Gouregine, an α-Gem-Dimethyltetradehydrocularine Alkaloid, and Other Aporphinoid Alkaloids from the Bark of Guatteria olivacea (Annonaceae) and Their In Vitro Cytotoxic Activities
Abstract
1. Introduction
2. Results and Discussion
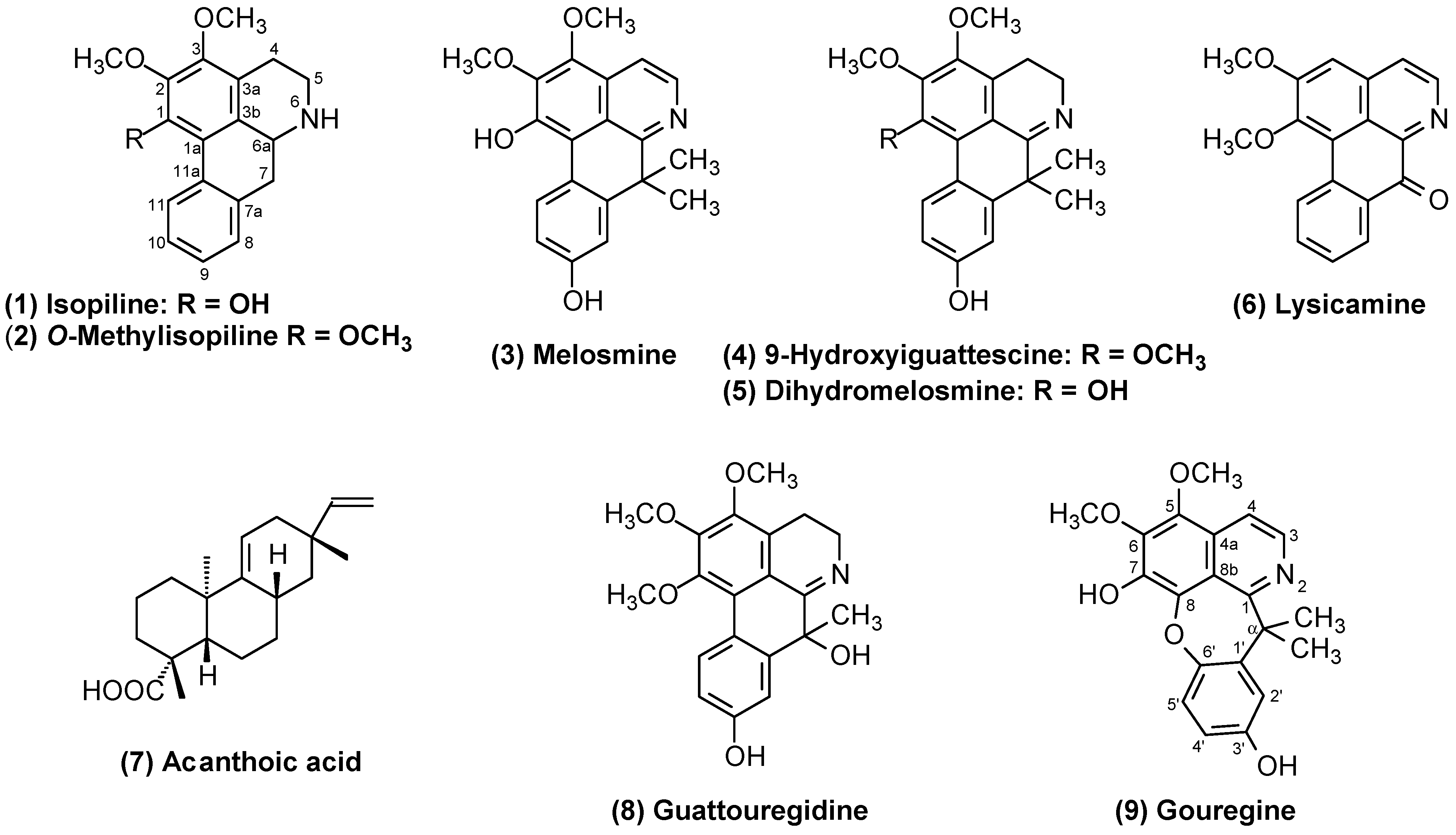
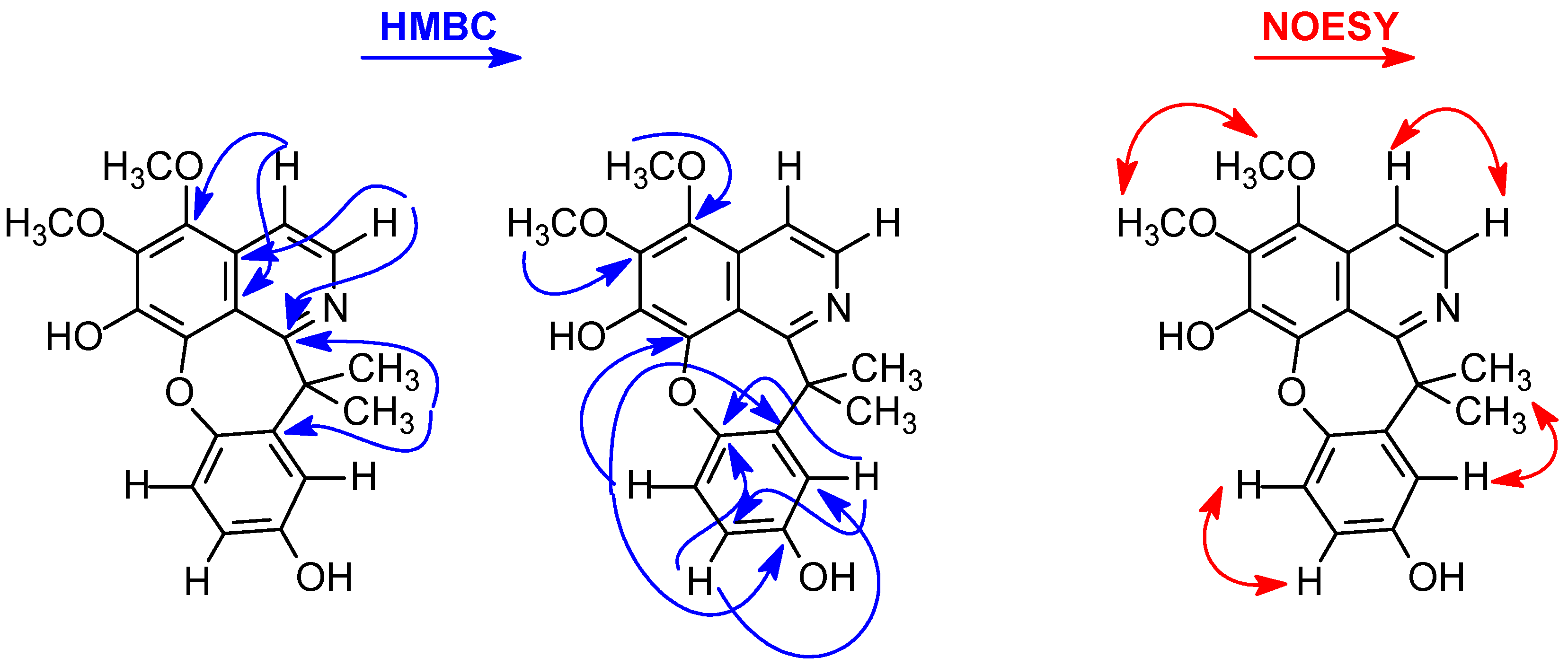
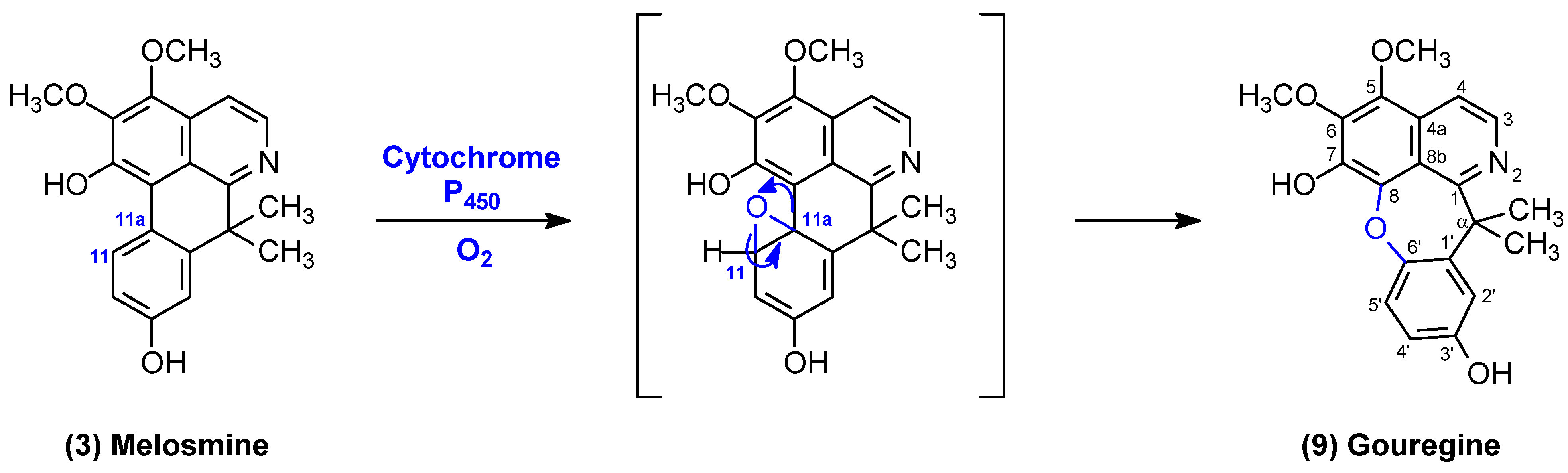
2.1. Structural Elucidation of Compounds
2.2. Cytotoxicity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Cells
3.3.2. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, P.J.M.; Westra, L.Y.T.; Guerrero, S.A.; Lobão, A.Q.; Scharf, U.; Zamora, N.A.; Erkens, R.H.J. Confronting a morphological nightmare: Revision of the Neotropical genus Guatteria (Annonaceae). Blumea 2015, 60, 1–219. [Google Scholar] [CrossRef]
- Erkens, R.H.J.; Westra, L.Y.T.; Maas, P.J.M. Increasing diversity in the species-rich genus Guatteria (Annonaceae). Blumea 2008, 53, 467–514. [Google Scholar] [CrossRef][Green Version]
- Lobão, A.Q.; Mello-Silva, R.; Maas, P.J.M.; Forzza, R.C. Taxonomic and nomenclatural notes on Guatteria australis (Annonaceae). Phytotaxa 2011, 20, 33–46. [Google Scholar] [CrossRef]
- Britto, A.C.; Oliveira, A.C.; Henriques, R.M.; Cardoso, G.M.; Bomfim, D.S.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Pinheiro, M.L.; Costa, E.V.; et al. In vitro and in vivo antitumor effects of the essential oil from the leaves of Guatteria friesiana. Planta Med. 2012, 78, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.E.N.; Ferraz, R.P.; Britto, A.C.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumor effect of the essential oil from leaves of Guatteria pogonopus (Annonaceae). Chem. Biodivers. 2013, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, C.A.; Serain, A.F.; Pascoal, A.C.; Andreazza, N.L.; Lourenço, C.C.; Ruiz, A.L.; Carvalho, J.E.; Souza, A.C.; Mesquita, J.T.; Tempone, A.G.; et al. Bioactivity and chemical composition of the essential oil from the leaves of Guatteria australis A. St.-Hil. Nat. Prod. Res. 2015, 29, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Benghi, T.G.S.; Nepel, A.; Marques, F.A.; Lobão, A.Q.