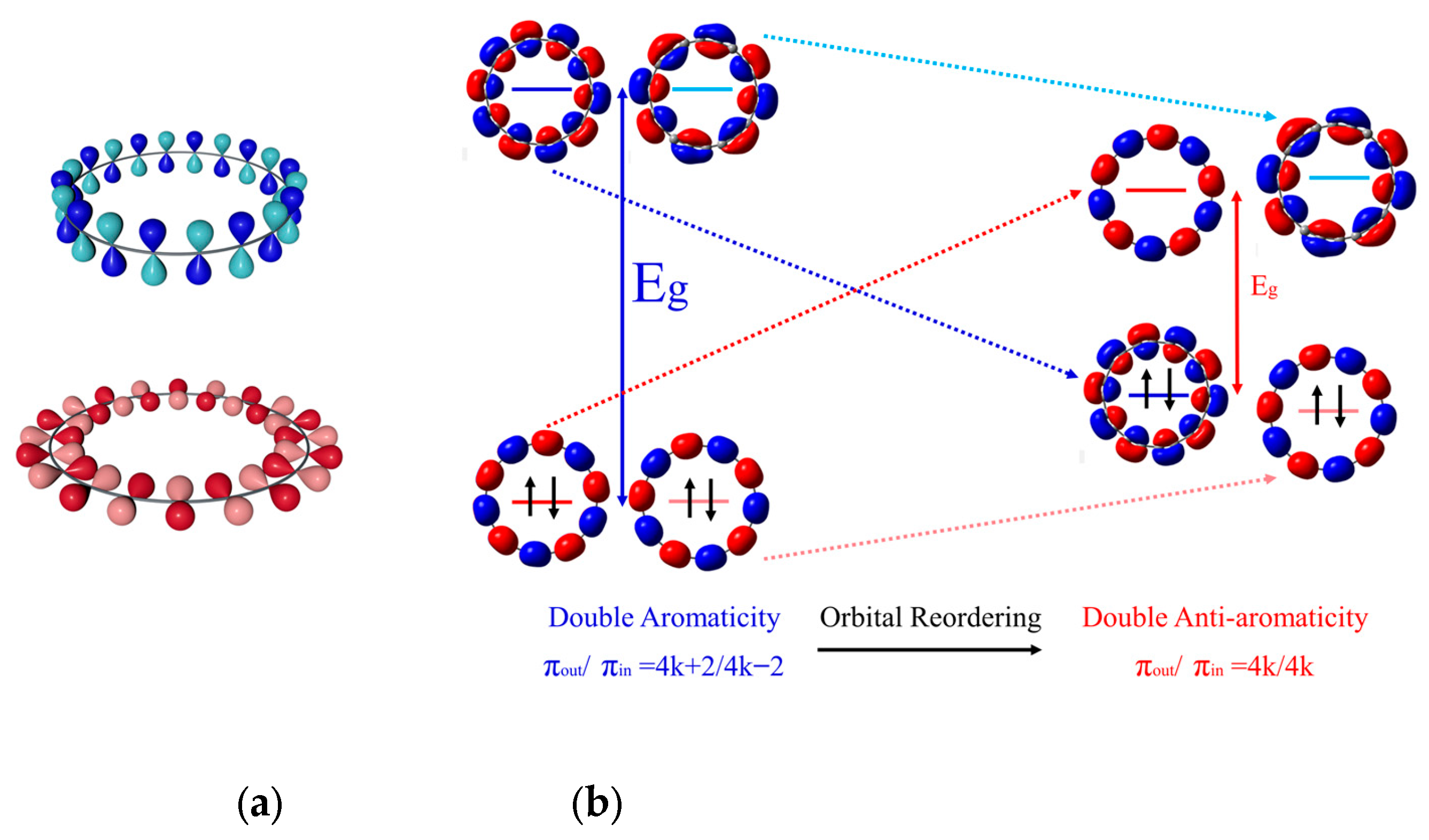
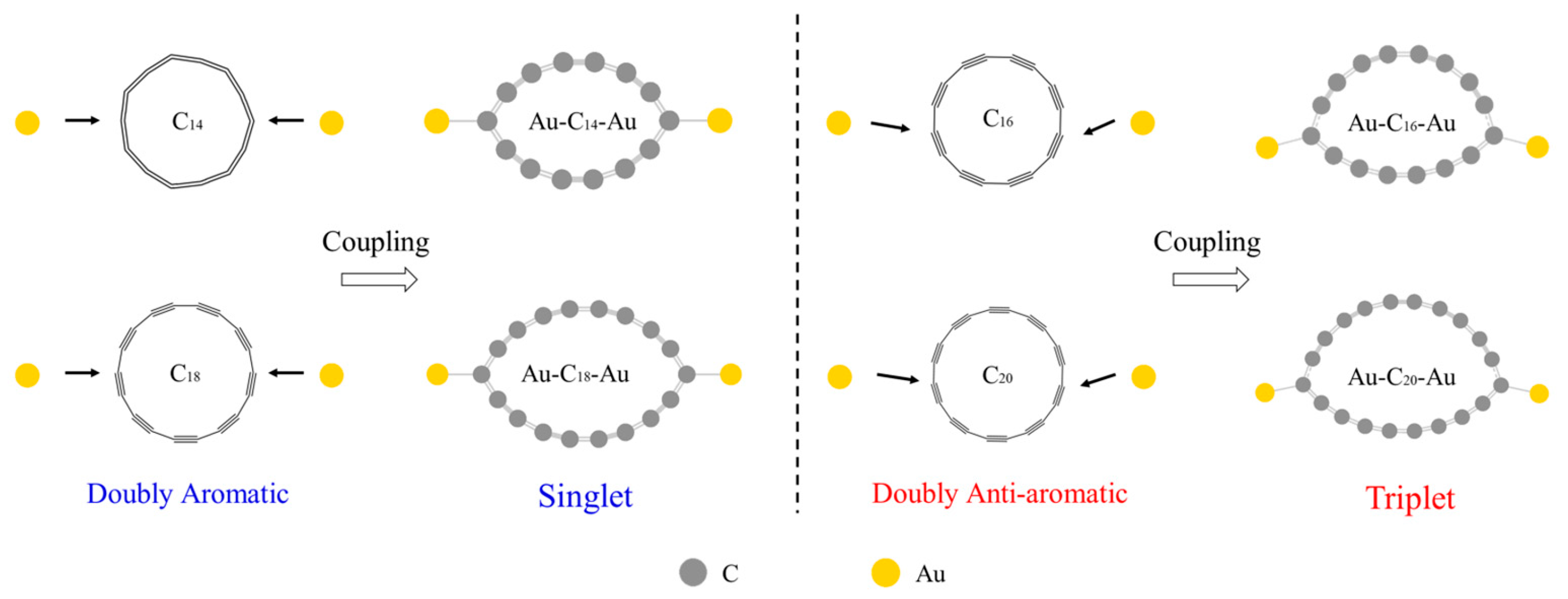
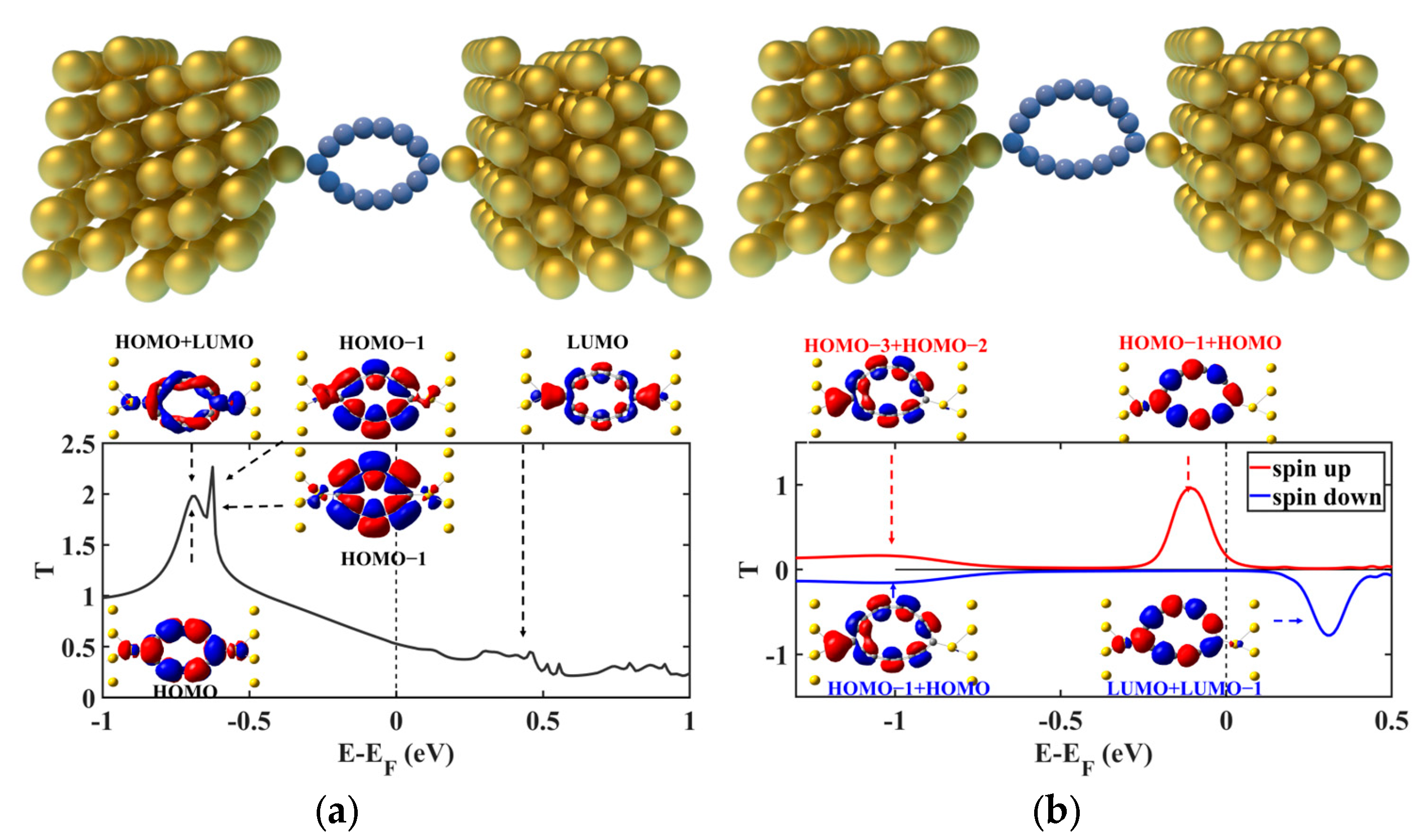
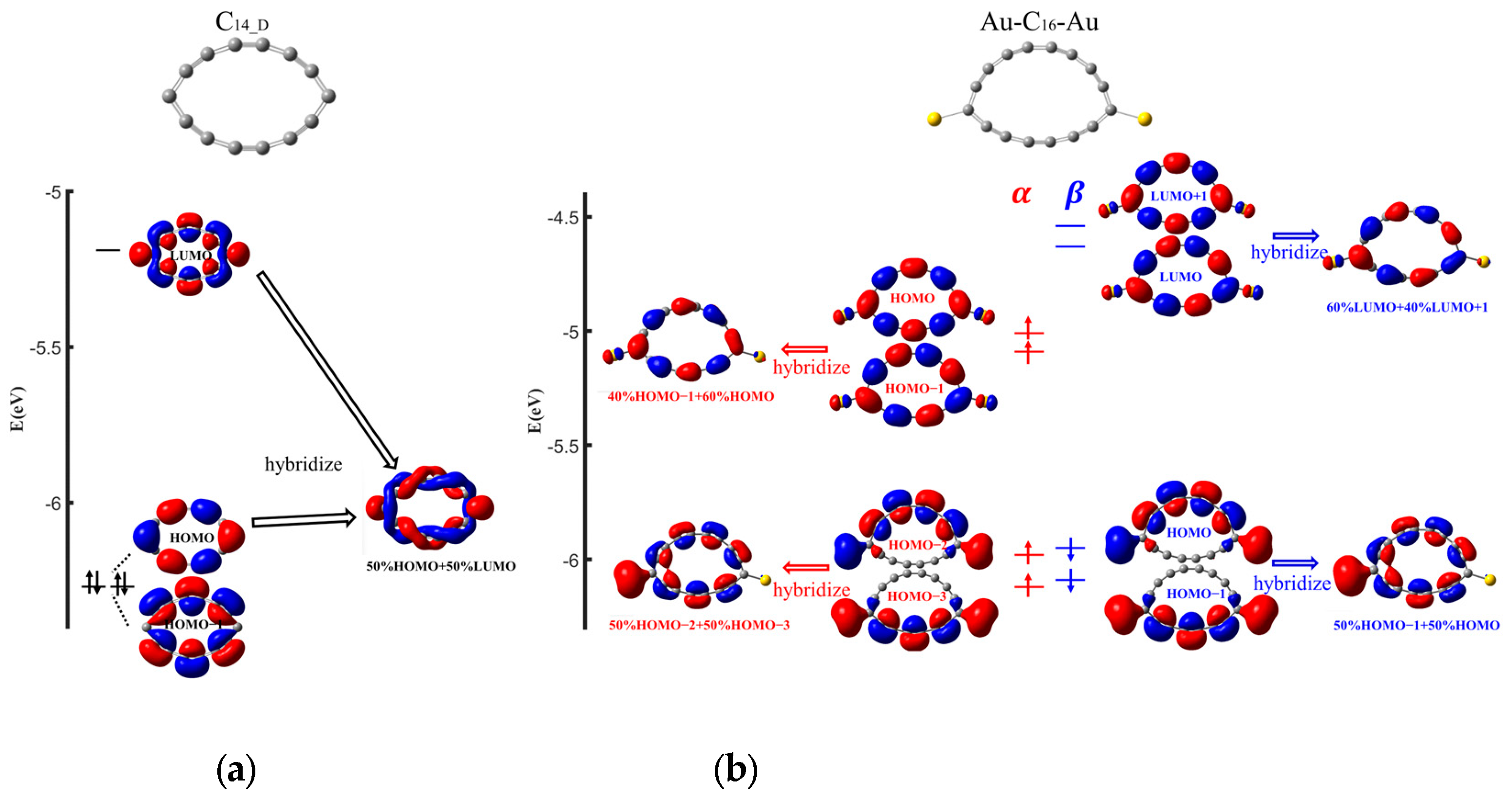
2. Results and Discussion
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Diederich, F.; Rubin, Y.; Knobler, C.B.; Whetten, R.L.; Schriver, K.E.; Houk, K.N.; Li, Y. All-carbon molecules: Evidence for the generation of cyclo[18]carbon from a stable organic precursor. Science 1989, 245, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- von Helden, G.; Gotts, N.G.; Bowers, M.T. Experimental evidence for the formation of fullerenes by collisional heating of carbon rings in the gas phase. Nature 1993, 363, 60–63. [Google Scholar] [CrossRef]
- McElvany, S.W.; Ross, M.M.; Goroff, N.S.; Diederich, F. Cyclocarbon coalescence: Mechanisms for tailor-made fullerene formation. Science 1993, 259, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Kivala, M. All-carbon scaffolds by rational design. Adv. Mater. 2010, 22, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Scriven, L.M.; Schulz, F.; Gawel, P.; Gross, L.; Anderson, H.L. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 2019, 365, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Albrecht, F.; Rončević, I.; Ettedgui, I.; Kumar, P.; Scriven, L.M.; Christensen, K.E.; Mishra, S.; Righetti, L.; Rossmannek, M.; et al. On-surface synthesis of a doubly anti-aromatic carbon allotrope. Nature 2023, 623, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zheng, W.; Kang, F.; Gao, W.; Xu, W. On-surface synthesis and characterization of anti-aromatic cyclo[12]carbon and cyclo[20]carbon. