The Utilization of Rice Husk as Both the Silicon Source and Mesoporous Template for the Green Preparation of Mesoporous TiO2/SiO2 and Its Excellent Catalytic Performance in Oxidative Desulfurization
Abstract
:1. Introduction
2. Results and Discussion
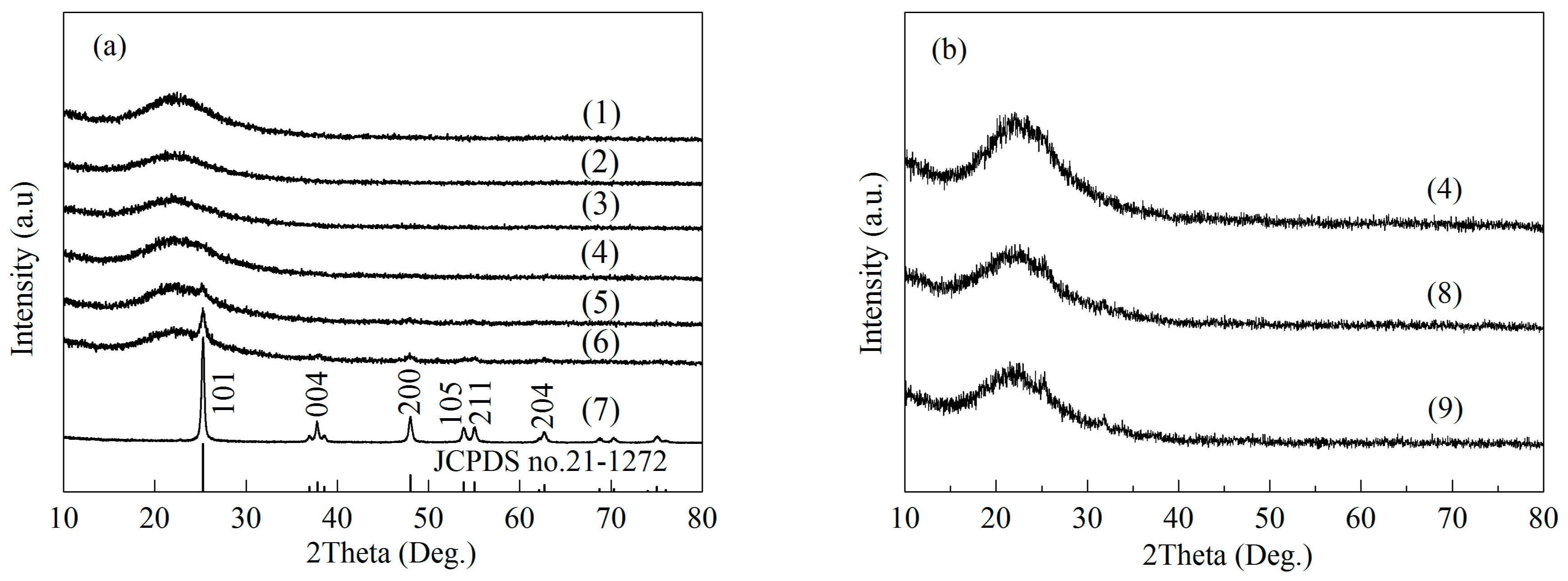
