Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review
Abstract
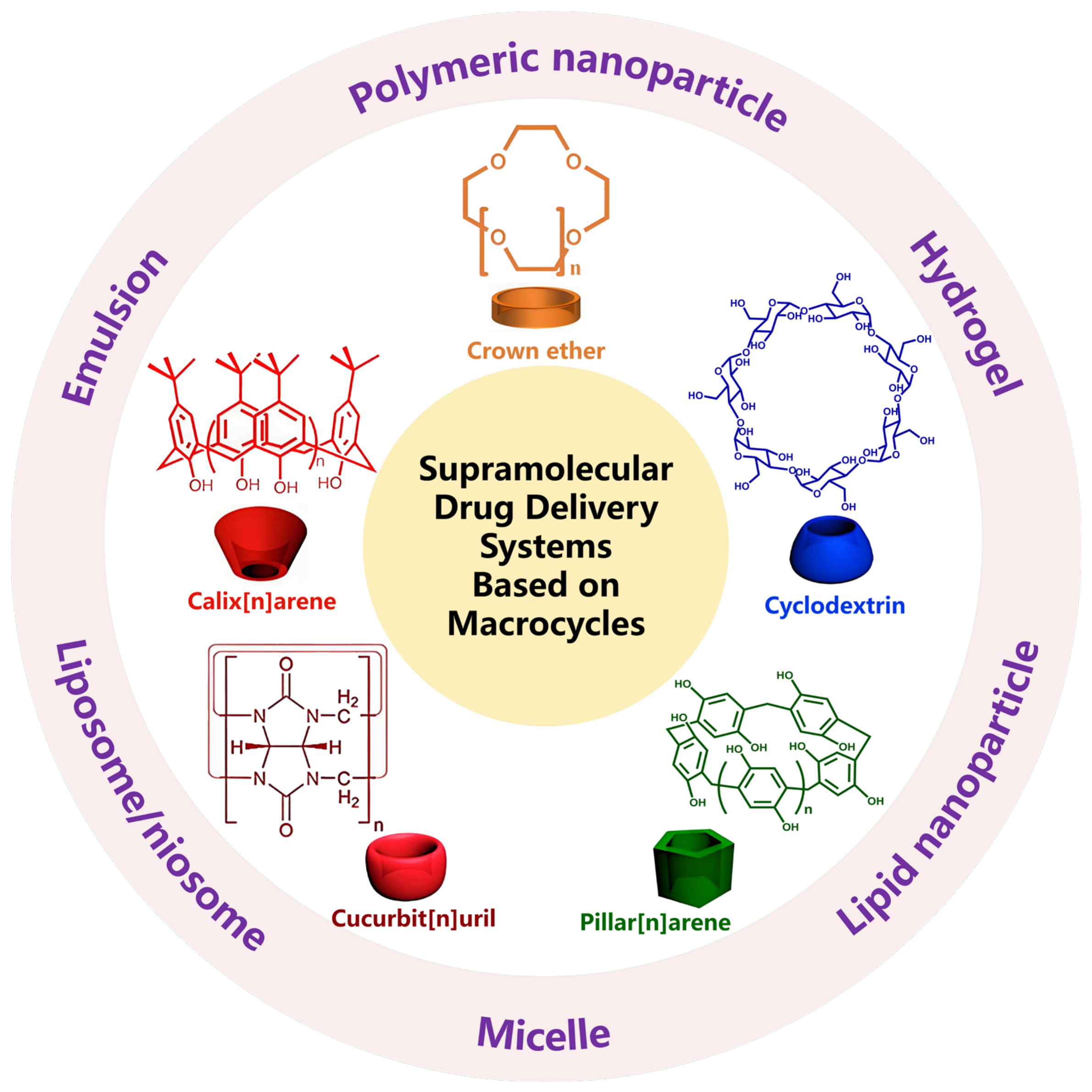
1. Introduction
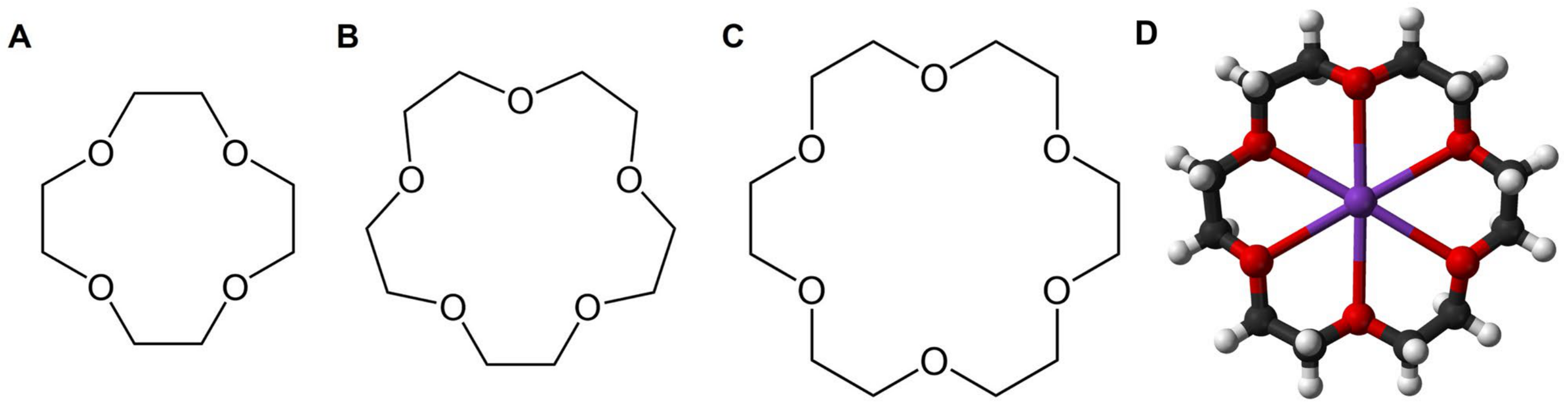
2. Crown Ether-Based DDSs
2.1. Ion Transporters or Ion Containers Based on CEs
2.2. CE-Based NDDSs
2.2.1. Improve the Encapsulation Efficiency of Nucleic Acid Drugs
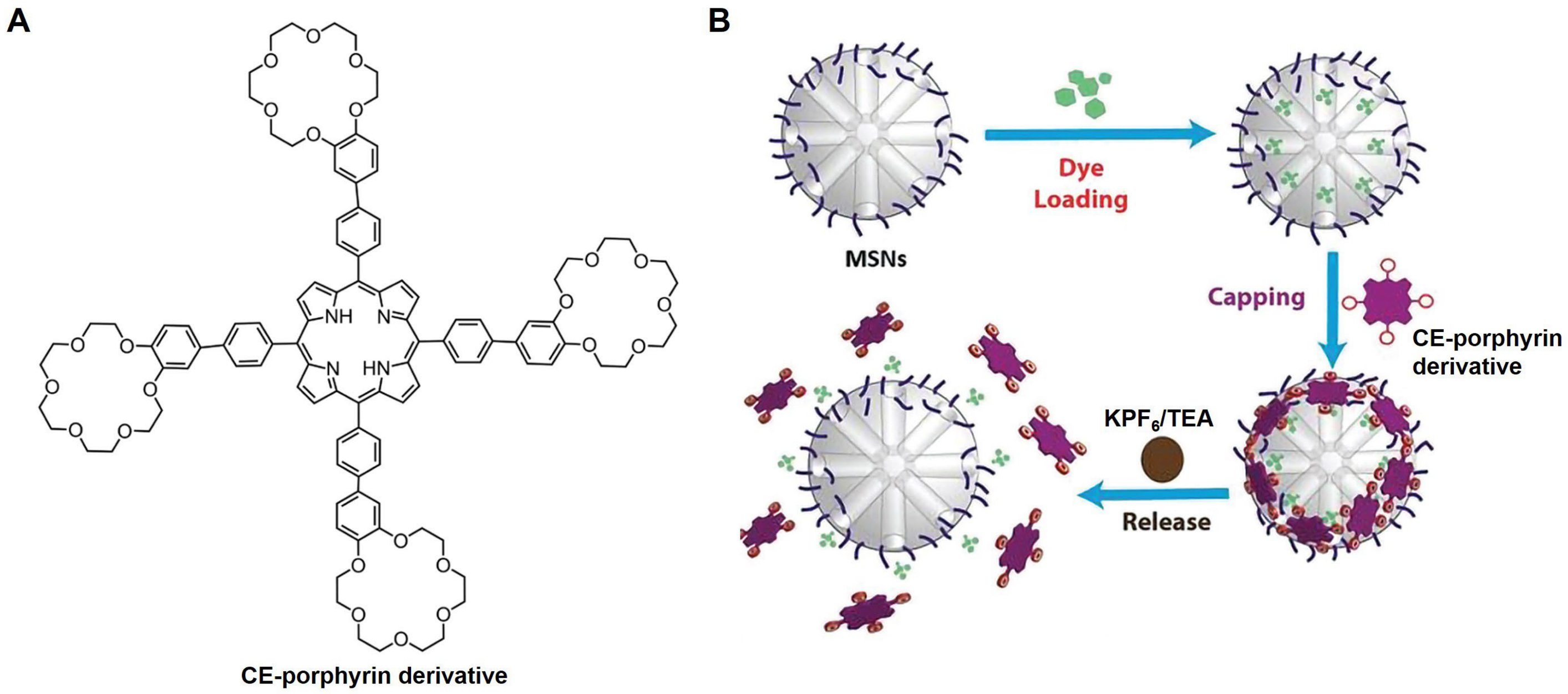
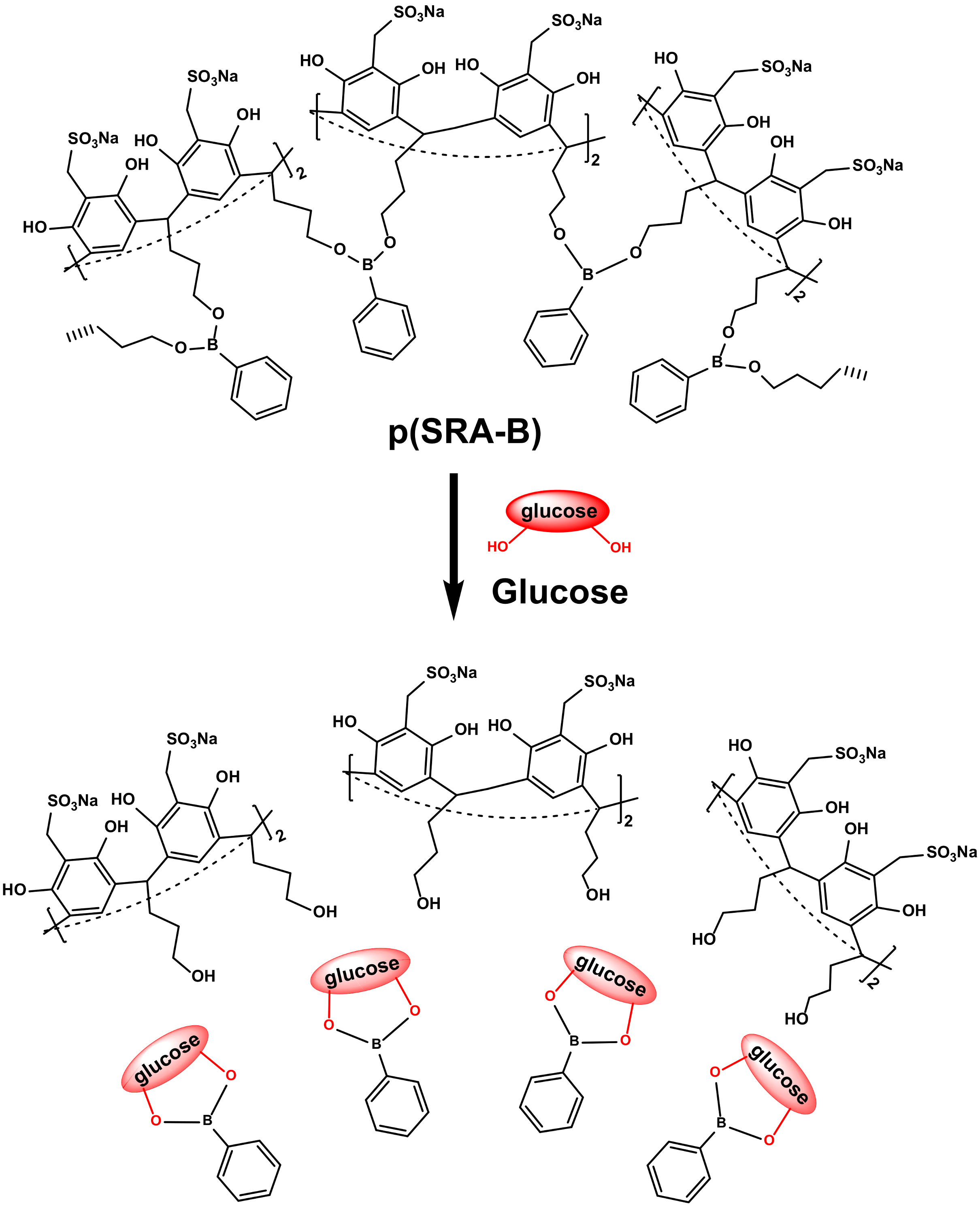
2.2.2. Construct Responsive DDSs
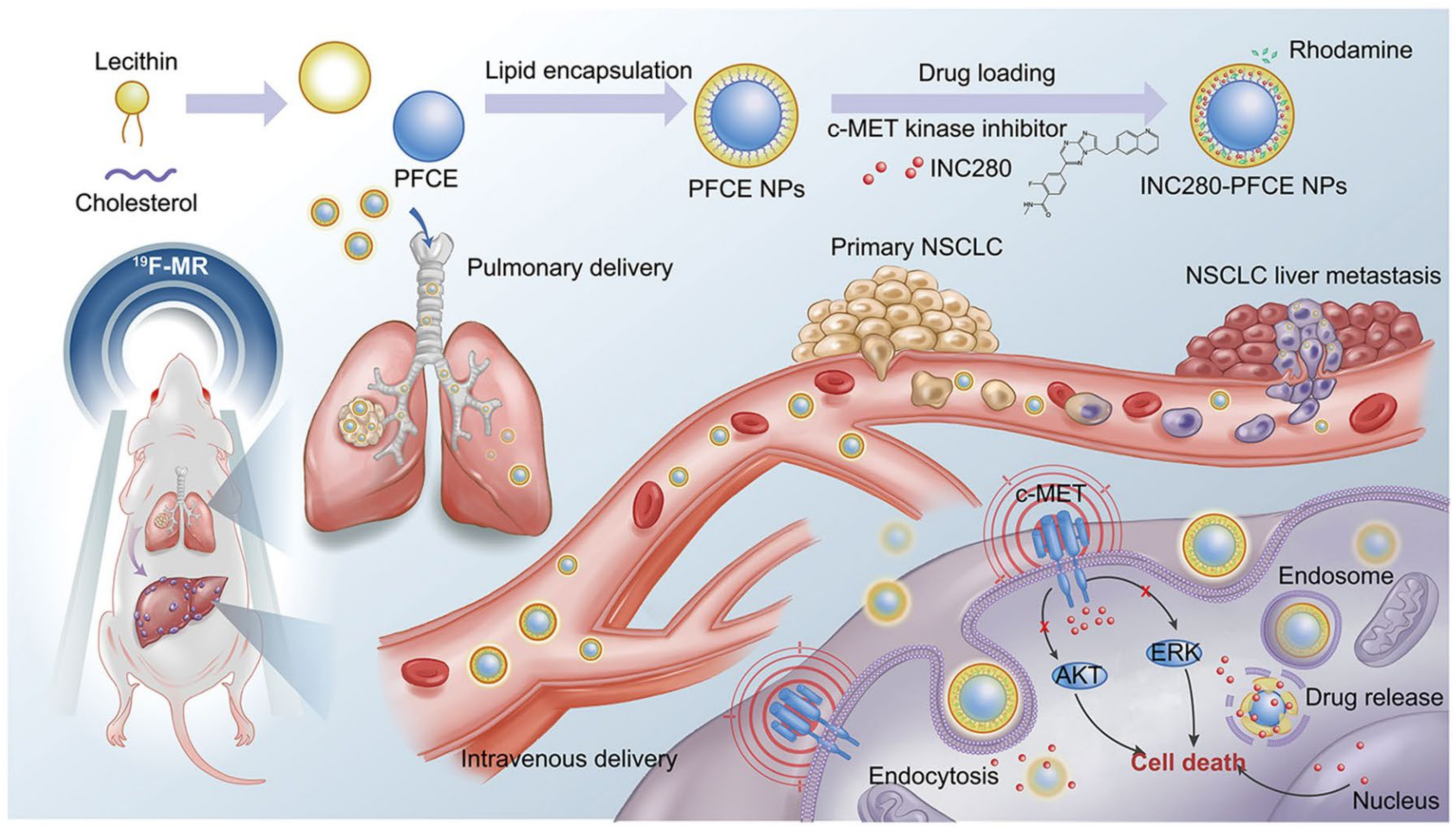
2.2.3. Perfluoro-CE-Based DDSs
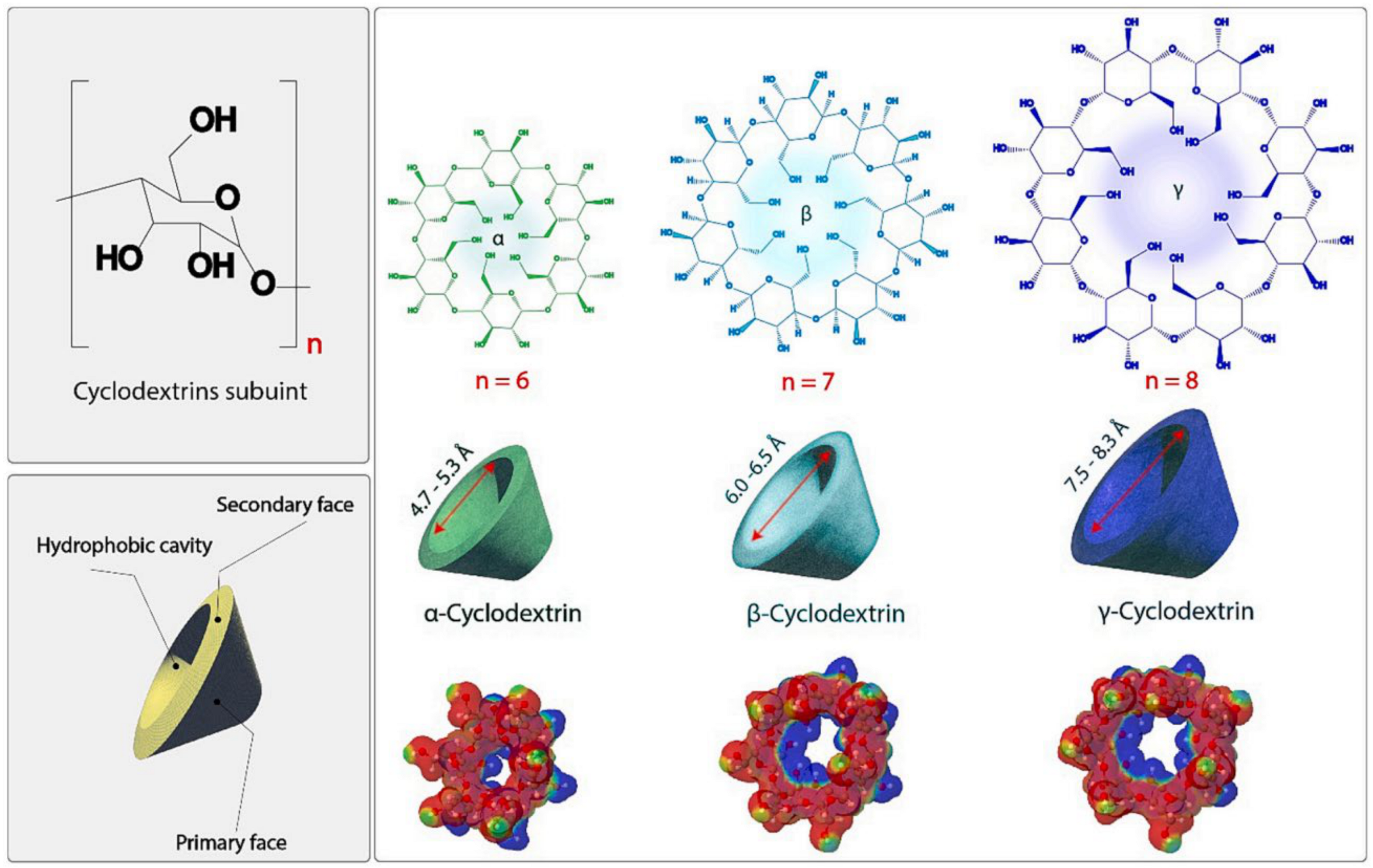
3. Cyclodextrin-Based DDSs
3.1. CD-Based Liposomes/Niosomes
