Polyvinylidene Fluoride-Based Nanowire-Imprinted Membranes with High Flux for Efficient and Selective Separation of Artemisinin/Artemether
Abstract
:1. Introduction
2. Experimental
2.1. Materials
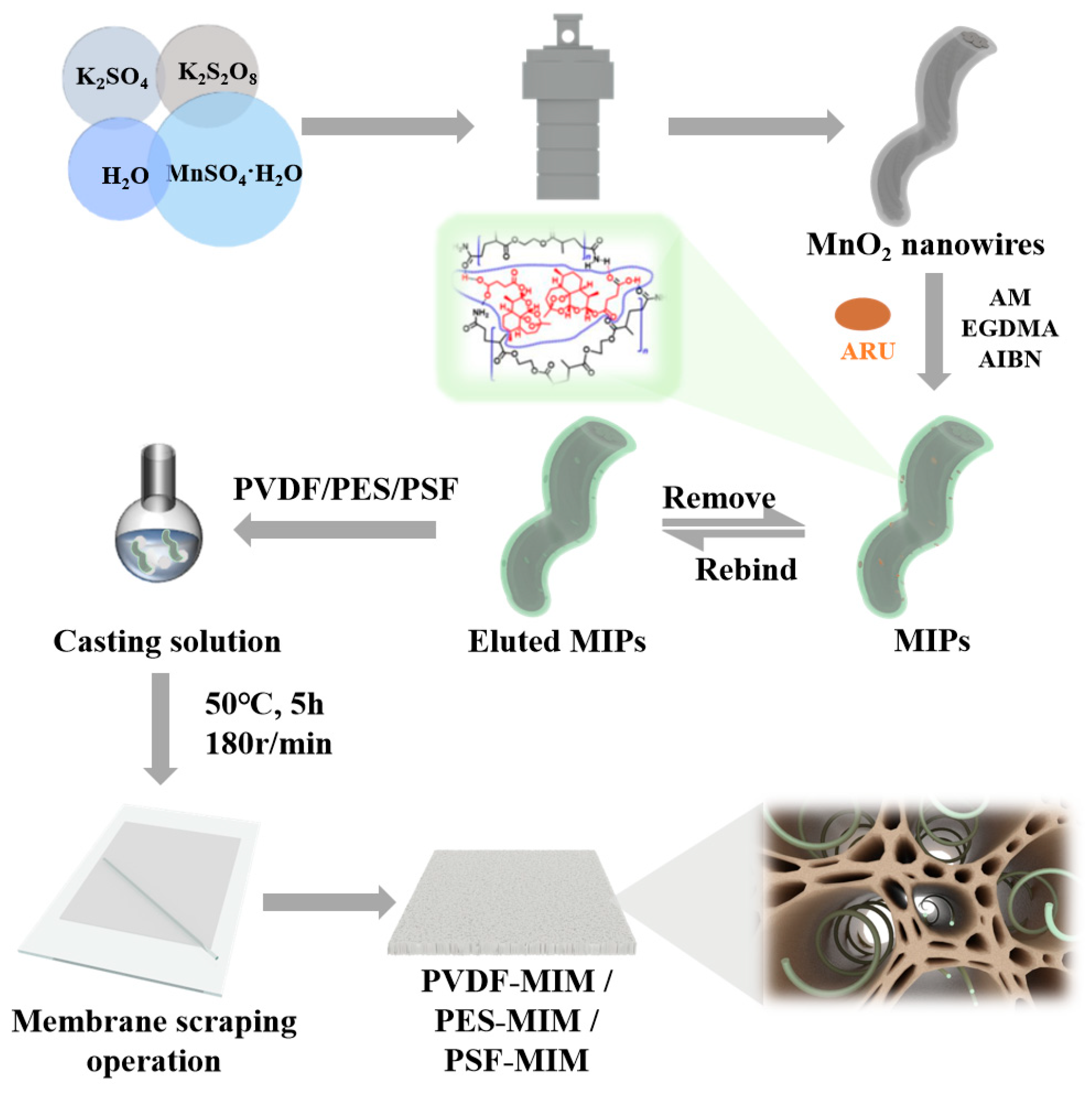
2.2. Synthetic Experiments
2.2.1. Synthesis of MnO2 Nanowires
2.2.2. Synthesis of Imprinted Polymers
2.2.3. Synthesis of MnO2-Nanowire-Blended Imprinting Membrane
2.3. Flux Test
2.4. Adsorption Experiment
2.4.1. MIPs/NIPs Adsorption
2.4.2. Optimal Concentration
2.4.3. Adsorption Kinetics
2.4.4. Sorption Isotherm
2.4.5. Adsorption Thermodynamics
2.4.6. Adsorption Selectivity
2.4.7. Dynamic Adsorption
2.4.8. HPLC
2.5. Characterization Method
3. Results and Discussions
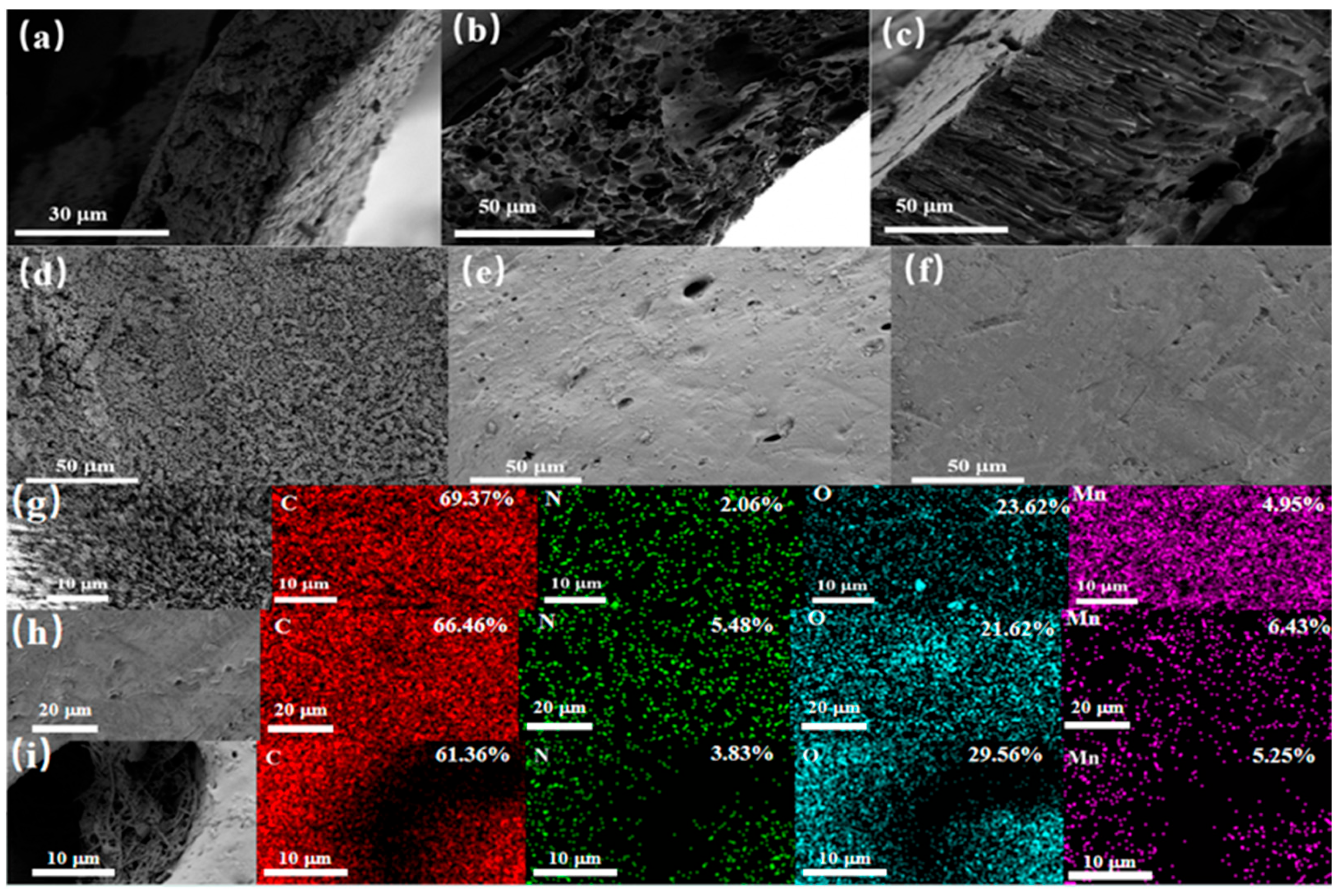
3.1. Characterization of Nanowire-Based Molecular Imprinting Polymers
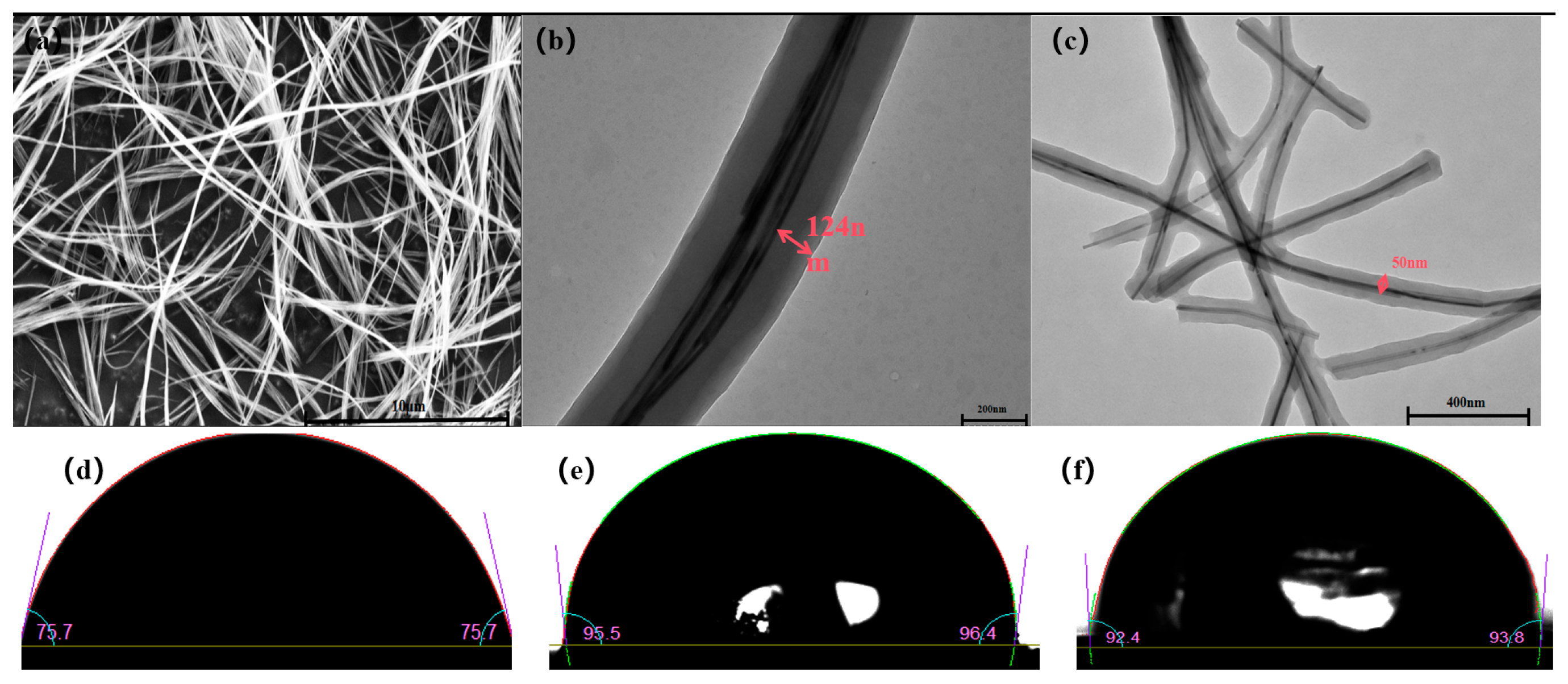
3.1.1. SEM, TEM, and Contact Angle Test
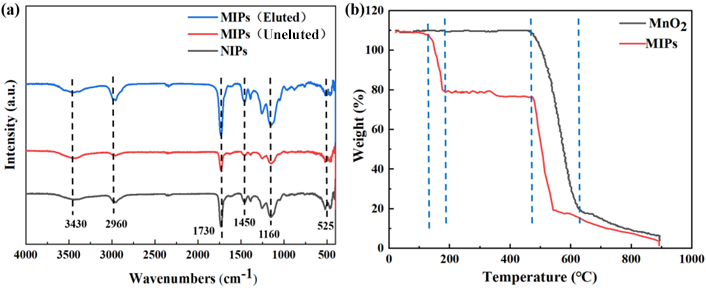
3.1.2. FT-IR, TG, and Adsorption Experiment
3.2. Characterization of Membranes
3.2.1. SEM and Physical Drawings
3.2.2. XRD and FT-IR Studies
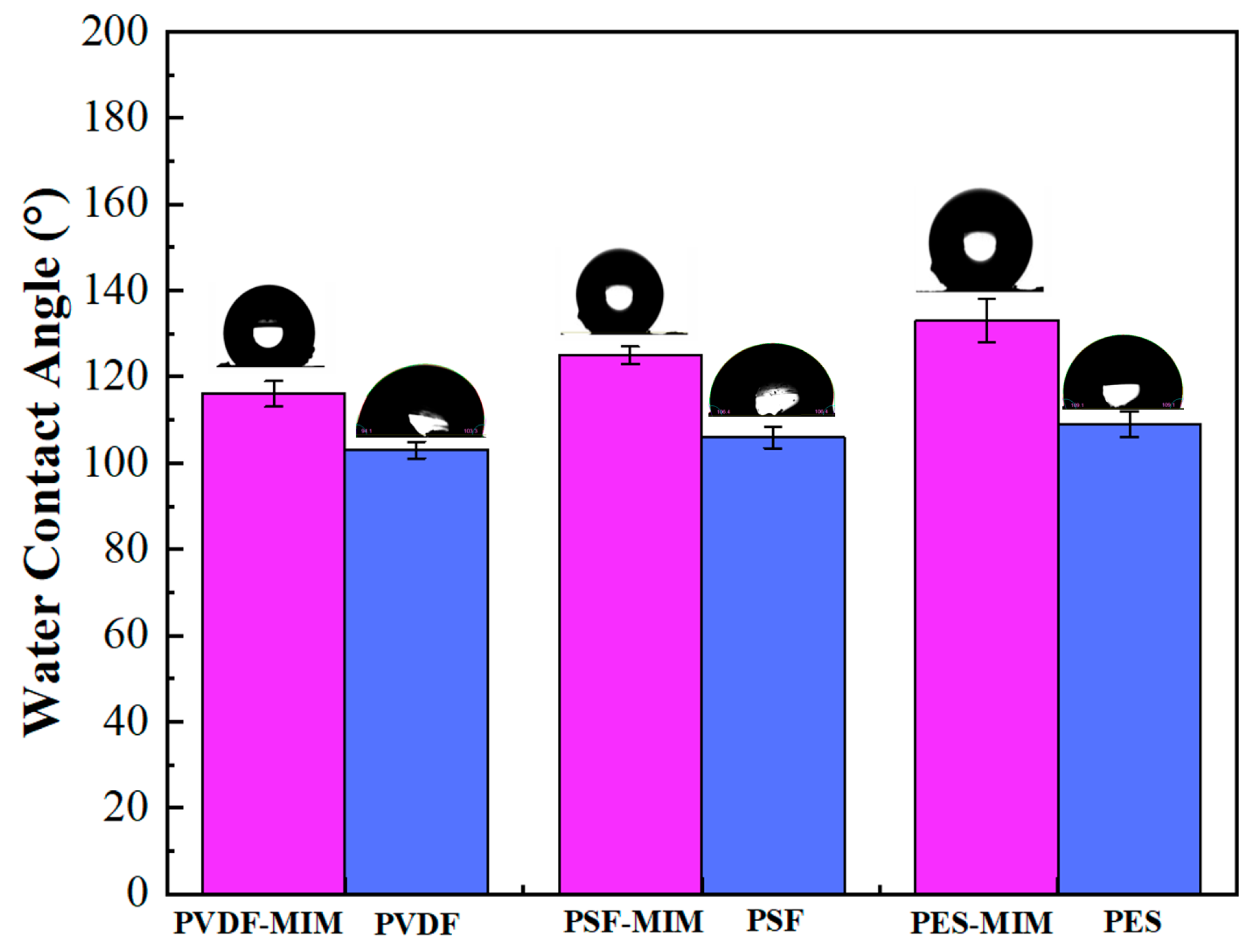
3.2.3. Analysis of Membrane Pore Size, Membrane Flux, and Contact Angle
3.3. Adsorption Performance
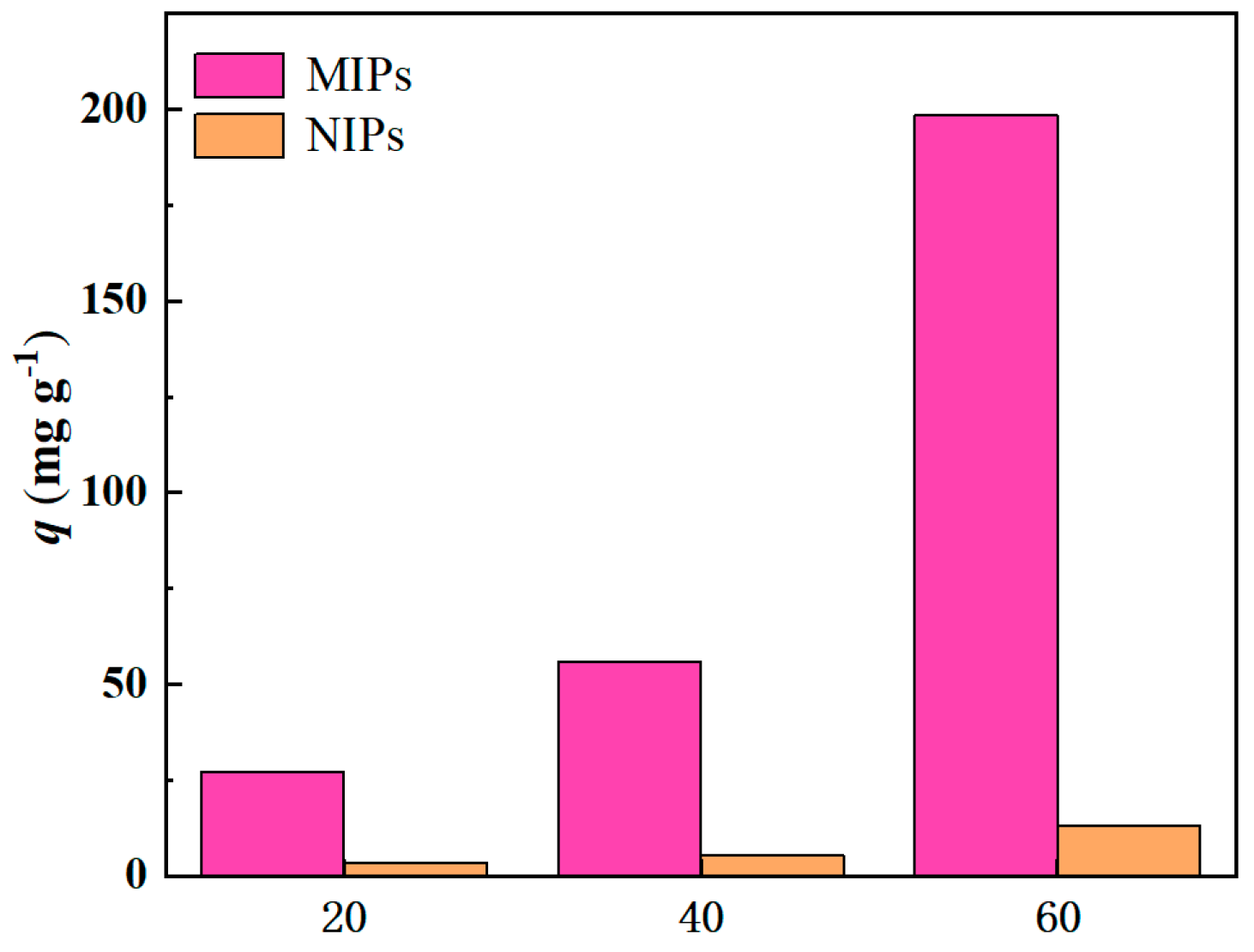
3.3.1. The Best Adsorption Concentration
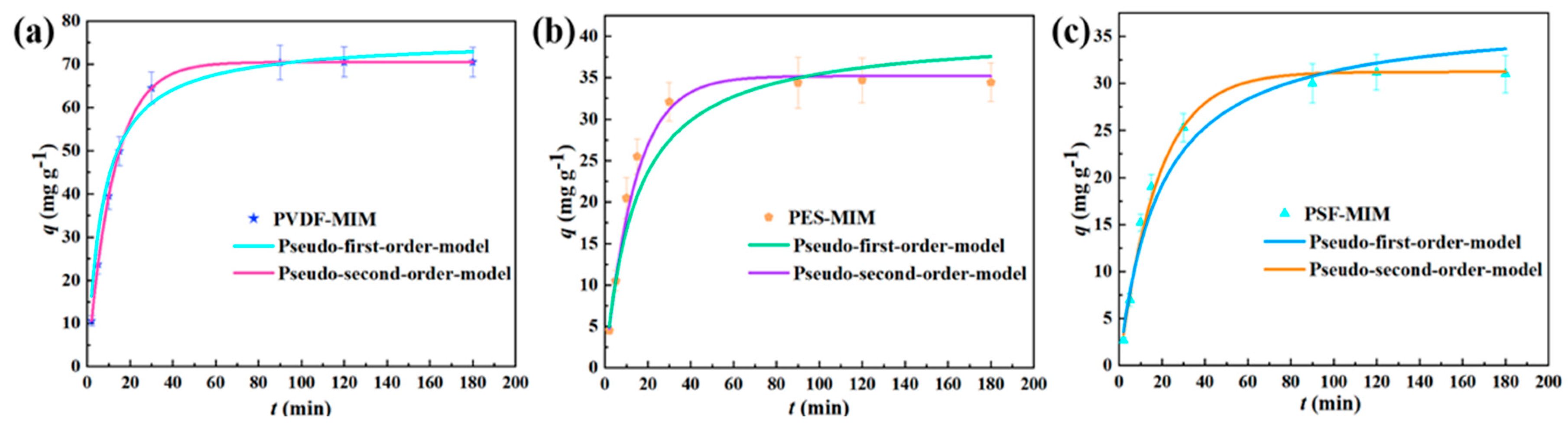
3.3.2. Adsorption Kinetics