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, W.; Gao, W.; Kang, F.; Zhao, M.; Xu, W. On-surface synthesis of aromatic cyclo[10]carbon and cyclo[14]carbon. Nature 2023, 623, 972–976. [Google Scholar] [CrossRef]
- Brémond, E.; Pérez-Jiménez, A.J.; Adamo, C.; Sancho-García, J.C. Stability of the polyynic form of C18, C22, C26, and C30 nanorings: A challenge tackled by range-separated double-hybrid density functionals. Phys. Chem. Chem. Phys. 2022, 24, 4515–4525. [Google Scholar] [CrossRef]
- Pereira, Z.S.; da Silva, E.Z. Spontaneous symmetry breaking in cyclo[18]carbon. J. Phys. Chem. A 2020, 124, 1152–1157. [Google Scholar] [CrossRef]
- Zhu, B.-C.; Liu, C.-J.; Deng, P.-J.; Zhao, J.; Zhang, J.; Zeng, L.; Liao, Y.-H.; Bao, L.; Bao, J. DFT-based study on the differences between odd and even Cn (n = 6–31) ring clusters. Results Phys. 2023, 52, 106852. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, L.; Zhang, X.; Ma, Y.; Jiang, Y.; Li, H. Parallel-self-assembling stack, center-capture effect, and reactivity-enhancing effect of n-layer (n = 1, 2, 3) cyclo[18]carbon. ACS Nano 2022, 16, 21345–21355. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Valiev, R.R.; Nasibullin, R.T.; Sundholm, D.; Kurten, T.; Ågren, H. Aromaticity of even-number cyclo[n]carbons (n = 6–100). J. Phys. Chem. A 2020, 124, 10849–10855. [Google Scholar] [CrossRef]