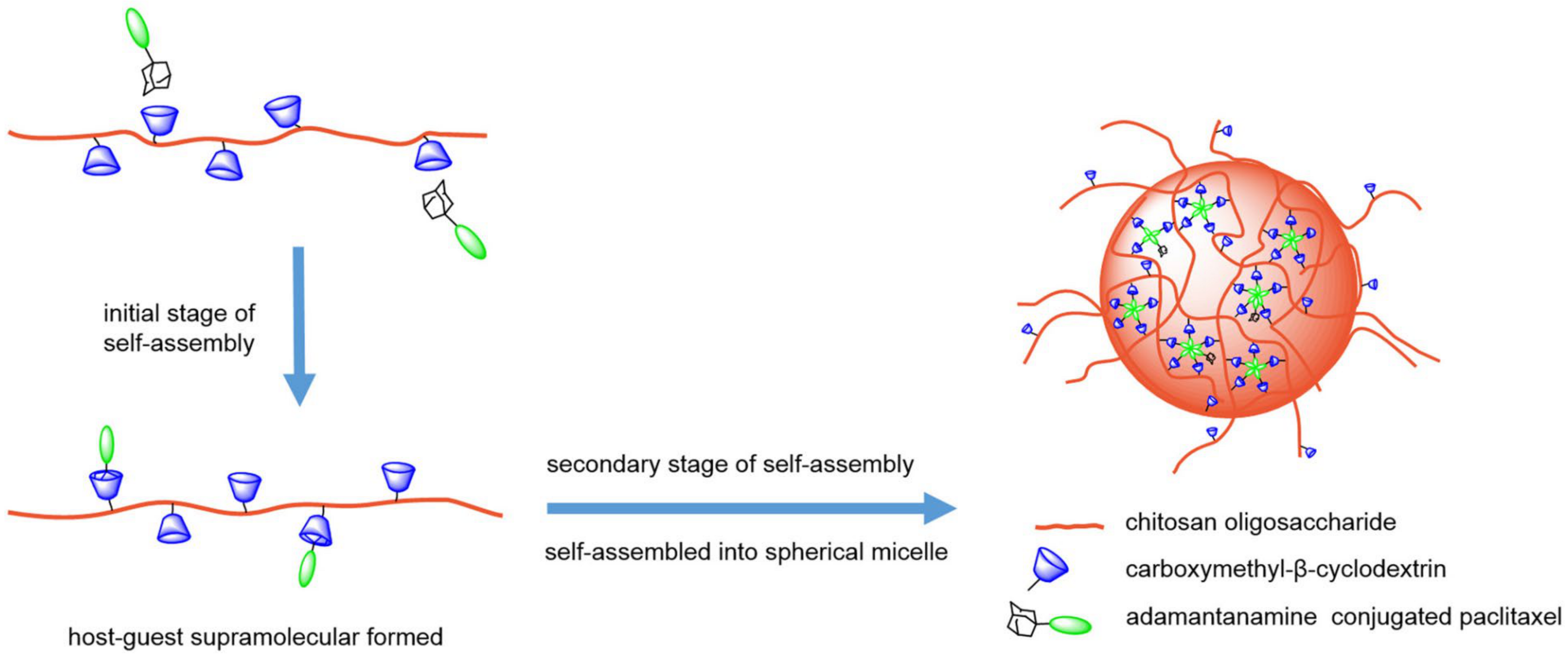
3.2. CD-Based Polymeric NPs
3.3. CD-Based Lipid NPs
3.4. CD-Based Emulsions
3.5. CD-Based Micelles
3.6. CD-Based Hydrogels
4. Cucurbit[n]uril-Based DDSs
4.1. CB[n]-Based Inclusion Complexes
4.2. CB[n]-Based NDDSs
4.3. Acyclic CB[n]-Based DDSs
4.4. CB[n]-Based Supramolecular–Organic Frameworks
5. Calix[n]arene-Based DDSs
5.1. C[n]A-Based Inclusion Complexes
5.2. C[n]A-Based NDDSs
5.3. Calix[4]resorcinarene-Based DDSs
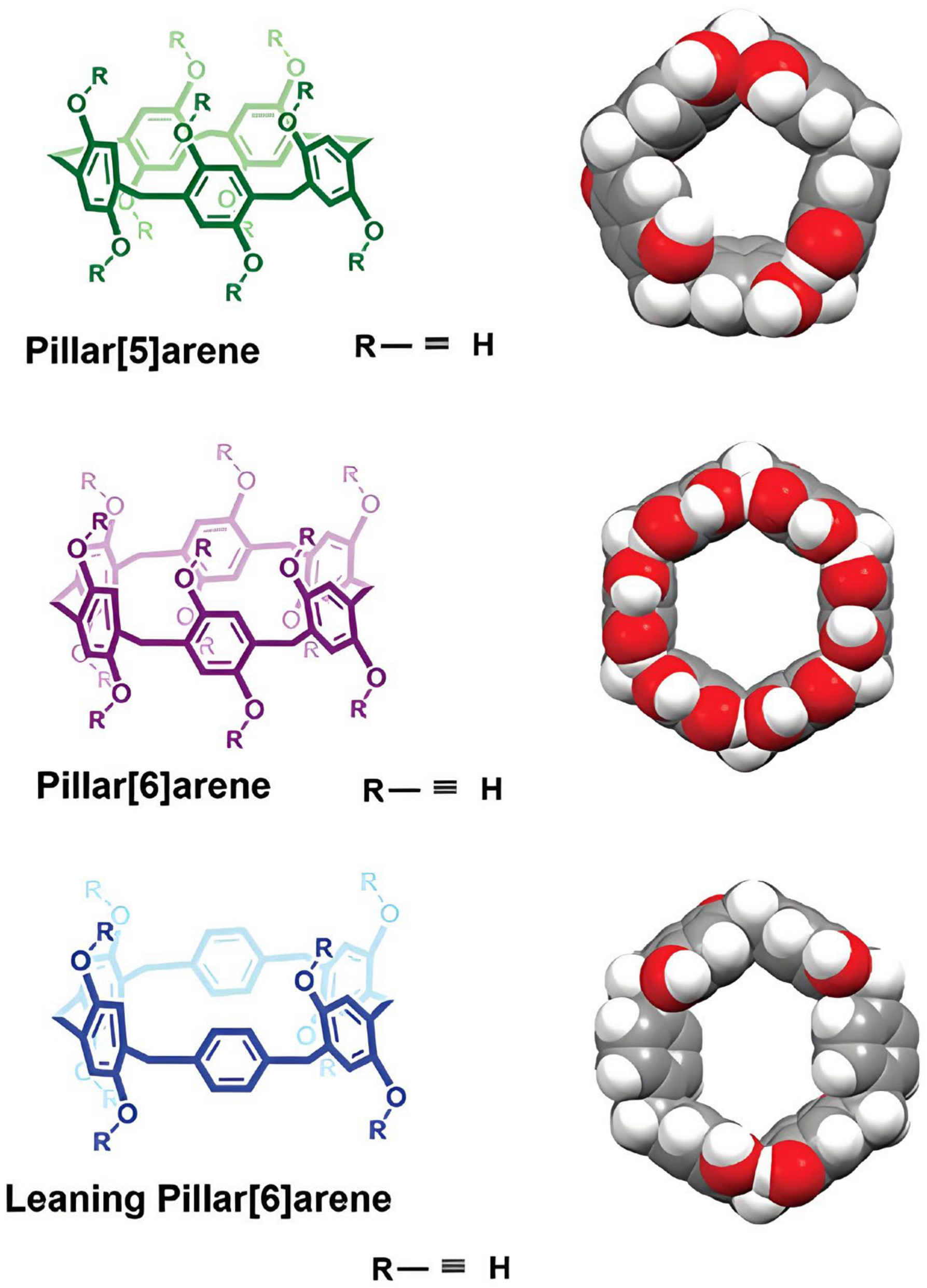
6. Pillar[n]arene-Based DDSs
6.1. P[n]A-Based Inclusion Complexes
6.2. P[n]A-Based NDDSs
7. Conclusions and Prospect
- I.