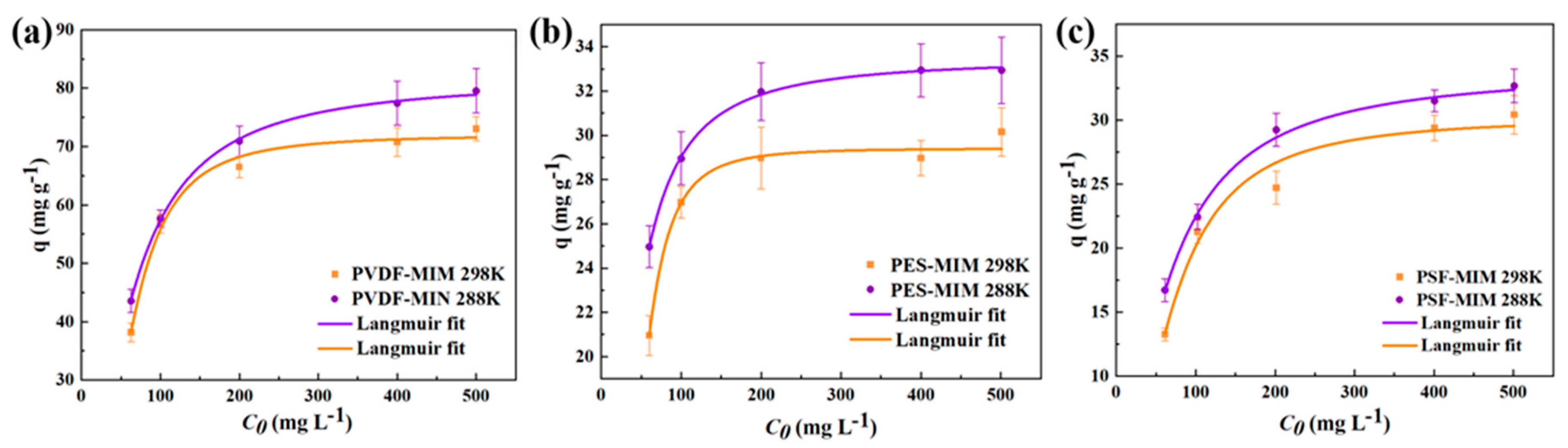
3.3.3. Sorption Isotherm
3.3.4. Thermodynamics of Adsorption
3.4. Adsorption Selectivity
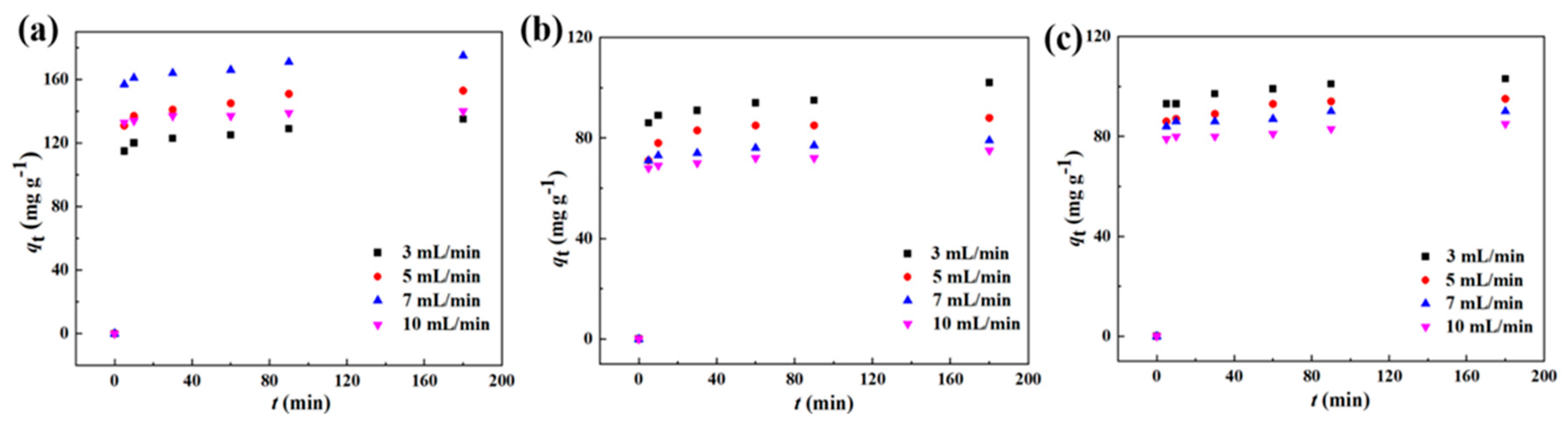
3.5. Dynamic Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laminou, I.M.; Issa, I.; Adehossi, E.; Maman, K.; Jackou, H.; Coulibaly, E.; Tohon, Z.B.; Ahmed, J.; Sanoussi, E.; Koko, D. Therapeutic efficacy and tolerability of artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Nige. Malar. J. 2024, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Bonepally, K.R.; Takahashi, N.; Matsuoka, N.; Koi, H.; Mizoguchi, H.; Hiruma, T.; Ochiai, K.; Suzuki, S.; Yamagishi, Y.; Oikawa, H.; et al. Rapid and systematic exploration of chemical space relevant to Artemisinins: An-ti-malarial Activities of Skeletally Diversified Tetracyclic Peroxides and 6-Aza-artemisinins. J. Org. Chem. 2020, 85, 9694–9712. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2011, 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Monatshefte Fuer Chemie/Chem. Mon. 2021, 152, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Esu, E.B.; Effa, E.E.; Opie, O.N.; Meremikwu, M.M. Artemether for severe malaria. Emerg. Rev. De La Soc. Esp. De Med. De Emerg. 2020, 32, 131–132. [Google Scholar]
- Wang, M.; Liu, X.; Zheng, X.; Luo, Y.; Gao, Y.; Chen, H. Synthesis of Artemisinin G from Artemisinin via Photocatalysis. Eur. J. Org. Chem. 2024, 27, e202301286. [Google Scholar] [CrossRef]