- Fowler, P.W.; Mizoguchi, N.; Bean, D.E.; Havenith, R.W.A. Double aromaticity and ring currents in all-carbon rings. Chem.—Eur. J. 2009, 15, 6964–6972. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Valiev, R.R.; Valiulina, L.I.; Kurtsevich, A.E.; Kurtén, T.; Sundholm, D.; Pittelkow, M.; Zhang, J.; Ågren, H. Odd-number cyclo[n]carbons sustaining alternating aromaticity. J. Phys. Chem. A 2022, 126, 2445–2452. [Google Scholar] [CrossRef]
- Dai, C.; Chen, D.; Zhu, J. Achieving adaptive aromaticity in cyclo[10]carbon by screening cyclo[n]carbon (n=8−24). Chem.—Asian J. 2020, 15, 2187–2191. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Valiev, R.R.; Kuklin, A.V.; Sundholm, D.; Ågren, H. Cyclo[18]carbon: Insight into electronic structure, aromaticity, and surface coupling. J. Phys. Chem. Lett. 2019, 10, 6701–6705. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Bonding character, electron delocalization, and aromaticity. Carbon 2020, 165, 468–475. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Ultrastrong regulation effect of the electric field on the all-carbon atomic ring cyclo[18]Carbon. ChemPhysChem 2021, 22, 386–395. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Yuan, A.; Wang, X.; Chen, Q.; Yan, X. Remarkable size effect on photophysical and nonlinear optical properties of all-carboatomic rings, cyclo[18]carbon and its analogues. Chem.—Asian J. 2021, 16, 2267–2271. [Google Scholar] [CrossRef]
- Nandi, A.; Solel, E.; Kozuch, S. Carbon tunneling in the automerization of cyclo[18]carbon. Chem.—Eur. J. 2020, 26, 625–628. [Google Scholar] [CrossRef]