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Carvalho, J.E.; Maia, B.H.L.N.S. In vitro antiproliferative and antibacterial activities of essential oils from four species of Guatteria. Chem. Biodivers. 2017, 14, e1700097. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.S.; Jesus, A.M.; Anjos, C.S.; Silva, T.B.; Santos, A.D.; Jesus, J.R.; Andrade, M.S.; Sampaio, T.S.; Gomes, W.F.; Alves, P.B.; et al. Evaluation of the cytotoxic activity of some Brazilian medicinal plants. Planta Med. 2012, 78, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Cytotoxic properties of the leaf essential oils of Guatteria blepharophylla and Guatteria hispida (Annonaceae). Flavour Frag. J. 2014, 29, 228–232. [Google Scholar] [CrossRef]
- Ferreira, A.K.R.; Lourenço, F.R.; Cláudia, M.; Young, M.; Lima, M.E.L.; Cordeiro, I.; Suffredini, I.B.; Lopes, P.S.; Moreno, P.R.H. Chemical composition and biological activities of Guatteria elliptica R. E. Fries (Annonaceae) essential oils. J. Essent. Oil Res. 2017, 30, 69–76. [Google Scholar] [CrossRef]
- Costa, R.G.A.; Anunciação, T.A.D.; Araujo, M.S.; Souza, C.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.D.; Koolen, H.H.F.; et al. In vitro and in vivo growth inhibition of human acute promyelocytic leukemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed. Pharmacother. 2020, 122, 109713. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.F.C.; Araújo, M.d.S.; Silva, V.R.; Santos, L.d.S.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.d.; Koolen, H.H.F.; Zengin, G.; et al. Antitumor Effect of Guatteria olivacea R. E. Fr. (Annonaceae) Leaf essential oil in liver cancer. Molecules 2022, 27, 4407. [Google Scholar] [CrossRef]
- Ribeiro, J.E.L.S.; Maas, P.J.M.; Maas, H.; Miralha, J.M.; Webber, A. Annonaceae. In Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de Uma Floresta de Terra-Firme na Amazônia Central; Ribeiro, J.E.L.S., Hopkins, M.J.G., Vicentini, A., Sothers, C.A., Costa, M.A.S., Brito, J.M., Souza, M.A.D., Martins, L.H.P., Lohmann, L.G., Assunção, P.A.C.L., et al., Eds.; DFID (Departamento for International Development), INPA: Manaus, Brazil, 1999; pp. 121–135. [Google Scholar]