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Perez-Criado, S.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 2017, 40, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Komartin, R.S.; Stroescu, M.; Chira, N.; Stan, R.; Stoica-Guzun, A. Optimization of oil extraction from Lallemantia iberica seeds using ultra-sound-assisted extraction. J. Food Meas. Charact. 2021, 15, 2010–2020. [Google Scholar] [CrossRef]
- Lou, X.; Zhu, A.; Wang, H.; Wu, J.; Zhou, L.; Long, F. Direct and ultrasensitive optofluidic-based immunosensing assay of aflatoxin M1 in dairy products using organic solvent extraction. Anal. Chim. Acta 2016, 940, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Mass transfer and kinetic modelling of supercritical CO2 extraction of fresh tea leaves (Camellia sinensis L). Braz. J. Chem. Eng. 2017, 34, 799–810. [Google Scholar] [CrossRef]
- Zu, Y.G.; Wang, L.; Zhao, X.; Li, Y.; Wu, W.; Zu, C.; Huang, Y.; Wu, M.; Feng, Z. Purification of Ginkgo biloba extract by antisolvent recrystallization. Chem. Eng. Technol. 2016, 39, 1301–1308. [Google Scholar] [CrossRef]
- Uno, T.; Niioka, T.; Hayakari, M.; Sugawara, K.; Tateishi, T. Simultaneous determination of warfarin enantiomers and its metabolite in human plasma by column-switching high-performance liquid chromatography with chiral separation. Ther. Drug Monit. 