- Iyakutti, K.; Surya, V.J.; Lakshmi, I.; Rajeswarapalanichamy, R.; Kawazoe, Y. 18 and 12—Member carbon rings (cyclo[n]carbons)—A density functional study. Mater. Sci. Eng. B 2021, 263, 114895. [Google Scholar] [CrossRef]
- Hassani, N.; Hassani, M.R.; Neek-Amal, M. Catalytic properties of cyclo-carbon clusters: An investigation on o2 activation and CO oxidation. Surf. Sci. 2022, 720, 122050. [Google Scholar] [CrossRef]
- Tang, C.; Xu, D.; Ouyang, G. Cross-plane transport in cyclo[18]carbon-based molecular devices. Appl. Phys. Lett. 2023, 122, 044101. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, X.; Xie, L.; Guan, Z.; Long, M.; Chen, T. Spin-resolved transport of multifunctional C(18)molecule-based nanodevices: A first-principles study. J. Phys. Condens. Matter 2023, 35, 395302. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Feng, Y.P.; Shen, L. Diverse transport behaviors in cyclo[18]carbon-based molecular devices. J. Phys. Chem. Lett. 2020, 11, 2611–2617. [Google Scholar] [CrossRef]
- Rojas, C.; León, A.; Pacheco, M.; Chico, L.; Orellana, P.A. Transport signatures of few-atom carbon rings. Phys. Chem. Chem. Phys. 2022, 24, 15973–15981. [Google Scholar] [CrossRef]
- Hou, L.; Hu, H.; Yang, G.; Ouyang, G. Giant switching effect and spintronic transport properties in cyclo[18]carbon-based molecular devices. Phys. Status Solidi—Rapid Res. Lett. 2021, 15, 2000582. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Meir, Y.; Wingreen, N.S. Landauer formula for the current through an interacting electron region. Phys. Rev. Lett. 1992, 68, 2512–2515. [Google Scholar] [CrossRef]