- Maas, P.J.M.; Maas, H.; Miralha, J.M.S.; Junikka, L. Flora da Reserva Ducke, Amazonas, Brasil: Annonaceae. Rodriguesia 2007, 58, 617–662. [Google Scholar] [CrossRef]
- Araújo, M.S.; Silva, F.M.A.; Koolen, H.H.F.; Costa, E.V. Isoquinoline-derived alkaloids from the bark of Guatteria olivacea (Annonaceae). Biochem. Syst. Ecol. 2020, 92, 104105. [Google Scholar]
- Bay, M.; Oliveira, J.V.S.; Sales Junior, P.A.; Murta, S.M.F.; Santos, A.R.; Bastos, I.S.; Orlandi, P.P.; Sousa Junior, P.T. In vitro trypanocidal and antibacterial activities of essential oils from four species of the Family Annonaceae. Chem. Biodivers. 2019, 16, e1900359. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Pinheiro, M.L.B.; Xavier, C.M.; Silva, J.R.A.; Amaral, A.C.F.; Souza, A.D.L.; Barison, A.; Campos, F.R.; Ferreira, A.G.; Machado, G.M.C.; et al. A pyrimidine-β-carboline and other alkaloids from Annona foetida with antileishmanial activity. J. Nat. Prod. 2006, 69, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Pinheiro, M.L.B.; Maia, B.H.L.H.S.; Marques, F.A.; Ruiz, A.L.T.G.; Marchetti, G.; De Carvalho, J.E.; Soares, M.B.P.; Costa, C.O.S.; Galvão, A.F.C.; et al. 7,7-Dimethylaporphine and other alkaloids from the bark of Guatteria friesiana. J. Nat. Prod. 2016, 79, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphinoid alkaloid, III. J. Nat. Prod. 1983, 46, 761–835. [Google Scholar] [CrossRef]
- Leboeuf, M.; Cortes, D.; Hocquemiller, R.; Cavé, A.; Chiaroni, A.; Riche, C. Structure de la gouregine, alcaloïde original apparent aux cularines. Tetrahedron 1982, 38, 2889–2896. [Google Scholar] [CrossRef]
- Leboeuf, M.; Hocquemiller, R.; Cavé, A. Alcaloïds des Annonacées XLVII*: Alcaloïdesde Guatteria ouregou**[Alkaloids from Annonaceae XLVII*: Alkaloids of Guatteria ouregou. Planta Med. 1983, 48, 234–245. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 978-0-470-74168-9. [Google Scholar]
- Da Silva, F.M.A.; Bataglion, G.; De Almeida, R.A.; Heerdt, G.; Souza, I.L.; Da Silva Filho, F.A.; Alencar, D.C.; Costa, E.V.; Souza, A.D.L.; Pinheiro, M.L.B.; et al. Positive electrospray ionization ion trap mass spectrometry and ab initio computational studies of the multi-pathway fragmentation of oxoaporphine alkaloids. Int. J. Mass Spectrom. 2017, 418, 30–36. [Google Scholar] [CrossRef]