2007, 29, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Masakazu, Y.; Kalsang, T.; Ştefan-Ovidiu, D. Molecularly imprinted membranes: Past, present, and future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar]
- Yang, H.; Liu, H.-B.; Tang, Z.-S.; Qiu, Z.-D.; Zhu, H.-X.; Song, Z.-X.; Jia, A.-L. Synthesis, performance, and application of molecularly imprinted membranes: A review. J. Environ. Chem. Eng. 2021, 9, 106352. [Google Scholar] [CrossRef]
- Chen, J.; Wei, M.; Meng, M. Advanced Development of Molecularly Imprinted Membranes for Selective Separation. Molecules 2023, 28, 5764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, S.; Wang, H.; Wang, J.; Xiao, S. Research progress and application prospects of molecularly imprinted membrane technology: A review. Mater. Res. Innov. 2023, 27, 449–463. [Google Scholar] [CrossRef]
- Bi, Y.; Dong, J.; Zhou, Y.; Zhang, M.; Chen, X.; Zhang, Y. Application of membrane separation technology in the purification of pharmaceutical components. Prep. Biochem. Biotechnol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The application of membrane separation technology in the pharmaceutical industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Song, J.; Yan, Y.; Gao, J.; Xing, W.; Meng, M.; Yan, Y.; Ma, Z.; Wu, Y. A “graphdiyne-like” anti-fouling TBBPA molecularly imprinted membrane synthesized based on the delayed phase inversion method: A concomitant permeability and selectivity. J. Membr. Sci. 2022, 659, 120808. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Liu, X.; Li, C.; Zeng, S.; Wang, H.; Zhang, S. Fabrication of multilayered molecularly imprinted membrane for selective recognition and separation of artemisinin. ACS Sustain. Chem. Eng. 2019, 7, 3127–3137. [Google Scholar] [CrossRef]
- Bian, W.; Zhang, R.; Chen, X.; Zhang, C.; Meng, M. Three-Dimensional Porous PVDF Foam Imprinted Membranes with High Flux and Selectivity toward Artemisinin/Artemether. Molecules 2023, 28, 7452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruan, H.; Zhang, J.; Huang, Y.; Guo, M.; Kong, D.; Luo, J.; Yang, M. Recyclable and selective PVDF-based molecularly imprinted membrane combining mussel-inspired biomimetic strategy for dimethomorph elimination. Chem. Eng. J. 2023, 478, 147322. [Google Scholar] [CrossRef]
- Meng, M.; Li, Y.; Peng, H.; Li, B.; Zhang, C.; Ren, J.; Ren, Q.; Liu, Y.; Pan, J. Hydrophilic imprinted MnO2 nanowires “coating” membrane with ultrahigh adsorption capacity for highly selective separation of Artemisinin/Artemether. Chem. Eng. J. 2023, 466, 143020. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Xue, M.; Han, Q.; Han, X. Enhanced separation properties of polyvinylidene membranes with MnO2 nanowire addition. Mater. Today Commun. 