A Novel Synthesis Method of Dumbbell-like (Gd1−xTbx)2O(CO3)2·H2O Phosphor for Latent Fingerprint
Abstract
:1. Introduction
2. Results and Discussion
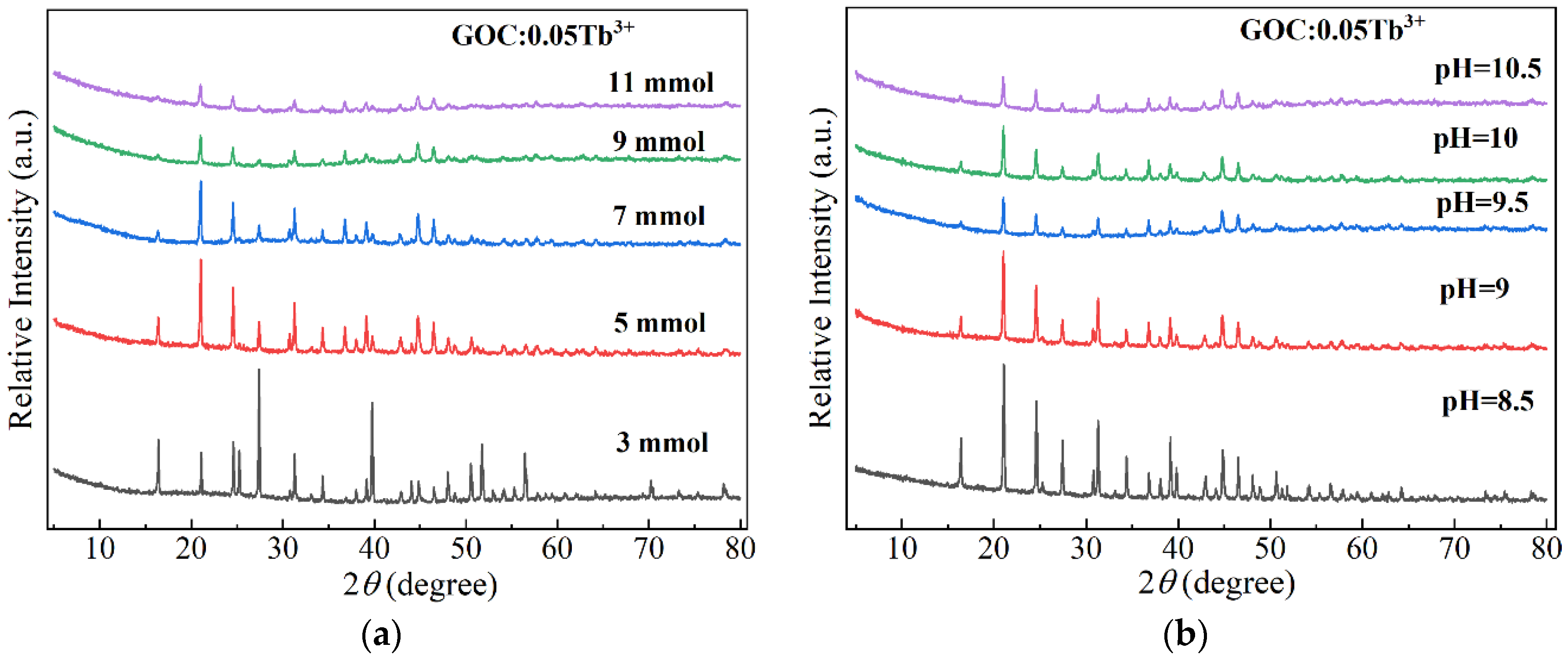
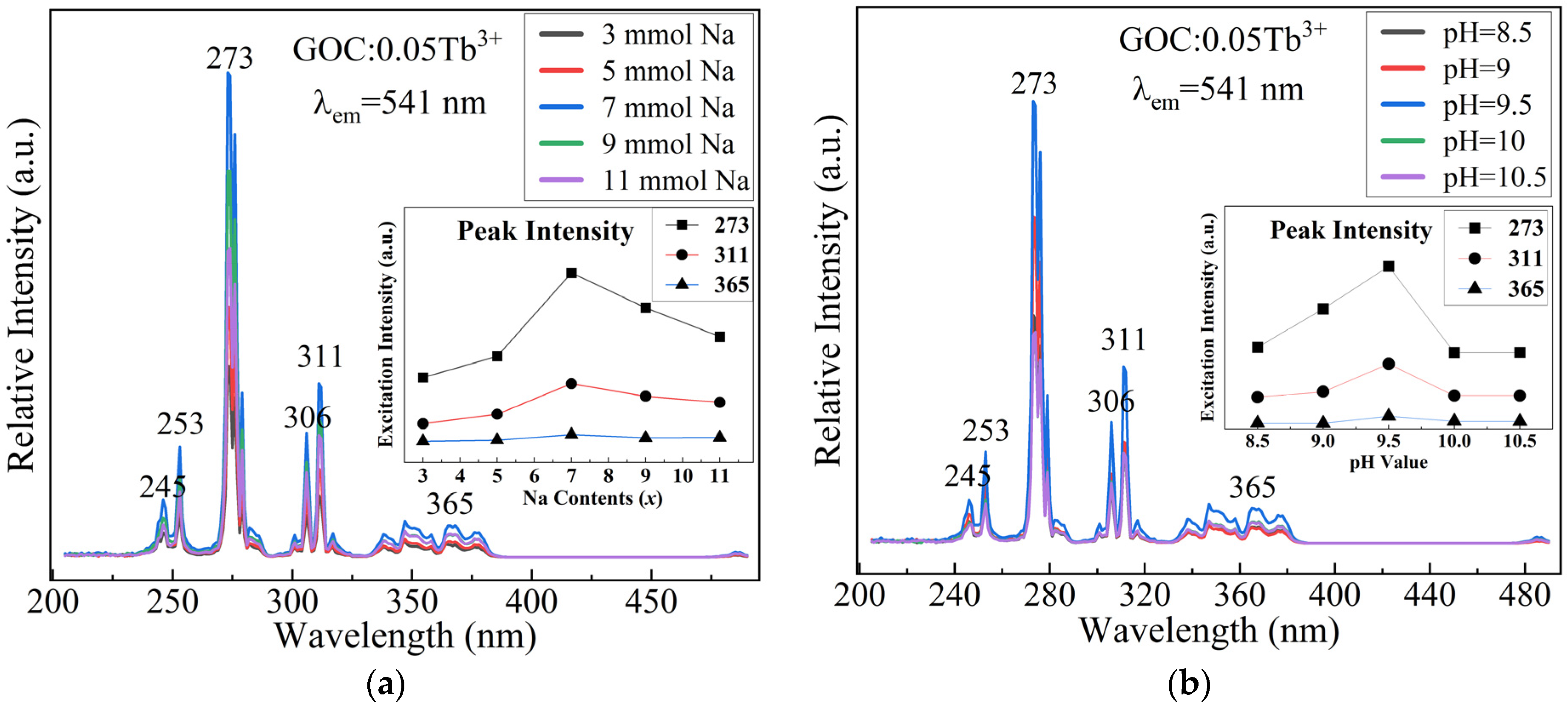
2.1. Optimization of Preparation Conditions
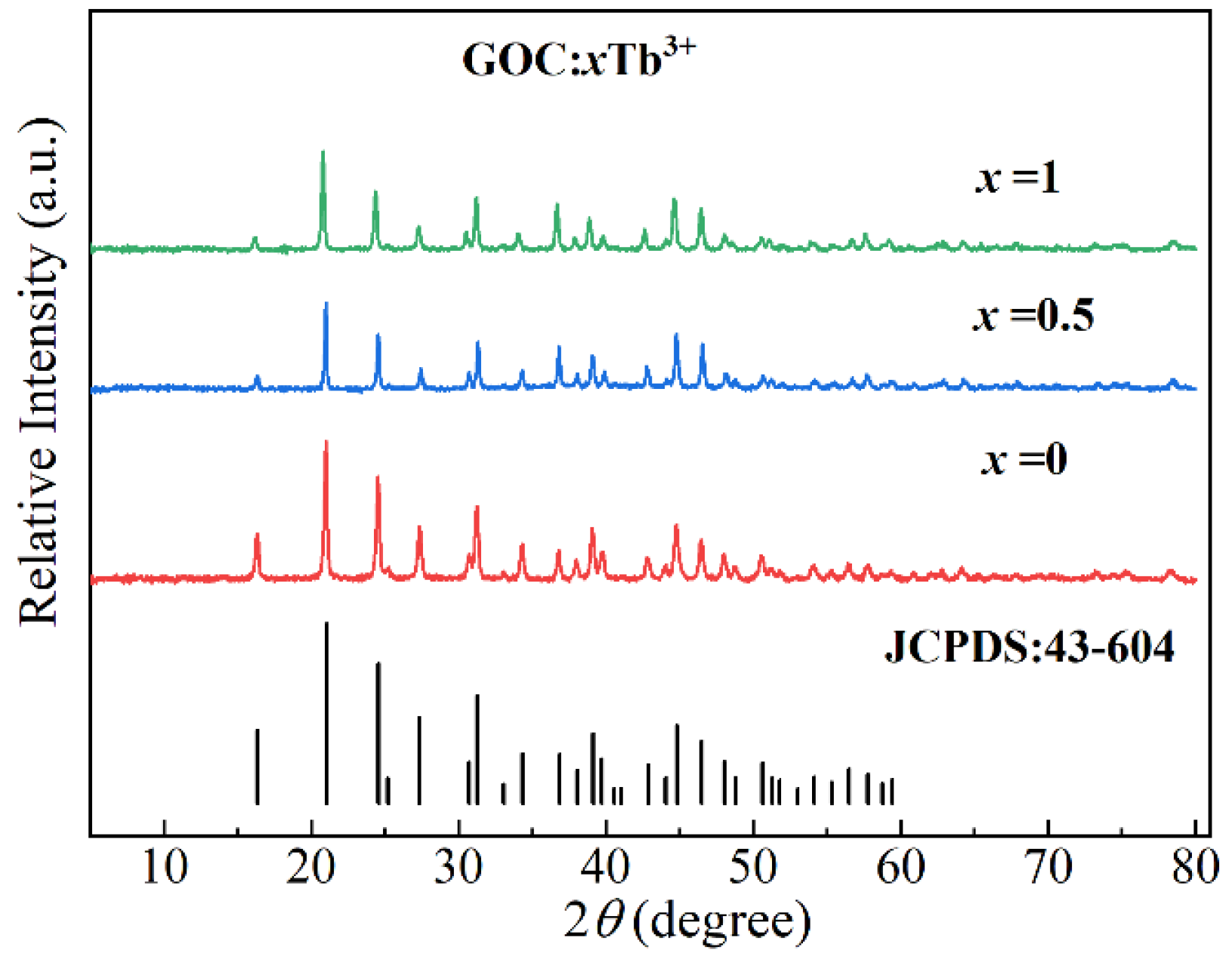
2.2. Crystal Structures
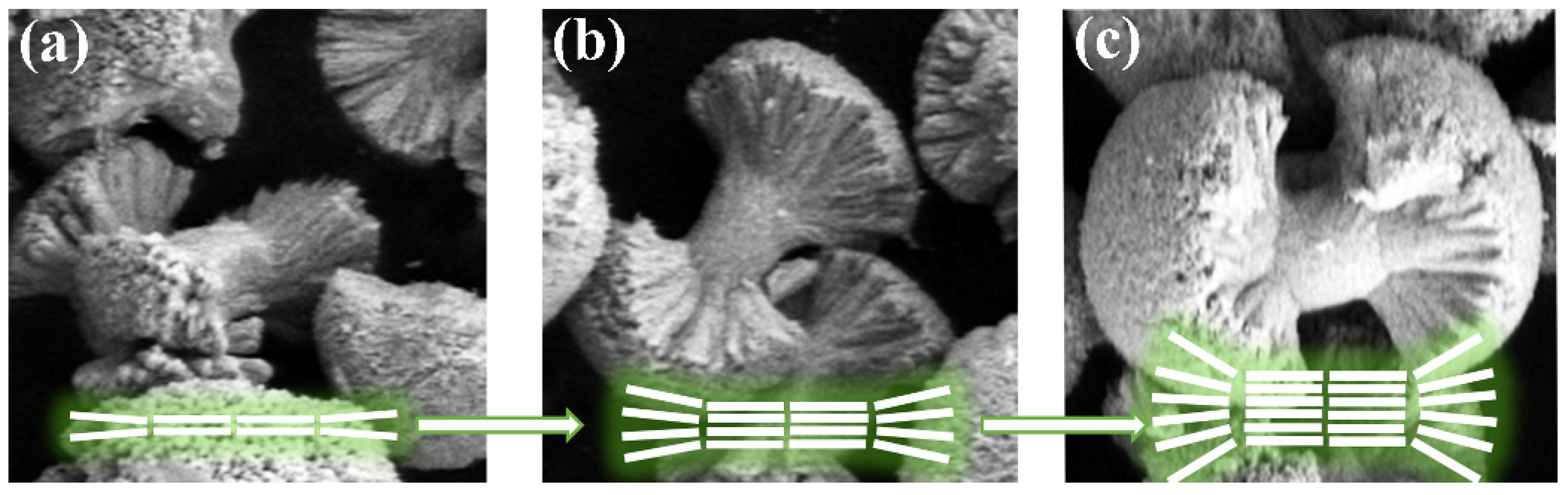
2.3. Microstructure
2.4. Luminescence Spectra
2.5. Decay Curve
2.6. Chromaticity Coordinates
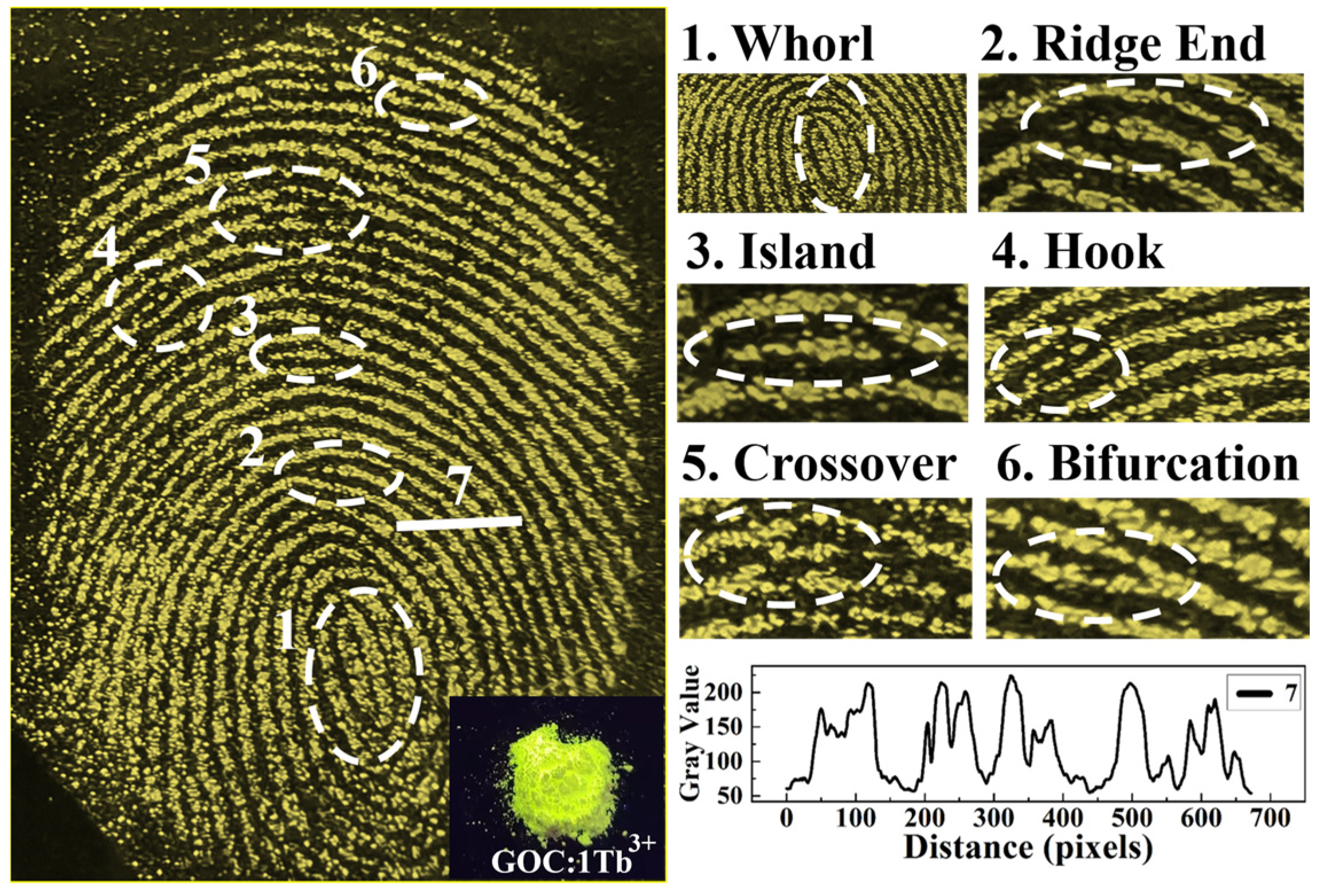
2.7. Latent Fingerprint
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouertani, G.; Maciejewska, K.; Piotrowski, W.; Horchani-Naifer, K.; Marciniak, L.; Ferhi, M. High thermal stability of warm white emitting single phase GdPO4:Dy3+/Sm3+ phosphor for UV excited wLEDs. J. Lumin. 2024, 265, 120228. [Google Scholar] [CrossRef]
- Priya, R.; Pandey, O.P.; Dhoble, S.J. Review on the synthesis, structural and photo-physical properties of Gd2O3 phosphors for various luminescent applications. Opt. Laser Technol. 2021, 135, 106663. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Lalli, D.; Marchese, L.; Platas-Iglesias, C.; Carniato, F. Bifunctional Paramagnetic and Luminescent Clays Obtained by Incorporation of Gd3+ and Eu3+ Ions in the Saponite Framework. Inorg. Chem. 2021, 60, 10749–10756. [Google Scholar] [CrossRef] [PubMed]