- Navarro, V.R.; Sette, I.M.F.; Da Cunha, E.V.L.; Silva, M.S.; Barbosa-Filho, J.M.; Maia, J.G.S. Alcalóides de Duguetia flagellaris Huber (Annonaceae). Rev. Bras. Plantas Med. 2001, 3, 23–29. [Google Scholar]
- de Oliveira Teles, M.N.; Dutra, L.M.; Barison, A.; Costa, E.V. Alkaloids from leaves of Annona salzmannii and Annona vepretorum (Annonaceae). Biochem. Syst. Ecol. 2015, 61, 465–469. [Google Scholar] [CrossRef]
- Kim, Y.H.; Chung, B.S.; Sankawa, U. Pimaradiene diterpenes from Acanthopanax koreanum. J. Nat. Prod. 1988, 51, 1080–1083. [Google Scholar] [CrossRef]
- Zabel, V.; Watson, W.H.; Phoebe, C.H., Jr.; Knapp, J.E.; Schiff, P.L., Jr.; Slatkin, D.J. Melosmine and melosmidine 7,7-dimethyltetradehydroaporphine alkaloids from Guatteria melosma. J. Nat. Prod. 1982, 45, 94–101. [Google Scholar] [CrossRef]
- Hocquemiller, R.; Rasamizafy, S.; Cavé, A.; Moretti, C. Alcaloïdes des Annonacees XXXVII: Alcaloïdes du Guatteria scandens. J. Nat. Prod. 1983, 46, 335–341. [Google Scholar] [CrossRef]
- Hocquemiller, R.; Debitus, C.; Roblot, F.; Cavé, A.; Jacquemin, H. Alcaloïdes des Annonacées, XLVIII. Alcaloïdes des Écorces de Guatteria discolor. J. Nat. Prod. 1984, 47, 353–362. [Google Scholar] [CrossRef]
- Hocquemiller, R.; Debitus, C.; Roblot, F.; Cavé, A. Guadiscine et guadiscoline, alcalöides aporphiniques originaux de Guatteria discolor, Annonaceae (1). Tetrahedron Lett. 1982, 41, 4247–4250. [Google Scholar] [CrossRef]
- Cortes, D.; Ramahatra, A.; Cavé, A.; Bayma, J.C.; Dadoun, H. Alcaloïdes des Annonacées, LVIII. Alcaloïdes des Ecorces de Guatteria schomburgkiana. J. Nat. Prod. 1985, 48, 254–259. [Google Scholar] [CrossRef]
- Mahiou, V.; Roblot, F.; Hocquemiller, R.; Cavé, A.; De Arias, A.R.; Inchausti, A.; Yaluff, G.; Fournet, A.; Angelo, A. New aporphine alkaloids from Guatteria foliosa. J. Nat. Prod. 1994, 57, 890–895. [Google Scholar] [CrossRef]