- The sensitivity and specificity of supramolecular systems towards lesion tissues or cells still need to be strengthened. At present, the system still has certain limitations in accurately identifying and locating specific lesion areas, which may result in unexpected drug distribution, thereby affecting treatment effectiveness. Therefore, future research needs to focus on improving the sensitivity and specificity of SDDSs to achieve more precise and effective drug delivery.
- II.
- Due to the dynamic and weak nature of non-covalent interactions, the stability of host–guest complexes or assemblies is often challenged. This instability may lead to drug leakage during delivery, which may result in a series of side effects and expose patients to unnecessary health risks. Therefore, when designing and optimizing SDDSs, it is necessary to fully consider the shortcomings of non-covalent interactions, and take corresponding measures to improve the stability of the system to ensure that drugs can reach the target site safely and effectively. Designing macrocycles and guests that are capable of attaining biotin/(strept–) avidin level affinity in aqueous environments is an attractive and long-term subject.
- III.
- There is another practical challenge; that is, the industrial quantitative production of macrocycles and supramolecular systems is difficult. It is crucial to achieve stable and efficient preparation processes for macrocycles and supramolecules in order to promote their widespread application in the pharmaceutical field. However, this process requires overcoming many technical difficulties and continuous exploration and innovation by researchers.
Author Contributions
Funding
Conflicts of Interest
References
- Tong, F.; Zhou, Y.; Xu, Y.; Chen, Y.; Yudintceva, N.; Shevtsov, M.; Gao, H. Supramolecular nanomedicines based on host-guest interactions of cyclodextrins. Exploration 2023, 3, 20210111. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jimenez, I.; Comenge, J.; Garcia-Fernandez, L.; Megson, Z.A.; Casals, E.; Puntes, V.F. Engineered inorganic nanoparticles for drug delivery applications. Curr. Drug Metab. 2013, 14, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Voltan, R.; Bortot, B.; Zauli, G.; Secchiero, P. Actively targeted nanocarriers for drug delivery to cancer cells. Expert Opin. Drug Deliv. 2019, 16, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.B.; Ozturk Atar, K.; Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 2021, 596, 120268. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qin, Y.; Lee, J.; Liao, H.; Wang, N.; Davis, T.P.; Qiao, R.; Ling, D. Stimuli-responsive nano-assemblies for remotely controlled drug delivery. J. Control. Release 2020, 322, 566–592. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, C. Stimuli-responsive drug delivery systems triggered by intracellular or subcellular microenvironments. Adv. Drug Deliv. Rev. 2023, 196, 114773. [Google Scholar] [CrossRef]
- Wu, J.R.; Wu, G.; Yang, Y.W. Pillararene-Inspired Macrocycles: From Extended Pillar[n]arenes to Geminiarenes. Acc. Chem. Res. 2022, 55, 3191–3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Du, X.; Cao, Y.; Ping, K.; Liu, D. Cucurbit [8]uril-based supramolecular theranostics. J. Nanobiotechnol. 2024, 22, 235. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
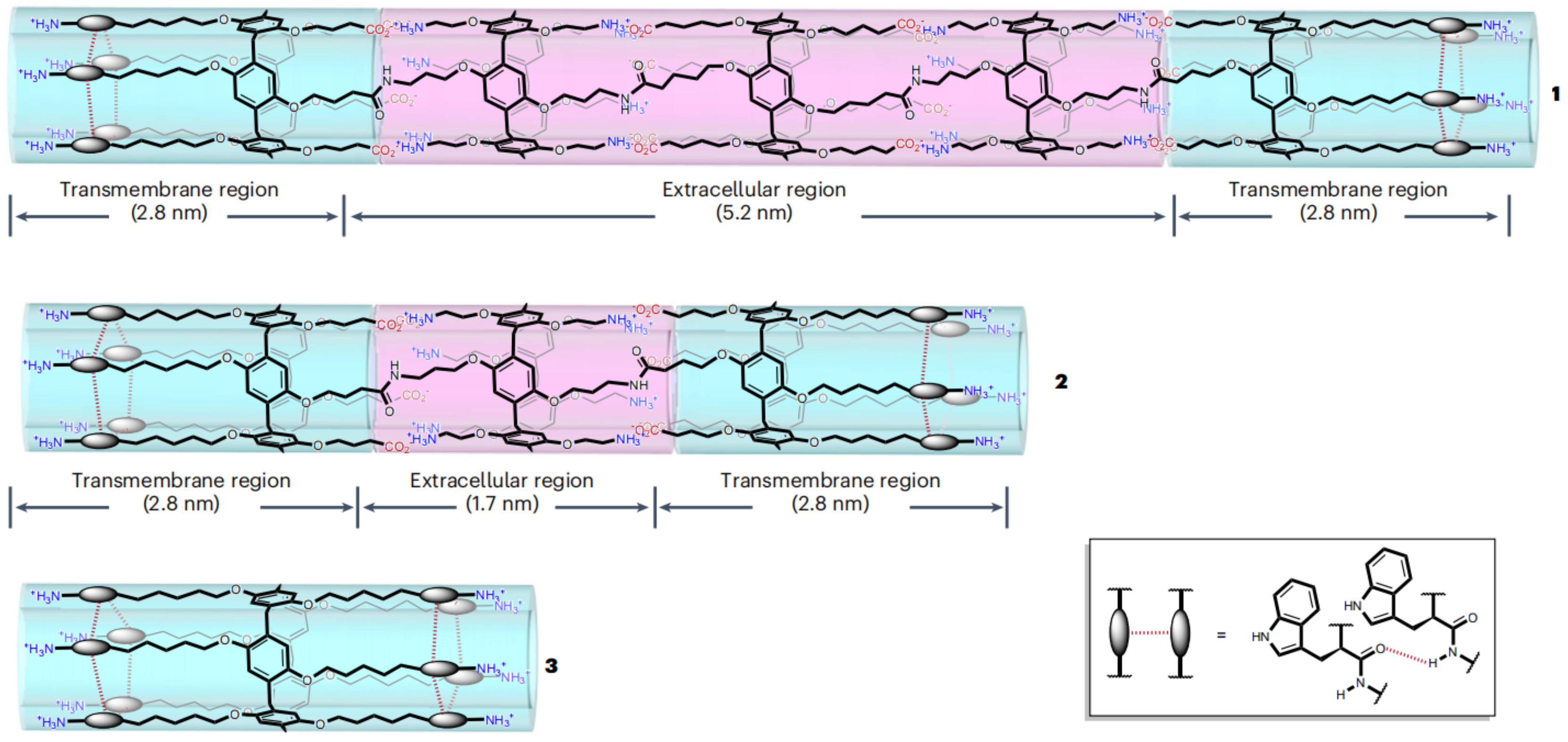
- Qian, Y.; Wu, Y.; Qiu, S.; He, X.; Liu, Y.; Kong, X.Y.; Tian, W.; Jiang, L.; Wen, L. A Bioinspired Free-Standing 2D Crown-Ether-Based Polyimine Membrane for Selective Proton Transport. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300167. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.M.; Gutsche, N.T.; King, A.P.; Baidoo, K.E.; Kelada, O.J.; Choyke, P.L.; Escorcia, F.E. Glypican-3-Targeted Alpha Particle Therapy for Hepatocellular Carcinoma. Molecules 2020, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.Y.; Liu, Y. High affinity crown ether complexes in water: Thermodynamic analysis, evidence of crystallography and binding of NAD+. J. Org. Chem. 2012, 77, 9766–9773. [Google Scholar] [CrossRef]
- You, X.R.; Ju, X.J.; He, F.; Wang, Y.; Liu, Z.; Wang, W.; Xie, R.; Chu, L.Y. Polymersomes with Rapid K(+)-Triggered Drug-Release Behaviors. ACS Appl. Mater. Interfaces 2017, 9, 19258–19268. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Rosmaninho, A.; Machado, S.; Selores, M. Bart syndrome. Int. J. Dermatol. 2014, 53, e54–e55. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Y.; Huang, L.M.; Quan, X.F.; Mao, J.H. Dent disease: Classification, heterogeneity and diagnosis. World J. Pediatr. 2021, 17, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.S.; Tan, H.L.; Wilde, A.A. Cardiac ion channels in health and disease. Heart Rhythm 2010, 7, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hatta, S.; Sakamoto, J.; Horio, Y. Ion channels and diseases. Med. Electron. Microsc. 2002, 35, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, P.; Gu, Z.; Luo, Y.; Luo, J. Mitochondrial Metal Ion Transport in Cell Metabolism and Disease. Int. J. Mol. Sci. 2021, 22, 7525. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, B.; Yan, X.; Wu, X.; Li, Z.; Gale, P.A.; Jiang, Y.-B. Crown ether-thiourea conjugates as ion transporters. Front. Chem. Sci. Eng. 2022, 16, 81–91. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Xiang, Y.; Pang, S.; Bao, C.; Zhu, L. A Synthetic Phospholipid Derivative Mediates Ion Transport Across Lipid Bilayers. Front. Chem. 2021, 9, 667472. [Google Scholar] [CrossRef]