- Farcasanu, A.S.; Todea, M.; Muresan-Pop, M.; Petrisor, D.M.; Simion, A.; Vulpoi, A.; Simon, S. Synthesis and structural characterization of silica particles doped with Dy and Gd paramagnetic ions as MRI contrast agents. Results Chem. 2022, 4, 100520. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Choi, J.-s.; Lee, J.-H.; Shin, T.-H.; Song, H.-T.; Kim, E.Y.; Cheon, J. Self-Confirming “AND” Logic Nanoparticles for Fault-Free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.-W.; Jhang, F.-Y.; Su, C.-H.; Yeh, C.-S. Fabrication of Gd2O(CO3)2·H2O/silica/gold hybrid particles as a bifunctional agent for MR imaging and photothermal destruction of cancer cells. J. Mater. Chem. 2009, 19, 2147–2153. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ahmad, N.; Labis, J.P. Highly colloidal luminescent porous Tb-doped gadolinium oxide nanoparticles: Photophysical and luminescent properties. J. Photochem. Photobiol. A 2019, 371, 10–16. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yu, Z.; Lou, X.; Zhang, C.; Jia, X.; Jia, G. Well-defined Gd(OH)CO3, Gd2O(CO3)2·H2O, and Gd2O3 compounds with multiform morphologies and adjustable particle sizes: Synthesis, formation process, and luminescence properties. Colloid Surf. A 2021, 624, 126834. [Google Scholar] [CrossRef]