- Xue, Y.; Datta, S.; Ratner, M.A. First-principles based matrix Green’s function approach to molecular electronic devices: General formalism. J. Chem. Phys. 2002, 281, 151–170. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.-L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef]
- Rocha, A.R.; García-suárez, V.M.; Bailey, S.W.; Lambert, C.J.; Ferrer, J.; Sanvito, S. Towards molecular spintronics. Nat. Mater. 2005, 4, 335–339. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Rong, C.; Zhong, A.; Liu, S. Toward understanding the isomeric stability of fullerenes with density functional theory and the information-theoretic approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef]
- Luo, C.; He, X.; Zhong, A.; Liu, S.; Zhao, D. What dictates alkane isomerization? A combined density functional theory and information-theoretic approach study. Theor. Chem. Acc. 2023, 142, 78. [Google Scholar] [CrossRef]
- Plattner, D.A.; Houk, K.N. C18 Is a polyyne. J. Am. Chem. Soc. 1995, 117, 4405–4406. [Google Scholar] [CrossRef]
- Arulmozhiraja, S.; Ohno, T. CCSD calculations on C14, C18, and C22 carbon clusters. J. Chem. Phys. 2008, 128, 114301. [Google Scholar] [CrossRef]
- Gaweł, P.; Foroutan-Nejad, C. Carbon rings push limits of chemical theories. Nature 2023, 623, 922–924. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Zhang, F.; Yang, B.; Du, P.; Chen, C.; Zang, Y.; Zhu, D. Highly efficient charge transport across carbon nanobelts. Sci. Adv. 2022, 8, eade4692. [Google Scholar] [CrossRef]