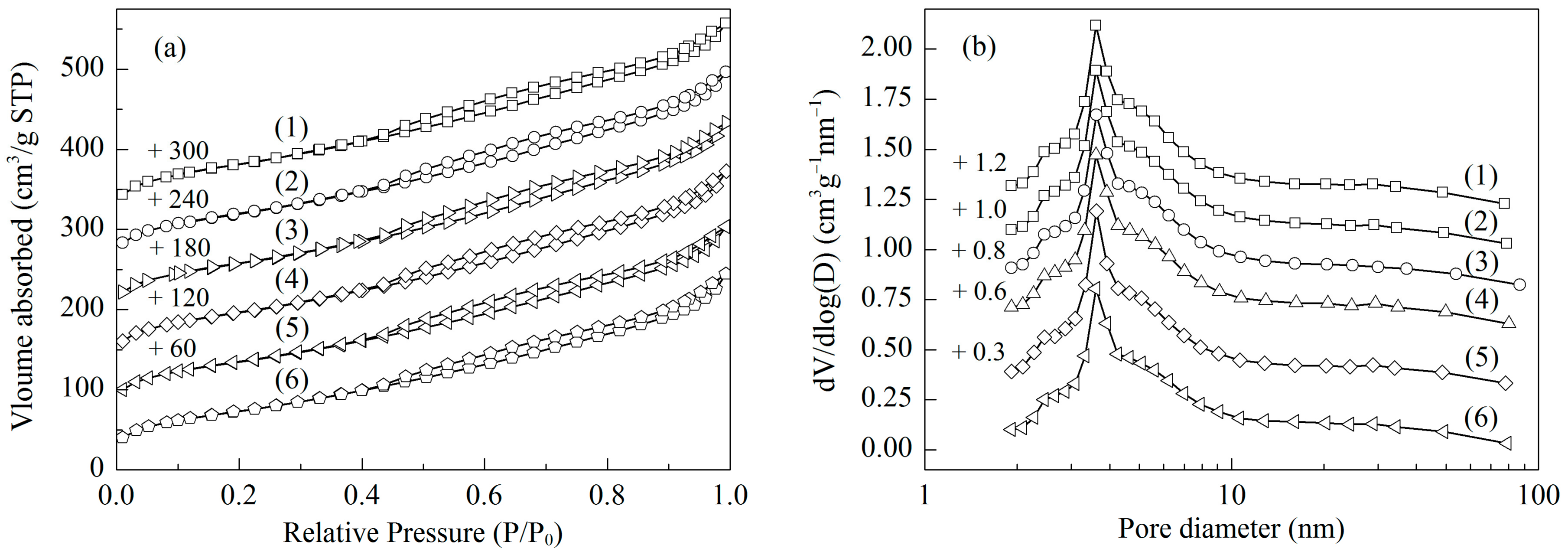
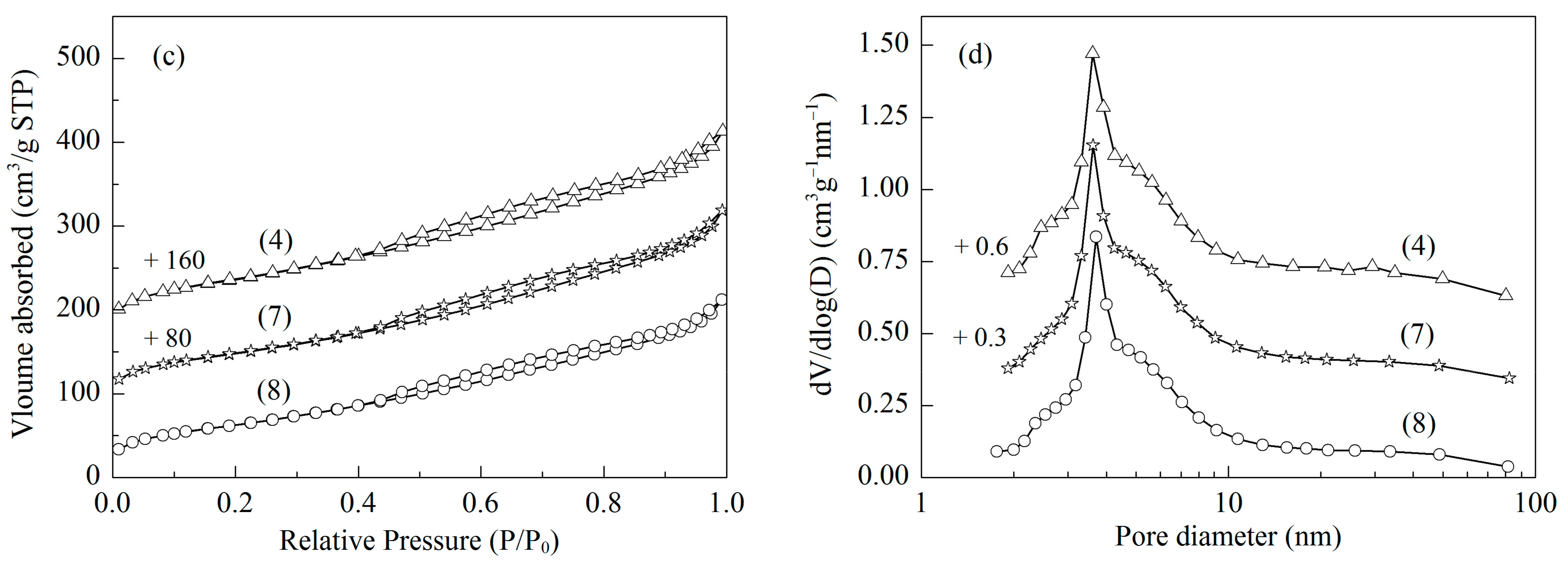
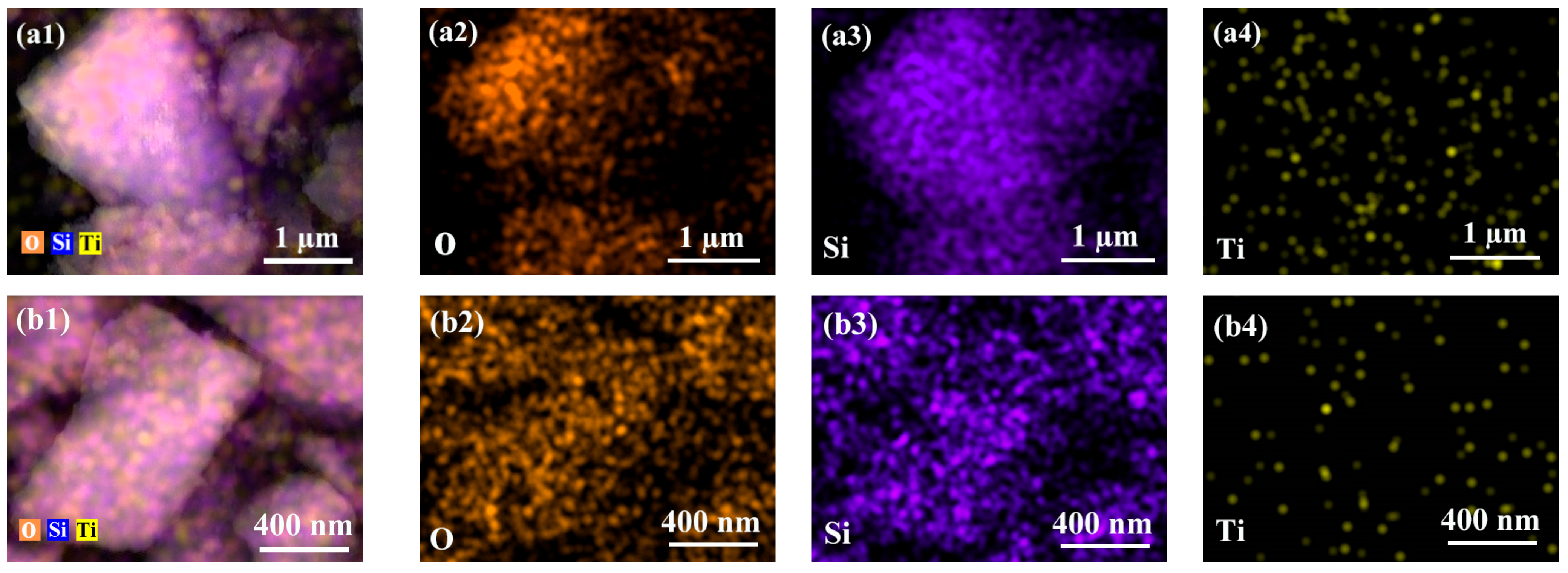
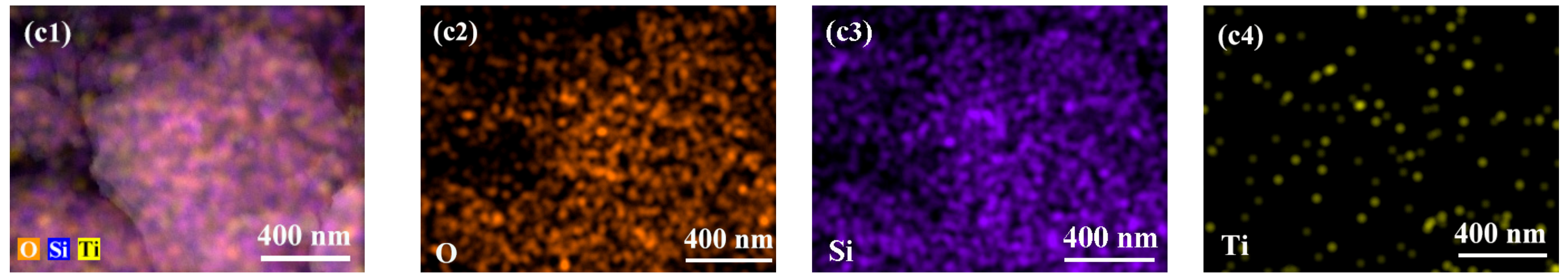
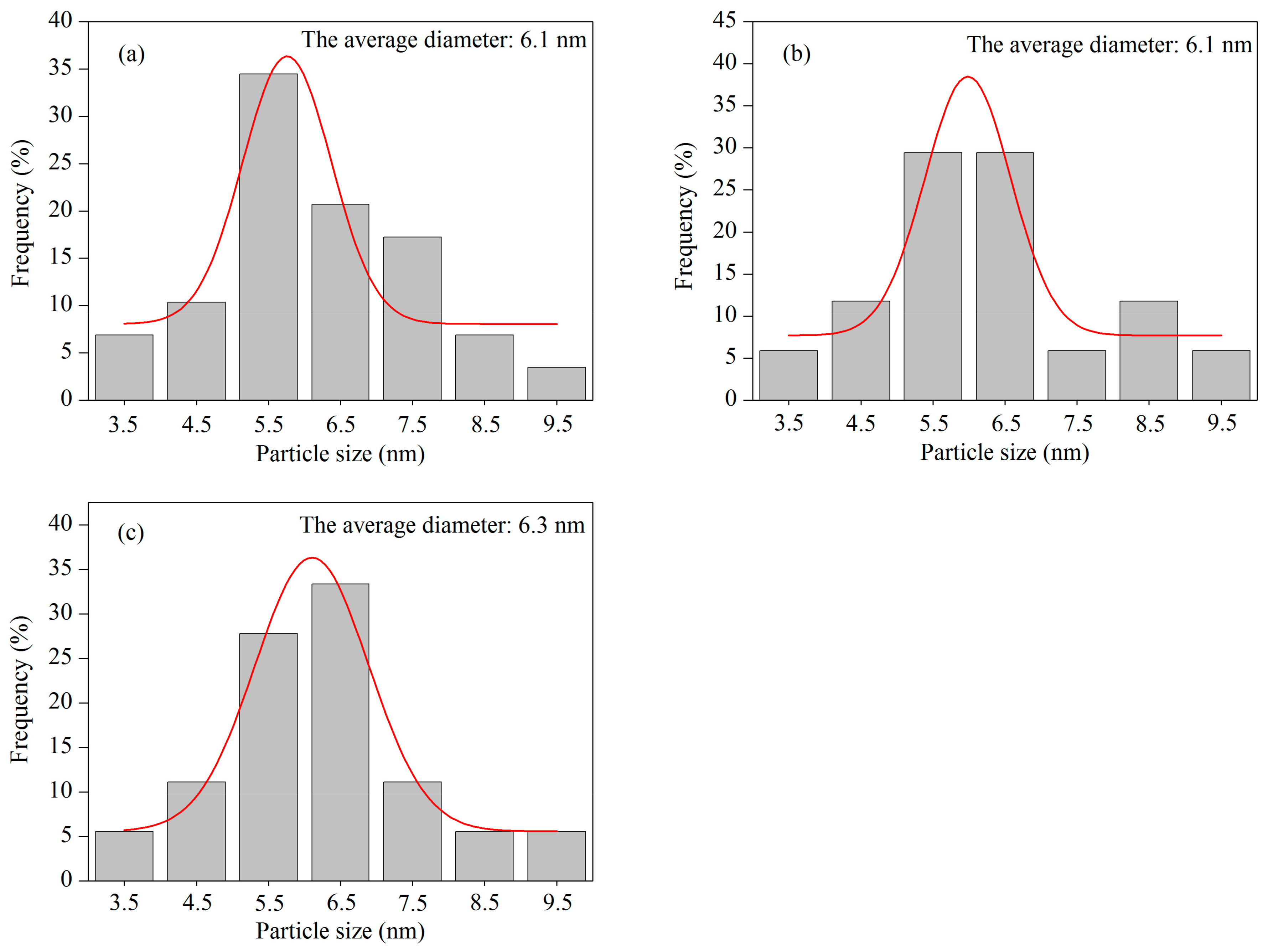
2.1. Structural Characterizations
2.2. Catalytic Performance on Oxidative Desulfurization
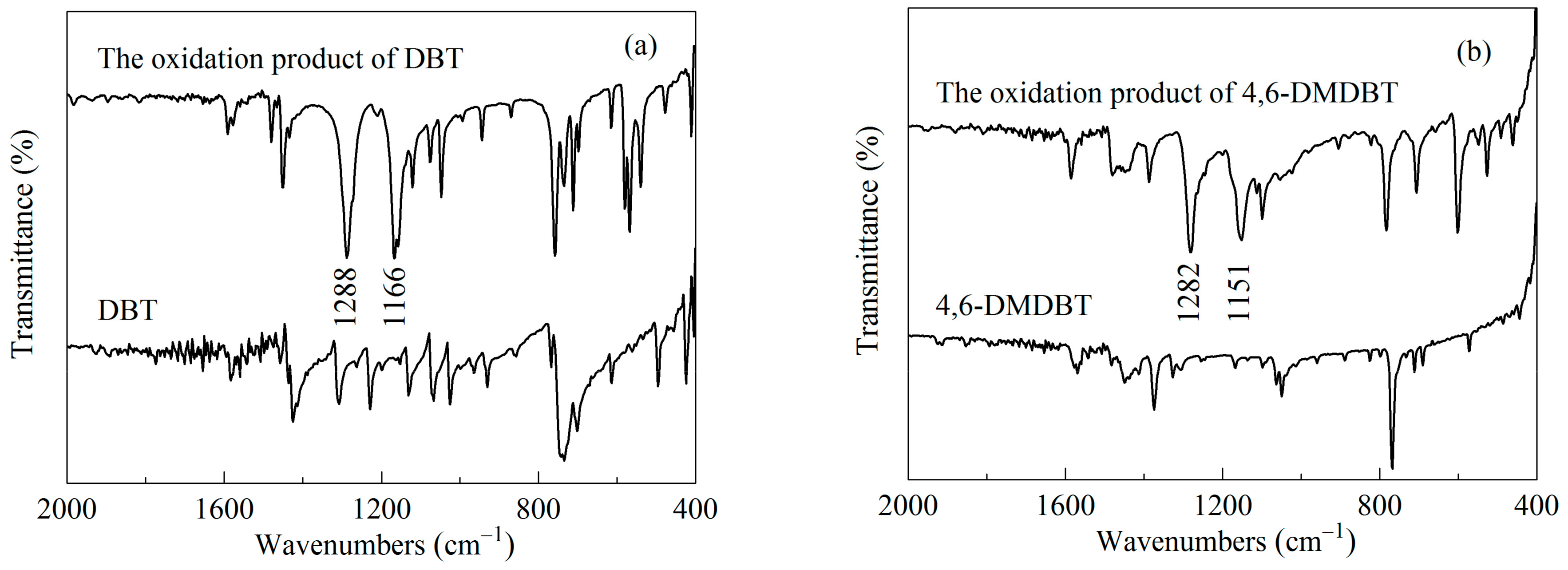