- Hu, W.-Y.; Liu, H.; Shao, Y.-Z. Fluorescein isothiocyanate embedded silica spheres in gadolinium carbonate shells as novel magnetic resonance imaging and fluorescence bi-modal contrast agents. New J. Chem. 2015, 39, 7363–7371. [Google Scholar] [CrossRef]
- Xu, W.; Park, J.Y.; Kattel, K.; Ahmad, M.W.; Bony, B.A.; Heo, W.C.; Jin, S.; Park, J.W.; Chang, Y.; Kim, T.J.; et al. Fluorescein-polyethyleneimine coated gadolinium oxide nanoparticles as T1 magnetic resonance imaging (MRI)–cell labeling (CL) dual agents. RSC Adv. 2012, 2, 10907–10915. [Google Scholar] [CrossRef]
- Straßer, M.; Schrauth, J.H.X.; Dembski, S.; Haddad, D.; Ahrens, B.; Schweizer, S.; Christ, B.; Cubukova, A.; Metzger, M.; Walles, H.; et al. Calcium fluoride based multifunctional nanoparticles for multimodal imaging. Beilstein J. Nanotech. 2017, 8, 1484–1493. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Tang, Q.; Li, Y. A new type of silica-coated Gd2(CO3)3:Tb nanoparticle as a bifunctional agent for magnetic resonance imaging and fluorescent imaging. Nanotechnology 2012, 23, 205103. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Yu, J.S. Facile template free synthesis of Gd2O(CO3)2·H2O chrysanthemum-like nanoflowers and luminescence properties of corresponding Gd2O3:RE3+ spheres. Dalton Trans. 