2023, 37, 107479. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, W.; Cao, R. Hydrophilic MnO2 nanowires coating with o-fluoroaniline for electrocatalytic water oxidation. Chin. J. Struct. Chem. 2023, 42, 100105. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, H.; Lee, H.; Lim, G.; Cho, S.J. A pore-size tunable superhydrophobic membrane for high-flux membrane distillation. J. Membr. Sci. 2022, 641, 119862. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Mohamed Idris, Z.; Hameed, B.H.; Ye, L.; Hajizadeh, S.; Mattiasson, B.; Din, A.T.M. Amino-functionalised silica-grafted molecularly imprinted polymers for chloramphenicol adsorption. J. Environ. Chem. Eng. 2020, 8, 103981. [Google Scholar] [CrossRef]
- Abu-Alsoud, G.F.; Hawboldt, K.A.; Bottaro, C.S. Comparison of four adsorption isotherm models for characterizing molecular recognition of individual phenolic compounds in porous tailor-made molecularly imprinted polymer films. ACS Appl. Mater. Interfaces 2020, 12, 11998–12009. [Google Scholar] [CrossRef] [PubMed]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Diankin, I.D.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Zhivulin, E.V. Controlled modification of polyvinylidene fluoride as a way for carbyne synthesis. Polym. Degrad. Stab. 2022, 203, 110054. [Google Scholar] [CrossRef]






| Blended Imprinted Membrane | m/g | m/g | m/g | m/g | m/g |