2.3. Reaction Mechanism of the ODS Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2/SiO2 Samples
3.3. Treatment of Samples with TBHP Aqueous Solution
3.4. Characterization
3.5. Catalytic Activity and Recycle Tests
3.5.1. Catalytic Activity
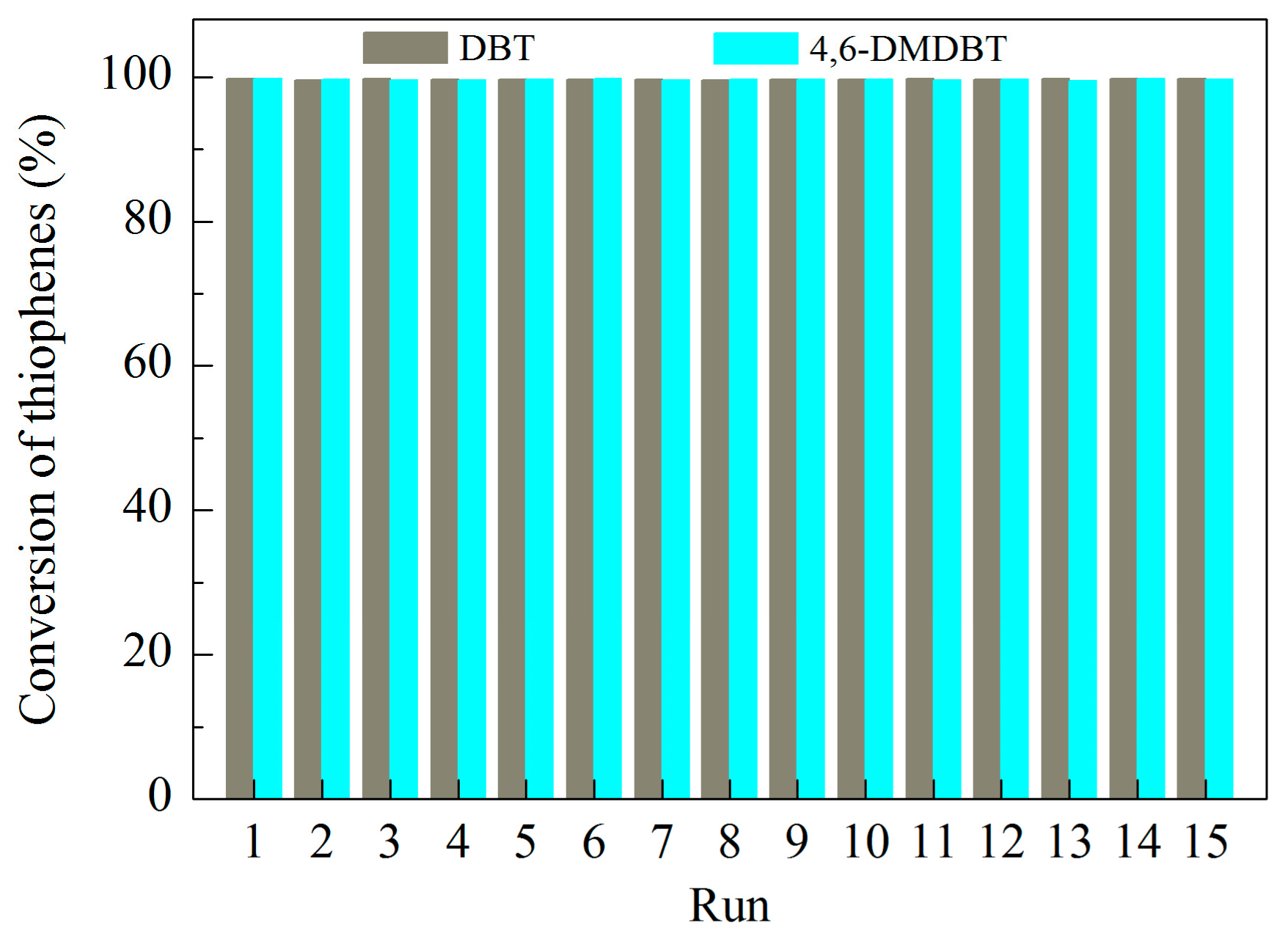
3.5.2. Recycle Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef]
- Announcement No. 16 of 2018 by the National Development and Reform Commission of the People’s Republic of China. Available online: https://www.ndrc.gov.cn/xxgk/zcfb/gg/201812/t20181229_961214_ext.html (accessed on 29 December 2018).