- Zhang, Z.; ElSohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. New sesquiterpenoids from the root of Guatteria multivenia. J. Nat. Prod. 2002, 65, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Da Cruz, P.E.O.; Pinheiro, M.L.B.; Marques, F.A.; Ruiz, A.L.T.G.; Marchetti, G.M.; De Carvalho, J.E.; Barison, A.; Maia, B.H.L.N.S. Aporphine and tetrahydroprotoberberine alkaloids from the leaves of Guatteria friesiana (Annonaceae) and their cytotoxic activities. J. Braz. Chem. Soc. 2013, 24, 788–796. [Google Scholar]
- Leboeuf, M.; Cortes, D.; Hocquemiller, R.; Cave, A. Guattouregidine, guattouregine et dihydromelosmine, alcaloïdes aporphiniques nouveaux de Guatteria ouregou Dun, Annonacées. C. R. Acad. Sci. 1982, 295, 191–194. [Google Scholar]
- De Souza, C.A.S.; Nardelli, V.B.; Paz, W.H.P.; Pinheiro, M.L.B.; Rodrigues, A.C.B.C.; Bomfim, L.M.; Soares, M.B.P.; Bezerra, D.P.; Chaar, J.S.; Koolen, H.H.F.; et al. Asarone-derived phenylpropanoids and isoquinoline-derived alkaloids from the bark of Duguetia pycnastera (Annonaceae) and their cytotoxicities. Quim. Nova 2020, 43, 1397–1403. [Google Scholar] [CrossRef]
- Omar, H.; Hashim, N.M.; Zajmi, A.; Nordin, N.; Abdelwahab, S.I.; Azizan, A.H.; Hadi, A.H.; Ali, H.M. Aporphine alkaloids from the leaves of Phoebe grandis (Nees) Mer. (Lauraceae) and their cytotoxic and antibacterial activities. Molecules 2013, 18, 8994–9009. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.T.; Michelli, A.P.P.; Caso, G.F.; De Oliveira, P.R.; Rodrigues-Junior, D.M.; Morale, M.G.; Machado Júnior, J.; Bortoluci, K.R.; Tamura, R.E.; Da Silva, T.R.C.; et al. Lysicamine Reduces Protein Kinase B (AKT) Activation and Promotes Necrosis in Anaplastic Thyroid Cancer. Pharmaceuticals 2023, 16, 1687. [Google Scholar] [CrossRef] [PubMed]
- Okayama, M.; Matsumoto, T.; Kitagawa, T.; Nakamura, S.; Ohta, T.; Yoshida, T.; Watanabe, T. Cytotoxic activities of alkaloid constituents from the climbing stems and rhizomes of Sinomenium acutum against cancer stem cells. J. Nat. Med. 2024, 78, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Majid, N.A.; Hashim, N.M.; Rahman, M.A.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Devel. Ther. 2015, 9, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.E.J.; Montoya, P.G.L.; Muñoz, D.K.; Arango, A.G.J. Actividad antiplasmodial de alcaloides aporfínicos de Rollinia pittieri Y Pseudomalmea boyacana (Annonaceae). Vitae 2006, 13, 49–54. [Google Scholar]