2013, 42, 11400–11410. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Jiu, H.; Wang, Y.; Fu, Y.; Zhang, J. Facile synthesis and luminescence properties of Gd2O3:Tb hollow microspheres. Micro Nano Lett. 2011, 6, 927–931. [Google Scholar] [CrossRef]
- Zhou, L.; Yin, W.; Ren, W.; Gu, Z.; Li, W.; Jin, S.; Yan, L.; Tian, G.; Hu, Z.; Zhao, Y. Controllable synthesis of Gd2O(CO3)2·H2O@silica–FITC nanoparticles with size-dependent optical and magnetic resonance imaging properties. New J. Chem. 2012, 36, 2599–2606. [Google Scholar] [CrossRef]
- Yang, M.; Gao, L.; Liu, K.; Luo, C.; Wang, Y.; Yu, L.; Peng, H.; Zhang, W. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta 2015, 131, 661–665. [Google Scholar] [CrossRef]
- Park, I.Y.; Kim, D.; Lee, J.; Lee, S.H.; Kim, K.-J. Effects of urea concentration and reaction temperature on morphology of gadolinium compounds prepared by homogeneous precipitation. Mater. Chem. Phys. 2007, 106, 149–157. [Google Scholar] [CrossRef]
- Siribbal, M.S.; Jurewicz, A.; Hassan, M.; Iqbal, S.; Hu, Z.; Uvdal, K.; Hussain, M.S.; Mathur, S.; Ilyas, S. Biocompatible Hollow Gadolinium Oxide Nanocarriers for the Transport of Bioactive Molecules to Cells. ACS Appl. Nano Mater. 2024, 7, 12408–12419. [Google Scholar] [CrossRef]