- Bleich, M.; Shan, Q.; Himmerkus, N. Calcium regulation of tight junction permeability. Ann. N. Y. Acad. Sci. 2012, 1258, 93–99. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Porfiryeva, N.N.; Chahal, S.; Salakhov, I.A.; Lacourt, C.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V. Crown Ethers: Novel Permeability Enhancers for Ocular Drug Delivery? Mol. Pharm. 2017, 14, 3528–3538. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Afifi, S.A.; El Sayed, N.S. Crown Ether Nanovesicles (Crownsomes) Repositioned Phenytoin for Healing of Corneal Ulcers. Mol. Pharm. 2020, 17, 3952–3965. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Pisani, M.; Mobbili, G.; Marini, M.; Gasbarri, C. Neutral liposomes containing crown ether-lipids as potential DNA vectors. Biochim. Biophys. Acta 2013, 1828, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Zhu, X.M.; Wang, Y.X.; Xuan, S.H.; You, Q.; Chan, W.H.; Wong, C.H.; Wang, F.; Yu, J.C.; Cheng, C.H.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, M. Synthesis and characterization of a porphyrin-crown ether conjugate as a potential intermediate for drug delivery application. J. Porphyr. Phthalocyanines 2021, 25, 95–101. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.; Liu, X.; Zeng, B.; Xu, Y.; Yuan, C.; Dai, L. K(+)-Responsive Crown Ether-Based Amphiphilic Copolymer: Synthesis and Application in the Release of Drugs and Au Nanoparticles. Polymers 2022, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Yamaguchi, T.; Nakao, S.I. A Molecular-Recognition Microcapsule for Environmental Stimuli-Responsive Controlled Release. Adv. Mater. 2002, 14, 386–389. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Sun, J.; Zou, H.; Sun, Y.; Luo, J.; Xie, Q.; Rong, A.; Wang, H.; Li, X.; et al. An Osimertinib-Perfluorocarbon Nanoemulsion with Excellent Targeted Therapeutic Efficacy in Non-small Cell Lung Cancer: Achieving Intratracheal and Intravenous Administration. ACS Nano 2022, 16, 12590–12605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, J.; Li, Y.; Luo, J.; Sun, J.; Li, D.; Wang, Y.; Wang, K.; Yang, L.; Wu, L.; et al. Single low-dose INC280-loaded theranostic nanoparticles achieve multirooted delivery for MET-targeted primary and liver metastatic NSCLC. Mol. Cancer 2022, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnurch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Liu, Y.H.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Niu, X.; Li, G. Liposome Nanoparticles as a Novel Drug Delivery System for Therapeutic and Diagnostic Applications. Curr. Drug Deliv. 2022, 20, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Kheilnezhad, B.; Hadjizadeh, A. Factors Affecting the Penetration of Niosome into the Skin, Their Laboratory Measurements and Dependency to the Niosome Composition: A Review. Curr. Drug Deliv. 2021, 18, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Hayashi, Y.; Higashi, T.; Motoyama, K. Recent advances in cyclodextrin delivery techniques. Expert Opin. Drug Deliv. 2015, 12, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Fraceto, L.F.; Grillo, R.; Sobarzo-Sanchez, E. Cyclodextrin inclusion complexes loaded in particles as drug carrier systems. Curr. Top. Med. Chem. 2014, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.R. Cyclodextrin Inclusion of Medicinal Compounds for Enhancement of their Physicochemical and Biopharmaceutical Properties. Curr. Top. Med. Chem. 2019, 19, 2357–2370. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin-drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, X.; Fang, A.; Li, H.; Zhou, Y.; Liu, Y.; Jiang, C.; Wu, J.; Song, X. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-beta-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug-Delivery System. Front. Pharmacol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Nascimento Vieira, A.L.; Franz-Montan, M.; Cabeca, L.F.; de Paula, E. Anaesthetic benefits of a ternary drug delivery system (Ropivacaine-in-Cyclodextrin-in-Liposomes): In-vitro and in-vivo evaluation. J. Pharm. Pharmacol. 2020, 72, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.P.; Cai, B.C.; Yang, X.X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Jain, A.; Bhola, M. Multivesicular liposomes bearing celecoxib-beta-cyclodextrin complex for transdermal delivery. Drug Deliv. 2007, 14, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.Y.; Dinarvand, R.; Esmaeili, M.; Mahjub, R.; Toliyat, T. Controlled-release drug delivery system based on fluocinolone acetonide-cyclodextrin inclusion complex incorporated in multivesicular liposomes. Pharm. Dev. Technol. 2015, 20, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Gupta, Y.; Jain, A.; Amin, S. Elastic liposomes bearing meloxicam-beta-cyclodextrin for transdermal delivery. Curr. Drug Deliv. 2008, 5, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Coll. Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Silva, O.F.; de Rossi, R.H.; Fernandez, M.A. Cyclodextrin modified niosomes to encapsulate hydrophilic compounds. RSC Adv. 2018, 8, 29909–29916. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Rinaldi, F.; Esposito, S.; Di Marzio, L.; Carafa, M. Niosomes encapsulating Ibuprofen-cyclodextrin complexes: Preparation and characterization. Curr. Drug Targets 2013, 14, 1070–1078. [Google Scholar] [CrossRef]
- Duchene, D.; Cavalli, R.; Gref, R. Cyclodextrin-based Polymeric Nanoparticles as Efficient Carriers for Anticancer Drugs. Curr. Pharm. Biotechnol. 2016, 17, 248–255. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Qiu, N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers 2023, 15, 1400. [Google Scholar] [CrossRef] [PubMed]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Garcia-Fuentes, M.; Mura, P.; Alonso, M.J. A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. Eur. J. Pharm. Biopharm. 2006, 63, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; El-Nahhas, S.A.; Ghorab, M.M.; Salama, A.H. Chitosan/cyclodextrin nanoparticles as drug delivery system. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 127–136. [Google Scholar] [CrossRef]
- Fulop, Z.; Saokham, P.; Loftsson, T. Sulfobutylether-beta-cyclodextrin/chitosan nano- and microparticles and their physicochemical characteristics. Int. J. Pharm. 2014, 472, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tang, B.; Tang, P.; Sun, Q.; Suo, Z.; Zhang, M.; Gan, N.; Yang, H.; Li, H. Chitosan/Sulfobutylether-beta-Cyclodextrin Nanoparticles for Ibrutinib Delivery: A Potential Nanoformulation of Novel Kinase Inhibitor. J. Pharm. Sci. 2020, 109, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-beta-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206. [Google Scholar] [CrossRef]
- Jingou, J.; Shilei, H.; Weiqi, L.; Danjun, W.; Tengfei, W.; Yi, X. Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Coll. Surf. B Biointerfaces 2011, 83, 103–107. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Z.; Chen, J.; Yang, X.; Li, Y.; Tang, J.; Du, X.; Tang, L.; Liang, D.; Zhu, X.; et al. Beta-Cyclodextrin-Grafted Chitosan Enhances Intestinal Drug Absorption and Its Preliminary Mechanism Exploration. AAPS PharmSciTech 2022, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.M.; Patra, S.; Swain, S.K. Host-guest drug delivery by beta-cyclodextrin assisted polysaccharide vehicles: A review. Int. J. Biol. Macromol. 2023, 240, 124338. [Google Scholar] [CrossRef]