- Jiang, Z.; Lü, H.; Zhang, Y.; Li, C. Oxidative desulfurization of fuel oils. Chin. J. Catal. 2011, 32, 707–715. [Google Scholar] [CrossRef]
- Haghighi, M.; Gooneh-Farahani, S. Insights to the oxidative desulfurization process of fossil fuels over organic and inorganic heterogeneous catalysts: Advantages and issues. Environ. Sci. Pollut. Res. 2020, 27, 39923–39945. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, G.; Zhao, H. Polyoxometalate as effective catalyst for the deep desulfurization of diesel oil. Catal. Today 2010, 149, 117–121. [Google Scholar] [CrossRef]
- Yan, X.M.; Mei, P.; Xiong, L.; Gao, L.; Yang, Q.; Gong, L. Mesoporous titania–silica–polyoxometalate nanocomposite materials for catalytic oxidation desulfurization of fuel oil. Catal. Sci. Technol. 2013, 3, 1985–1992. [Google Scholar] [CrossRef]
- Huai, J.; Liu, X.; Zhang, Y.; Yang, Y.; Gou, Y.; Qin, L.; Shan, J.; Zheng, Y.; Li, H. Synthesis of hierarchical TS-1 and its recycling catalytic property for oxidative desulfurization. J. Renew. Mater. 2022, 10, 1711–1726. [Google Scholar] [CrossRef]
- Du, Q.; Guo, Y.; Wu, P.; Liu, H.; Chen, Y. Facile synthesis of hierarchical TS−1 zeolite without using mesopore templates and its application in deep oxidative desulfurization. Microporous Mesoporous Mater. 2019, 275, 61–68. [Google Scholar] [CrossRef]
- Manriquez-Ramirez, M.E.; Valdez, M.T.; Castro, L.V.; Flores, M.E.; Ortiz-Islas, E. Application of CeO2-V2O5 catalysts in the oxidative desulfurization of 4,6-dimethyl dibenzothiophene as a model reaction to remove sulfur from fuels. Mater. Res. Bull. 2022, 153, 111864. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, J.; Gao, X.; An, X.; Leng, Y.; Zhu, L.; Zhu, W.; Li, H. In situ fabrication of hollow silica confined defective molybdenum oxide for enhanced catalytic oxidative desulfurization of diesel fuels. Fuel 2021, 305, 121470. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Abu Bakar, W.A.W.; Ali, R.; Abdul Kadir, A.A. Catalytic oxidative desulfurization of diesel oil by Co/Mn/Al2O3 catalysts—Tert-butyl hydroperoxide (TBHP) system: Preparation, characterization, reaction, and mechanism. Clean Technol. Environ. Policy 2015, 17, 1487–1497. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhao, R.; Zhang, J.; Zhang, L.; Zhang, W.; Hu, J.; Li, H. The Green Preparation of Mesoporous WO3/SiO2 and Its Application in Oxidative Desulfurization. Catalysts 2024, 14, 103. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Jian, P. Synthesis of amorphous TiO2-SiO2 composite oxides and their catalytic activity for oxidative desulfurization of gasoline. Ind. Catal. 2014, 22, 869–873. [Google Scholar]