- Anh, T.K.; Huong, N.T.; Thao, D.T.; Lien, P.T.; Van Nghia, N.; Phuong, H.T.; Tomala, R.; Minh, L.Q. High monodisperse nanospheres Gd2O3:Yb3+,Er3+ with strong upconversion emission fabricated by synergistic chemical method. J. Nanopart. Res. 2021, 23, 264. [Google Scholar] [CrossRef]
- Cichos, J.; Karbowiak, M.; Hreniak, D.; Stręk, W. Synthesis and characterization of monodisperse Eu3+ doped gadolinium oxysulfide nanocrystals. J. Rare Earth 2016, 34, 850–856. [Google Scholar] [CrossRef]
- Tamrakar, R.K.; Upadhyay, K.; Butoliya, S.; Taunk, P.B. Enhanced luminescence intensity of Y2O3:Er3+ phosphor by using combustion preparation process and incorporating Gd3+ ions. Chem. Pap. 2023, 77, 4469–4472. [Google Scholar] [CrossRef]
- Savinkina, E.V.; Karavaev, I.A.; Grigoriev, M.S.; Buzanov, G.A.; Davydova, M.N. A series of urea complexes with rare-earth nitrates: Synthesis, structure and thermal decomposition. Inorg. Chim. Acta 2022, 532, 120759. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, K.; Rai, S.B. Multifunctional Er3+–Yb3+ codoped Gd2O3 nanocrystalline phosphor synthesized through optimized combustion route. Appl. Phys. B 2009, 94, 165–173. [Google Scholar] [CrossRef]