- Merkle, H.P. Drug delivery’s quest for polymers: Where are the frontiers? Eur. J. Pharm. Biopharm. 2015, 97, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, Y.; Watanabe, H.; Kometani, M.; Hasegawa, T. Effect of androgens on phalloidin-induced liver toxicity in mice. Toxicol. Lett. 1988, 40, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M.E. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum Harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics 2022, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CD-CUR inclusion complex co-loaded in thermosensitive hydrogel PLGA-PEG-PLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Huang, J.; Liu, J.; Zhu, Y.; Chen, J.; Chen, J.; Lei, L.; Guan, Z.; Ban, J.; Lu, Z. Nanoformulation of Paclitaxel: Exploring the Cyclodextrin/PLGA Nano Delivery Carrier to Slow Down Paclitaxel Release, Enhance Accumulation in Vivo. J. Cancer 2023, 14, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Rahimi, F.; Iranshahi, M.; Kahroba, H.; Zarebkohan, A.; Talebi, M.; Salehi, R.; Mousavi, H.Z. Co-delivery of doxorubicin and conferone by novel pH-responsive beta-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci. Rep. 2021, 11, 21425. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, J.; Xu, R.; Li, Q.; Shen, Q.; Zhu, G. Nanoparticles based on polymers modified with pH-sensitive molecular switch and low molecular weight heparin carrying Celastrol and ferrocene for breast cancer treatment. Int. J. Biol. Macromol. 2021, 183, 2215–2226. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Chen, Y.; Dou, Y.; Tao, H.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Structure–Property Correlations of Reactive Oxygen Species-Responsive and Hydrogen Peroxide-Eliminating Materials with Anti-Oxidant and Anti-Inflammatory Activities. Chem. Mater. 2017, 29, 8221–8238. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, H.; Lin, Y.; Hu, Y.; An, H.; Zhang, D.; Feng, S.; Hu, H.; Wang, R.; Li, X.; et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Li, S.; Liu, R.; Jin, T.; Dou, Y.; Zhou, Z.; Zhang, J. A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment. Theranostics 2019, 9, 3732–3753. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hu, Y.; Peng, S.; Han, S.; Tao, H.; Zhang, Q.; Xu, X.; Zhang, J.; Hu, H. Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis. Biomaterials 2016, 105, 167–184. [Google Scholar] [CrossRef] [PubMed]
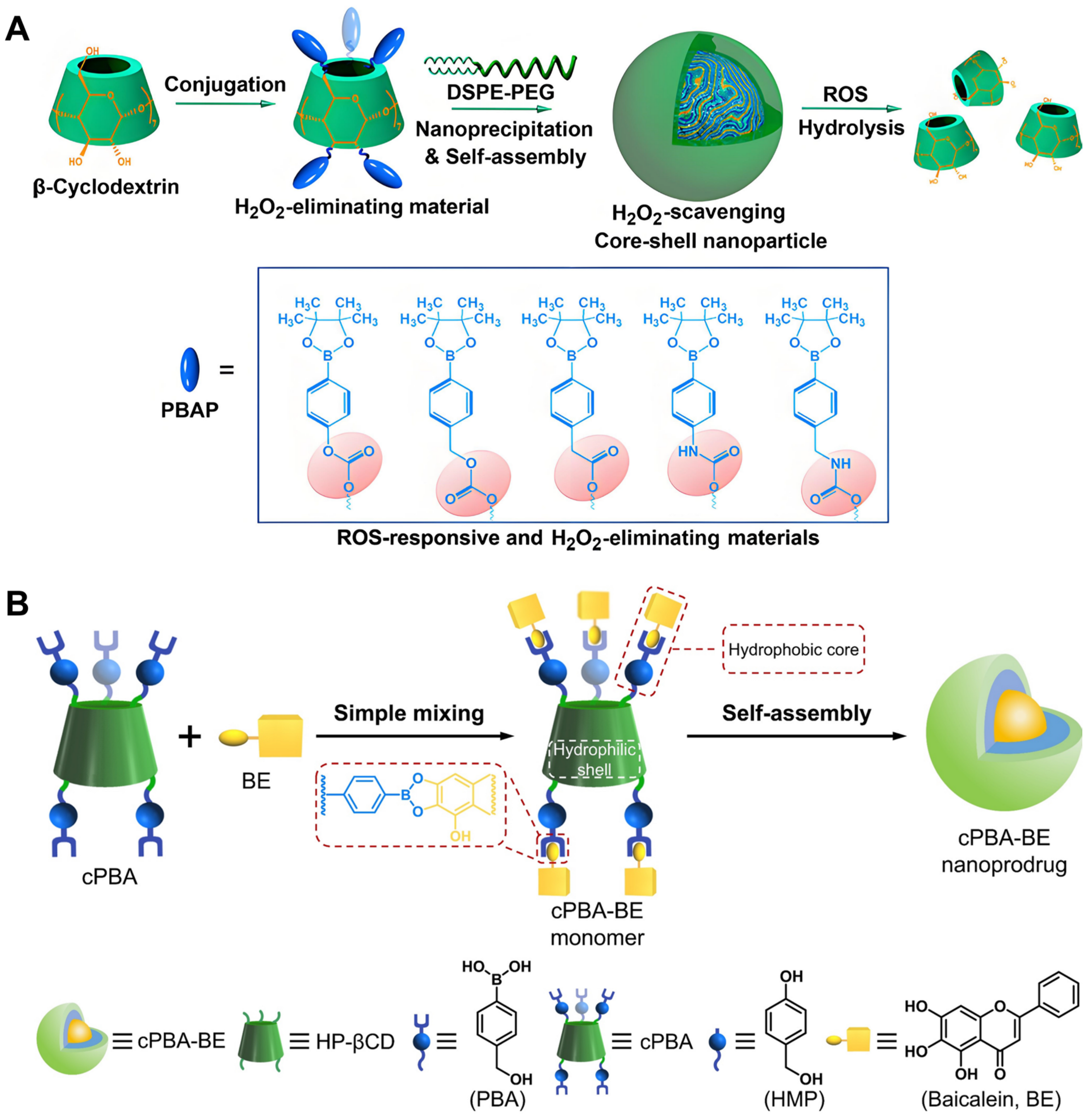
- Zhang, Q.; Li, S.; Cai, L.; Zhu, Y.; Duan, X.; Jiang, P.; Zhong, L.; Guo, K.; Tong, R. Microenvironment Activatable Nanoprodrug Based on Gripper-like Cyclic Phenylboronic Acid to Precisely and Effectively Alleviate Drug-induced Hepatitis. Theranostics 2021, 11, 8301–8321. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, N.A.M.; Zulfakar, M.H. Nanostructured Lipid Carriers for the Delivery of Natural Bioactive Compounds. Curr. Drug Deliv. 2023, 20, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues da Silva, G.H.; Moura, L.D.; Carvalho, F.V.; Geronimo, G.; Mendonca, T.C.; Lima, F.F.; de Paula, E. Antineoplastics Encapsulated in Nanostructured Lipid Carriers. Molecules 2021, 26, 6929. [Google Scholar] [CrossRef]
- Baek, J.S.; Kim, J.H.; Park, J.S.; Cho, C.W. Modification of paclitaxel-loaded solid lipid nanoparticles with 2-hydroxypropyl-beta-cyclodextrin enhances absorption and reduces nephrotoxicity associated with intravenous injection. Int. J. Nanomed. 2015, 10, 5397–5405. [Google Scholar] [CrossRef]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Combined Approach of Cyclodextrin Complexationand Nanostructured Lipid Carriers for the Development of a Pediatric Liquid Oral Dosage Form of Hydrochlorothiazide. Pharmaceutics 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Ettireddy, S.; Youssef, A.A.A.; Puchchakayala, G. Development and In vivo Pharmacokinetic and Pharmacodynamic Evaluation of an Oral Innovative Cyclodextrin Complexed Lipid Nanoparticles of Irbesartan Formulation for Enhanced Bioavailability. Nanotheranostics 2023, 7, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.Q.; da Silva, J.K.R.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Lipid nanoparticles as carriers of cyclodextrin inclusion complexes: A promising approach for cutaneous delivery of a volatile essential oil. Coll. Surf. B Biointerfaces 2019, 182, 110382. [Google Scholar] [CrossRef]
- Permana, A.D.; Sam, A.; Marzaman, A.N.F.; Rahim, A.; Nainu, F.; Bahar, M.A.; Asri, R.M.; Chabib, L. Solid lipid nanoparticles cyclodextrin-decorated incorporated into gellan gum-based dry floating in situ delivery systems for controlled release of bioactive compounds of safflower (Carthamus tinctorius. L): A proof of concept study in biorelevant media. Int. J. Biol. Macromol. 2023, 237, 124084. [Google Scholar] [CrossRef] [PubMed]
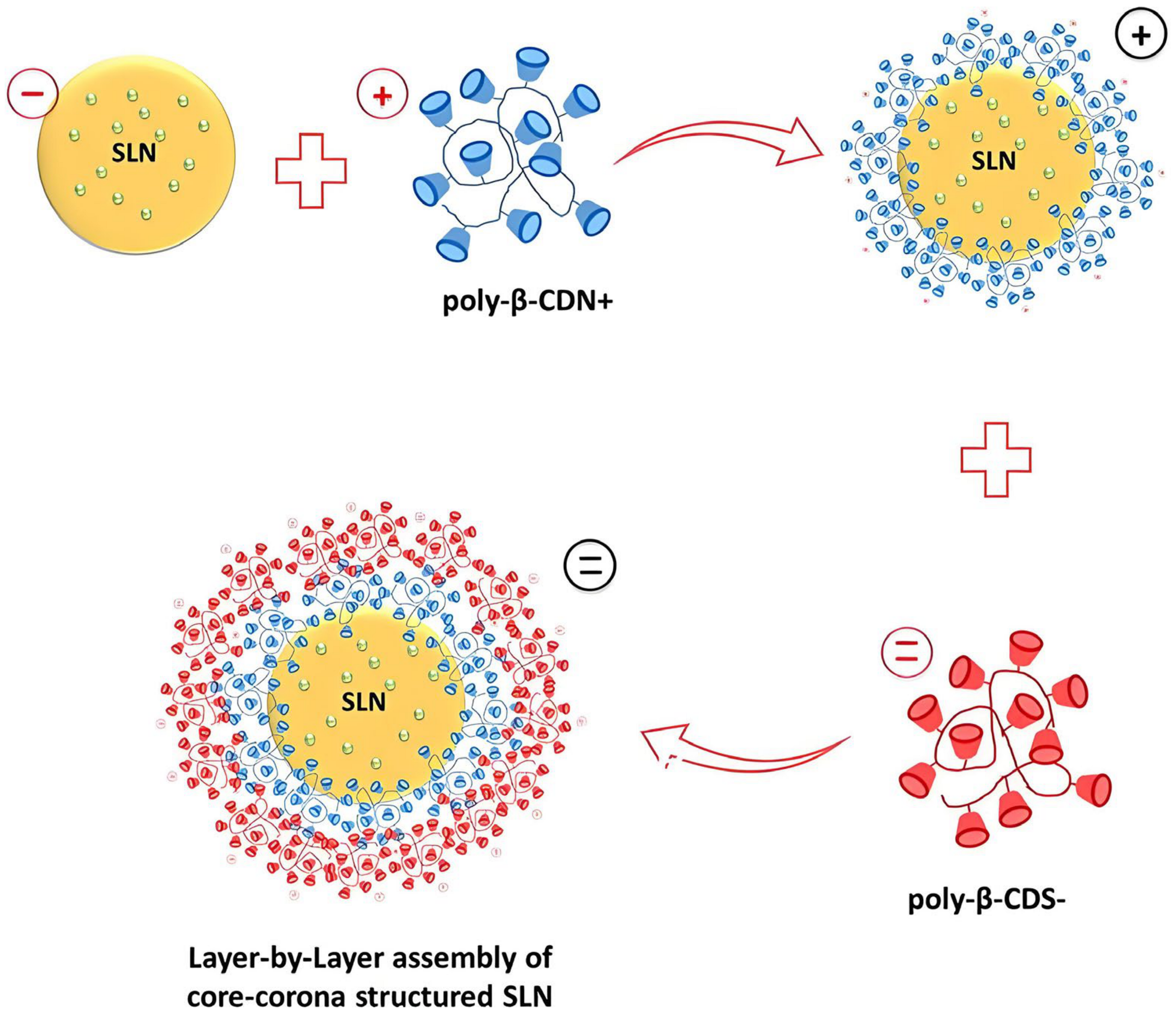
- Amasya, G.; Bakar-Ates, F.; Wintgens, V.; Amiel, C. Layer by layer assembly of core-corona structured solid lipid nanoparticles with beta-cyclodextrin polymers. Int. J. Pharm. 2021, 592, 119994. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, Y.; Ma, Z.; Zhou, L. Pickering Emulsion Stabilized by beta-Cyclodextrin and Cinnamaldehyde/beta-Cyclodextrin Composite. Foods 2023, 12, 2366. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, J.; Yi, T. A Novel Solid Nanocrystals Self-Stabilized Pickering Emulsion Prepared by Spray-Drying with Hydroxypropyl-beta-cyclodextrin as Carriers. Molecules 2021, 26, 1809. [Google Scholar] [CrossRef]
- Taguchi, H.; Tanaka, H.; Hashizaki, K.; Saito, Y.; Fujii, M. Application of Pickering Emulsion with Cyclodextrin as an Emulsifier to a Transdermal Drug Delivery Vehicle. Biol. Pharm. Bull. 2019, 42, 116–122. [Google Scholar] [CrossRef]