- Liu, X.; Liu, X.; Shan, J.; Huai, J.; Yang, H.; Yan, X.; Zheng, Y.; Li, H. Synthesis of amorphous mesoporous TiO2-SiO2 and its excellent catalytic performance in oxidative desulfurization. Inorg. Chem. Commun. 2021, 123, 108336. [Google Scholar] [CrossRef]
- Bazyari, A.; Khodadadi, A.A.; Mamaghani, A.H.; Beheshtian, J.; Thompson, L.T.; Mortazavi, Y. Microporous titania-silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization. Appl. Catal. B 2016, 180, 65–77. [Google Scholar] [CrossRef]
- Ghahramaninezhad, M.; Ahmadpour, A. Carboxylmethyl-β-cyclodextrin as a good modifier agent for oxidation of dibenzothiophene. Surf. Interfaces 2022, 28, 101612. [Google Scholar] [CrossRef]
- Bazani, H.A.G.; Thomé, A.; Affeldt, R.F.; Probst, L.F.D. SBA-15 obtained from rice husk ashes wet-impregnated with metals (Al, Co, Ni) as efficient catalysts for 1,4-dihydropyridine three-component reaction. New J. Chem. 2022, 46, 7899–7909. [Google Scholar] [CrossRef]
- Cui, J.; Cheng, F.; Lin, J.; Yang, J.; Jiang, K.; Wen, Z.; Sun, J. High surface area C/SiO2 composites from rice husks as a high-performance anode for lithium ion batteries. Powder Technol. 2017, 311, 1–8. [Google Scholar] [CrossRef]
- Grisolia, A.; Dell’Olio, G.; Spadafora, A.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. Hybrid polymer-silica nanostructured materials for environmental remediation. Molecules 2023, 28, 5105. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, S.; Li, L.; Wang, M. From waste to catalyst: Growth mechanisms of ZSM-5 zeolite from coal fly ash & rice husk ash and its performance as catalyst for tetracycline degradation in fenton-like oxidation. Environ. Pollut. 2024, 345, 123509. [Google Scholar]
- Pham, L.K.H.; Alsaiari, M.; Thao, B.T.T.; Hieu, N.H.; Duy, N.P.H.; Vo, D.V.N.; Witoon, T.; Nguyen, V.C.; Kongparakul, S.; Samart, C.; et al. High selective hydrocarbon and hydrogen products from catalytic pyrolysis of rice husk: Role of the ordered mesoporous silica derived from rice husk ash for Ni-nanocatalyst performance. J. Anal. Appl. Pyrolysis 2024, 178, 106383. [Google Scholar] [CrossRef]