- Lv, Y.; Lin, J.; Song, K.; Song, X.; Zang, H.; Zang, Y.; Zhu, D. Single cycloparaphenylene molecule devices: Achieving large conductance modulation via tuning radial π-conjugation. Sci. Adv. 2021, 7, eabk3095. [Google Scholar] [CrossRef]
- Xin, N.; Guan, J.; Zhou, C.; Chen, X.; Gu, C.; Li, Y.; Ratner, M.A.; Nitzan, A.; Stoddart, J.F.; Guo, X. Concepts in the design and engineering of single-molecule electronic devices. Nat. Rev. Phys. 2019, 1, 211–230. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, W. High-efficiency switching effect and negative differential conductance in cyclo[18]carbon–graphene nanoribbon junction. J. Appl. Phys. 2020, 128, 194303. [Google Scholar] [CrossRef]
- Paulsson, M.; Brandbyge, M. Transmission eigenchannels from nonequilibrium Green’s functions. Phys. Rev. B 2007, 76, 115117. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theoret. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Binning, R.C., Jr.; Curtiss, L.A. Compact contracted basis sets for third-row atoms: Ga–Kr. J. Comput. Chem. 1990, 11, 1206–1216. [Google Scholar] [CrossRef]
- Fuentealba, P.; Preuss, H.; Stoll, H.; Von Szentpály, L. A proper account of core-polarization with pseudopotentials: Single valence-electron alkali compounds. Chem. Phys. Lett. 1982, 89, 418–422. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Chem. Phys. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent developments and applications. J. Chem. Phys. 2020, 152, 204108–204131. [Google Scholar] [CrossRef] [PubMed]