- Través, P.G.; Pimentel-Santillana, M.; Rico, D.; Rodriguez, N.; Miethke, T.; Castrillo, A.; Theodorakis, E.A.; Martín-Sanz, P.; Palladino, M.A.; Boscá, L. Anti-inflammatory actions of acanthoic acid-related diterpenes involve activation of the PI3K p110γ/δ subunits and inhibition of NF-κB. Chem. Biol. 2014, 21, 955–966. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Saehlim, N.; Arsakhant, P.; Sittithumcharee, G.; Okada, S.; Saeeng, R. A novel synthetic acanthoic acid analogues and their cytotoxic activity in cholangiocarcinoma cells. Bioorg. Med. Chem. 2021, 29, 115886. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.H.; Lam, T.; Vong, B.G.; Través, P.G.; Hortelano, S.; Chowdhury, C.; Bahjat, F.R.; Lloyd, G.K.; Moldawer, L.L.; Boscá, L.; et al. A new family of synthetic diterpenes that regulates cytokine synthesis by inhibiting IkappaBalpha phosphorylation. Chembiochem. 2005, 6, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Ling, T.; Chowdhury, C.; Chao, T.H.; Bahjat, F.R.; Lloyd, G.K.; Moldawer, L.L.; Palladino, M.A.; Theodorakis, E.A. Synthesis of a novel family of diterpenes and their evaluation as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2003, 13, 3217–3221. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.Y.; Jiang, Y.C.; Cui, Z.Y.; Lian, L.H.; Nan, J.X.; Wu, Y.L. Acanthoic acid, unique potential pimaradiene diterpene isolated from Acanthopanax koreanum Nakai (Araliaceae): A review on its pharmacology, molecular mechanism, and structural modification. Phytochemistry 2022, 200, 113247. [Google Scholar] [CrossRef]
- Kim, K.N.; Ham, Y.M.; Moon, J.Y.; Kim, M.J.; Jung, Y.H.; Jeon, Y.J.; Lee, N.H.; Kang, N.; Yang, H.M.; Kim, D.; et al. Acanthoic acid induces cell apoptosis through activation of the p38 MAPK pathway in HL-60 human promyelocytic leukaemia. Food Chem. 2012, 135, 2112–2117. [Google Scholar] [CrossRef]
- ATCC Animal Cell Culture Guide. Get Time-Tested Tips for Culturing ATCC Animal Cells. 2024. Available online: https://www.atcc.org/resources/culture-guides/animal-cell-culture-guide (accessed on 3 June 2024).