- Bi, M.; Guo, Y.; Wang, S.; Zhang, B.; Jiang, Y.; Li, H.; Xu, Q.; Chen, L.; Zhao, Q.; Han, N. Titanium dioxide-modified nanosized TS-1 zeolite-supported phosphotungstic acid as a catalyst for deep catalytic oxidative desulfurization of fuel oil. New J. Chem. 2023, 47, 12027–12036. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Titania–silica as catalysts: Molecular structural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Peng, X.; Lin, M.; Zhu, B.; Duan, Q.; Luo, Y.; Shu, X. Relationship between Ti-Si coordination and catalytic activity for titanosilicates. Acta Pet. Sin. (Pet. Process. Sect.) 2019, 35, 860–867. [Google Scholar]
- Bai, R.; Sun, Q.; Song, Y.; Wang, N.; Zhang, T.; Wang, F.; Zou, Y.; Feng, Z.; Miao, S.; Yu, J. Intermediate-crystallization promoted catalytic activity of titanosilicate zeolites. J. Mater. Chem. A 2018, 6, 8757–8762. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.H.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 750–753. [Google Scholar] [CrossRef]
- Socrates, G. Sulphur and selenium compounds. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001; pp. 211–218. [Google Scholar]
- Serrano, D.P.; Sanz, R.; Pizarro, P.; Moreno, I.; Medina, S. Hierarchical TS−1 zeolite as an efficient catalyst for oxidative desulphurization of hydrocarbon fractions. Appl. Catal. B Environ. 2014, 146, 35–42. [Google Scholar] [CrossRef]
- Luo, Q.; Zhou, Q.; Lin, Y.; Wu, S.; Liu, H.; Du, C.; Zhong, Y.; Yang, C. Fast and deep oxidative desulfurization of dibenzothiophene with catalysts of MoO3-TiO2@MCM-22 featuring adjustable Lewis and Bronsted acid sites. Catal. Sci. Technol. 2019, 9, 6166–6179. [Google Scholar] [CrossRef]
- Ghahramaninezhad, M.; Ahmadpour, A. A new simple protocol for the synthesis of nanohybrid catalyst for oxidative desulfurization of dibenzothiophene. Environ. Sci. Pollut. Res. 2020, 27, 4104–4114. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, Y.; Zhang, Z.; Li, N.; Fan, Y. Shape-controlled synthesis of the metal-organic framework MIL-125 towards a highly enhanced catalytic performance for the oxidative desulfurization of 4,6-dimethyldibenzothiophene. Dalton Trans. 2020, 49, 10052–10057. [Google Scholar] [CrossRef]
- Lu, S.; Zhong, H.; Mo, D.; Hu, Z.; Zhou, H.; Yao, Y. A H-titanate nanotube with superior oxidative desulfurization selectivity. Green Chem. 2017, 19, 1371–1377. [Google Scholar] [CrossRef]