- Papior, N.; Lorente, N.; Frederiksen, T.; García, A.; Brandbyge, M. Improvements on non-equilibrium and transport Green function techniques: The next-generation transiesta. Comput. Phys. Commun. 2017, 212, 8–24. [Google Scholar] [CrossRef]
- Soler, J.; Artacho, E.; Gale, J.; Garcia, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, H.; Lin, D.; Li, S.; Wang, Y.; Sanvito, S.; Hou, S. Robust covalent pyrazine anchors forming highly conductive and polarity-tunable molecular junctions with carbon electrodes. Phys. Chem. Chem. Phys. 2022, 24, 21337–21347. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Pan, H.; Wang, Y.; Li, J.; Dong, Y.; Wang, Y.; Hou, S. Exploring Aromaticity Effects on Electronic Transport in Cyclo[n]carbon Single-Molecule Junctions. Molecules 2024, 29, 3827. https://doi.org/10.3390/molecules29163827
Yang P, Pan H, Wang Y, Li J, Dong Y, Wang Y, Hou S. Exploring Aromaticity Effects on Electronic Transport in Cyclo[n]carbon Single-Molecule Junctions. Molecules. 2024; 29(16):3827. https://doi.org/10.3390/molecules29163827
Chicago/Turabian StyleYang, Peiqi, Haoyang Pan, Yudi Wang, Jie Li, Yangyu Dong, Yongfeng Wang, and Shimin Hou. 2024. "Exploring Aromaticity Effects on Electronic Transport in Cyclo[n]carbon Single-Molecule Junctions" Molecules 29, no. 16: 3827. https://doi.org/10.3390/molecules29163827
APA StyleYang, P., Pan, H., Wang, Y., Li, J., Dong, Y., Wang, Y., & Hou, S. (2024). Exploring Aromaticity Effects on Electronic Transport in Cyclo[n]carbon Single-Molecule Junctions. Molecules, 29(16), 3827. https://doi.org/10.3390/molecules29163827