|---|---|---|---|---|---|
| PVDF | PSF | PES | DMSO | MIPs | |
| PVDF-MIM | 0.42 | - | - | 5.58 | 0.01 |
| PSF-MIM | - | 0.6 | - | 5.40 | 0.01 |
| PES-MIM | - | - | 0.6 | 5.40 | 0.01 |
| Membrane | Pseudo-First-Order Kinetic Equation | Pseudo-Second-Order Kinetic Equation | |||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg g−1) | k1 (min−1) | R2 | qe,exp (mg g−1) | qe,cal (mg g−1) | k2 (g mg−1 min−1) | R2 | |
| PVDF-MIM | 75.85 | 0.0081 | 0.83 | 70.50 | 70.49 | 0.0821 | 0.99 |
| PES-MIM | 40.56 | 0.0017 | 0.80 | 34.60 | 35.10 | 0.0723 | 0.99 |
| PSF-MIM | 37.19 | 0.0014 | 0.81 | 31.20 | 31.23 | 0.0560 | 0.99 |
| Models | Parameter | PVDF-MIM | PES-MIM | PSF-MIM |
|---|---|---|---|---|
| Langmuir model | R2 | 0.99 | 0.99 | 0.99 |
| KL (L mg−1) | 6.69 × 10−6 | 4.42 × 10−5 | 3.94 × 10−4 | |
| qm,cal (mg g−1) | 74.90 | 34.56 | 33.41 | |
| Freundich model | R2 | 0.96 | 0.95 | 0.97 |
| KF (mg g−1) | 78.87 | 40.02 | 43.66 | |
| n | 1.86 | 1.31 | 1.1 |
| Membrane | (kJ mol−1) | |||
|---|---|---|---|---|
| (kJ mol−1) | (kJ mol−1 k−1) | 15 °C | 25 °C | |
| PVDF-MIM | −46.72 ± 2.10 | −0.17 ± 0.03 | −2.81 ± 0.3 | −1.32 ± 0.20 |
| PES-MIM | −2.53 ± 0.32 | −0.012 ± 0.001 | −0.85 ± 0.25 | −0.85 ± 0.15 |
| PSF-MIM | −9.20 ± 1.5 | −0.036 ± 0.004 | −1.31 ± 0.17 | −0.98 ± 0.10 |
| q (mg g−1) | Kd (L g−1) | α | |||
|---|---|---|---|---|---|
| Membrane | ART | ARE | ARE | ARE | |