- Kuboniwa, S.; Hoshina, T. Luminescent Properties of Tb3+ in Oxygen-Dominated Compounds. J. Phys. Soc. Jpn. 1972, 32, 1059–1068. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, Y.; Wang, Q. Slow release realized in 40 min? Assembly of lanthanide hydroxycarbonates and oxycarbonates based on multiple irradiations. J. Nanopart. Res. 2014, 16, 2552. [Google Scholar] [CrossRef]
- Geng, B.; Zhan, F.; Jiang, H.; Xing, Z.; Fang, C. Facile Production of Self-Assembly Hierarchical Dumbbell-Like CoOOH Nanostructures and Their Room-Temperature CO-Gas-Sensing Properties. Cryst. Growth Des. 2008, 8, 3497–3500. [Google Scholar] [CrossRef]
- Li, D.; Qian, J.; Huang, L.; Zhang, Y.; Zhu, G. Quantum Cutting in KGd(CO3)2:Tb3+ Green Phosphor. Nanomaterials 2023, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Kokate, R.; Nair, G.B.; Dhoble, S.J.; Jeong, H.; Jeong, H.; Boddula, R.; Singh, V. UV and VUV induced luminescence in Tb3+ doped Ca3La3(BO3)5 phosphors for PDP applications. Optik 2021, 241, 166633. [Google Scholar] [CrossRef]
- Choubey, S.R.; Gedam, S.C.; Dhoble, S.J. Resonant and non-resonant energy transfer from Ce3+→X (X=Tb3+, Eu3+ or Dy3+) in NaMgSO4F material. Luminescence 2017, 32, 253–256. [Google Scholar] [CrossRef]