- Cheng, J.H.; Hu, Y.N.; Luo, Z.G.; Chen, W.; Chen, H.M.; Peng, X.C. Preparation and properties of octenyl succinate beta-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 2017, 218, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaur, K.; Uppal, S.; Mehta, S.K. Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability. Ultrason. Sonochem. 2017, 37, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.; Li, X. Preparation, in-vitro release and antioxidant potential of formulation of apigenin with hydroxypropyl-β-cyclodextrin modified microemulsion. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 93–102. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.; Raupach, K.; El-Hagin, N.; Valenta, C. Development of sucrose stearate-based nanoemulsions and optimisation through gamma-cyclodextrin. Eur. J. Pharm. Biopharm. 2011, 79, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Haberfeld, S.; Hartl, A.; Valenta, C. Effect of gamma-cyclodextrin on the in vitro skin permeation of a steroidal drug from nanoemulsions: Impact of experimental setup. Int. J. Pharm. 2012, 423, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.; Zimmermann, A.M.; Vojnikovic, E.; Valenta, C. Enhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesterone nanoemulsions. Int. J. Pharm. 2010, 393, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery, Biotechnology and Ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Qin, D.; Liu, W.; Song, C.; Lou, K.; Wang, W.; Gao, F. Beta-Cyclodextrin-Based Inclusion Complexation Bridged Biodegradable Self-Assembly Macromolecular Micelle for the Delivery of Paclitaxel. PLoS ONE 2016, 11, e0150877. [Google Scholar] [CrossRef]
- Li, F.; Cao, D.; Gu, W.; Cui, L.; Qiu, Z.; Liu, Z.; Li, D.; Guo, X. Delivery of miR-34a-5p by Folic Acid-Modified β-Cyclodextrin-Grafted Polyethylenimine Copolymer Nanocarriers to Resist KSHV. ACS Appl. Nano Mater. 2023, 6, 10826–10836. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Z.; Yu, C.Y.; Wei, H. Engineered cyclodextrin-based supramolecular hydrogels for biomedical applications. J. Mater. Chem. B 2023, 12, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Dey, S.; Pal, S. Chemical modification of beta-cyclodextrin towards hydrogel formation. Carbohydr. Polym. 2023, 306, 120576. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.; Yu, J.; Song, X.Y.; Chen, J.; Shi, Y.P. Tunable temperature-responsive supramolecular hydrogels formed by prodrugs as a codelivery system. ACS Appl. Mater. Interfaces 2014, 6, 10623–10630. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Z.; Guo, H.; Li, H.; Chen, Y.; Guan, S. A Double-Layer Hydrogel Dressing with High Mechanical Strength and Water Resistance Used for Drug Delivery. Molecules 2023, 28, 499. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Selvapalam, N.; Oh, D.H. Cucurbiturils—A New Family of Host Molecules. J. Incl. Phenom. Macrocycl. Chem. 2004, 50, 31–36. [Google Scholar]
- Zhang, X.X.; Krakowiak, K.E.; Xue, G.; Bradshaw, J.S.; Izatt, R.M. A Highly Selective Compound for Lead: Complexation Studies of Decamethylcucurbit [5]uril with Metal Ions. Ind. Eng. Chem. Res. 2000, 39, 3516–3520. [Google Scholar] [CrossRef]
- Barooah, N.; Mohanty, J.; Bhasikuttan, A.C. Cucurbituril-Based Supramolecular Assemblies: Prospective on Drug Delivery, Sensing, Separation, and Catalytic Applications. Langmuir 2022, 38, 6249–6264. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhang, Y.M.; Yu, H.J.; Liu, Y. Cucurbituril-Based Biomacromolecular Assemblies. Angew. Chem. Int. Ed. Engl. 2021, 60, 3870–3880. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Zeng, J.-P.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Molecular capsules formed by three different cucurbit [5]urils and some lanthanide ions. J. Mol. Struct. 2009, 929, 167–173. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Li, S.; Wang, L.H.; Zhang, J.; Wang, R. A systematic evaluation of the biocompatibility of cucurbit [7]uril in mice. Sci. Rep. 2018, 8, 8819. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Chen, J.M.; Jiang, L.; Yao, J.; Lu, T.B. The delivery of triamterene by cucurbit [7]uril: Synthesis, structures and pharmacokinetics study. Mol. Pharm. 2013, 10, 4698–4705. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.I.; Biedermann, F.; Mustafina, K.R.; Assaf, K.I.; Hennig, A.; Nau, W.M. Nanomolar Binding of Steroids to Cucurbit[n]urils: Selectivity and Applications. J. Am. Chem. Soc. 2016, 138, 13022–13029. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, H.; Bardelang, D.; Xiao, J.; Zheng, Y.; Wang, R. Supramolecular formulation of nitidine chloride can alleviate its hepatotoxicity and improve its anticancer activity. Food Chem. Toxicol. 2017, 109, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zheng, Y.; Parker, R.M.; Lan, Y.; Wu, Y.; Coulston, R.J.; Zhang, J.; Scherman, O.A.; Abell, C. Microfluidic Droplet-Facilitated Hierarchical Assembly for Dual Cargo Loading and Synergistic Delivery. ACS Appl. Mater. Interfaces 2016, 8, 8811–8820. [Google Scholar] [CrossRef] [PubMed]
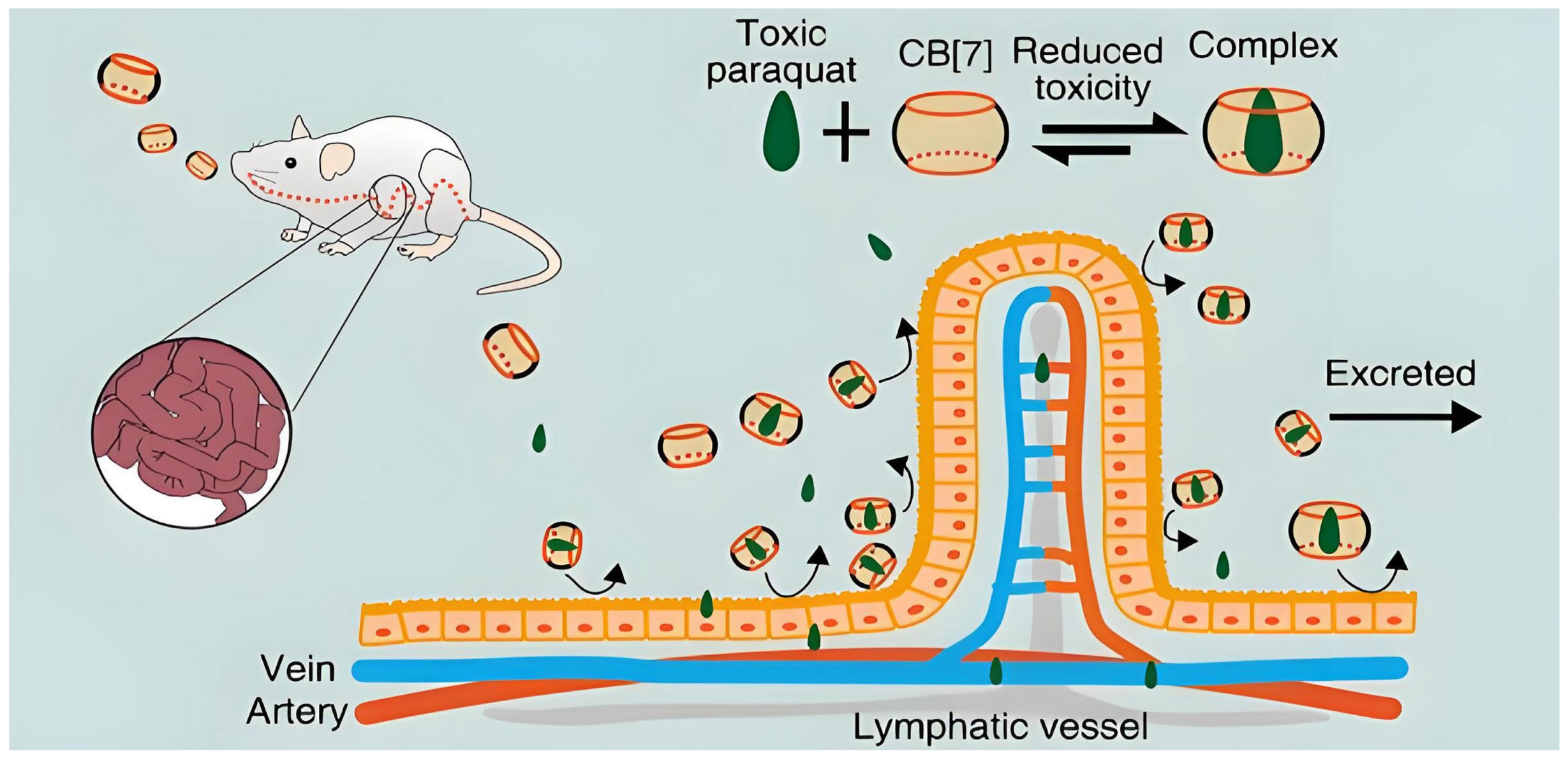
- Zhang, X.; Xu, X.; Li, S.; Li, L.; Zhang, J.; Wang, R. A Synthetic Receptor as a Specific Antidote for Paraquat Poisoning. Theranostics 2019, 9, 633–645. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Q.; Li, L.; Shangguan, L.; Li, C.; Li, S.; Huang, F.; Zhang, J.; Wang, R. Supramolecular therapeutics to treat the side effects induced by a depolarizing neuromuscular blocking agent. Theranostics 2019, 9, 3107–3121. [Google Scholar] [CrossRef]
- Kuok, K.I.; In Ng, P.C.; Ji, X.; Wang, C.; Yew, W.W.; Chan, D.P.C.; Zheng, J.; Lee, S.M.Y.; Wang, R. Supramolecular strategy for reducing the cardiotoxicity of bedaquiline without compromising its antimycobacterial efficacy. Food Chem. Toxicol. 2018, 119, 425–429. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Niu, Y.; Andrew, G.L.; Bardelang, D.; Chen, X.; Wang, R. Alleviation of Hepatotoxicity of Arecoline (Areca Alkaloid) by a Synthetic Receptor. ChemistrySelect 2017, 2, 2219–2223. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, W.; Chen, H.; Zeng, J. Targeting Polyamine Metabolism for Control of Human Viral Diseases. Infect. Drug Resist. 2020, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Olsen, M.E.; Vignuzzi, M.; Connor, J.H. Polyamines and Their Role in Virus Infection. Microbiol. Mol. Biol. Rev. 2017, 81, e00029-17. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Zhang, X.; Ding, Y.; Li, S.; Qiu, Y.; Wang, R.; Zhou, X. Cucurbit [7]uril as a Broad-Spectrum Antiviral Agent against Diverse RNA Viruses. Virol. Sin. 2021, 36, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
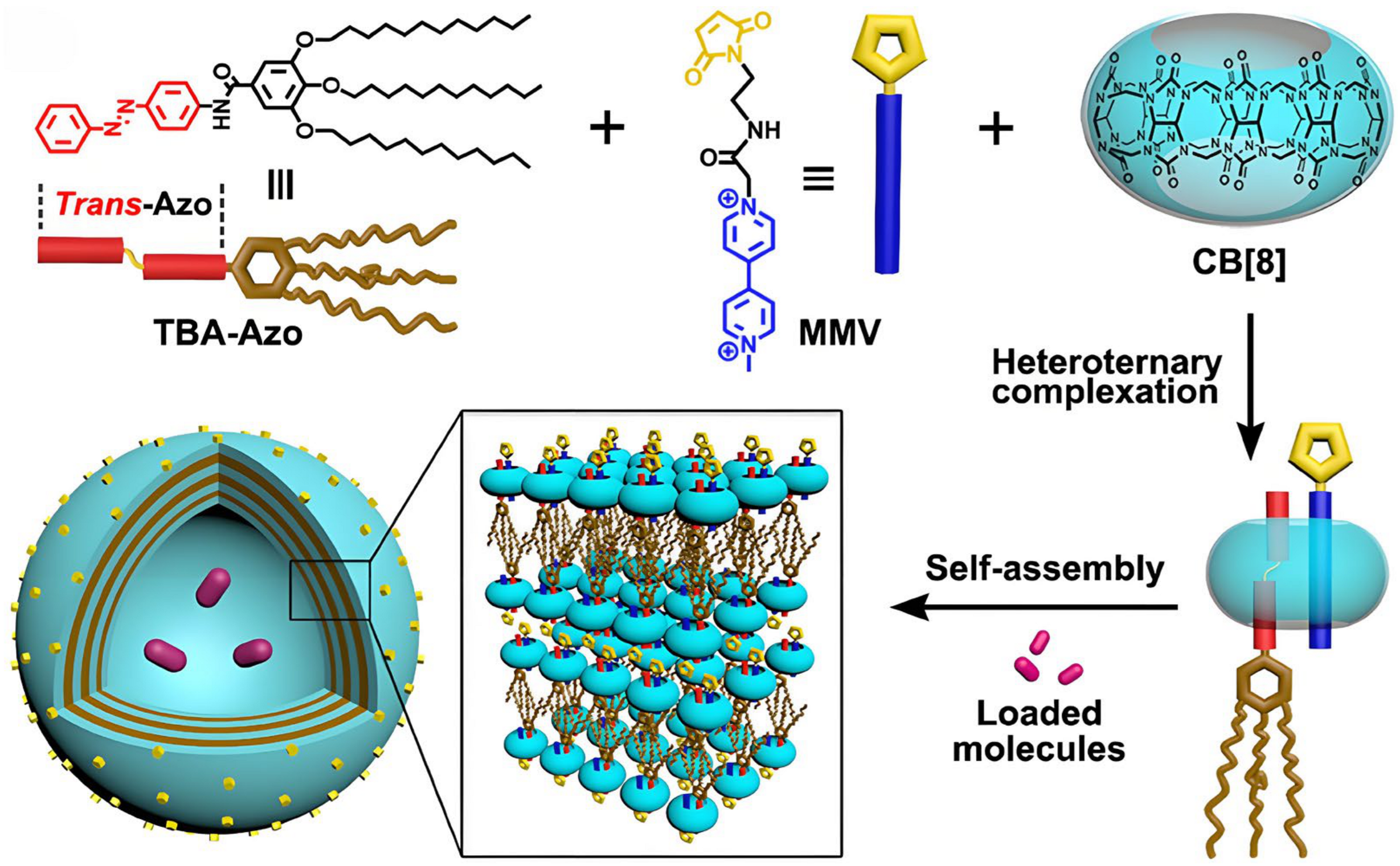
- Hu, C.; Ma, N.; Li, F.; Fang, Y.; Liu, Y.; Zhao, L.; Qiao, S.; Li, X.; Jiang, X.; Li, T.; et al. Cucurbit [8]uril-Based Giant Supramolecular Vesicles: Highly Stable, Versatile Carriers for Photoresponsive and Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 4603–4613. [Google Scholar] [CrossRef]