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]-thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]

| Position | 3 | 5 | 8 | Position | 9 | ||||
|---|---|---|---|---|---|---|---|---|---|
| δC mult. | δH mult. (J in Hz) | δC mult. | δH mult. (J in Hz) | δC mult. | δH mult. (J in Hz) | δC mult. | δH mult. (J in Hz) | ||
| 1 | 144.4, qC | 144.5, qC | 144.0, qC | 1 | 160.2, qC | ||||
| 1a | 111.3, qC | 116.7, qC | 116.0, qC | 3 | 137.9, CH | 8.17 d (5.9) | |||
| 2 | 142.2, qC | 142.6, qC | 142.9, qC | 4 | 114.2, CH | 7.74 d (5.9) | |||
| 3 | 142.8, qC | 146.7, qC | 147.0, qC | 4a | 127.2, qC | ||||
| 3a | 127.2, qC | 122.8, qC | 122.4, qC | 5 | 142.2, qC | ||||
| 3b | 119.4, qC | 119.1, qC | 117.4, qC | 6 | 142.7, qC | ||||
| 4ax 4eq | 112.3, CH | 7.68 d (5.6) | 19.4, CH2 | 2.61 t (7.2) | 19.5, CH2 | 2.42 td (15.3; 5.5) 2.94 dddd (16.5; 15.3; 4.7; 0.8) | 7 | 135.8, qC | |
| 5ax 5eq | 140.8, CH | 8.46 d (5.6) | 46.0, CH2 | 3.62 t (7.2) | 45.6, CH2 | 3.20 dddd (16.5; 15.3; 5.5; 0.8) 4.09 dd (15.3; 5.5) | 8 | 140.0, qC | |
| 6a | 163.0, qC | 173.0 qC | 170.7, qC | 8a | 116.9, qC | ||||
| 7 | 42.1 qC | 42.9, qC | 72.7, qC | 1′ | 140.2, qC | ||||
| 7a | 147.0, qC | 145.1, qC | 144.1, qC | 2′ | 113.8, CH | 6.95 d (2.9) | |||
| 8 | 113.4, CH | 7.17 d (2.7) | 111.8, CH | 7.01 d (2.6) | 111.5, CH | 7.30 d (2.7) | 3′ | 153.9, qC | |
| 9 | 155.5, qC | 156.0, qC | 155.5, qC | 4′ | 114.8, CH | 6.71 dd (8.5; 2.9) | |||
| 10 | 113.7, CH | 6.86 dd (8.8; 2.7) | 113.4, CH | 6.78 dd (8.7; 2.6) | 127.5, CH | 6.80 dd (8.7; 2.7) | 5′ | 122.3, CH | 7.11 d (8.5) |
| 11 | 129.0, CH | 8.87 d (8.8) | 129.8, CH | 8.45 d (8.7) | 129.6, CH | 8.44 d (8.7) | 6′ | 149.9, qC | |
| 11a | 121.7, qC | 121.9, qC | 120.9, qC | α | 45,6, qC | ||||
| 1-OH | α-(CH3)2 | 27.5, CH3 | 1.87 s | ||||||
| 2-OCH3 | 61.2, CH3 | 4.17 s | 61.0 | 4.04 s | 61.1 | 4.04 s | 5-OCH3 | 61.6, CH3 | 3.94 s |
| 3-OCH3 | 61.1, CH3 | 3.99 s | 60.6 | 3.86 s | 60.7 | 3.87 s | 6-OCH3 | 61.2, CH3 | 4.13 s |
| 9-OH | 7-OH | ||||||||
| 7-(CH3)2 | 32.6, CH3 | 1.73 s | 27.3 | 1.47 s | 33.3 | 1.47 s | 3′-OH | ||
| 7-OH | |||||||||
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC mult. | δH mult. (J in Hz) | δC mult. | δH mult. (J in Hz) | |
| 1 | 145.5, qC | 150.3, qC | ||
| 1a | 115.4, qC | 122.3, qC | ||
| 2 | 138.9, qC | 145.6, qC | ||
| 3 | 148.9, qC | 150.6, qC | ||
| 3a | 130.1, qC | 131.0, qC | ||
| 3b | 117.7, qC | 122.4, qC | ||
| 4ax 4eq | 22.4, CH2 | 2.84 m | 23.2, CH2 | 2.84 m |
| 5ax 5eq | 42.2, CH2 | 2.99 m 3.54 m | 42.5, CH2 | 2.97 m 3.48 ddd (12.2; 5.0; 1.9) |
| 6a | 53.5, CH | 3.97 m | 53.7, CH | 3.87 d (13.8; 4.7) |
| 7ax 7eq | 36.0, CH2 | 2.88 t (13.8) 2.99 dd (13.8; 4.5) | 36.5, CH2 | 2.82 t (13.8) 2.92 dd (13.8; 4.7) |
| 7a | 134.3, qC | 135.0, qC | ||
| 8 | 127.8, CH | 7.21 br d (7.2) | 127.83, CH | 7.23 dd (7.3; 1.7) |
| 9 | 126.8, CH | 7.17 td (7.2; 1.2) | 127.09, CH | 7.19 td (7.3; 1.3) |
| 10 | 126.9, CH | 7.31 ddd (7.9; 7.2; 1.1) | 127.06, CH | 7.29 ddd (7.9; 7.3; 1.7) |