- Bonino, F.; Damin, A.; Ricchiardi, G.; Ricci, M.; Spanò, G.; D’Aloisio, R.; Zecchina, A.; Lamberti, C.; Prestipino, C.; Bordiga, S. Ti-Peroxo Species in the TS-1/H2O2/H2O System. J. Phys. Chem. B 2004, 108, 3573–3583. [Google Scholar] [CrossRef]





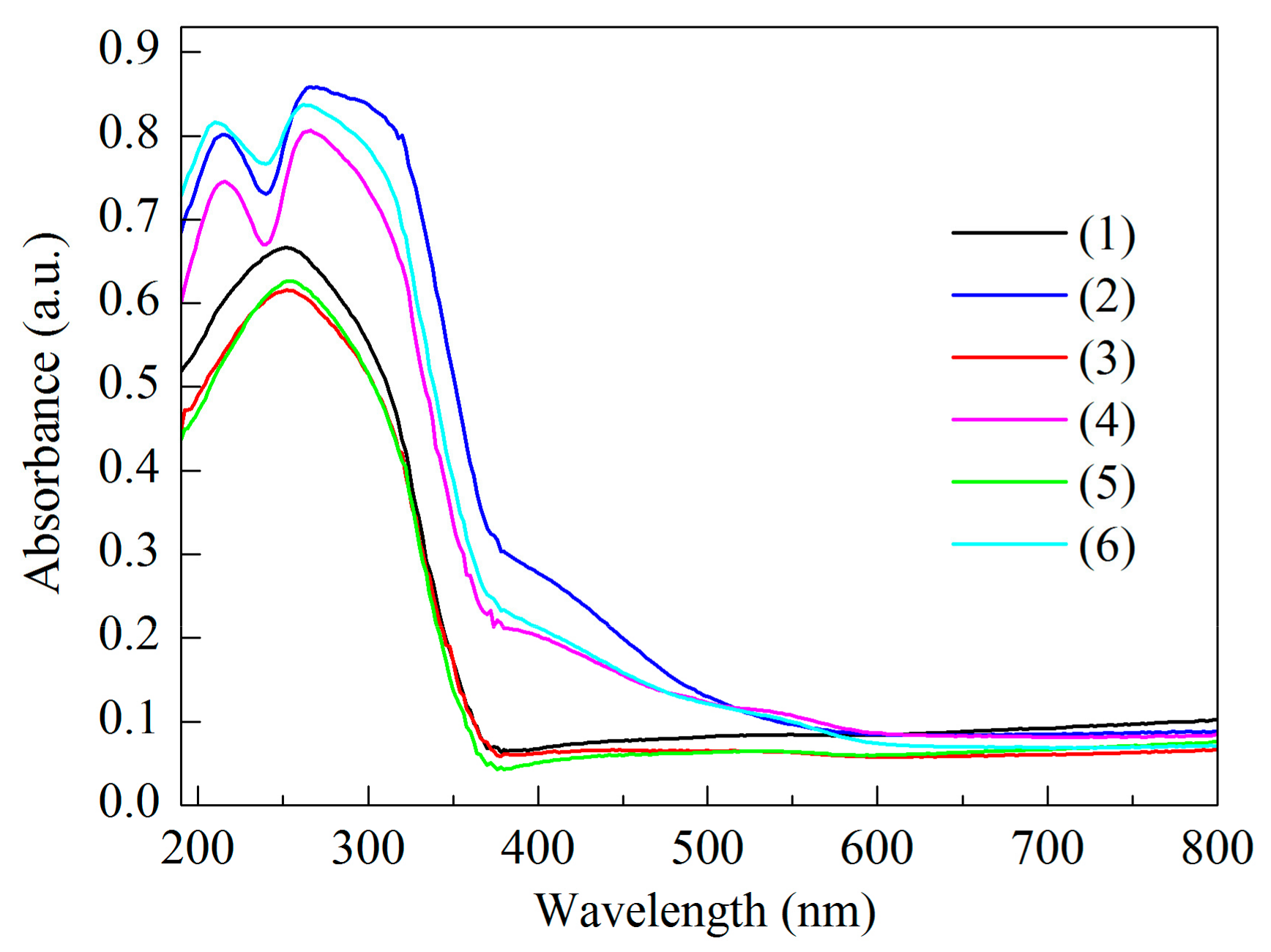
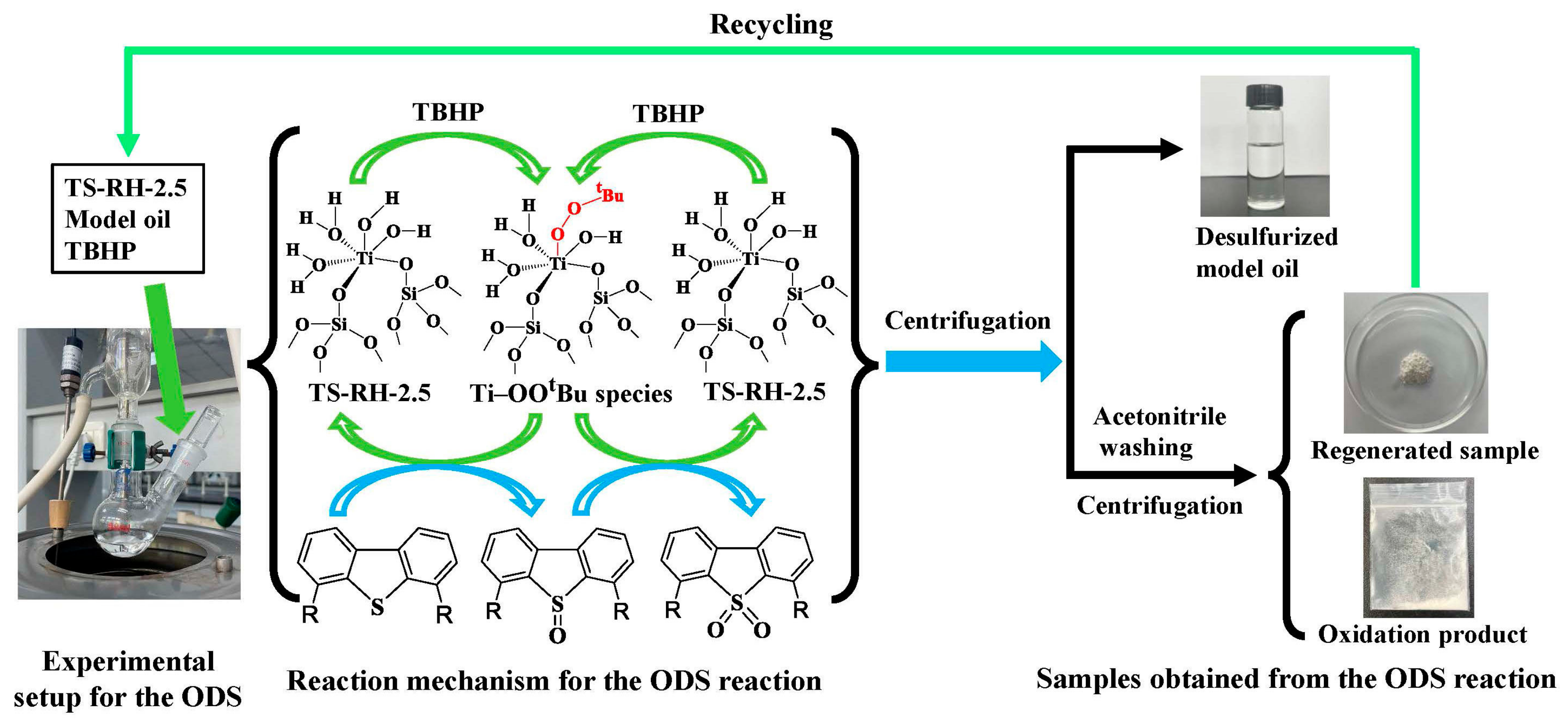
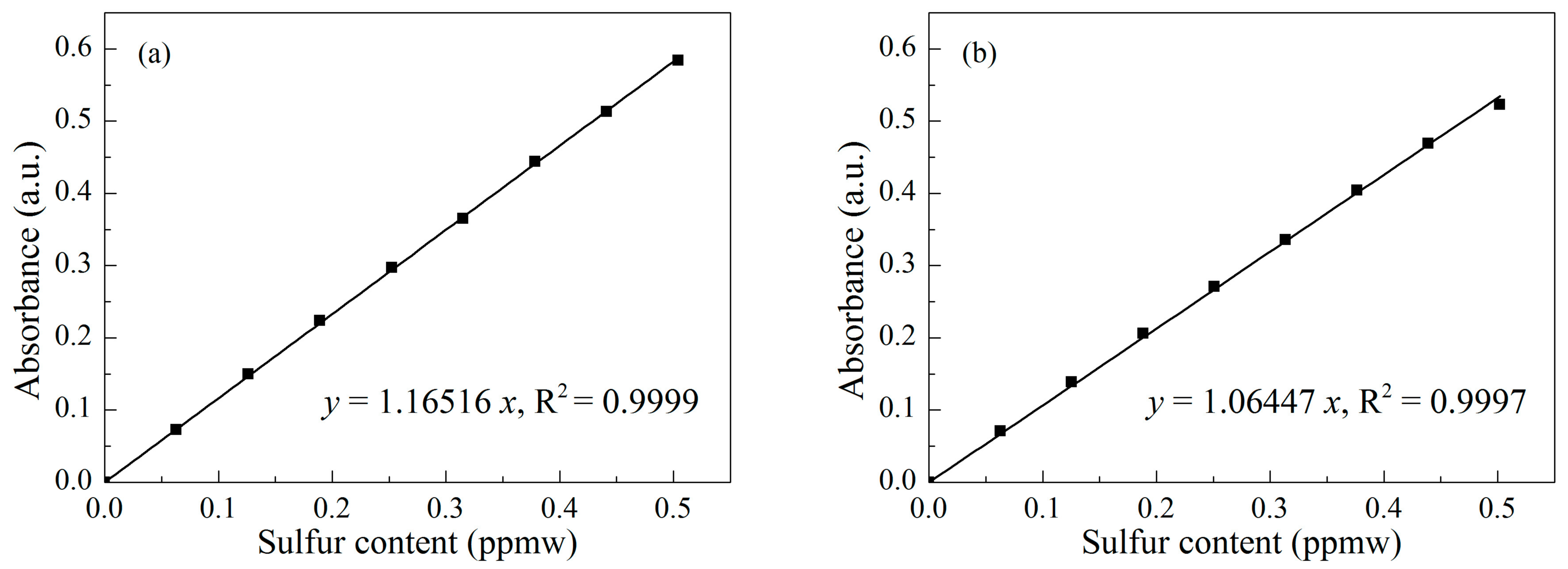

| Sample | SBET (m2/g) | Vmeso (cm3/g) | XDBT (%) | X4,6-DMDBT (%) |
|---|---|---|---|---|
| SiO2-RH | 294.4 | 0.39 | 33.9 | 24.8 |
| Pure TiO2 | 3.3 | 0.01 | 32.3 | 22.6 |
| TS-RH-0.5 | 286.6 | 0.38 | 97.7 | 98.1 |
| TS-RH-1.0 | 281.5 | 0.38 | 98.8 | 98.7 |
| TS-RH-2.5 | 277.6 | 0.38 | 99.8 | 99.7 |
| TS-RH-5.0 | 271.0 | 0.37 | 99.7 | 99.8 |
| TS-RH-10 | 264.8 | 0.37 | 99.8 | 99.8 |
| TS-RH-2.5c a | 246.9 | 0.34 | - | - |
| TS-RH-2.5c b | 229.5 | 0.34 | - | - |
| Sample | Temp. (K) | S Content (ppm) | Cyclic Number | XDBT a (%) | X4,6-DMDBT a (%) | Ref. |
|---|---|---|---|---|---|---|
| TS-0.2 | 333 | 1000, 500 b | 5 | 99.0 | 99.8 | [14] |
| TS-50 | 353 | 500 | 2 | 100.0 | – | [15] |
| TS-4/CM-β-CD | 298 | 500 | 3 | 99.0 | – | [16] |
| MT-1:4 | 373 | 500 | 8 | 94.6 | – | [29] |
| TS-4 | 298 | 500 | 3 | 98.0 | – | [30] |
| MIL-125-TRO | 333 | 250 | 3 | – | 92.3 | [31] |
| H-TiNTs | 313 | 320 | 4 | ~98.0 | – | [32] |
| TS-RH-2.5 | 333 | 1000 | 15 | 99.8 | 99.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, L.; Hu, J.; Zhang, W.; Xiang, X.; Cheng, H.; Qin, L.; Li, H. The Utilization of Rice Husk as Both the Silicon Source and Mesoporous Template for the Green Preparation of Mesoporous TiO2/SiO2 and Its Excellent Catalytic Performance in Oxidative Desulfurization. Molecules 2024, 29, 3856. https://doi.org/10.3390/molecules29163856
Liu X, Zhang L, Hu J, Zhang W, Xiang X, Cheng H, Qin L, Li H. The Utilization of Rice Husk as Both the Silicon Source and Mesoporous Template for the Green Preparation of Mesoporous TiO2/SiO2 and Its Excellent Catalytic Performance in Oxidative Desulfurization. Molecules. 2024; 29(16):3856. https://doi.org/10.3390/molecules29163856
Chicago/Turabian StyleLiu, Xiaoxue, Lanfen Zhang, Jian Hu, Wei Zhang, Xiaorong Xiang, Huiqing Cheng, Li Qin, and Hao Li. 2024. "The Utilization of Rice Husk as Both the Silicon Source and Mesoporous Template for the Green Preparation of Mesoporous TiO2/SiO2 and Its Excellent Catalytic Performance in Oxidative Desulfurization" Molecules 29, no. 16: 3856. https://doi.org/10.3390/molecules29163856