- Ding, Y.F.; Wei, J.; Li, S.; Pan, Y.T.; Wang, L.H.; Wang, R. Host-Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 28665–28670. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, S.; Ding, Y.F.; Yin, H.; Wang, L.H.; Wang, R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater. Sci. 2018, 6, 1031–1039. [Google Scholar] [CrossRef]
- Deng, C.L.; Murkli, S.L.; Isaacs, L.D. Supramolecular hosts as in vivo sequestration agents for pharmaceuticals and toxins. Chem. Soc. Rev. 2020, 49, 7516–7532. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Isaacs, L. Synthesis and Recognition Properties of Enantiomerically Pure Acyclic Cucurbit[n]uril-Type Molecular Containers. Org. Lett. 2015, 17, 4038–4041. [Google Scholar] [CrossRef]
- Ma, D.; Hettiarachchi, G.; Nguyen, D.; Zhang, B.; Wittenberg, J.B.; Zavalij, P.Y.; Briken, V.; Isaacs, L. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat. Chem. 2012, 4, 503–510. [Google Scholar] [CrossRef]
- Ganapati, S.; Grabitz, S.D.; Murkli, S.; Scheffenbichler, F.; Rudolph, M.I.; Zavalij, P.Y.; Eikermann, M.; Isaacs, L. Molecular Containers Bind Drugs of Abuse in Vitro and Reverse the Hyperlocomotive Effect of Methamphetamine in Rats. ChemBioChem 2017, 18, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, B.; Hoffmann, U.; Sundrup, M.G.; Eikermann, M.; Isaacs, L. Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 11358–11362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, J.; Liu, Y.; Wang, S.; Zhou, W.; Li, Z.T.; Zhang, D.W.; Ma, D. Acyclic cucurbit[n]uril-based nanosponges significantly enhance the photodynamic therapeutic efficacy of temoporfin in vitro and in vivo. J. Mater. Chem. B 2023, 11, 9027–9034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, Y.; Lv, P.; Liao, X.; Zhao, Y.; Yang, B. Functional supramolecular micelles driven by the amphiphilic complex of biotin-acyclic cucurbituril and cannabidiol for cell-targeted drug delivery. Int. J. Pharm. 2022, 625, 122048. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Tian, J.; Wang, H.; Zhang, D.W.; Li, Z.T. Loading-free supramolecular organic framework drug delivery systems (sof-DDSs) for doxorubicin: Normal plasm and multidrug resistant cancer cell-adaptive delivery and release. Chin. Chem. Lett. 2017, 28, 893–899. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Mao, W.; Zhao, Z.; Wang, Z.K.; Liu, Y.Y.; Wu, Y.; Wang, H.; Zhang, D.W.; Li, Z.T.; Ma, D. Self-assembled nanoparticles based on supramolecular-organic frameworks and temoporfin for an enhanced photodynamic therapy in vitro and in vivo. J. Mater. Chem. B 2022, 10, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.Z.; Wang, Z.K.; Zhou, W.; Wang, H.; Zhang, Y.C.; Zhang, D.W.; Ma, D.; Li, Z.T. Supramolecular organic frameworks improve the safety of clinically used porphyrin photodynamic agents and maintain their antitumor efficacy. Biomaterials 2022, 284, 121467. [Google Scholar] [CrossRef] [PubMed]
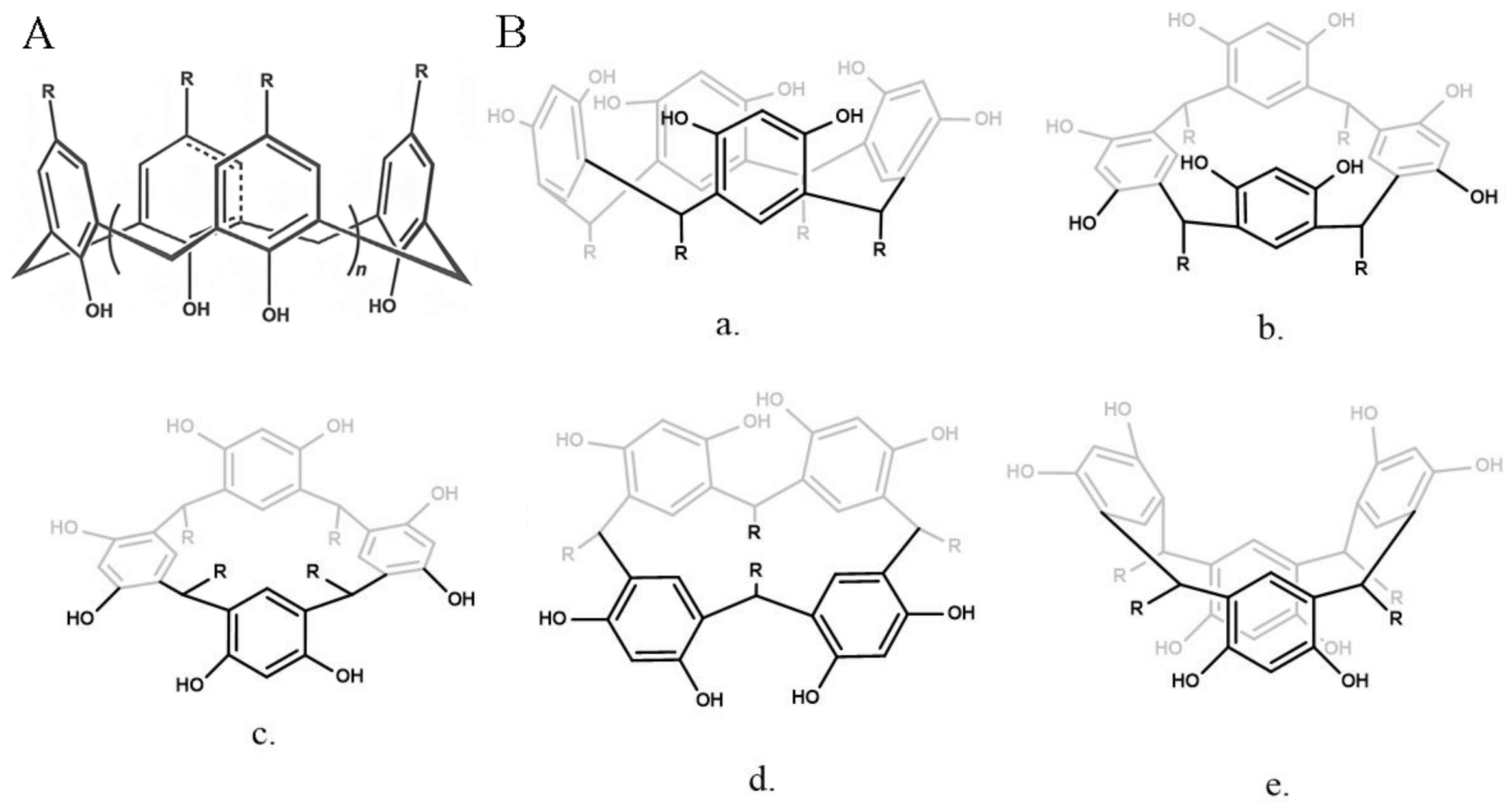
- Basilotta, R.; Mannino, D.; Filippone, A.; Casili, G.; Prestifilippo, A.; Colarossi, L.; Raciti, G.; Esposito, E.; Campolo, M. Role of Calixarene in Chemotherapy Delivery Strategies. Molecules 2021, 26, 3963. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Ashraf, M.U.; Muhammad, G.; Tahir, M.N.; Bukhari, S.N.A. Calixarene: A Versatile Material for Drug Design and Applications. Curr. Pharm. Des. 2017, 23, 2377–2388. [Google Scholar] [CrossRef]
- Bartocci, A.; Gillet, N.; Jiang, T.; Szczepaniak, F.; Dumont, E. Molecular Dynamics Approach for Capturing Calixarene-Protein Interactions: The Case of Cytochrome C. J. Phys. Chem. B 2020, 124, 11371–11378. [Google Scholar] [CrossRef]
- Curtis, A.D.M. Simple Calix[n]arenes and Calix [4]resorcinarenes as Drug Solubilizing Agents. J. Nanomed. Res. 2015, 2, 28. [Google Scholar] [CrossRef]
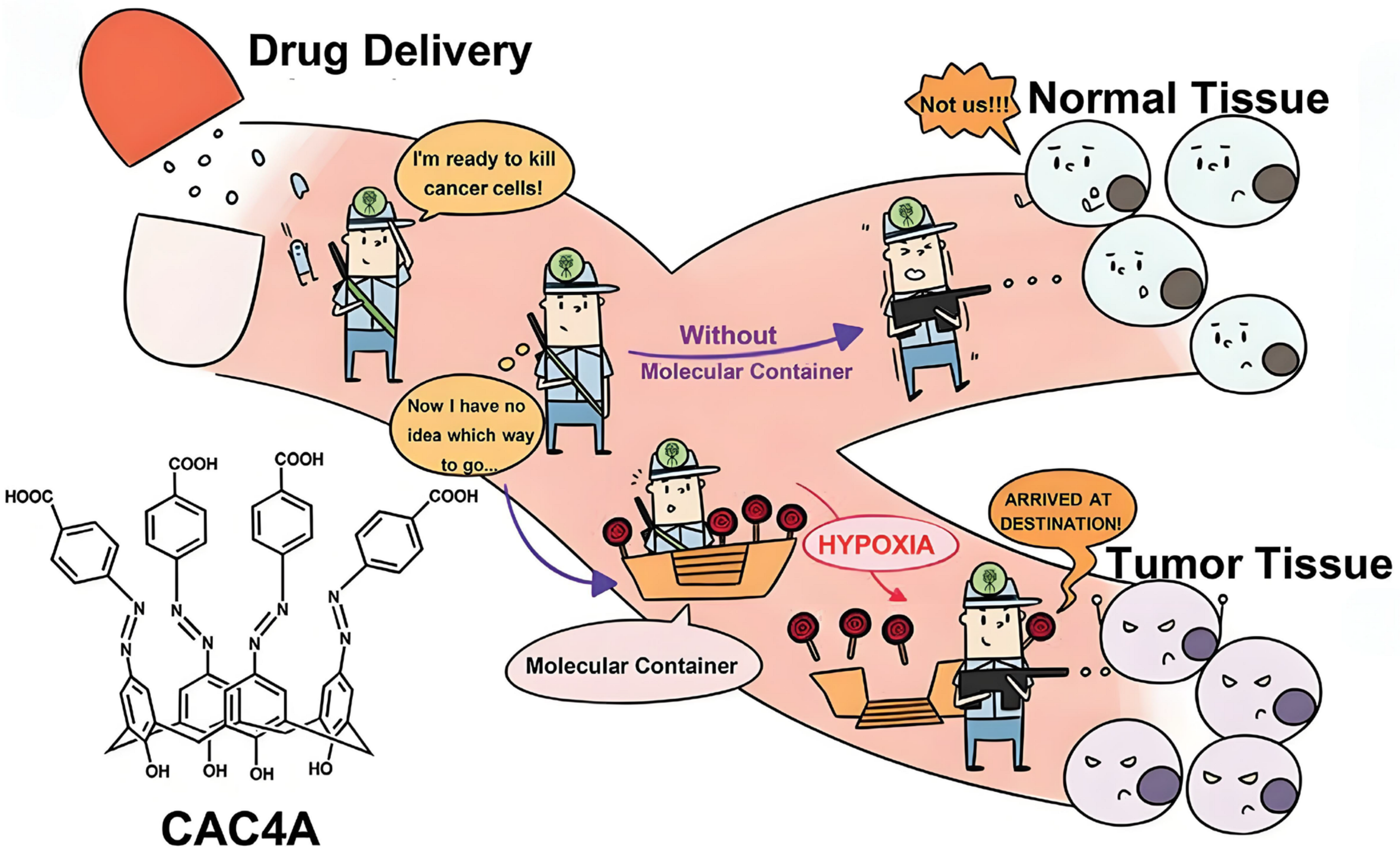
- Zhang, T.X.; Zhang, Z.Z.; Yue, Y.X.; Hu, X.Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.S. A General Hypoxia-Responsive Molecular Container for Tumor-Targeted Therapy. Adv. Mater. 2020, 32, e1908435. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Li, C.Z.; Han, H.; Geng, W.C.; Zhang, S.X.; Jiang, Z.T.; Zhao, Q.Y.; Cai, K.; Guo, D.S. Expanding the Hydrophobic Cavity Surface of Azocalix [4]arene to Enable Biotin/Avidin Affinity with Controlled Release. Angew. Chem. Int. Ed. Engl. 2024, 63, e202402139. [Google Scholar] [CrossRef] [PubMed]
- Lomazzi, M.; Franceschi, V.; Bagnacani, V.; Vezzoni, C.A.; Donofrio, G.; Casnati, A.; Sansone, F. A Structure-Activity Investigation on Modified Analogues of an Argininocalixarene Based Non-Viral Gene Vector. Eur. J. Org. Chem. 2021, 2021, 4076–4087. [Google Scholar] [CrossRef]
- Li, M.; Mao, L.; Chen, M.; Li, M.; Wang, K.; Mo, J. Characterization of an Amphiphilic Phosphonated Calixarene Carrier Loaded with Carboplatin and Paclitaxel: A Preliminary Study to Treat Colon Cancer in Vitro and in Vivo. Front. Bioeng. Biotechnol. 2019, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Drakalska, E.; Momekova, D.; Manolova, Y.; Budurova, D.; Momekov, G.; Genova, M.; Antonov, L.; Lambov, N.; Rangelov, S. Hybrid liposomal PEGylated calix [4]arene systems as drug delivery platforms for curcumin. Int. J. Pharm. 2014, 472, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Budurova, D.; Momekova, D.; Momekov, G.; Shestakova, P.; Penchev, H.; Rangelov, S. PEG-Modified tert-Octylcalix [8]arenes as Drug Delivery Nanocarriers of Silibinin. Pharmaceutics 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Chen, F.-Y.; Han, B.-B.; Zhang, H.-Q.; Zhao, L.; Zhang, Z.-R.; Li, J.-J.; Zhang, B.-D.; Zhang, Y.-N.; Yue, Y.-X.; et al. CASTING: A Potent Supramolecular Strategy to Cytosolically Deliver STING Agonist for Cancer Immunotherapy and SARS-CoV-2 Vaccination. CCS Chem. 2023, 5, 885–901. [Google Scholar] [CrossRef]
- Xu, L.; Chai, J.; Wang, Y.; Zhao, X.; Guo, D.-S.; Shi, L.; Zhang, Z.; Liu, Y. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 2022, 15, 7295–7303. [Google Scholar] [CrossRef]
- Bono, N.; Pennetta, C.; Sganappa, A.; Giupponi, E.; Sansone, F.; Volonterio, A.; Candiani, G. Design and synthesis of biologically active cationic amphiphiles built on the calix [4]arene scaffold. Int. J. Pharm. 2018, 549, 436–445. [Google Scholar] [CrossRef]