- Singh, V.; Rao, B.R.V.; Bhat, A.A.; Devi, C.B.A.; Rao, A.S.; Joo, J.B. Characterization of Luminescent Li3Ba2Gd3Mo8O32:Tb3+ Phosphors Prepared via Sol–Gel Method. J. Electron. Mater. 2024, 53, 3723–3732. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Gupta, I.; Dalal, A.; Kumar, V.; Singh, D. Preparation, structural and photometric properties of single-phased Gd3Al5O12:Tb3+ green-emitting phosphors for solid state lighting purpose. Mat. Sci. Eng. B 2023, 288, 116189. [Google Scholar] [CrossRef]
- Ou, M.; Mutelet, B.; Martini, M.; Bazzi, R.; Roux, S.; Ledoux, G.; Tillement, O.; Perriat, P. Optimization of the synthesis of nanostructured Tb3+-doped Gd2O3 by in-situ luminescence following up. J. Colloid Interf. Sci. 2009, 333, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Dehouche, Z.; Ireland, T.G.; Fern, G.R. Improved photovoltaic performance of monocrystalline silicon solar cell through luminescent down-converting Gd2O2S:Tb3+ phosphor. Prog. Photovolt. 2019, 27, 640–651. [Google Scholar] [CrossRef]
- Nandanwar, C.M.; Kokode, N.S.; Yerpude, A.N.; Dhoble, S.J. Luminescence properties of BiPO4:Ln (Ln=Dy3+, Tb3+ and Sm3+) orthophosphate phosphors for near-UV-based solid-state lighting. Bull. Mater. Sci. 2023, 46, 51. [Google Scholar] [CrossRef]

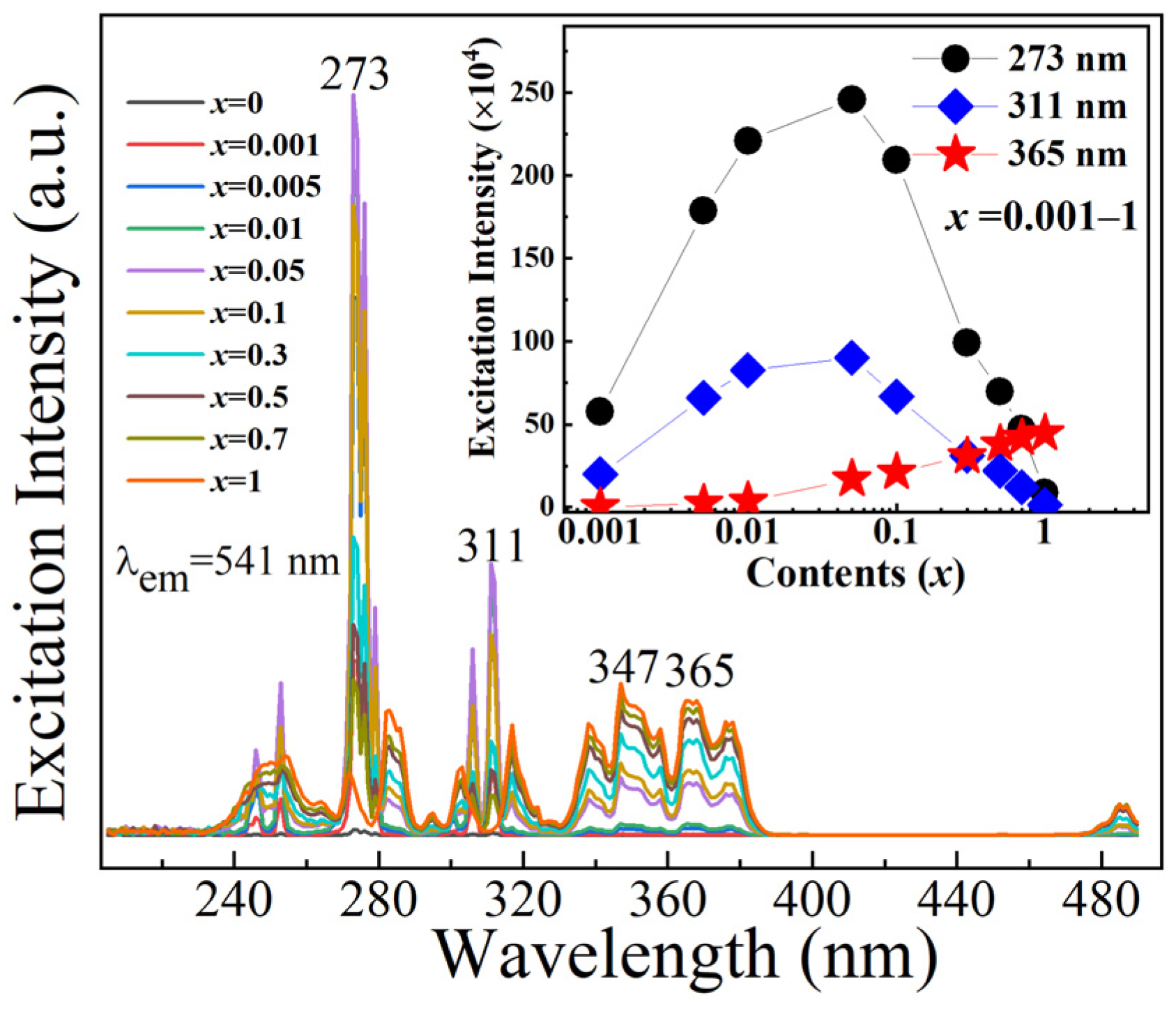
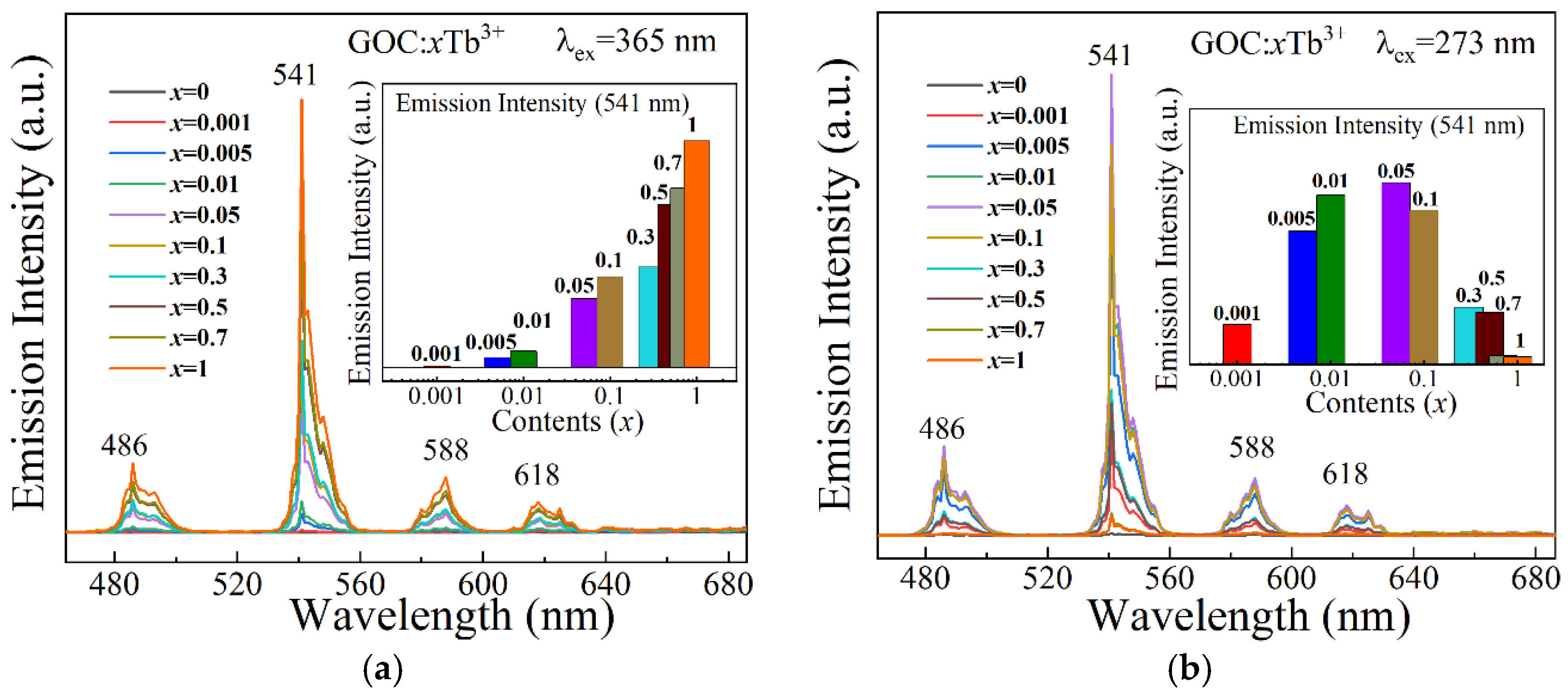
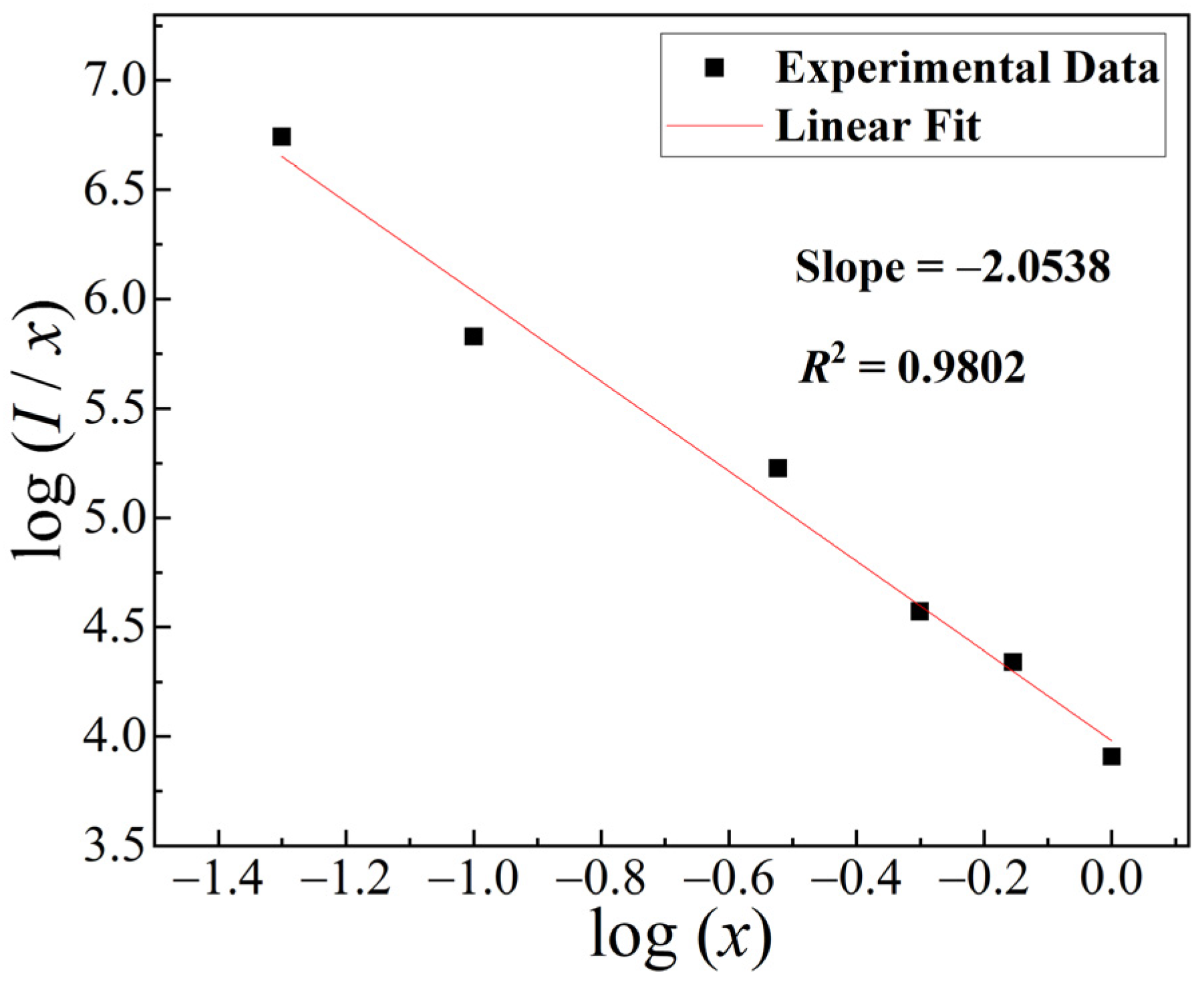
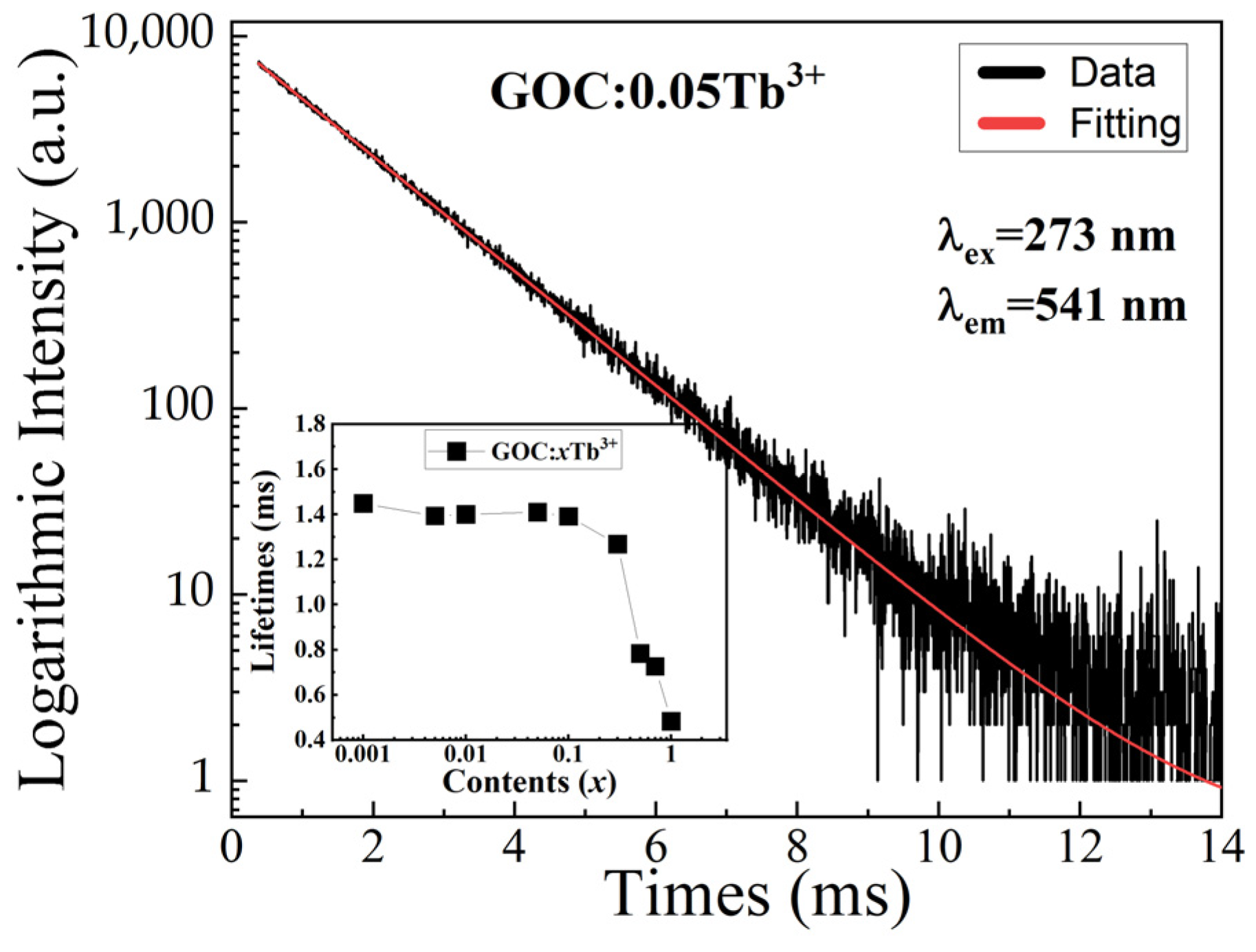

| x | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.3 | 0.5 | 0.7 | 1 |