| 11 | 127.8, CH | 8.31 br dd (7.9; 0.8) | 127.81, CH | 8.27 br dd (7.9; 0.8) |
| 11a | 131.9, qC | 131.9, qC | ||
| 1-OCH3 (OH) | 60.6, CH3 | 3.73 s | ||
| 2-OCH3 | 60.8, CH3 | 3.95 s | 60.9, CH3 | 3.95 s |
| 3-OCH3 | 59.9, CH3 | 3.87 s | 60.4, CH3 | 3.91 s |
| Compounds | IC50, in µg mL−1 (µmol/L), and 95% CI a | |||
|---|---|---|---|---|
| HepG2 | KG-1a | HCT116 | MRC-5 | |
| Isopiline (1) | N.T. | N.T. | >25 (>84.07) | >25 (>84.07) |
| O-Methylisopiline (2) | N.T. | N.T. | >25 (>80.28) | >25 (>80.28) |
| Melosmine (3) | N.T. | N.T. | 16.77 (49.70) 10.42–27.02 | 23.72 (70.30) 18.79–33.97 |
| 9-hydroxyiguattescine (4) | >25 (>70.73) | N.T. | >25 (>70.73) | >25 (>70.73) |
| Dihydromelosmine (5) | >25 (>73.66) | N.T. | >25 (>73.66) | >25 (>73.66) |
| Lysicamine (6) | N.T. | N.T. | 6.64 (22.79) 5.35–8.24 | 17.24 (59.18) 11.04–29.22 |
| Acanthoic acid (7) | 14.63 (48.37) 10.67–20.07 | N.T. | 21.25 (70.25) 13.25–34.10 | >25 (>82.65) |
| Guattouregidine (8) | >25 (>73.23) | N.T. | >25 (>73.23) | >25 (>73.23) |
| Gouregine (9) | >25 (>70.74) | >25 (>70.74) | N.T. | >25 (>70.74) |
| Doxorubicin b | 0.04 (0.07) 0.03–0.05 | 0.22 (0.40) 0.15–0.33 | 0.20 (0.36) 0.11–0,35 | 1.42 (2.61) 0.22–2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.V.; Freitas, J.G.C.; Manickchand, S.P.; Araújo, M.d.S.; Silva, V.R.; Santos, L.d.S.; Koolen, H.H.F.; Silva, F.M.A.d.; Soares, M.B.P.; Bezerra, D.P. Gouregine, an α-Gem-Dimethyltetradehydrocularine Alkaloid, and Other Aporphinoid Alkaloids from the Bark of Guatteria olivacea (Annonaceae) and Their In Vitro Cytotoxic Activities. Molecules 2024, 29, 3834. https://doi.org/10.3390/molecules29163834
Costa EV, Freitas JGC, Manickchand SP, Araújo MdS, Silva VR, Santos LdS, Koolen HHF, Silva FMAd, Soares MBP, Bezerra DP. Gouregine, an α-Gem-Dimethyltetradehydrocularine Alkaloid, and Other Aporphinoid Alkaloids from the Bark of Guatteria olivacea (Annonaceae) and Their In Vitro Cytotoxic Activities. Molecules. 2024; 29(16):3834. https://doi.org/10.3390/molecules29163834
Chicago/Turabian StyleCosta, Emmanoel V., José Guilherme C. Freitas, Steve Pereira Manickchand, Morgana de S. Araújo, Valdenizia R. Silva, Luciano de S. Santos, Hector Henrique Ferreira Koolen, Felipe M. A. da Silva, Milena Botelho Pereira Soares, and Daniel P. Bezerra. 2024. "Gouregine, an α-Gem-Dimethyltetradehydrocularine Alkaloid, and Other Aporphinoid Alkaloids from the Bark of Guatteria olivacea (Annonaceae) and Their In Vitro Cytotoxic Activities" Molecules 29, no. 16: 3834. https://doi.org/10.3390/molecules29163834
APA StyleCosta, E. V., Freitas, J. G. C., Manickchand, S. P., Araújo, M. d. S., Silva, V. R., Santos, L. d. S., Koolen, H. H. F., Silva, F. M. A. d., Soares, M. B. P., & Bezerra, D. P. (2024). Gouregine, an α-Gem-Dimethyltetradehydrocularine Alkaloid, and Other Aporphinoid Alkaloids from the Bark of Guatteria olivacea (Annonaceae) and Their In Vitro Cytotoxic Activities. Molecules, 29(16), 3834. https://doi.org/10.3390/molecules29163834