- Bandela, A.K.; Hinge, V.K.; Yarramala, D.S.; Rao, C.P. Versatile, Reversible, and Reusable Gel of a Monocholesteryl Conjugated Calix [4]arene as Functional Material to Store and Release Dyes and Drugs Including Doxorubicin, Curcumin, and Tocopherol. ACS Appl. Mater. Interfaces 2015, 7, 11555–11566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiong, H.; Yao, S.Y.; Wang, S.; Li, S.; Chang, J.; Zhai, Z.; Guo, D.S.; Fan, C.; Gao, C. Hypoxia and Matrix Metalloproteinase 13-Responsive Hydrogel Microspheres Alleviate Osteoarthritis Progression in Vivo. Small 2024, 20, e2308599. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Saifullah, S.; Imran, M.; Nisar, J.; Javed, I.; Shah, M.R. Synthesis and biocompatibility of self-assembling multi-tailed resorcinarene-based supramolecular amphiphile. Coll. Polym. Sci. 2020, 298, 331–339. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Syakaev, V.V.; Shalaeva, Y.V.; Sapunova, A.S.; Voloshina, A.D.; Gubaidullin, A.T.; Bazanova, O.B.; Babaev, V.M.; Nizameev, I.R.; et al. The pH-responsive calix [4]resorcinarene-mPEG conjugates bearing acylhydrazone bonds: Synthesis and study of the potential as supramolecular drug delivery systems. Coll. Surf. A Physicochem. Eng. Asp. 2020, 589, 124453. [Google Scholar] [CrossRef]
- Sergeeva, T.Y.; Mukhitova, R.K.; Bakhtiozina, L.R.; Nizameev, I.R.; Kadirov, M.K.; Sapunova, A.S.; Voloshina, A.D.; Ziganshina, A.Y.; Antipin, I.S. Doxorubicin delivery by polymer nanocarrier based on N-methylglucamine resorcinarene. Supramol. Chem. 2020, 32, 150–161. [Google Scholar] [CrossRef]
- Sergeeva, T.Y.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Sapunova, A.S.; Voloshina, A.D.; Mukhametzyanov, T.A.; Ziganshina, A.Y.; Antipin, I.S. A Glucose-Responsive Polymer Nanocarrier Based on Sulfonated Resorcinarene for Controlled Insulin Delivery. ChemPlusChem 2019, 84, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Santra, S.; Majee, A.; Ranu, B.C. Pillararenes as Promising Carriers for Drug Delivery. Int. J. Mol. Sci. 2023, 24, 5167. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.Y.; Yang, Y.W. Pillar[n]arene-Based Supramolecular Switches in Solution and on Surfaces. Adv. Mater. 2020, 32, e2003263. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xia, J.; Huang, Y.; Yao, Y. The design strategy for pillararene based active targeted drug delivery systems. Chem. Commun. 2023, 59, 12091–12099. [Google Scholar] [CrossRef]
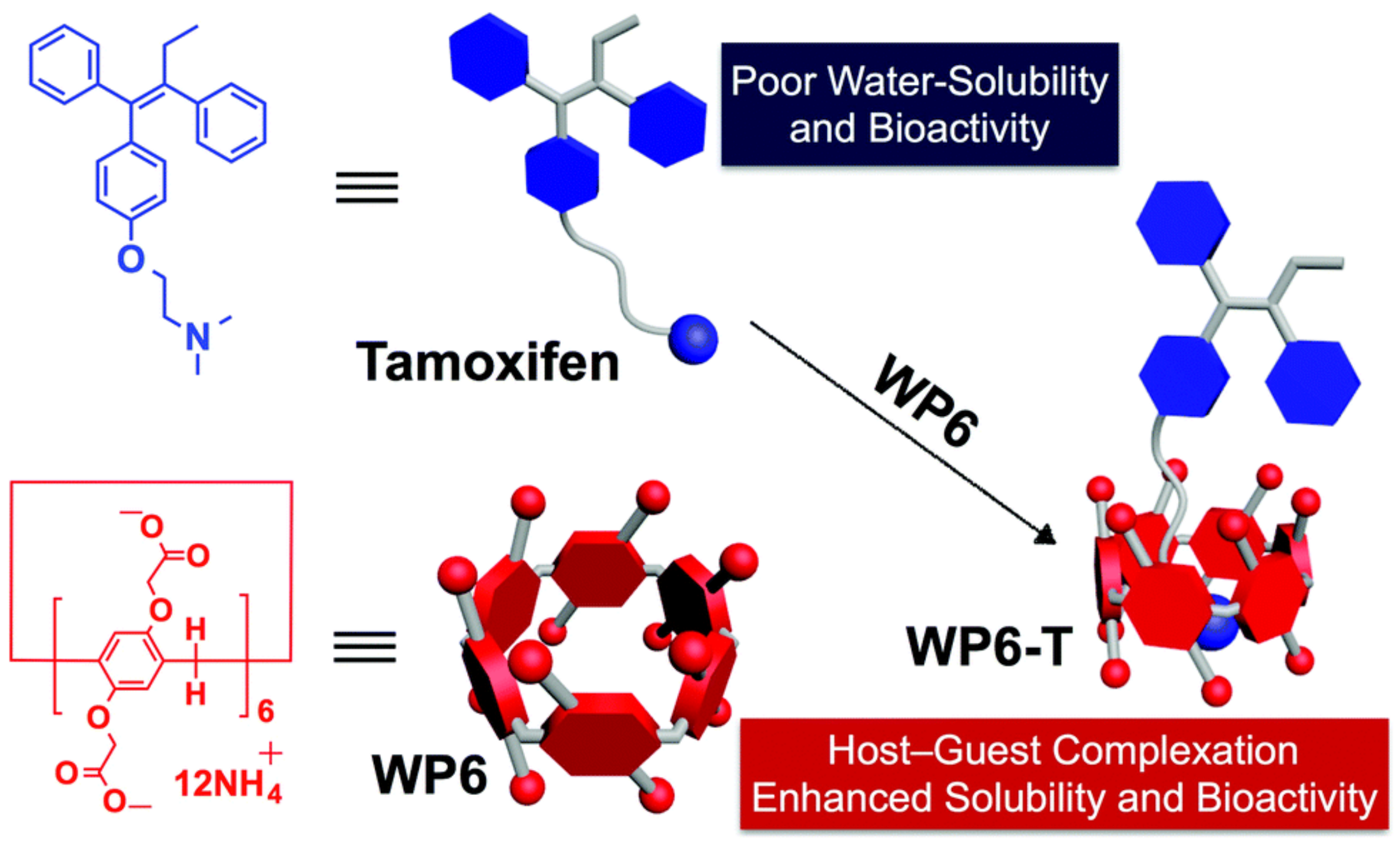
- Shangguan, L.; Chen, Q.; Shi, B.; Huang, F. Enhancing the solubility and bioactivity of anticancer drug tamoxifen by water-soluble pillar [6]arene-based host-guest complexation. Chem. Commun. 2017, 53, 9749–9752. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular Chemotherapy: Carboxylated Pillar [6]arene for Decreasing Cytotoxicity of Oxaliplatin to Normal Cells and Improving Its Anticancer Bioactivity against Colorectal Cancer. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, X.; Wei, J.; Lu, S.; Bardelang, D.; Wang, R. Recent advances in supramolecular antidotes. Theranostics 2021, 11, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, X.; Zhang, Z.; Han, C.; Mao, Z.; Gao, C.; Huang, F. Pillar [6]arene/paraquat molecular recognition in water: High binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 2012, 134, 19489–19497. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ni, H.; Meng, Z.; Wang, J.; Huang, X.; Dong, Y.; Sun, C.; Zhang, Y.; Cui, L.; Li, J.; et al. Supramolecular trap for catching polyamines in cells as an anti-tumor strategy. Nat. Commun. 2019, 10, 3546. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, J.; Zhao, Q.; Lu, B.; Yao, Y. Dual-Responsive Drug-Delivery System Based on PEG-Functionalized Pillararenes Containing Disulfide and Amido Bonds for Cancer Theranostics. ChemBioChem 2023, 24, e202300513. [Google Scholar] [CrossRef] [PubMed]
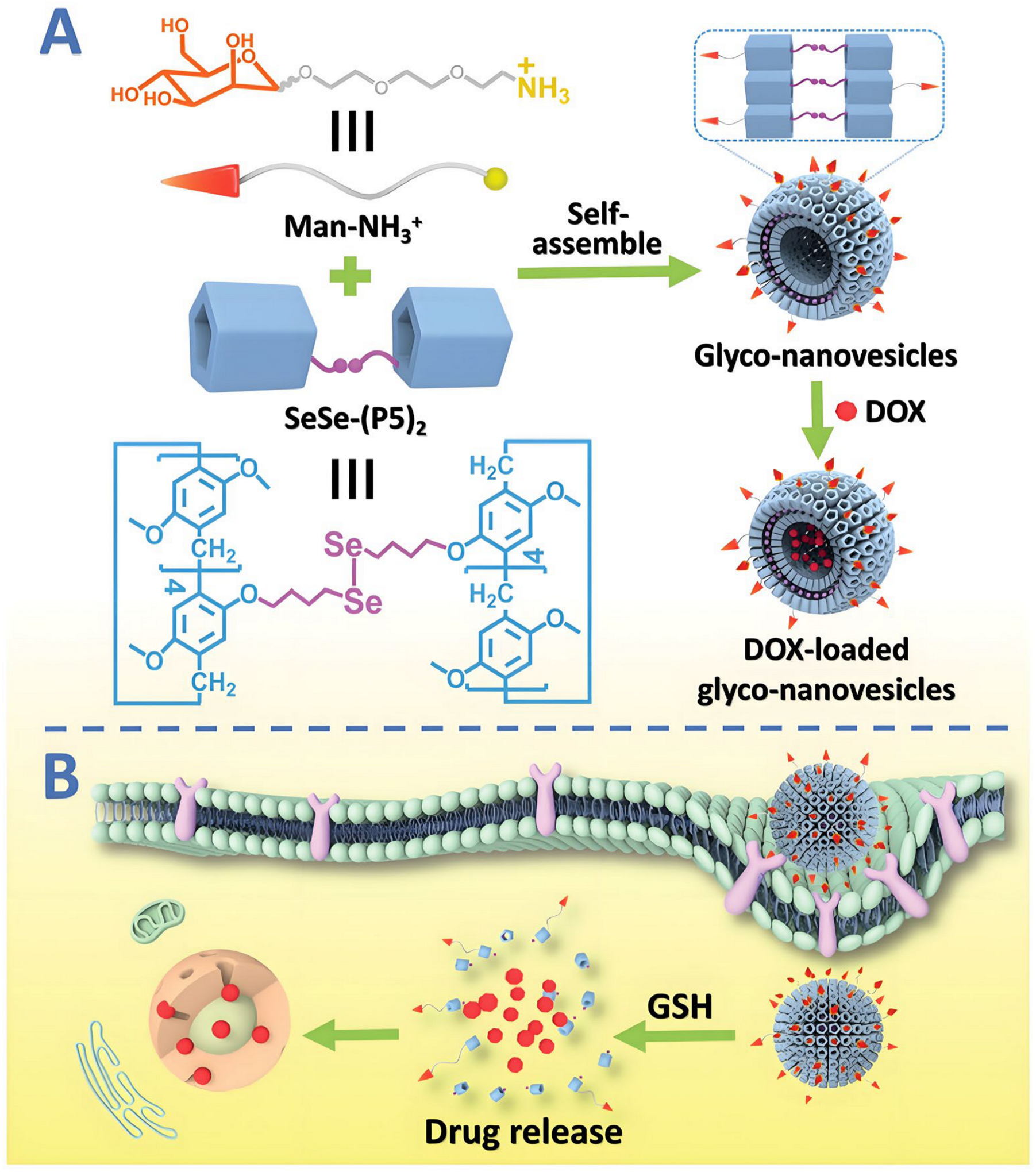
- Wang, Y.; Jin, M.; Chen, Z.; Hu, X.; Pu, L.; Pei, Z.; Pei, Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar [5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, S.; Ke, X.; Li, X.; Du, X. Phosphonated Pillar [5]arene-Valved Mesoporous Silica Drug Delivery Systems. ACS Appl. Mater. Interfaces 2017, 9, 19638–19645. [Google Scholar] [CrossRef]
- Sun, Y.L.; Yang, Y.W.; Chen, D.X.; Wang, G.; Zhou, Y.; Wang, C.Y.; Stoddart, J.F. Mechanized silica nanoparticles based on pillar [5]arenes for on-command cargo release. Small 2013, 9, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
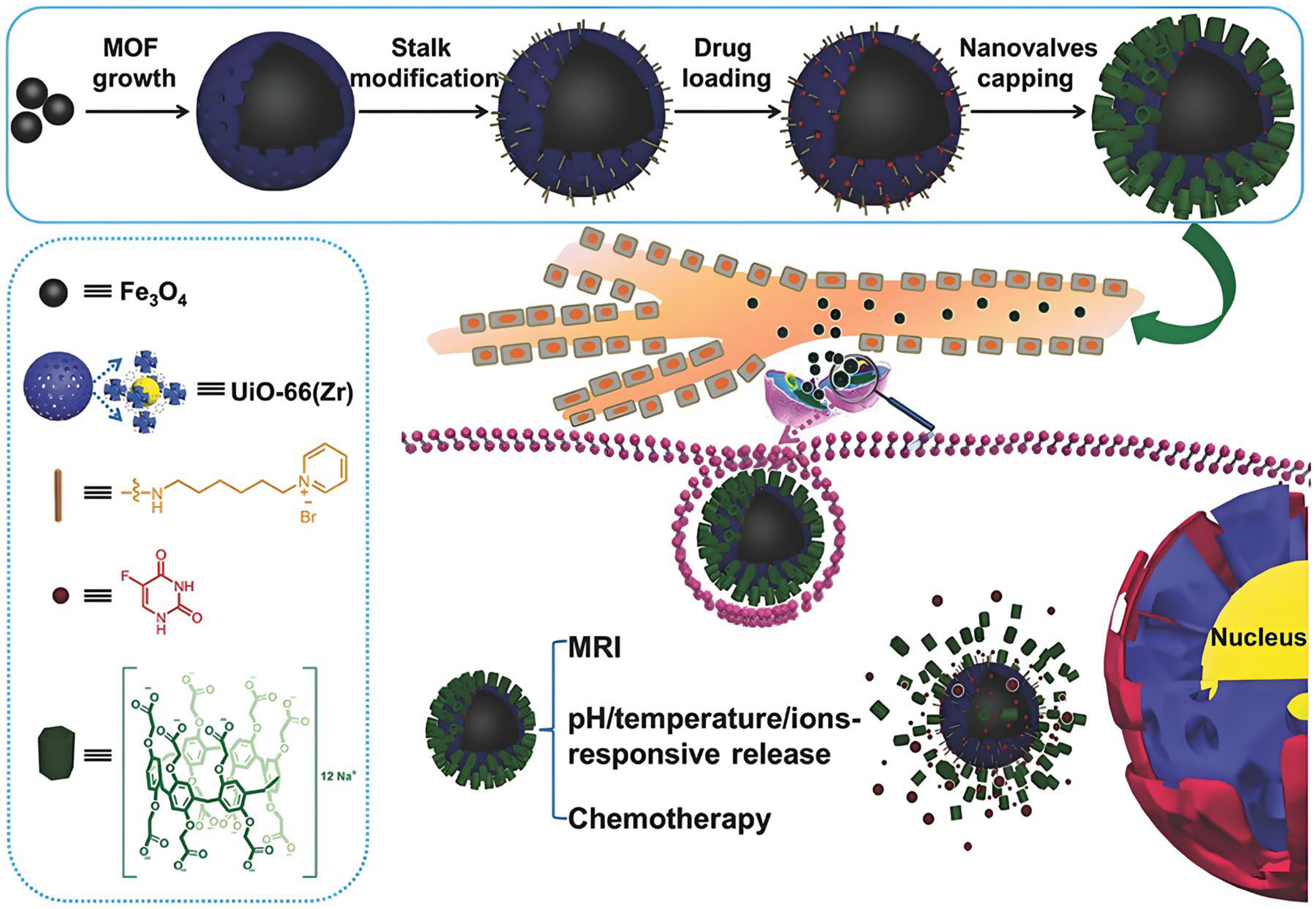
- Wu, M.-X.; Gao, J.; Wang, F.; Yang, J.; Song, N.; Jin, X.; Mi, P.; Tian, J.; Luo, J.; Liang, F.; et al. Multistimuli Responsive Core–Shell Nanoplatform Constructed from Fe3O4@MOF Equipped with Pillar [6]arene Nanovalves. Small 2018, 14, 1704440. [Google Scholar] [CrossRef]
- Barran-Berdon, A.L.; Martinez-Negro, M.; Garcia-Rio, L.; Domenech, O.; Tros de Ilarduya, C.; Aicart, E.; Junquera, E. A biophysical study of gene nanocarriers formed by anionic/zwitterionic mixed lipids and pillar [5]arene polycationic macrocycles. J. Mater. Chem. B 2017, 5, 3122–3131. [Google Scholar] [CrossRef]
- Geng, W.C.; Huang, Q.; Xu, Z.; Wang, R.; Guo, D.S. Gene delivery based on macrocyclic amphiphiles. Theranostics 2019, 9, 3094–3106. [Google Scholar] [CrossRef] [PubMed]
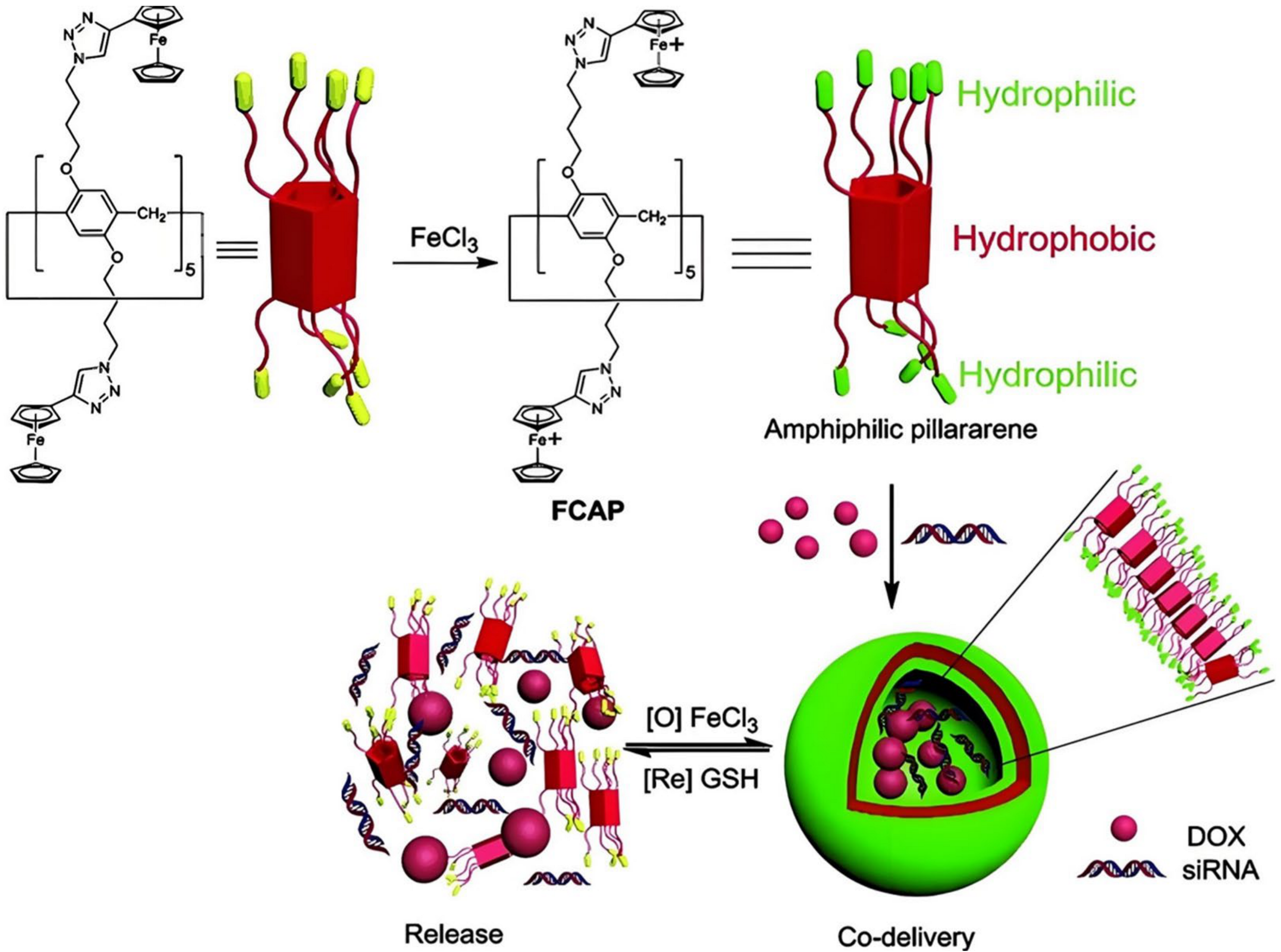
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic vesicles based on amphiphilic pillar [5]arene capped with ferrocenium: A redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. Engl. 2014, 53, 13126–13130. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Hu, Y.F.; Lin, T.; Zhuang, G.W.; Wang, Y.L.; Chen, W.X.; Li, Z.T.; Hou, J.L. Constructing artificial gap junctions to mediate intercellular signal and mass transport. Nat. Chem. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, P.; Feng, H.; Zeng, R.; Li, S.; Zhang, Q. Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review. Molecules 2024, 29, 3828. https://doi.org/10.3390/molecules29163828
Yang Y, Li P, Feng H, Zeng R, Li S, Zhang Q. Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review. Molecules. 2024; 29(16):3828. https://doi.org/10.3390/molecules29163828
Chicago/Turabian StyleYang, Yanrui, Pengcheng Li, Haibo Feng, Rui Zeng, Shanshan Li, and Qixiong Zhang. 2024. "Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review" Molecules 29, no. 16: 3828. https://doi.org/10.3390/molecules29163828
APA StyleYang, Y., Li, P., Feng, H., Zeng, R., Li, S., & Zhang, Q. (2024). Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review. Molecules, 29(16), 3828. https://doi.org/10.3390/molecules29163828