| PVDF-MIM | 70.1 ± 5.5 | 22.0 ± 1.8 | 2.344 ± 0.30 | 0.28 ± 0.02 | 8.37 ± 1.01 |
| PES-MIM | 30.3 ± 2.1 | 39.8 ± 2.7 | 0.435 ± 0.09 | 0.661 ± 0.04 | 0.66 ± 0.10 |
| PSF-MIM | 29.2 ± 2.5 | 33.1 ± 2.5 | 0.41 ± 0.05 | 0.49 ± 0.03 | 0.84 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, M.; Ren, J.; Zhang, C.; Du, W.; Wang, J. Polyvinylidene Fluoride-Based Nanowire-Imprinted Membranes with High Flux for Efficient and Selective Separation of Artemisinin/Artemether. Molecules 2024, 29, 3868. https://doi.org/10.3390/molecules29163868
Meng M, Ren J, Zhang C, Du W, Wang J. Polyvinylidene Fluoride-Based Nanowire-Imprinted Membranes with High Flux for Efficient and Selective Separation of Artemisinin/Artemether. Molecules. 2024; 29(16):3868. https://doi.org/10.3390/molecules29163868
Chicago/Turabian StyleMeng, Minjia, Jiajia Ren, Chuanxun Zhang, Wanqi Du, and Jixiang Wang. 2024. "Polyvinylidene Fluoride-Based Nanowire-Imprinted Membranes with High Flux for Efficient and Selective Separation of Artemisinin/Artemether" Molecules 29, no. 16: 3868. https://doi.org/10.3390/molecules29163868
APA StyleMeng, M., Ren, J., Zhang, C., Du, W., & Wang, J. (2024). Polyvinylidene Fluoride-Based Nanowire-Imprinted Membranes with High Flux for Efficient and Selective Separation of Artemisinin/Artemether. Molecules, 29(16), 3868. https://doi.org/10.3390/molecules29163868