|---|---|---|---|---|---|---|---|---|---|
| λex = 273 nm | 15.33% | 36.99% | 55.60% | 57.50% | 44.25% | 37.37% | 27.96% | 22.45% | 14.11% |
| λex = 311 nm | 3.34% | 17.35% | 40.90% | 42.77% | 23.38% | 18.04% | 13.56% | 7.51% | 2.49% |
| λex = 365 nm | ~0 | 0.19% | 3.38% | 16.43% | 17.66% | 25.85% | 29.65% | 31.01% | 33.73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Qian, J.; Sun, S.; Li, Z.; Li, D. A Novel Synthesis Method of Dumbbell-like (Gd1−xTbx)2O(CO3)2·H2O Phosphor for Latent Fingerprint. Molecules 2024, 29, 3846. https://doi.org/10.3390/molecules29163846
Huang L, Qian J, Sun S, Li Z, Li D. A Novel Synthesis Method of Dumbbell-like (Gd1−xTbx)2O(CO3)2·H2O Phosphor for Latent Fingerprint. Molecules. 2024; 29(16):3846. https://doi.org/10.3390/molecules29163846
Chicago/Turabian StyleHuang, Lei, Jian Qian, Shijian Sun, Zheng Li, and Dechuan Li. 2024. "A Novel Synthesis Method of Dumbbell-like (Gd1−xTbx)2O(CO3)2·H2O Phosphor for Latent Fingerprint" Molecules 29, no. 16: 3846. https://doi.org/10.3390/molecules29163846
APA StyleHuang, L., Qian, J., Sun, S., Li, Z., & Li, D. (2024). A Novel Synthesis Method of Dumbbell-like (Gd1−xTbx)2O(CO3)2·H2O Phosphor for Latent Fingerprint. Molecules, 29(16), 3846. https://doi.org/10.3390/molecules29163846





